Abstract
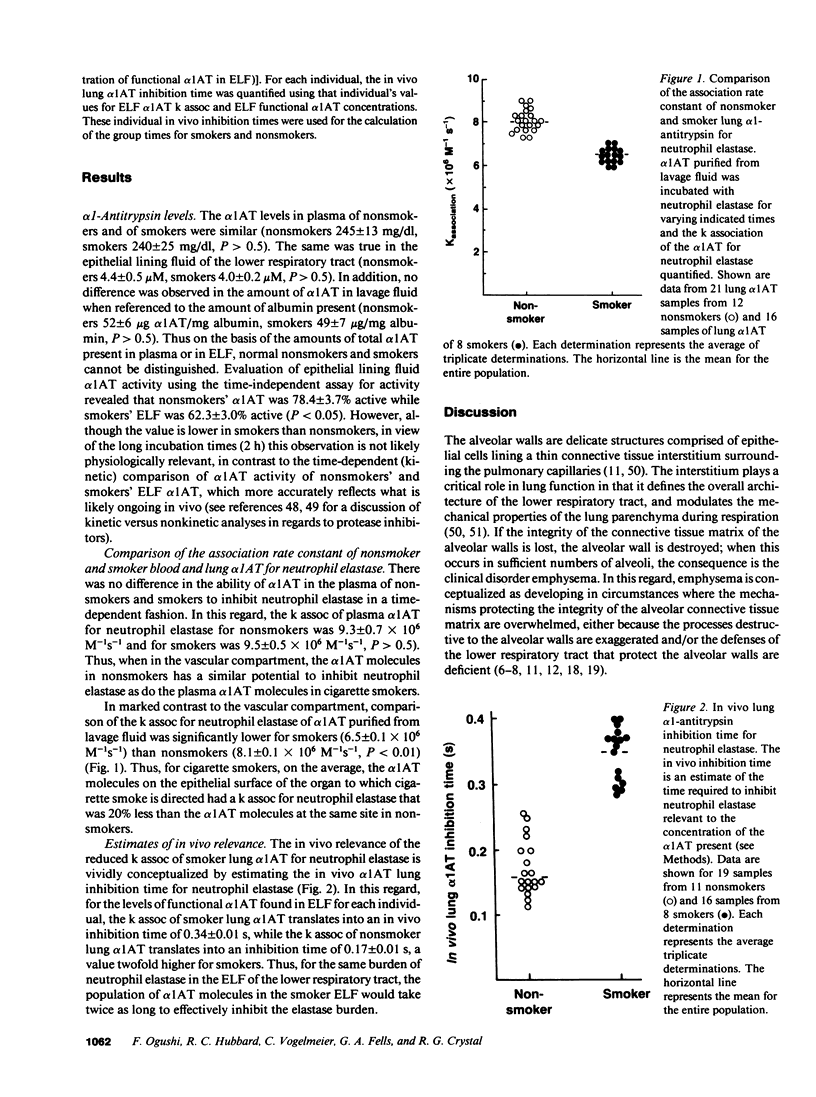
The increased risk of developing emphysema among individuals who smoke cigarettes and who have normal levels of alpha 1-antitrypsin (alpha 1AT) is hypothesized to result from a decrease in the antineutrophil elastase capacity of the lower respiratory tract alpha 1AT of smokers compared with nonsmokers. To evaluate this hypothesis we compared the time-dependent kinetics of the inhibition of neutrophil elastase by lung alpha 1AT from healthy, young cigarette smokers (n = 8) and nonsmokers (n = 12). alpha 1-antitrypsin was purified from lavage fluid using affinity and molecular sieve chromatography, and the association rate constant (k assoc) for neutrophil elastase quantified. The k assoc of smoker plasma alpha 1AT (9.5 +/- 0.5 X 10(6) M-1s-1) was similar to that of nonsmoker plasma (9.3 +/- 0.7 X 10(6) M-1s-1, P greater than 0.5). In marked contrast, the k assoc of smoker lower respiratory tract alpha 1AT was significantly lower than that of nonsmoker alpha 1AT (6.5 +/- 0.4 X 10(6) M-1s-1 vs. 8.1 +/- 0.5 X 10(6) M-1s-1, P less than 0.01). Furthermore, the smoker lower respiratory tract alpha 1AT k assoc was significantly less than that of autologous plasma (P less than 0.01). When considered in the context of the concentration of alpha 1AT in the lower respiratory tract epithelial lining fluid, the inhibition time for neutrophil elastase of smoker lung alpha 1AT was twofold greater than that of nonsmoker lung alpha 1AT (smoker: 0.34 +/- 0.05 s vs. nonsmoker: 0.17 +/- 0.05 s, P less than 0.01). Consequently, for concentrations of alpha 1AT in the lower respiratory tract it takes twice as long for an equivalent amount of neutrophil elastase to be inhibited in the smoker's lung compared with the nonsmoker's lung. These observations support the concept that cigarette smoking is associated with a decrease in the lower respiratory tract neutrophil elastase inhibitory capacity, thus increasing the vulnerability of the lung to elastolytic destruction and thereby increasing the risk for the development of emphysema.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud R. T., Fera T., Richter A., Tabona M. Z., Johal S. Acute effect of smoking on the functional activity of alpha1-protease inhibitor in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1985 Jan;131(1):79–85. doi: 10.1164/arrd.1985.131.1.79. [DOI] [PubMed] [Google Scholar]
- Auerbach O., Hammond E. C., Garfinkel L., Benante C. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972 Apr 20;286(16):853–857. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Beatty K., Matheson N., Travis J. Kinetic and chemical evidence for the inability of oxidized alpha 1-proteinase inhibitor to protect lung elastin from elastolytic degradation. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):731–736. doi: 10.1515/bchm2.1984.365.2.731. [DOI] [PubMed] [Google Scholar]
- Bieth J. G. In vivo significance of kinetic constants of protein proteinase inhibitors. Biochem Med. 1984 Dec;32(3):387–397. doi: 10.1016/0006-2944(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Bieth J. G. Pathophysiological interpretation of kinetic constants of protease inhibitors. Bull Eur Physiopathol Respir. 1980;16 (Suppl):183–197. doi: 10.1016/b978-0-08-027379-2.50020-x. [DOI] [PubMed] [Google Scholar]
- Boudier C., Pelletier A., Pauli G., Bieth J. G. The functional activity of alpha 1-proteinase inhibitor in bronchoalveolar lavage fluids from healthy human smokers and non-smokers. Clin Chim Acta. 1983 Aug 31;132(3):309–315. doi: 10.1016/0009-8981(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Bruce R. M., Cohen B. H., Diamond E. L., Fallat R. J., Knudson R. J., Lebowitz M. D., Mittman C., Patterson C. D., Tockman M. S. Collaborative study to assess risk of lung disease in Pi MZ phenotype subjects. Am Rev Respir Dis. 1984 Sep;130(3):386–390. doi: 10.1164/arrd.1984.130.3.386. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Miller F., Hoidal J. R., Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Cohen A. B., James H. L. Reduction of the elastase inhibitory capacity of alpha 1-antitrypsin by peroxides in cigarette smoke: an analysis of brands and filters. Am Rev Respir Dis. 1982 Jul;126(1):25–30. doi: 10.1164/arrd.1982.126.1.25. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Damiano V. V., Tsang A., Kucich U., Abrams W. R., Rosenbloom J., Kimbel P., Fallahnejad M., Weinbaum G. Immunolocalization of elastase in human emphysematous lungs. J Clin Invest. 1986 Aug;78(2):482–493. doi: 10.1172/JCI112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS P., PFITZER E. A., TOLKER E., BABYAK M. A., KASCHAK M. EXPERIMENTAL EMPHYSEMA: ITS PRODUCTION WITH PAPAIN IN NORMAL AND SILICOTIC RATS. Arch Environ Health. 1965 Jul;11:50–58. doi: 10.1080/00039896.1965.10664169. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Crystal R. G. The connective tissue of lung. Am Rev Respir Dis. 1975 Nov;112(5):657–711. doi: 10.1164/arrd.1975.112.5.657. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones P., Whimster W. The etiology and management of disabling emphysema. Am Rev Respir Dis. 1978 Feb;117(2):343–378. doi: 10.1164/arrd.1978.117.2.343. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Cigarette smoking and lung destruction. Accumulation of neutrophils in the lungs of cigarette smokers. Am Rev Respir Dis. 1983 Nov;128(5):833–838. doi: 10.1164/arrd.1983.128.5.833. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H., Lee D. K., Drew R. T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979 Dec 14;206(4424):1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H. Possible mechanisms of emphysema in smokers: cigarette smoke condensate suppresses protease inhibition in vitro. Am Rev Respir Dis. 1977 Jul;116(1):65–72. doi: 10.1164/arrd.1977.116.1.65. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Janoff A., George-Nascimento C., Rosenberg S. A genetically engineered, mutant human alpha-1-proteinase inhibitor is more resistant than the normal inhibitor to oxidative inactivation by chemicals, enzymes, cells, and cigarette smoke. Am Rev Respir Dis. 1986 Mar;133(3):353–356. doi: 10.1164/arrd.1986.133.3.353. [DOI] [PubMed] [Google Scholar]
- Janoff A., Raju L., Dearing R. Levels of elastase activity in bronchoalveolar lavage fluids of healthy smokers and nonsmokers. Am Rev Respir Dis. 1983 May;127(5):540–544. doi: 10.1164/arrd.1983.127.5.540. [DOI] [PubMed] [Google Scholar]
- Janoff A., White R., Carp H., Harel S., Dearing R., Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979 Oct;97(1):111–136. [PMC free article] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Fagerhol M. Properties of isolated human alpha1-antitrypsins of Pi types M, S and Z. Eur J Biochem. 1978 Feb 1;83(1):143–153. doi: 10.1111/j.1432-1033.1978.tb12078.x. [DOI] [PubMed] [Google Scholar]
- Jochum M., Pelletier A., Boudier C., Pauli G., Bieth J. G. The concentration of leukocyte elastase-alpha 1-proteinase inhibitor complex in bronchoalveolar lavage fluids from healthy human subjects. Am Rev Respir Dis. 1985 Oct;132(4):913–914. doi: 10.1164/arrd.1985.132.4.913. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Karlinsky J. B., Snider G. L. Animal models of emphysema. Am Rev Respir Dis. 1978 Jun;117(6):1109–1133. doi: 10.1164/arrd.1978.117.6.1109. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Black L. F. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis. 1974 Aug;110(2):176–194. doi: 10.1164/arrd.1974.110.2.176. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Pierce J., Persson U., Thulin E. Purification of alpha1-antitrypsin from plasma through thiol-disulfide interchange. Eur J Biochem. 1975 Sep 1;57(1):107–113. doi: 10.1111/j.1432-1033.1975.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Ludwig P. W., Schwartz B. A., Hoidal J. R., Niewoehner D. E. Cigarette smoking causes accumulation of polymorphonuclear leukocytes in alveolar septum. Am Rev Respir Dis. 1985 Jun;131(6):828–830. doi: 10.1164/arrd.1985.131.6.828. [DOI] [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. O., Lebowitz M. D., Knudson R. J., Burrows B. A community study of the relation of alpha1-antitrypsin levels to obstructive lung diseases. N Engl J Med. 1975 Feb 6;292(6):278–281. doi: 10.1056/NEJM197502062920602. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., Rice D. B. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974 Oct 10;291(15):755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- Olsen G. N., Harris J. O., Castle J. R., Waldman R. H., Karmgard H. J. Alpha-1-antitrypsin content in the serum, alveolar macrophages, and alveolar lavage fluid of smoking and nonsmoking normal subjects. J Clin Invest. 1975 Feb;55(2):427–430. doi: 10.1172/JCI107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Richter A. M., Abboud R. T., Johal S. S., Fera T. A. Acute effect of smoking on superoxide production by pulmonary alveolar macrophages. Lung. 1986;164(4):233–242. doi: 10.1007/BF02713647. [DOI] [PubMed] [Google Scholar]
- Saltini C., Hance A. J., Ferrans V. J., Basset F., Bitterman P. B., Crystal R. G. Accurate quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Oct;130(4):650–658. doi: 10.1164/arrd.1984.130.4.650. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Calore J. D., McGowan S. E., Bernardo J., Snider G. L., Franzblau C. Functional alpha 1-protease inhibitor in the lower respiratory tract of cigarette smokers is not decreased. Science. 1983 Sep 16;221(4616):1187–1189. doi: 10.1126/science.6612333. [DOI] [PubMed] [Google Scholar]
- Stone P. J. The elastase-antielastase hypothesis of the pathogenesis of emphysema. Clin Chest Med. 1983 Sep;4(3):405–412. [PubMed] [Google Scholar]
- Straus S. D., Fells G. A., Wewers M. D., Courtney M., Tessier L. H., Tolstoshev P., Lecocq J. P., Crystal R. G. Evaluation of recombinant DNA-directed E.coli produced alpha 1-antitrypsin as an anti-neutrophil elastase for potential use as replacement therapy of alpha 1-antitrypsin deficiency. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1177–1184. doi: 10.1016/0006-291x(85)91739-5. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Hayakawa S., Adachi T., Ito Y., Hirano K., Sawaki S. A simple one-step purification of human alpha 1-proteinase inhibitor by immunoadsorbent column chromatography. J Biochem Biophys Methods. 1981 Dec;5(5):243–249. doi: 10.1016/0165-022x(81)90034-8. [DOI] [PubMed] [Google Scholar]
- The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985 Jul;132(1):182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Herzyk D. J., Gadek J. E. Comparison of smoker and nonsmoker lavage fluid for the rate of association with neutrophil elastase. Am J Respir Cell Mol Biol. 1989 Nov;1(5):423–429. doi: 10.1165/ajrcmb/1.5.423. [DOI] [PubMed] [Google Scholar]
- Zaslow M. C., Clark R. A., Stone P. J., Calore J. D., Snider G. L., Franzblau C. Human neutrophil elastase does not bind to alpha 1-protease inhibitor that has been exposed to activated human neutrophils. Am Rev Respir Dis. 1983 Sep;128(3):434–439. doi: 10.1164/arrd.1983.128.3.434. [DOI] [PubMed] [Google Scholar]
- el Yamani J., Hayem A., Lafitte J. J., Gressier B., Mizon J. Functional and immunoreactive alpha 1 proteinase inhibitor in bronchoalveolar lavages: methodological studies. Bull Eur Physiopathol Respir. 1986 Jul-Aug;22(4):359–363. [PubMed] [Google Scholar]


