Abstract
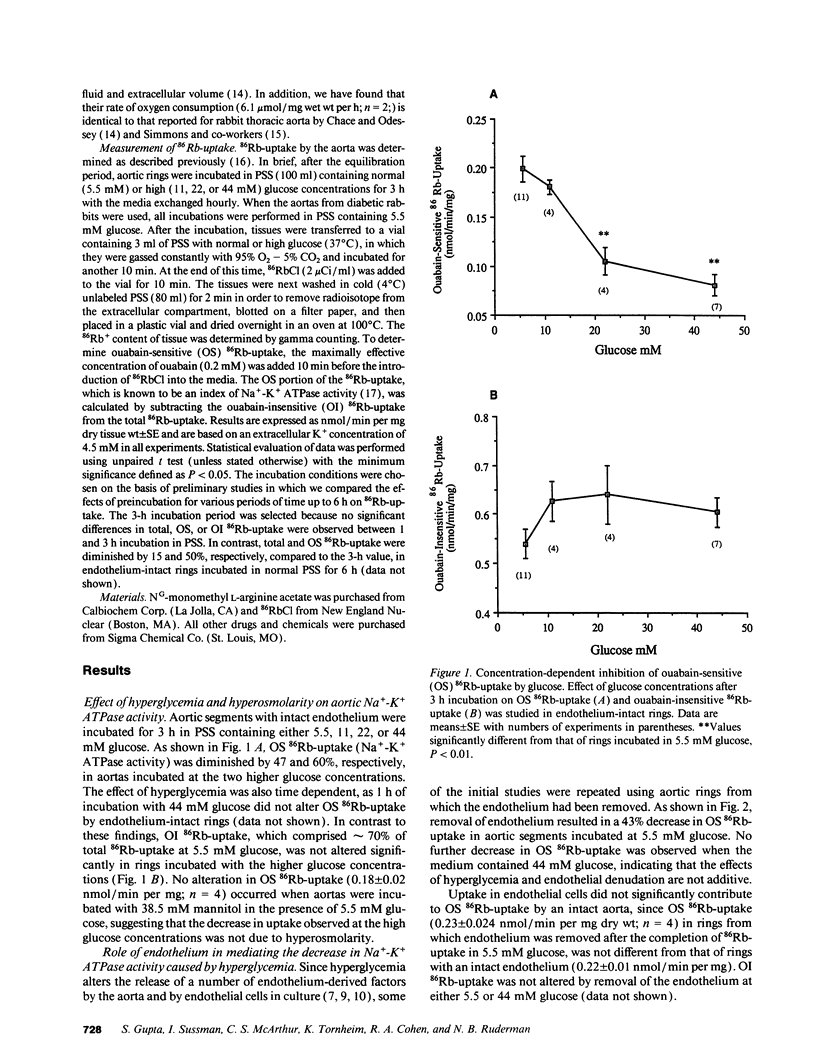
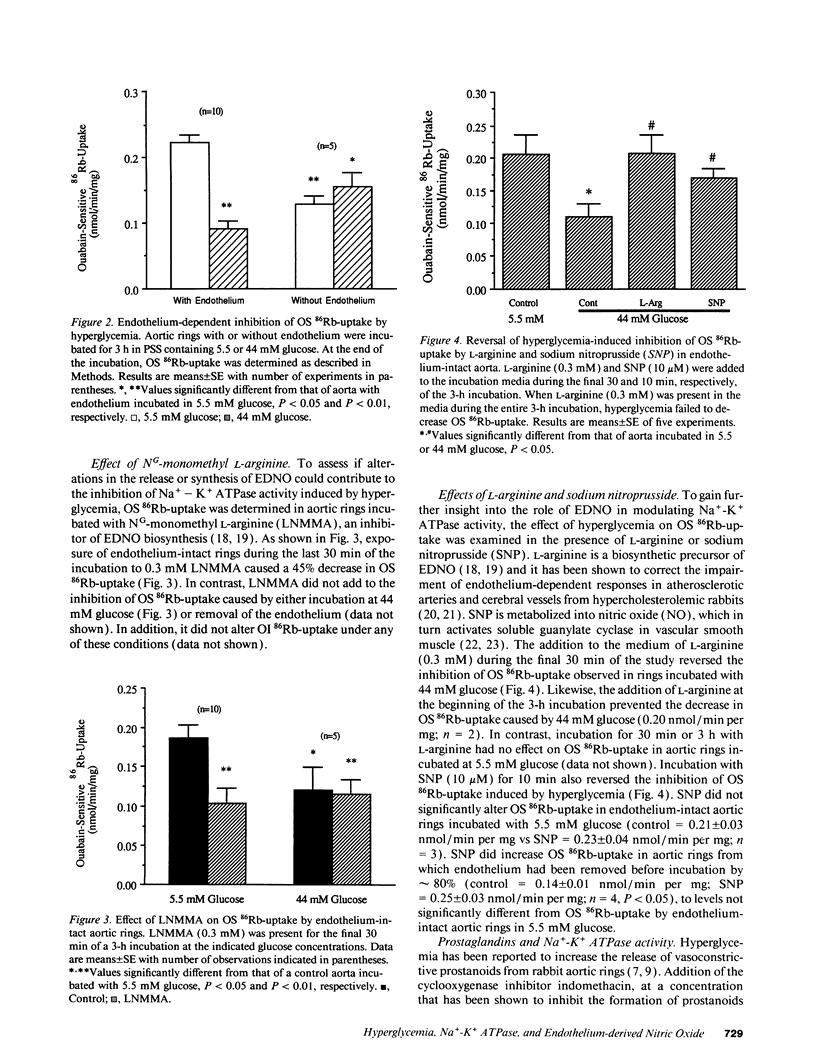
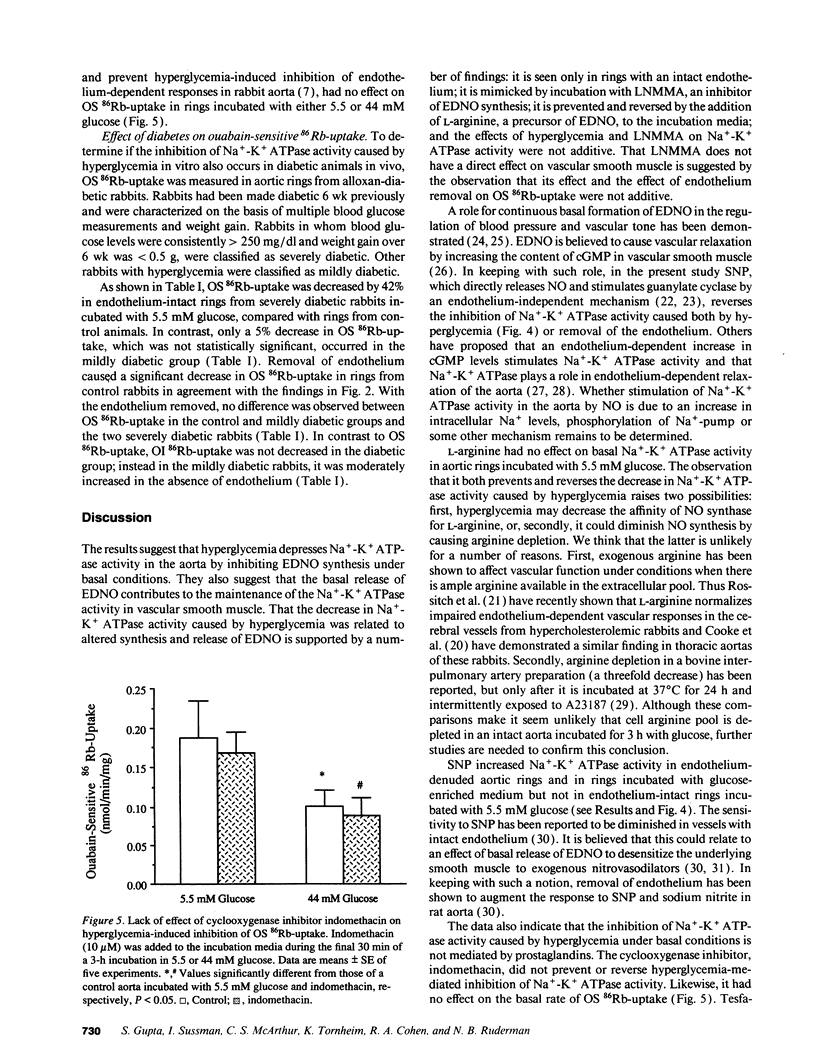
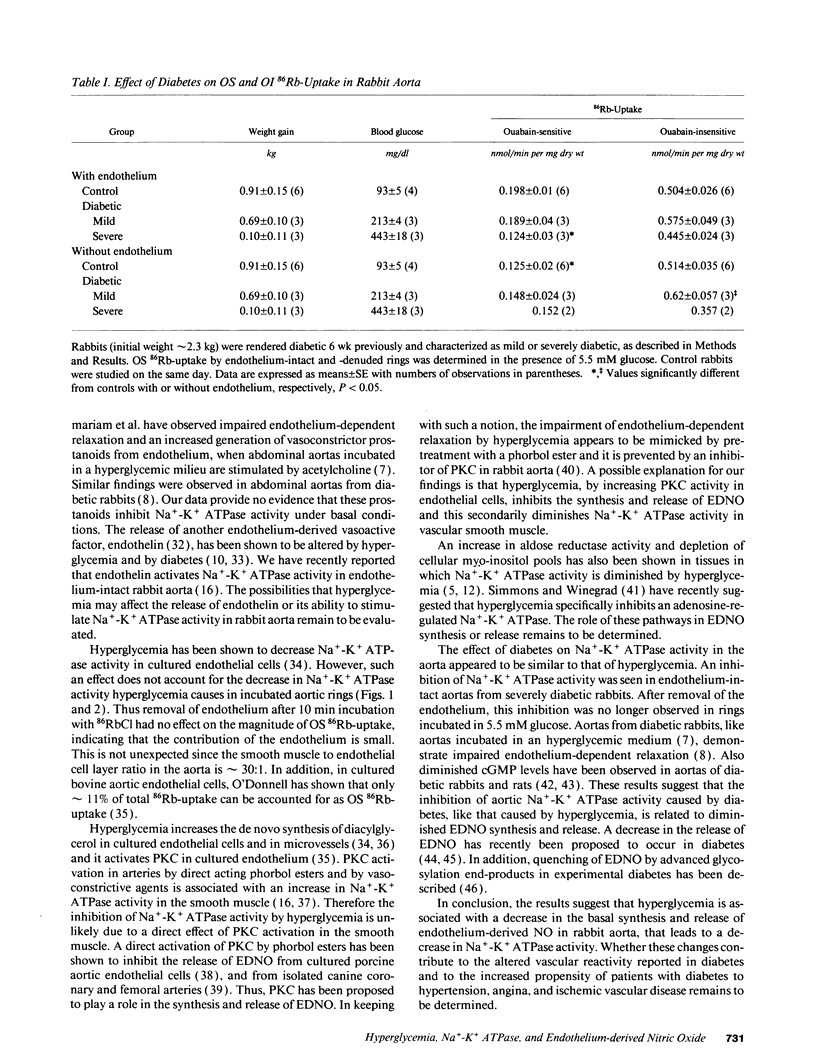
Hyperglycemia has been shown to diminish Na(+)-K+ ATPase activity in rabbit aorta. To examine the basis for this effect, aortic rings were incubated for 3 h in Krebs-Henseleit solution containing 5.5 or 44 mM glucose, and Na(+)-K+ ATPase activity was then quantified on the basis of ouabain-sensitive (OS) 86Rb-uptake. Incubation with 44 mM glucose medium caused a 60% decrease in Na(+)-K+ ATPase activity in rings with intact endothelium (from 0.22 +/- 0.01 to 0.091 +/- 0.006 nmol/min per mg dry wt; P less than 0.01). Similar decreases (45%; P less than 0.01) in Na(+)-K+ ATPase activity were seen when rings incubated with 5.5 mM glucose were exposed to NG-monomethyl L-arginine (300 microM), an inhibitor of endothelium-derived nitric oxide (EDNO) synthesis or when the endothelium was removed (43% decrease). The decrease in Na(+)-K+ ATPase activity induced by hyperglycemia was totally reversed upon adding to the medium either L-arginine, a precursor of EDNO biosynthesis or sodium nitroprusside, which bypasses endothelium and directly activates the soluble guanylate cyclase in vascular smooth muscle. A decrease in Na(+)-K+ ATPase activity (42%; P less than 0.05), only seen in the presence of endothelium, was also observed in aortas taken directly from alloxan-induced diabetic rabbits. These studies suggest that the decrease in vascular Na(+)-K+ ATPase activity induced by hyperglycemia is related, at least in part, to a decrease in the basal release of EDNO. They also suggest that alterations in basal EDNO release and possibly Na(+)-K+ ATPase activity contribute to the impairment in vascular relaxation caused by hyperglycemia and diabetes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiru T., Watanabe Y., Kamata K., Miyata N., Kasuya Y. Decrease in endothelium-dependent relaxation and levels of cyclic nucleotides in aorta from rabbits with alloxan-induced diabetes. Res Commun Chem Pathol Pharmacol. 1990 Apr;68(1):13–25. [PubMed] [Google Scholar]
- Akera T., Yamamoto S., Temma K., Kim D. H., Brody T. M. Is ouabain-sensitive rubidium or potassium uptake a measure of sodium pump activity in isolated cardiac muscle? Biochim Biophys Acta. 1981 Feb 6;640(3):779–790. doi: 10.1016/0005-2736(81)90108-5. [DOI] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme E., Graf H., Schultz G. Effects of sodium nitroprusside and other smooth muscle relaxants on cyclic GMP formation in smooth muscle and platelets. Adv Cyclic Nucleotide Res. 1978;9:131–143. [PubMed] [Google Scholar]
- Chace K. V., Odessey R. The utilization by rabbit aorta of carbohydrates, fatty acids, ketone bodies, and amino acids as substrates for energy production. Circ Res. 1981 Jun;48(6 Pt 1):850–858. doi: 10.1161/01.res.48.6.850. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Andon N. A., Girerd X. J., Hirsch A. T., Creager M. A. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation. 1991 Mar;83(3):1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Interaction between Na+,K+ exchanges and the direct inhibitory effect of acetylcholine on canine femoral arteries. Circ Res. 1980 Jun;46(6):826–836. doi: 10.1161/01.res.46.6.826. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. L-arginine causes whereas L-argininosuccinic acid inhibits endothelium-dependent vascular smooth muscle relaxation. Biochem Biophys Res Commun. 1989 Jun 15;161(2):536–543. doi: 10.1016/0006-291x(89)92632-6. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Gupta S., Ruderman N. B., Cragoe E. J., Jr, Sussman I. Endothelin stimulates Na(+)-K(+)-ATPase activity by a protein kinase C-dependent pathway in rabbit aorta. Am J Physiol. 1991 Jul;261(1 Pt 2):H38–H45. doi: 10.1152/ajpheart.1991.261.1.H38. [DOI] [PubMed] [Google Scholar]
- Hilton P. J. Cellular sodium transport in essential hypertension. N Engl J Med. 1986 Jan 23;314(4):222–229. doi: 10.1056/NEJM198601233140407. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. Simulating the diabetic environment modifies in vitro prostacyclin synthesis. Diabetes. 1983 Mar;32(3):217–221. doi: 10.2337/diab.32.3.217. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H. Ion movements through the sodium pump. Annu Rev Physiol. 1985;47:535–544. doi: 10.1146/annurev.ph.47.030185.002535. [DOI] [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Navran S. S., Adair S. E., Jemelka S. K., Seidel C. L., Allen J. C. Sodium pump stimulation by activation of two alpha adrenergic receptor subtypes in canine blood vessels. J Pharmacol Exp Ther. 1988 May;245(2):608–613. [PubMed] [Google Scholar]
- O'Donnell M. E. Regulation of Na-K-Cl cotransport in endothelial cells by atrial natriuretic factor. Am J Physiol. 1989 Jul;257(1 Pt 1):C36–C44. doi: 10.1152/ajpcell.1989.257.1.C36. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Schwartz K., Murad F. Effects of Na+,K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur J Pharmacol. 1985 Apr 2;110(2):203–209. doi: 10.1016/0014-2999(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossitch E., Jr, Alexander E., 3rd, Black P. M., Cooke J. P. L-arginine normalizes endothelial function in cerebral vessels from hypercholesterolemic rabbits. J Clin Invest. 1991 Apr;87(4):1295–1299. doi: 10.1172/JCI115132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Desiderio D., Luisi A., Johns A., Sybertz E. J. Phorbol dibutyrate inhibits release and action of endothelium-derived relaxing factor(s) in canine blood vessels. J Pharmacol Exp Ther. 1989 Jun;249(3):858–863. [PubMed] [Google Scholar]
- Ruderman N. B., Haudenschild C. Diabetes as an atherogenic factor. Prog Cardiovasc Dis. 1984 Mar-Apr;26(5):373–412. doi: 10.1016/0033-0620(84)90011-2. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki Y., Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol. 1985 Aug 7;114(1):93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y., Su C., Lee T. J., Kolm P., Cline W. H., Jr, Nickols G. A. Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J Pharmacol Exp Ther. 1986 Dec;239(3):861–866. [PubMed] [Google Scholar]
- Simmons D. A., Kern E. F., Winegrad A. I., Martin D. B. Basal phosphatidylinositol turnover controls aortic Na+/K+ ATPase activity. J Clin Invest. 1986 Feb;77(2):503–513. doi: 10.1172/JCI112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I. Elevated extracellular glucose inhibits an adenosine-(Na+,K+)-ATPase regulatory system in rabbit aortic wall. Diabetologia. 1991 Mar;34(3):157–163. doi: 10.1007/BF00418269. [DOI] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I. Mechanism of glucose-induced (Na+, K+)-ATPase inhibition in aortic wall of rabbits. Diabetologia. 1989 Jul;32(7):402–408. doi: 10.1007/BF00271258. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Lang D. Release of endothelium-derived relaxing factor from pig cultured aortic endothelial cells, as assessed by changes in endothelial cell cyclic GMP content, is inhibited by a phorbol ester. Br J Pharmacol. 1990 Mar;99(3):565–571. doi: 10.1111/j.1476-5381.1990.tb12969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki I., Cohen R. A., Chobanian A. V., Brecher P. Effect of endothelial cell denudation on fatty acid metabolism by rabbit aorta. Am J Physiol. 1990 Aug;259(2 Pt 2):H442–H447. doi: 10.1152/ajpheart.1990.259.2.H442. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Miyamori I., Yoneda T., Takeda R. Production of endothelin-1 from the mesenteric arteries of streptozotocin-induced diabetic rats. Life Sci. 1991;48(26):2553–2556. doi: 10.1016/0024-3205(91)90611-e. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Cohen R. A. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991 May;87(5):1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Cohen R. A. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991 May;87(5):1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Jakubowski J. A., Cohen R. A. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989 Nov;257(5 Pt 2):H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Williamson J. R., Easom R. A., Chang K., Sherman W. R., Turk J. Diacylglycerol accumulation and microvascular abnormalities induced by elevated glucose levels. J Clin Invest. 1991 Jan;87(1):31–38. doi: 10.1172/JCI114988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Ohnaka K., Takayanagi R., Umeda F., Nawata H. Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett. 1990 Jul 2;267(1):16–18. doi: 10.1016/0014-5793(90)80276-o. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


