Abstract
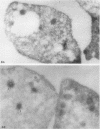
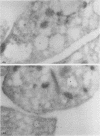
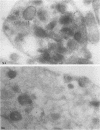
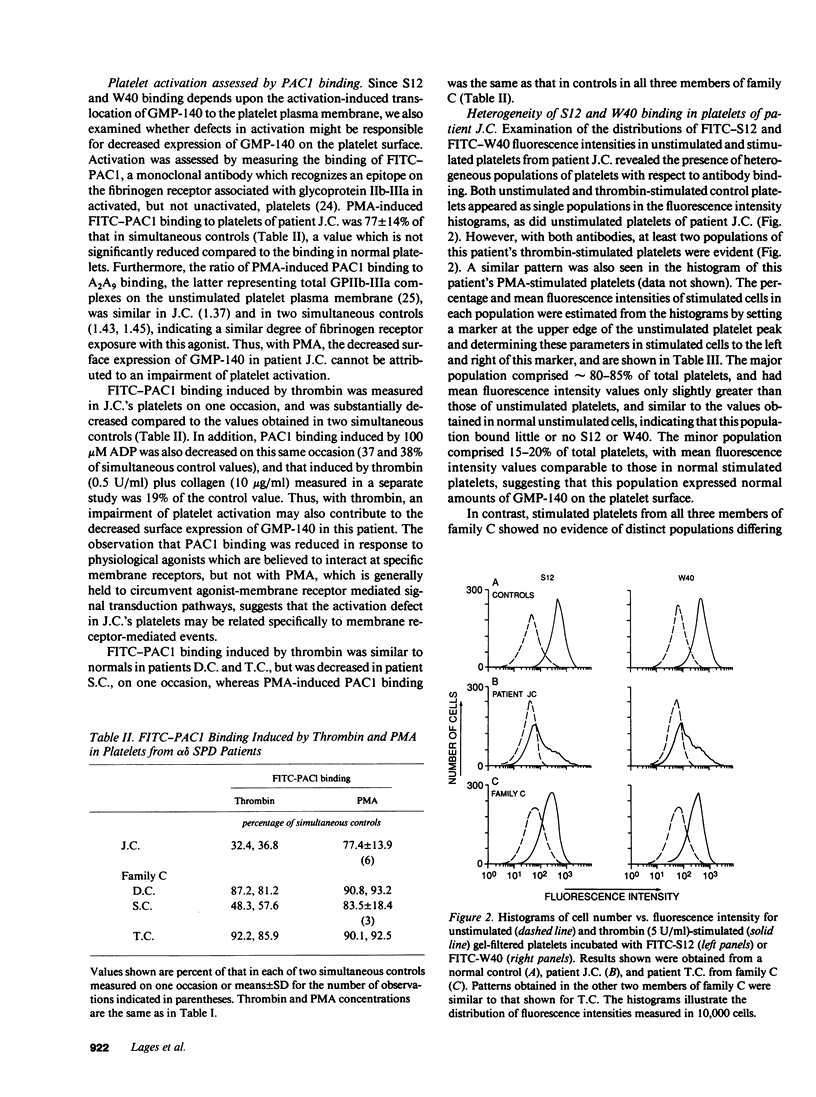
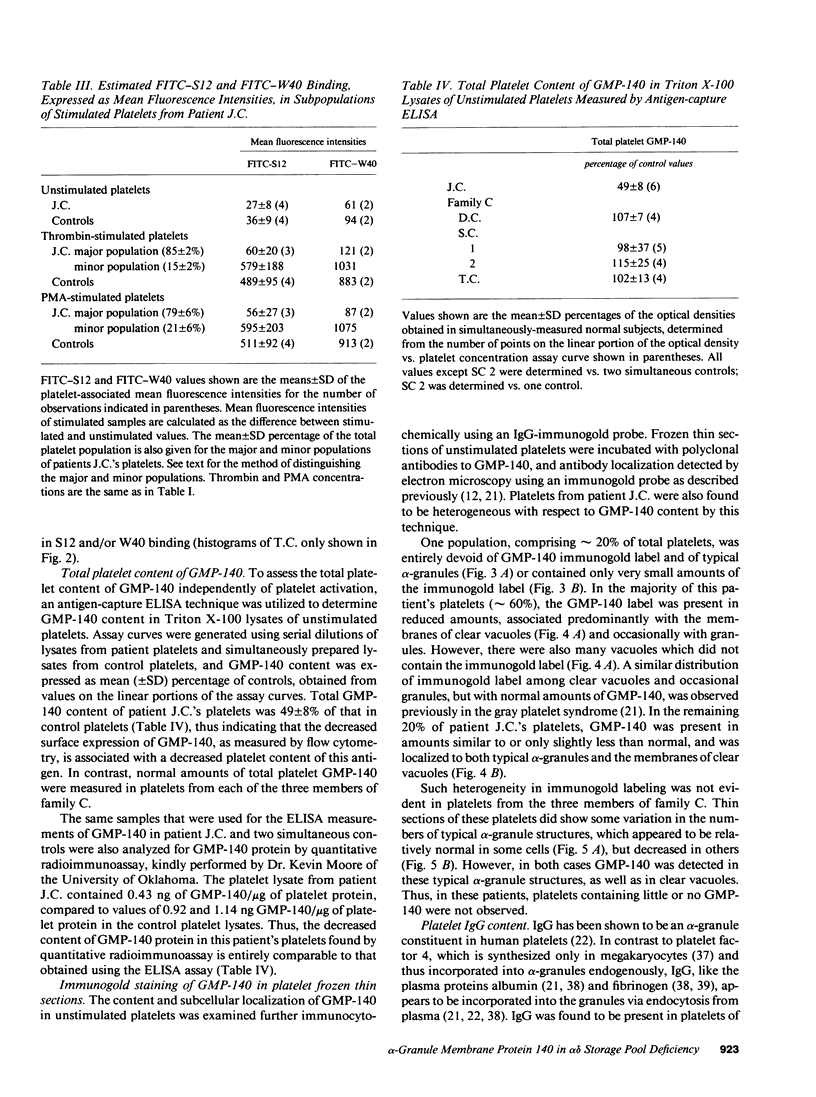
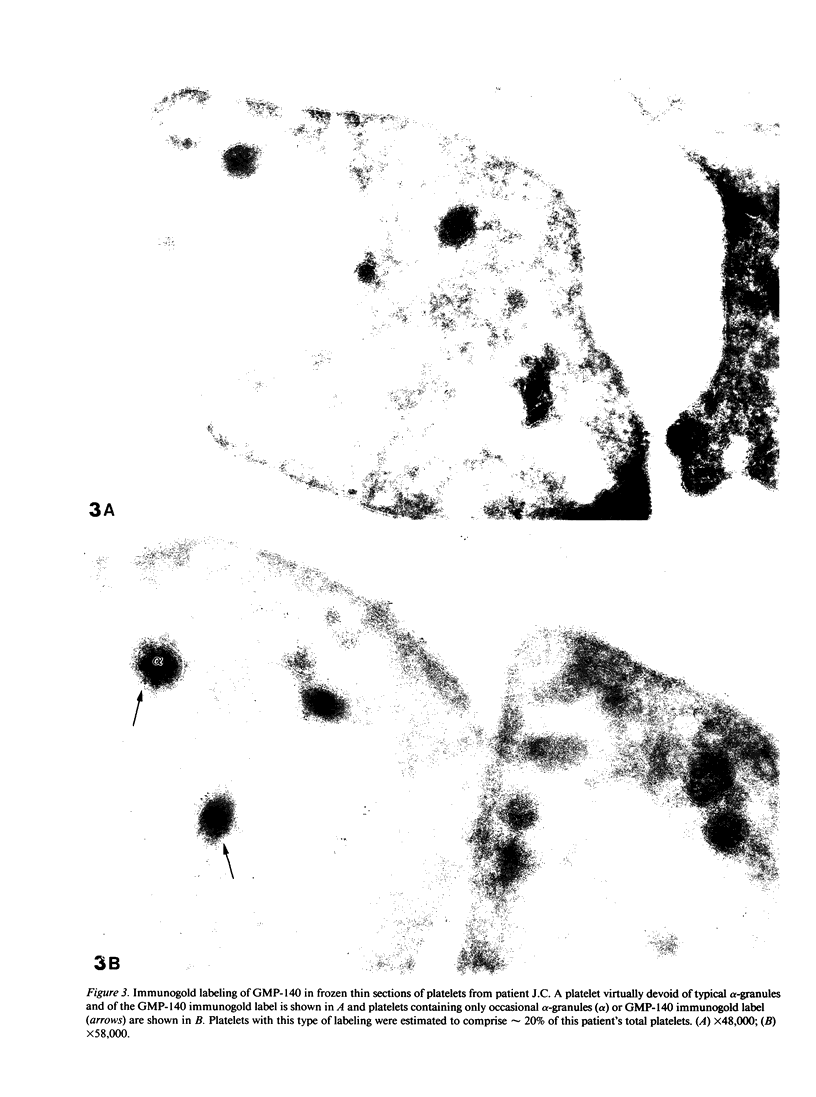
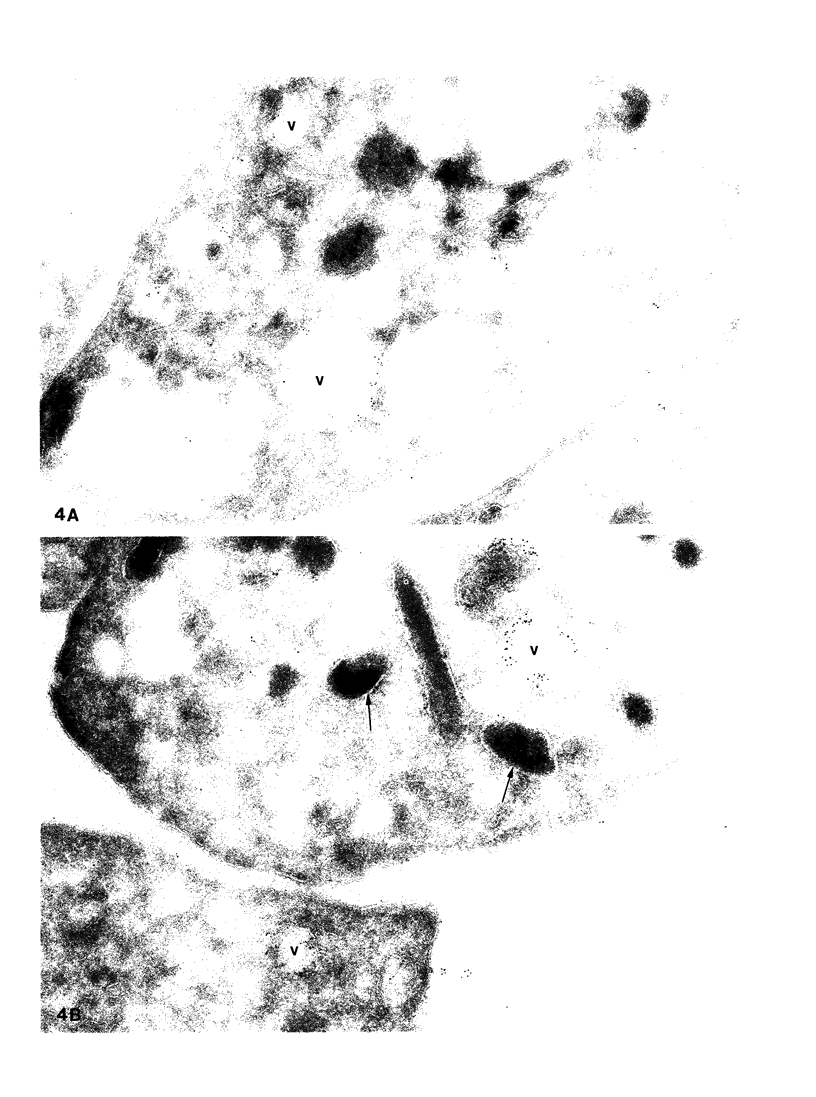
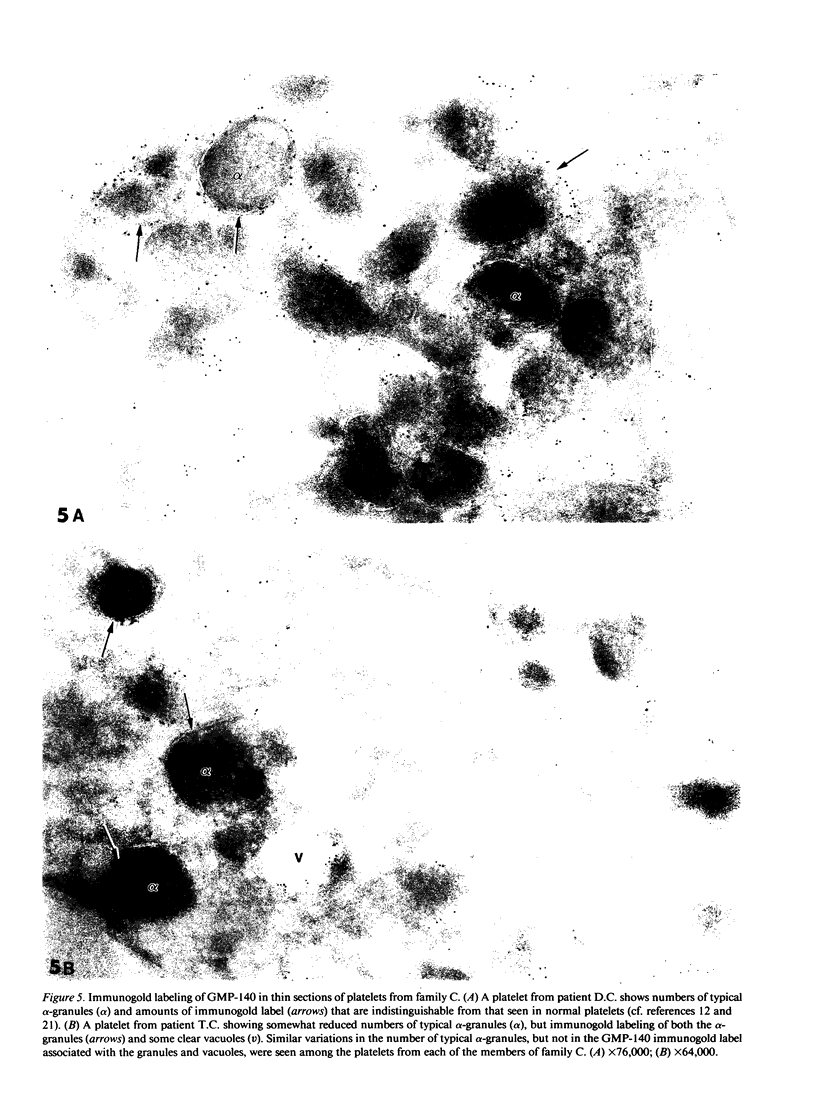
To determine whether alpha-granule membranes are present in platelets of patients with storage pool deficiencies of both alpha and dense granules (alpha delta-SPD), we examined the content and surface expression of the alpha-granule membrane protein GMP-140 in one patient (J.C.) with a severe alpha-granule deficiency and in three members of a family (family C) with milder alpha-granule deficiencies. Surface expression of GMP-140 in stimulated platelets, assessed by flow cytometric measurements of the binding of two anti-GMP-140 monoclonal antibodies, was 24-38% of normal values in platelets from patient J.C., vs. 60-95% of normal values in family C. Total platelet content of GMP-140, determined in platelet lysates by antigen-capture ELISA, was 49% of normal in patient J.C., but normal in the members of family C. Platelets of patient J.C. were found to be heterogeneous with respect to GMP-140 content and surface expression by both flow cytometry and immunogold electron microscopy. Approximately 80% of her platelets expressed little or no GMP-140 after stimulation, whereas the remaining 20% expressed normal amounts of GMP-140 and showed extensive immunogold labeling of typical alpha-granules and clear vacuoles. No such heterogeneity was found in platelets from family C. These findings in the severe alpha delta-SPD patient are in clear contrast to the observations of normal GMP-140 content in the three other alpha delta-SPD patients, and in patients with the gray platelet syndrome, reported previously by others. These results illustrate the phenotypic heterogeneity of alpha-granule deficiencies in human platelets, and suggest that a defect in granule formation in the megakaryocytes may account for the alpha-granule defect in at least one form of alpha delta-SPD.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Friedlander L. M., Shohet S. B. Abnormalities in granule formation in acute myelogenous leukemia. Blood. 1977 May;49(5):693–704. [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead J. H., Stenberg P. E., McEver R. P., Shuman M. A., Bainton D. F. Immunohistochemical localization of membrane and alpha-granule proteins in human megakaryocytes: application to plastic-embedded bone marrow biopsy specimens. Blood. 1986 Feb;67(2):285–293. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman C. L., Yeo E. L., Wencel-Drake J. D., Furie B. C., Ginsberg M. H., Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986 Jul;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Saucerman S., Levine S. P., Knieriem L. K., Bainton D. F. Immunoglobulin G is a platelet alpha granule-secreted protein. J Clin Invest. 1985 Nov;76(5):2020–2025. doi: 10.1172/JCI112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger S. A., McEver R. P. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990 Feb 1;75(3):550–554. [PubMed] [Google Scholar]
- Handagama P. J., Shuman M. A., Bainton D. F. Incorporation of intravenously injected albumin, immunoglobulin G, and fibrinogen in guinea pig megakaryocyte granules. J Clin Invest. 1989 Jul;84(1):73–82. doi: 10.1172/JCI114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Setkowsky C. A., Lages B., Day H. J., Weiss H. J., Scrutton M. C. Content and thrombin-induced release of acid hydrolases in gel-filtered platelets from patients with storage pool disease. Blood. 1975 Jul;46(1):131–142. [PubMed] [Google Scholar]
- Holmsen H., Weiss H. J. Further evidence for a deficient storage pool of adenine nucleotides in platelets from some patients with thrombocytopathia--"storage pool disease". Blood. 1972 Feb;39(2):197–209. [PubMed] [Google Scholar]
- Holmsen H., Weiss H. J. Hereditary defect in the platelet release reaction caused by a deficiency in the storage pool of platelet adenine nucleotides. Br J Haematol. 1970 Nov;19(5):643–649. doi: 10.1111/j.1365-2141.1970.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T., Fishback B., Johnson M. M., Egan J. J., Weiss H. J. Platelet membrane glycoproteins in thrombasthenia, Bernard-Soulier syndrome, and storage pool disease. J Lab Clin Med. 1979 Apr;93(4):652–660. [PubMed] [Google Scholar]
- Johnston G. I., Kurosky A., McEver R. P. Structural and biosynthetic studies of the granule membrane protein, GMP-140, from human platelets and endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1816–1823. [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Lages B., Dangelmaier C. A., Holmsen H., Weiss H. J. Specific correction of impaired acid hydrolase secretion in storage pool-deficient platelets by adenosine diphosphate. J Clin Invest. 1988 Jun;81(6):1865–1872. doi: 10.1172/JCI113532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E., Celi A., Gilbert G. E., Furie B. C., Erban J. K., Bonfanti R., Wagner D. D., Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989 Oct 20;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- Levy-Toledano S., Caen J. P., Breton-Gorius J., Rendu F., Cywiner-Golenzer C., Dupuy E., Legrand Y., Maclouf J. Gray platelet syndrome: alpha-granule deficiency. Its influence on platelet function. J Lab Clin Med. 1981 Dec;98(6):831–848. [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- Rosa J. P., George J. N., Bainton D. F., Nurden A. T., Caen J. P., McEver R. P. Gray platelet syndrome. Demonstration of alpha granule membranes that can fuse with the cell surface. J Clin Invest. 1987 Oct;80(4):1138–1146. doi: 10.1172/JCI113171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo R., Nakeff A., Huang S. S., Ginsberg M., Deuel T. F. New synthesis of a platelet-specific protein: platelet factor 4 synthesis in a megakaryocyte-enriched rabbit bone marrow culture system. J Cell Biol. 1983 Feb;96(2):515–520. doi: 10.1083/jcb.96.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrit H. F., Weiss H. J., Phillips G. B. The phospholipid and fatty acid composition of platelets in patients with primary defects of platelet function. Lipids. 1972 Jan;7(1):60–67. doi: 10.1007/BF02531271. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Walsh P. N. Albumin density gradient separation and washing of platelets and the study of platelet coagulant activities. Br J Haematol. 1972 Feb;22(2):205–217. doi: 10.1111/j.1365-2141.1972.tb08801.x. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Ames R. P. Ultrastructural findings in storage pool disease and aspirin-like defects of platelets. Am J Pathol. 1973 Jun;71(3):447–466. [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Chervenick P. A., Zalusky R., Factor A. A familialdefect in platelet function associated with imapired release of adenosine diphosphate. N Engl J Med. 1969 Dec 4;281(23):1264–1270. doi: 10.1056/NEJM196912042812303. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Lages B. Platelet malondialdehyde production and aggregation responses induced by arachidonate, prostaglandin-G2, collagen, and epinephrine in 12 patients with storage pool deficiency. Blood. 1981 Jul;58(1):27–33. [PubMed] [Google Scholar]
- Weiss H. J., Lages B. The response of platelets to epinephrine in storage pool deficiency--evidence pertaining to the role of adenosine diphosphate in mediating primary and secondary aggregation. Blood. 1988 Nov;72(5):1717–1725. [PubMed] [Google Scholar]
- Weiss H. J., Pietu G., Rabinowitz R., Girma J. P., Rogers J., Meyer D. Heterogeneous abnormalities in the multimeric structure, antigenic properties, and plasma-platelet content of factor VIII/von Willebrand factor in subtypes of classic (type I) and variant (type IIA) von Willebrand's disease. J Lab Clin Med. 1983 Mar;101(3):411–425. [PubMed] [Google Scholar]
- Weiss H. J., Rogers J. Thrombocytopathia due to abnormalities in platelet release reaction--studies on six unrelated patients. Blood. 1972 Feb;39(2):187–196. [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Platelet adhesion and thrombus formation on subendothelium in platelets deficient in glycoproteins IIb-IIIa, Ib, and storage granules. Blood. 1986 Feb;67(2):322–330. [PubMed] [Google Scholar]
- Weiss H. J., Witte L. D., Kaplan K. L., Lages B. A., Chernoff A., Nossel H. L., Goodman D. S., Baumgartner H. R. Heterogeneity in storage pool deficiency: studies on granule-bound substances in 18 patients including variants deficient in alpha-granules, platelet factor 4, beta-thromboglobulin, and platelet-derived growth factor. Blood. 1979 Dec;54(6):1296–1319. [PubMed] [Google Scholar]
- White J. G. Platelet granule disorders. Crit Rev Oncol Hematol. 1986;4(4):337–377. doi: 10.1016/s1040-8428(86)80027-0. [DOI] [PubMed] [Google Scholar]
- White J. G. Ultrastructural studies of the gray platelet syndrome. Am J Pathol. 1979 May;95(2):445–462. [PMC free article] [PubMed] [Google Scholar]
- Yeo E., Furie B. C., Furie B. PADGEM protein in human erythroleukemia cells. Blood. 1989 Feb 15;73(3):722–728. [PubMed] [Google Scholar]