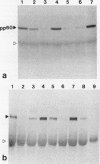
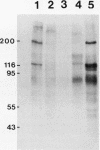
Abstract
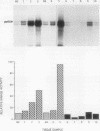
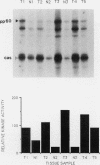
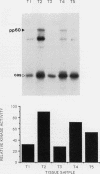
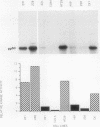
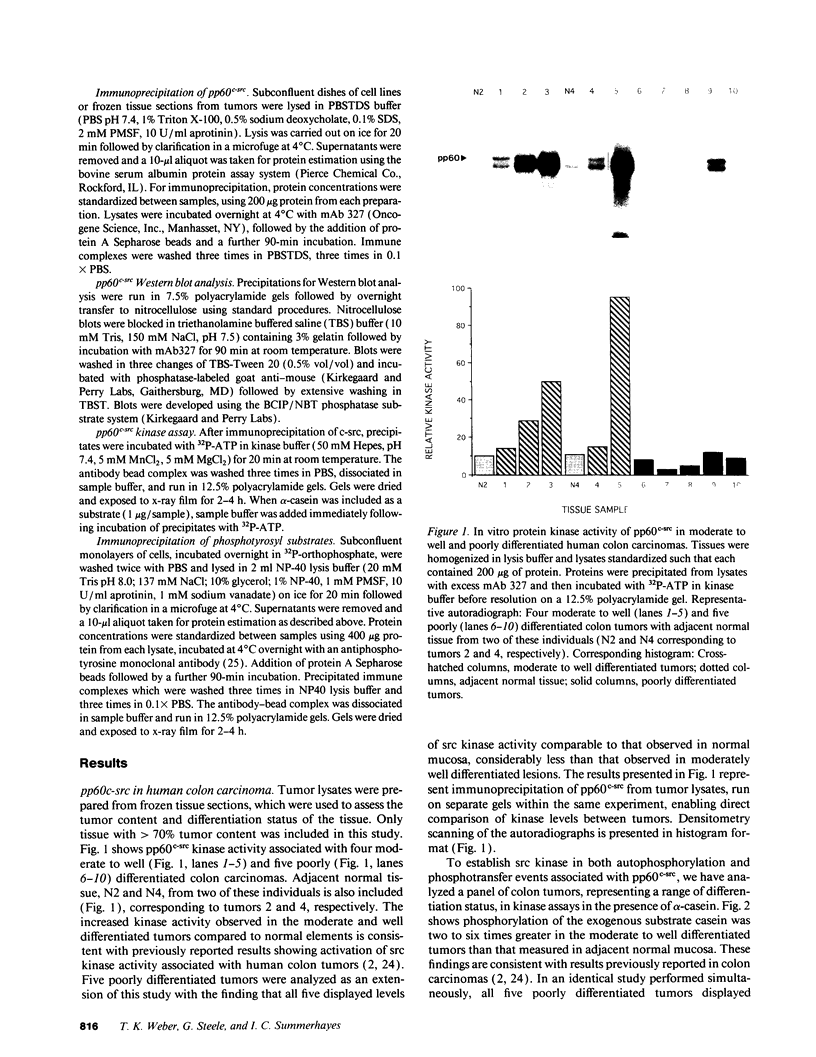
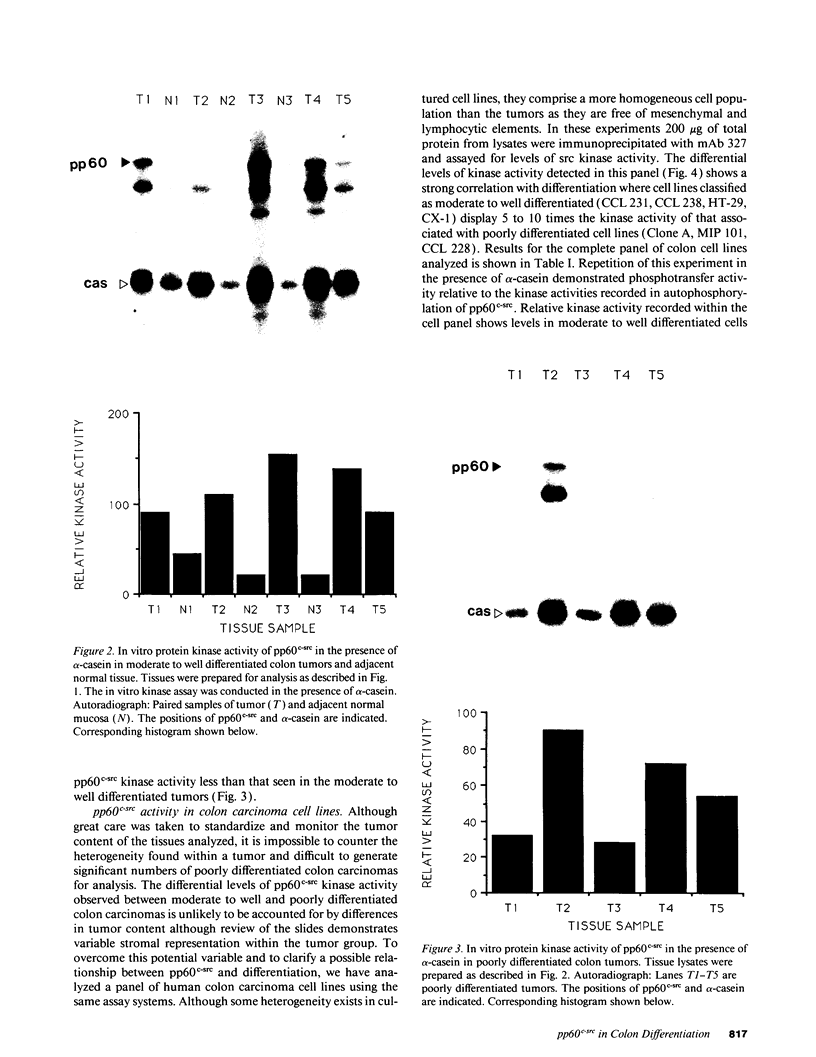
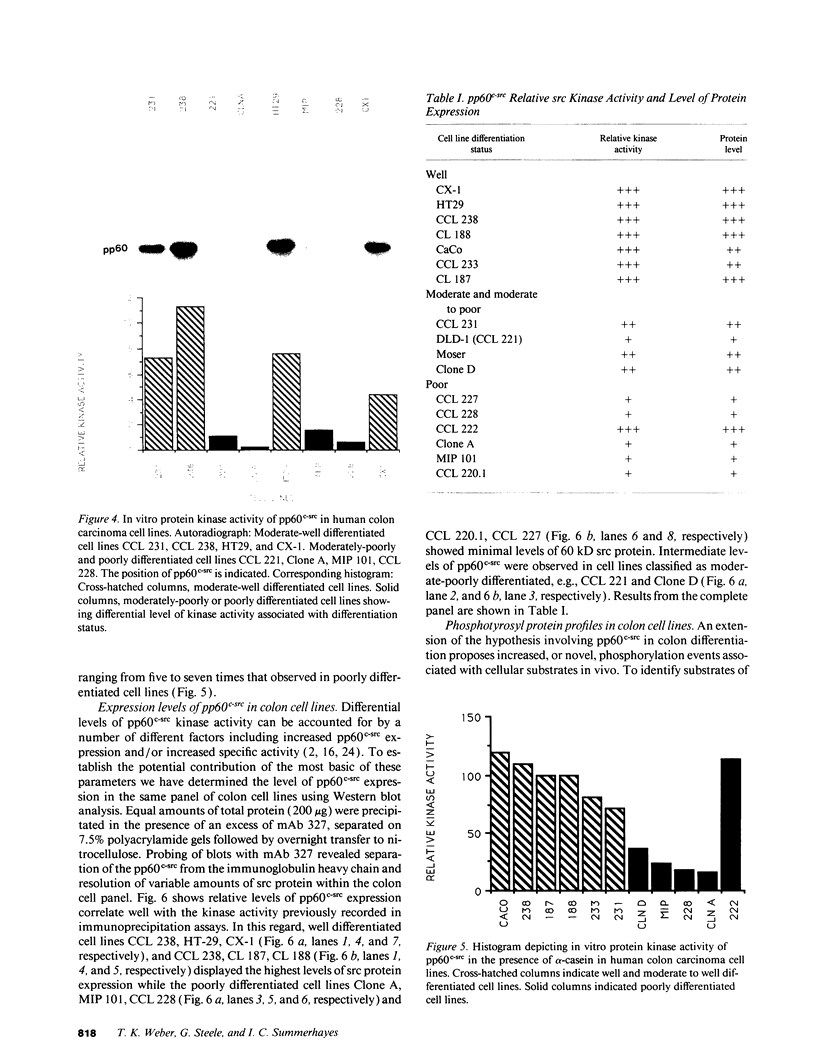
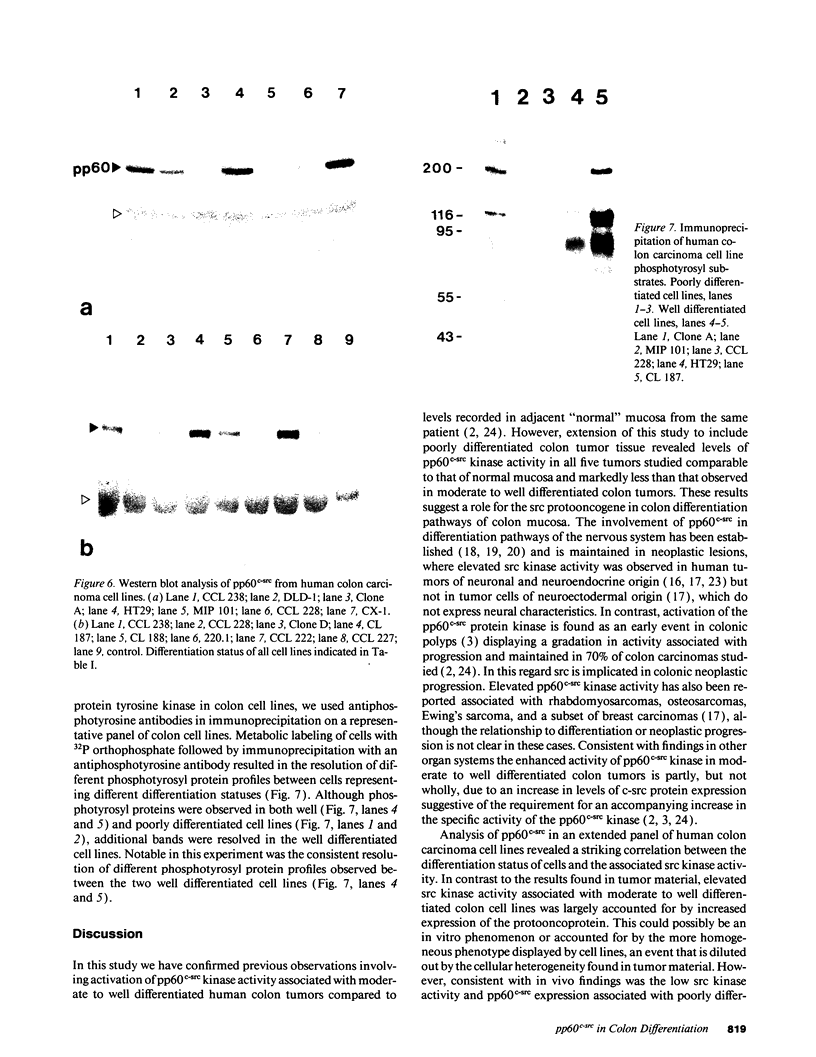
The results presented in this report demonstrate increased pp60c-src kinase activity associated with moderate to well differentiated colon tumors, corroborating previous observations by other groups. Extension of this analysis to include a small number of poorly differentiated colon carcinomas revealed src kinase activity comparable to that observed in normal colonic mucosa, considerably less than that observed in moderate/well differentiated lesions. Correlations of src kinase activity with differentiation was confirmed within a panel of colon cell lines where increased activity, associated with moderate/well differentiated lines, was accompanied by increased expression of pp60c-src protein. Use of an antiphosphotyrosine antibody in immunoprecipitation revealed the presence of novel phosphotyrosyl cellular substrates in human colon cell lines displaying elevated pp60c-src kinase activity. These observations suggest a role for the src protooncogene in colonic differentiation pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagrodia S., Chackalaparampil I., Kmiecik T. E., Shalloway D. Altered tyrosine 527 phosphorylation and mitotic activation of p60c-src. Nature. 1991 Jan 10;349(6305):172–175. doi: 10.1038/349172a0. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bjelfman C., Meyerson G., Cartwright C. A., Mellström K., Hammerling U., Påhlman S. Early activation of endogenous pp60src kinase activity during neuronal differentiation of cultured human neuroblastoma cells. Mol Cell Biol. 1990 Jan;10(1):361–370. doi: 10.1128/mcb.10.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Rosen N., Israel M. A. Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7275–7279. doi: 10.1073/pnas.82.21.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Cotton P., Lustig A., Yonemoto W., Lipsich L., Coussens P., Barrett J. N., Nonner D., Keane R. W. Characterization of the altered form of the c-src gene product in neuronal cells. Genes Dev. 1987 May;1(3):287–296. doi: 10.1101/gad.1.3.287. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Hutchinson M. A., Eckhart W. Structural and functional modification of pp60c-src associated with polyoma middle tumor antigen from infected or transformed cells. Mol Cell Biol. 1985 Oct;5(10):2647–2652. doi: 10.1128/mcb.5.10.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kamps M. P., Meisler A. I., Pipas J. M., Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989 Jun;83(6):2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Meisler A. I., Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Cowan W. M., Hunter T., Eckhart W. pp60c-src expression in the developing rat brain. Proc Natl Acad Sci U S A. 1988 May;85(10):3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Piwnica-Worms H., Harvey R. W., Roberts T. M., Smith A. E. The carboxy terminus of pp60c-src is a regulatory domain and is involved in complex formation with the middle-T antigen of polyomavirus. Mol Cell Biol. 1988 Apr;8(4):1736–1747. doi: 10.1128/mcb.8.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Rothberg P. G., Diehl R. E., Morse C. C., Spandorfer J. M., Astrin S. M. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol Cell Biol. 1985 Aug;5(8):1969–1976. doi: 10.1128/mcb.5.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman M. D., Scott J. K., Watt R. A., Astrin S. M. The c-myc protein is constitutively expressed at elevated levels in colorectal carcinoma cell lines. Oncogene. 1988 Apr;2(4):367–378. [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Carlson M., Thliveris A., Albertsen H., Gelbert L., Samowitz W., Groden J., Stevens J., Spirio L., Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991 Aug 9;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hamilton S. R., Hedge P., Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991 Mar 15;251(4999):1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström K., Bjelfman C., Hammerling U., Påhlman S. Expression of c-src in cultured human neuroblastoma and small-cell lung carcinoma cell lines correlates with neurocrine differentiation. Mol Cell Biol. 1987 Dec;7(12):4178–4184. doi: 10.1128/mcb.7.12.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen N., Bolen J. B., Schwartz A. M., Cohen P., DeSeau V., Israel M. A. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986 Oct 15;261(29):13754–13759. [PubMed] [Google Scholar]