Abstract
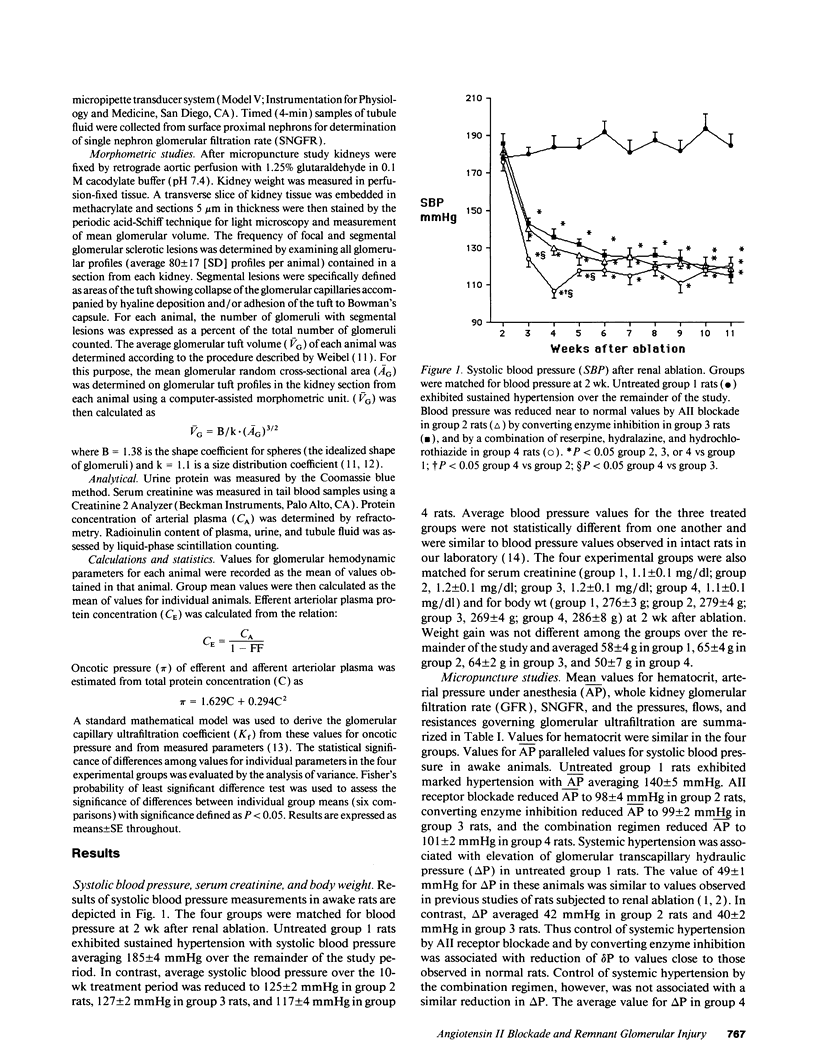
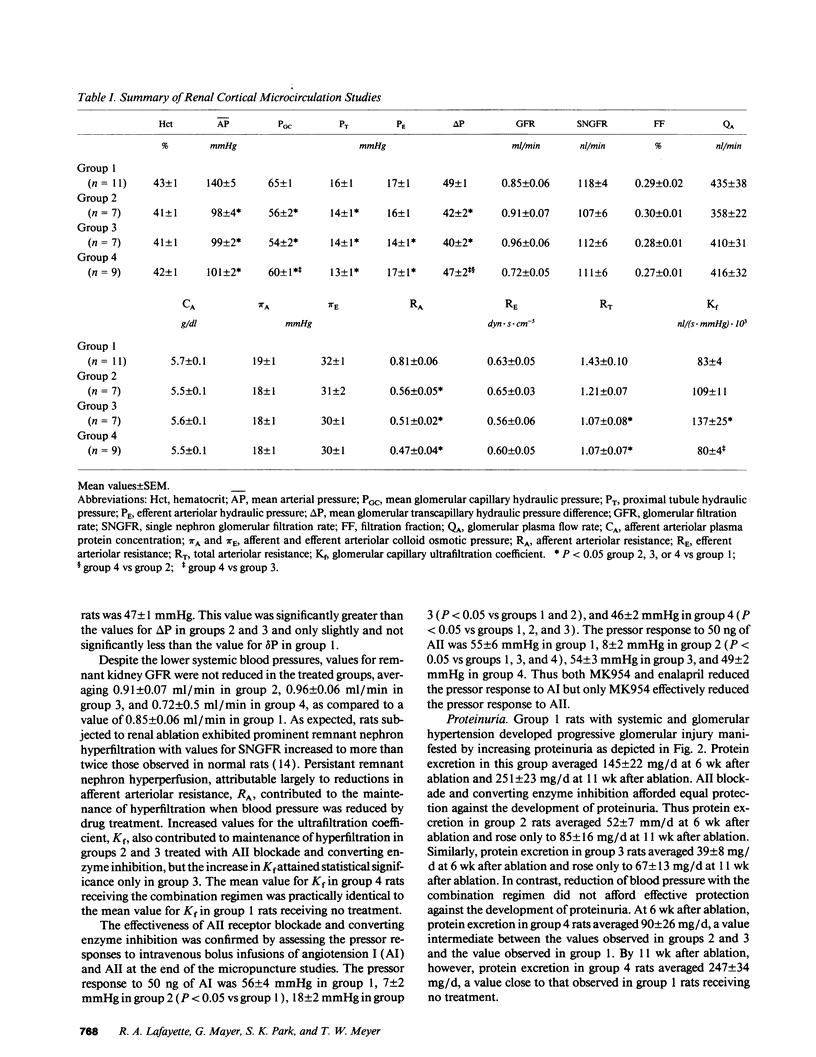
The effects of angiotensin II (AII) blockade were compared with the effects of angiotensin converting enzyme inhibition in rats with reduced nephron number. Rats were subjected to five-sixths renal ablation and divided into four groups with similar values for blood pressure and serum creatinine after 2 wk. Group 1 then served as untreated controls, while group 2 received the AII receptor antagonist MK954 (which has previously been designated DuP753), group 3 received the converting enzyme inhibitor enalapril, and group 4 received a combination of reserpine, hydralazine, and hydrochlorothiazide. Micropuncture and morphologic studies were performed 10 wk later. Converting enzyme inhibition, AII receptor blockade, and the combination regimen were equally effective in reversing systemic hypertension (time-averaged systolic blood pressure: group 1, 185 +/- 5 mmHg; group 2, 125 +/- 2 mmHg; group 3, 127 +/- 2 mmHg; group 4, 117 +/- 4 mmHg). Micropuncture studies showed that glomerular transcapillary pressure was reduced significantly by converting enzyme inhibition and by AII blockade but not by the combination regimen (delta P: group 1, 49 +/- 1 mmHg; group 2, 42 +/- 1 mmHg; group 3, 40 +/- 2 mmHg, group 4, 47 +/- 1 mmHg). Reduction of systemic blood pressure was associated with the development of markedly less proteinuria and segmental glomerular sclerosis in rats receiving enalapril and MK954 but not in rats receiving the combination regimen (prevalence of glomerular sclerotic lesions: group 1, 41 +/- 4%; group 2, 9 +/- 1%; group 3, 9 +/- 1%; group 4, 33 +/- 6%). These results indicate that the effects of converting enzyme inhibition on remnant glomerular function and structure depend on reduction in AII activity and are not attributable simply to normalization of systemic blood pressure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Meyer T. W., Rennke H. G., Brenner B. M. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985 Aug;76(2):612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Rennke H. G., Brenner B. M. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986 Jun;77(6):1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierwaltes W. H., Carretero O. A. Kinin antagonist reverses converting enzyme inhibitor-stimulated vascular prostaglandin I2 synthesis. Hypertension. 1989 Jun;13(6 Pt 2):754–758. doi: 10.1161/01.hyp.13.6.754. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Gutkowska J., Thibault G., Garcia R., Genest J., Cantin M. Distinct localization of atrial natriuretic factor and angiotensin II binding sites in the glomerulus. Am J Physiol. 1986 Oct;251(4 Pt 2):F594–F602. doi: 10.1152/ajprenal.1986.251.4.F594. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero O. A., Scicli A. G. Kinins paracrine hormone. Kidney Int Suppl. 1988 Oct;26:S52–S59. [PubMed] [Google Scholar]
- Chapman A. B., Johnson A., Gabow P. A., Schrier R. W. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990 Oct 18;323(16):1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- Danckwardt L., Shimizu I., Bönner G., Rettig R., Unger T. Converting enzyme inhibition in kinin-deficient brown Norway rats. Hypertension. 1990 Oct;16(4):429–435. doi: 10.1161/01.hyp.16.4.429. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Osterby R., Nozawa M., Gundersen H. J. Development of glomerular lesions in experimental long-term diabetes in the rat. Kidney Int. 1982 May;21(5):689–695. doi: 10.1038/ki.1982.82. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. N., Schambelan M., Kaysen G. A. Modulation of albuminuria by dietary protein and converting enzyme inhibition. Am J Physiol. 1987 Oct;253(4 Pt 2):F719–F725. doi: 10.1152/ajprenal.1987.253.4.F719. [DOI] [PubMed] [Google Scholar]
- Hutchison F. N., Martin V. I. Effects of modulation of renal kallikrein-kinin system in the nephrotic syndrome. Am J Physiol. 1990 May;258(5 Pt 2):F1237–F1244. doi: 10.1152/ajprenal.1990.258.5.F1237. [DOI] [PubMed] [Google Scholar]
- Ingelfinger J. R., Pratt R. E., Ellison K., Dzau V. J. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest. 1986 Nov;78(5):1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Reversing glomerular hypertension stabilizes established glomerular injury. Kidney Int. 1987 Mar;31(3):752–759. doi: 10.1038/ki.1987.62. [DOI] [PubMed] [Google Scholar]
- Meyer T. W., Rennke H. G. Progressive glomerular injury after limited renal infarction in the rat. Am J Physiol. 1988 Jun;254(6 Pt 2):F856–F862. doi: 10.1152/ajprenal.1988.254.6.F856. [DOI] [PubMed] [Google Scholar]
- Miller P. L., Meyer T. W. Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990 Dec;63(6):862–866. [PubMed] [Google Scholar]
- Miller P. L., Rennke H. G., Meyer T. W. Glomerular hypertrophy accelerates hypertensive glomerular injury in rats. Am J Physiol. 1991 Sep;261(3 Pt 2):F459–F465. doi: 10.1152/ajprenal.1991.261.3.F459. [DOI] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar L. G., Rosivall L. Contribution of the renin-angiotensin system to the control of intrarenal hemodynamics. Kidney Int. 1984 Jun;25(6):857–868. doi: 10.1038/ki.1984.102. [DOI] [PubMed] [Google Scholar]
- Olson J. L., Heptinstall R. H. Nonimmunologic mechanisms of glomerular injury. Lab Invest. 1988 Nov;59(5):564–578. [PubMed] [Google Scholar]
- Pelayo J. C., Westcott J. Y. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. J Clin Invest. 1991 Jul;88(1):101–105. doi: 10.1172/JCI115264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaleb N. E., Rouissi N., Nantel F., D'Orléans-Juste P., Regoli D. DuP 753 is a specific antagonist for the angiotensin receptor. Hypertension. 1991 Apr;17(4):480–484. doi: 10.1161/01.hyp.17.4.480. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E., Kren S. M., Hostetter T. H. Effect of dietary protein on the renin-angiotensin system in subtotally nephrectomized rats. Kidney Int. 1990 Aug;38(2):240–248. doi: 10.1038/ki.1990.192. [DOI] [PubMed] [Google Scholar]
- Scholey J. W., Miller P. L., Rennke H. G., Meyer T. W. Effect of converting enzyme inhibition on the course of adriamycin-induced nephropathy. Kidney Int. 1989 Nov;36(5):816–822. doi: 10.1038/ki.1989.267. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Blumenfeld J. D., Bell G. M., Pecker M. S., Sommers S. C., Laragh J. H. On the renal basis for essential hypertension: nephron heterogeneity with discordant renin secretion and sodium excretion causing a hypertensive vasoconstriction-volume relationship. J Hypertens. 1988 Oct;6(10):763–777. doi: 10.1097/00004872-198811000-00001. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F. Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci. 1991 Feb;12(2):55–62. doi: 10.1016/0165-6147(91)90498-h. [DOI] [PubMed] [Google Scholar]
- Warren D. J., Ferris T. F. Renin secretion in renal hypertension. Lancet. 1970 Jan 24;1(7639):159–162. doi: 10.1016/s0140-6736(70)90404-6. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Chiu A. T., Duncia J. V., Carini D. J., Wexler R. R., Johnson A. L., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. IX. Antihypertensive activity in rats of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990 Feb;252(2):726–732. [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Chiu A. T., Duncia J. V., Carini D. J., Wexler R. R., Johnson A. L., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990 Feb;252(2):719–725. [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Jr, Chiu A. T., Duncia J. V., Carini D. J., Wexler R. R., Johnson A. L., Timmermans P. B. Hypotensive action of DuP 753, an angiotensin II antagonist, in spontaneously hypertensive rats. Nonpeptide angiotensin II receptor antagonists: X. Hypertension. 1990 May;15(5):459–468. doi: 10.1161/01.hyp.15.5.459. [DOI] [PubMed] [Google Scholar]
- Zatz R., Dunn B. R., Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986 Jun;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]


