Abstract
Fluoroquinolone resistance in Mycobacterium tuberculosis has become increasingly important. A review of mutations in DNA gyrase, the fluoroquinolone target, is needed to improve the molecular detection of resistance. We performed a systematic review of studies reporting mutations in DNA gyrase genes in clinical M. tuberculosis isolates. From 42 studies that met inclusion criteria, 1220 fluoroquinolone-resistant M. tuberculosis isolates underwent sequencing of the quinolone resistance-determining region (QRDR) of gyrA; 780 (64%) had mutations. The QRDR of gyrB was sequenced in 534 resistant isolates; 17 (3%) had mutations. Mutations at gyrA codons 90, 91 or 94 were present in 654/1220 (54%) resistant isolates. Four different GyrB numbering systems were reported, resulting in mutation location discrepancies. We propose a consensus numbering system. Most fluoroquinolone-resistant M. tuberculosis isolates had mutations in DNA gyrase, but a substantial proportion did not. The proposed consensus numbering system can improve molecular detection of resistance and identification of novel mutations.
Keywords: gyrA, gyrB, QRDRs, M. tuberculosis
Introduction
Fluoroquinolones play an increasingly important role in the treatment of tuberculosis (TB). They are used to treat multidrug-resistant tuberculosis (MDR-TB; defined as resistance to at least isoniazid and rifampicin) and are also recommended to treat drug-susceptible TB in patients with intolerance to first-line antibiotics.1 Fluoroquinolones kill bacteria by altering DNA gyrase and DNA topoisomerase IV. Since Mycobacterium tuberculosis does not have topoisomerase IV, fluoroquinolones target DNA gyrase in M. tuberculosis.2 DNA gyrase consists of two A and two B subunits encoded by the gyrA and gyrB genes, respectively.3 The quinolone resistance-determining region (QRDR) is comprised of conserved areas within gyrA and gyrB in which mutations conferring fluoroquinolone resistance have been reported in most bacterial species, including M. tuberculosis.3–5
There are several fluoroquinolone resistance mechanisms in bacteria. Although the primary fluoroquinolone resistance mechanism in M. tuberculosis is related to mutations in the QRDR of DNA gyrase genes,6–12 fluoroquinolone resistance can also be conferred by increased fluoroquinolone efflux or DNA mimicry.13–15 However, the latter two mechanisms have been described only in laboratory strains, not clinical M. tuberculosis isolates. In many studies, more than 90% of fluoroquinolone-resistant M. tuberculosis strains have mutations in the QRDR of gyrA or gyrB,16–27 so we focused our review on mutations in DNA gyrase.
Conventional phenotypic M. tuberculosis drug susceptibility testing (DST) methods for fluoroquinolones require several weeks to complete and are not standardized. Therefore, molecular diagnostic tests for rapid detection of fluoroquinolone resistance at the point-of-care are urgently needed. In addition to fluoroquinolone mono-resistant M. tuberculosis isolates, such tests could be used to rapidly identify extensively drug-resistant tuberculosis (XDR-TB; defined as resistance to at least isoniazid and rifampicin plus a fluoroquinolone and a second-line injectable agent such as capreomycin, amikacin and kanamycin).28–30 A line probe assay has been developed to detect fluoroquinolone-resistance in M. tuberculosis, but its sensitivity ranges from 75.6% to 89.5% when compared with conventional phenotypic susceptibility methods,19,31,32 and 87% to 100% when compared with DNA sequencing.31,33
The purpose of this review was to characterize all DNA gyrase gene mutations described in M. tuberculosis clinical strains and to distinguish those associated with fluoroquinolone resistance and those reported in fluoroquinolone-susceptible strains. During the review process we found four different numbering systems used for the GyrB subunit, resulting in discrepancies regarding the location of resistance mutations. Some authors used the Escherichia coli numbering system; others used one of three M. tuberculosis numbering systems, which differ according to the position of the start codon.2,3,34 Most authors did not specify the numbering system used. Even the comprehensive compilation of mutations in the online database http://www.tbdreamdb.com,35 which focuses on the QRDR and uses one consistent numbering system, classifies some mutations as inside the QRDR of GyrB when they are indeed outside the QRDR. The different numbering systems make it difficult to confirm previously described mutations and clearly identify novel mutations. We therefore sought to clarify the location of these mutations.
To our knowledge this is the most extensive review to date of mutations described in the gyrA and gyrB genes of fluoroquinolone-resistant and -susceptible strains of M. tuberculosis. It is also the first work to propose a standard numbering system of M. tuberculosis GyrA and GyrB, which will allow for accurate comparison of resistance mutations by laboratories around the world.
Methods
Definitions
Mutations were defined as nucleotide base-pair changes that resulted in substitutions in amino acids (i.e. non-synonymous), regardless of whether the mutation was reported in a fluoroquinolone-resistant or -susceptible M. tuberculosis isolate. Of those mutations reported in fluoroquinolone-resistant isolates, we distinguished between mutations documented to confer fluoroquinolone resistance by biochemical or genetic experiments and those without such evidence.18,36 Polymorphisms were defined as non-synonymous nucleotide base-pair changes known to not be associated with or confer fluoroquinolone resistance. Base-pair changes that did not result in a change in amino acid (i.e. synonymous) were not included in this review. The three-letter abbreviation nomenclature was used to represent amino acids. Substitutions were noted as follows: Xxx##Yyy, where Xxx represents the wild-type amino acid, ## the codon number and Yyy the substituted amino acid.
Search strategy
A computerized search identified peer-reviewed primary research studies reporting fluoroquinolone-resistant or fluoroquinolone-susceptible isolates of M. tuberculosis in which mutations in DNA gyrase genes were identified. The search was limited to studies in English published between 1 January 1990 and 30 June 2010. Figure S1 (available as Supplementary data at JAC Online) illustrates the study selection methodology. Full text articles were screened using the Medical Literature Analysis and Retrieval System Online (MEDLINE) using the keywords ‘fluoroquinolone resistance’, ‘M. tuberculosis’, ‘gyrA’, ‘gyrB’, ‘DNA gyrase’, ‘mutations’, ‘drug resistance’, ‘fluoroquinolone susceptibility’, ‘second-line drug resistance’, ‘quinolone resistant’ and ‘ofloxacin resistance’ in different combinations.
The inclusion criteria consisted of (i) publications in which genotypic susceptibility methods were compared with a solid or liquid-based phenotypic resistance reference standard and (ii) DNA gyrase gene mutations were identified in M. tuberculosis isolates obtained from human clinical specimens.
Papers were excluded if they were reviews, letters, duplicates or if the title indicated that the study was not relevant to our study. The online database http://www.tbdreamdb.com35 was excluded because it is a compilation of mutations previously reported in the literature rather than a primary source document. Abstracts of the remaining papers were reviewed and studies with irrelevant content were excluded. If the abstract did not provide enough information to include or exclude the article, the entire article was reviewed. Articles were also excluded if they lacked data on amino acid changes or phenotypic susceptibility testing. The bibliographies of the included publications were reviewed, and additional articles not previously identified were added as appropriate.
Data acquisition
Data abstracted from journal articles that met the inclusion criteria were organized in three ways: (i) all mutations reported in gyrA, (ii) all mutations reported in gyrB and (iii) all combinations of mutations (in gyrA and/or gyrB) reported in a single M. tuberculosis isolate. Although substitutions Glu21Gln, Ser95Thr and Gly668Asp in GyrA result in amino acid changes, they were omitted from the summary tables because they are common polymorphisms that do not correlate with drug resistance.3,37
When more than one mutation was observed in one strain (double or triple mutation), we noted two scenarios: (i) either mutation was observed as a single mutation elsewhere or (ii) the mutations were never observed independent of one another. In both scenarios, the mutations were listed as single mutations and as multiple mutations. Mutations that were never observed independently of one another are noted in the tables. This process was designed to capture every mutation without undermining the potential effect that combinations of mutations may have on fluoroquinolone resistance.
For this review, all of the substitutions in GyrB were standardized to the re-annotated genome numbering system of M. tuberculosis GyrB, where the QRDR of M. tuberculosis GyrB ranges from codon 461 to 499.34 Regarding the QRDR of GyrA, some publications used the E. coli numbering system to describe substitution location.38–41 In this systematic review, all substitution locations in GyrA were standardized to the genome M. tuberculosis numbering system, in which the QRDR of GyrA ranges from codon 74 to 113.3
We reported the number of clinical isolates tested, the region sequenced (entire gyrA or gyrB genes or only the QRDRs of gyrA or gyrB), along with the genotypic and phenotypic susceptibility methods used to determine fluoroquinolone resistance for each study. The number of isolates containing a specific mutation was determined and the fluoroquinolone MIC associated with this mutation was included if available. Fluoroquinolone activity (measured as 50% inhibitory concentration) against M. tuberculosis with specific DNA gyrase mutants was also reviewed.
Quality control
Two authors (F. M. and A. W. K.) independently reviewed and abstracted the data. The data were reviewed for accuracy and compared with particular attention to the numbering systems used. Two additional authors (Y. F. van der H. and A. A.) adjudicated differences between the authors and reviewed the data for accuracy.
Results
Numbering systems for M. tuberculosis GyrA and GyrB subunits
GyrA
The first studies investigating the molecular basis of fluoroquinolone resistance in M. tuberculosis were based on E. coli39 or M. tuberculosis gyrA3 gene sequences. Since the GyrA QRDR is located at the N-terminal part of the GyrA subunit and the M. tuberculosis GyrA start codon is seven amino acids before the E. coli GyrA (Figure S2, available as Supplementary data at JAC Online), the amino acids at positions 88, 90, 91 and 94 in the M. tuberculosis numbering system correspond to the amino acids at positions 81, 83, 84 and 87 in the E. coli numbering system, respectively. When these differences were accounted for, there were no discrepancies in the location of substitutions reported in the GyrA QRDR in any of the studies reviewed.
GyrB
In contrast, four distinct numbering systems have been used to report substitutions in the GyrB QRDR. As a result, in several instances amino acid changes occurring at what was actually the same position were reported as occurring at different positions. For example, substitution Asp472Ala33 (2000; Table 1) is the same as substitution Asp500Ala42 (1998; Table 1), but the authors used different numbering systems. Codons 472 and 500 both correspond to position 426 in the E. coli numbering system (Table 1 and Figure S3, available as Supplementary data at JAC Online). Similarly, substitution Asn533Thr26 (1994; Table 1) is the same as Asn538Thr42 (1998; Table 1); both correspond to codon 464 in the E. coli numbering system. Additionally, discrepancies in the numbering system resulted in differences in classifying mutations as being inside or outside of the QRDR of GyrB. For example, mutations at codons 500 and 501 in the proposed numbering system (2002; Table 1) were designated as inside the QRDR in three studies, but are definitely located outside the QRDR of GyrB.33,42,43
Table 1.
Comparison of the three GyrB numbering systems in M. tuberculosis described in the literature, the corresponding region in E. coli and the proposed consensus numbering system
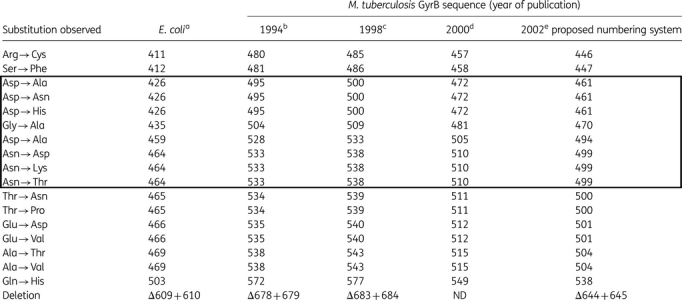 |
Most discrepancies arose from a lack of consensus regarding the gyrB start codon. The first sequence of M. tuberculosis gyrB (published in 1994) described the GyrB QRDR extending from codon 495 to 5333 (see gi|1107468|gb|AAA83016.1| in Figure S3). This numbering system was based on the GyrB E. coli sequence. This numbering system was used for the first studies reporting fluoroquinolone resistance in M. tuberculosis.36 When the entire genome of M. tuberculosis was published in 1998, the start codon of gyrB was 28 codons upstream of the codon that had been used earlier. Due to the absence of some amino acids in E. coli that are present in M. tuberculosis, the first codon of the GyrB QRDR was five codons upstream of the codon that had been used earlier; the QRDR therefore ranged from amino acid 500 to 5382 (see gi|1552558|emb|CAB02426.1 in Figure S3). Of the studies reviewed, most authors used the 500–538 numbering system to identify mutation location. However, the first gyrB mutations described in clinical M. tuberculosis strains were identified using yet another numbering system, which did not correspond to any of the previously published sequences. The publications using this numbering system did not provide references to justify this numbering sytem.7,18,21,33,43–45 In 2002, a re-annotation of the M. tuberculosis genome proposed a new start codon for gyrB.34 This numbering system is closer to the E. coli numbering system, thus shifting the QRDR to residues 461–49934 (see P0C5C5|1-675 in Figure S3). This latter proposed numbering system appears to be the most accurate. Despite the absence of experiments defining the gyrB start codon, the alignment of M. tuberculosis GyrB sequence and 50 bacterial species (other than M. tuberculosis) from five phyla whose sequences were obtained from the SWISS-PROT/TrEMBL (http://expasy.org/sprot) and NCBI (http://www.ncbi.nlm.nih.gov) databases demonstrated that the start codon chosen by Camus et al.34 was the most accurate (C. Mayer, personal data). This is the numbering system that we propose future studies use as the reference regarding fluoroquinolone resistance in M. tuberculosis.
Table 1 compares the three M. tuberculosis GyrB numbering systems used in the literature as well as the E. coli GyrB numbering system. The consensus numbering system that we propose is also included.
Findings
Forty-two publications met the inclusion criteria. From these studies, a total of 2482 M. tuberculosis isolates were assessed for genotypic mutations: 1220 (49%) were phenotypically fluoroquinolone resistant and 1262 (51%) were fluoroquinolone susceptible. The phenotypic and genotypic resistance methods used and the regions of M. tuberculosis DNA sequenced are provided in Table 2. Twenty-seven studies sequenced the QRDR of gyrA, 13 sequenced the QRDR of both gyrA and gyrB, 1 sequenced the entire gyrA and 1 sequenced the entire gyrA and gyrB genes (Table 2).
Table 2.
Clinical M. tuberculosis isolates studied: fluoroquinolone susceptibility and molecular detection methods used in each primary study included in the review
| Type of collection | No. of FQR | No. of FQS | Susceptibility method (OFX breakpoints) | Molecular detection method | DNA region studieda | Percentage FQR with mutations | Study reference |
|---|---|---|---|---|---|---|---|
| ND | 4 | 0 | PMS on 7H11 (2 mg/L) | PCR seq | QRDR_A | 100 | 25 |
| ND | 8 | 1 | ACM on LJ (2 mg/L) | PCR seq | QRDR_A | 100 | 27 |
| ND | 87 | 0 | ACM on LJ | PCR seq | QRDR_A | 100 | 46 |
| HIV-infected TB cases | 1 | 0 | MIC in liquid media (7H12 + Bactec) | PCR seq | QRDR_A | 100 | 60 |
| MDR | 52 | 55 | ACM on LJ (2 mg/L) | microchips | QRDR_A | 98 | 17 |
| ND | 71 | 47 | ACM on LJ (8 mg/L) | PCR seq | QRDR_A | 97.2 | 24 |
| XDR | 26 | 0 | PML (MGIT 960 + Bactec 460) | PCR seq | QRDR_A | 96.2 | 22 |
| MDR | 35 | 108 | ACM on LJ (2.4 mg/L) + PML (MGIT960) | PCR seq | QRDR_A | 94.3 | 20 |
| MDR | 11 | 43 | PMS on 7H11 (2 mg/L) | MAS-PCR | QRDR_A | 91 | 53 |
| Fluoroquinolone, AMK, CAP and/or EMB resistance | 32 | 74 | PML (MGIT 960) + PMS on LJ (2 mg/L) | PCR seq + MTBDRsl | QRDR_A | 90.6 | 19 |
| ND | 19 | 9 | MIC on 7H11 (2 mg/L) | PCR seq + line probe assay | QRDR_A | 89.5 | 31 |
| ND | 30 | 0 | PMS on LJ (2 mg/L) | PCR seq | QRDR_A | 86.7 | 61 |
| XDR | 13 | 0 | MIC in solid medium (5 mg/L) | PCR seq | QRDR_A | 85 | 62 |
| ND | 13 | 6 | growth inhibition in liquid culture (ID50) | PCR seq | QRDR_A | 84.6 | 63 |
| Drug resistant | 71 | 179 | ACM (4.8 mg/L) | PCR-SSCP/MPAC + PCR seq | QRDR_A | 78 | 54 |
| ND | 42 | 40 | PMS on LJ (2 mg/L) | LNA-PCR | QRDR_A | 76.2 | 48 |
| ND | 10 | 92 | disc proportion method | pyrosequencing | QRDR_A | 70 | 55 |
| ND | 110 | 0 | MIC (5 mg/L) | PCR seq, DHPLC | QRDR_A | 67.5 | 47 |
| ND | 12 | 0 | PMS (2 mg/L) | PCR seq | QRDR_A | 66.7 | 12 |
| ND | 55 | 83 | ACM on LJ (2 mg/L) | PCR seq | QRDR_A | 58 | 6 |
| ND | 4 | 0 | PMS on LJ + MIC in solid medium (2 mg/L) | PCR seq | QRDR_A | 50 | 11 |
| ND | 1 | 0 | PMS on LJ (2 mg/L) | PCR seq | QRDR_A | ND | 39 |
| ND | 0 | 16 | PML in 7H9 | PCR seq | QRDR_A | ND | 40 |
| ND | 2 | 0 | MIC on LJ | PCR seq | QRDR_A | ND | 64 |
| ND | 2 | 0 | MIC on 7H10 (2 mg/L) | PCR seq | QRDR_A | ND | 38 |
| ND | 3 | 3 | MIC (2 mg/L) | non-radioactive PCR-SSCP, PCR seq | QRDR_A | ND | 41 |
| ND | 16 | 0 | PML, Bactec 460 | PCR-SSCP, PCR seq | QRDR_A | ND | 65 |
| ND | 15 | 39 | PML (Bactec) (2 mg/L) | non-radioactive PCR-SSCP, PCR seq | QRDR_AB | 100 | 3 |
| ND | 4 | 4 | MIC in 7H11 (2 mg/L) | PCR seq | QRDR_AB | 100 | 18 |
| ND | 24 | 28 | PMS on LJ (2 mg/L) | PCR seq + MTBDRsl | QRDR_AB | 100 | 33 |
| MDR | 26 | 49 | PMS (2 mg/L) | PCR seq | QRDR_AB | 96 | 16 |
| FQR and MDR | 48 | 23 | ACM (2 mg/L) | PCR seq | QRDR_AB | 91.7 | 21 |
| Consecutive FQR | 109 | 0 | PMS on LJ (2 mg/L) | PCR seq | QRDR_AB | 82.6 | 42 |
| ND | 60 | 2 | ACM on 7H11 | PCR seq | QRDR_AB | 73.3 | 43 |
| FQR | 35 | 0 | PMS and ACM | PCR seq + SSCP | QRDR_AB | 60 | 8 |
| ND | 10 | 131 | MIC on 7H11 (2 mg/L) | PCR seq | QRDR_AB | 50 | 9 |
| ND | 14 | 28 | MIC in solid medium (2 mg/L) | PCR seq | QRDR_AB | 42.8 | 66 |
| ND | 68 | 0 | PMS (2 mg/L) | PCR seq | QRDR_AB | 10.3 | 10 |
| ND | 48 | 52 | Etest (4 mg/L) | PCR seq | QRDR_AB | 2 | 7 |
| ND | 1 | 0 | MIC in 7H9 (2 mg/L) | PCR seq | QRDR_AB | ND | 45 |
| ND | 3 | 135 | PMS on 7H10 | PCR seq | entire gyrA | 100 | 23 |
| ND | 25 | 15 | PMS on 7H11 (2 mg/L) + REMA | PCR seq | entire gyrA and gyrB | 100 | 26 |
| Total number of isolates | 1220 | 1262 |
ACM, absolute concentration method on Lowenstein–Jensen; AMK, amikacin; CAP, capreomycin; EMB, ethambutol; MIC, MIC by dilution method in solid or liquid medium; ND, no data; No. of FQR, number of fluoroquinolone-resistant strains as defined in the study; No. of FQS, number of fluoroquinolone-susceptible strains as defined in the study; OFX, ofloxacin; PCR seq, PCR and DNA sequencing; PML, proportion method in liquid medium (Bactec 460 or MGIT 960); PMS, proportion method on solid medium (Lowenstein–Jensen, 7H10 or 7H11); QRDR_A, QRDR gyrase A; QRDR_AB, QRDR gyrase A and B; REMA, resazurin microtitre assay.
aSome studies that sequenced the QRDR of gyrA also included some codons near the QRDR, but not the entire gyrA.
There were gyrA or gyrB mutations identified in 806/1220 (66%) phenotypically resistant isolates and 19/1262 (2%) phenotypically susceptible isolates. Specific mutations identified in each gyrA and gyrB gene are described in the following sections.
Among the 2482 isolates tested, 44 distinct mutations were identified. Of the 44 mutations, 26 (59%) were in GyrA and 18 (41%) in GyrB; they occurred at 24 different codons. Of the 44 mutations, 34 (77%) occurred in phenotypically resistant M. tuberculosis isolates, 5 (11%) in phenotypically susceptible isolates and 5 (11%) in both resistant and susceptible isolates. These findings are discussed in greater detail below and in Tables 3–5.
Table 3.
GyrA substitutions reported in fluoroquinolone-resistant and -susceptible clinical M. tuberculosis isolates
 |
The QRDR is enclosed in the bold box and ranges from codon 74 to codon 113.
Substitutions not demonstrated to confer fluoroquinolone resistance in M. tuberculosis are in italics.18
Substitutions demonstrated to confer fluoroquinolone resistance in M. tuberculosis are in bold.18,64
Substitutions not italicized or in bold have not been assessed for conferring resistance.
aPolymorphisms Glu21Gln, Ser95Thr and Gly668Asp were not included.37
Table 4.
GyrB substitutions reported in fluoroquinolone-resistant and -susceptible clinical M. tuberculosis isolates
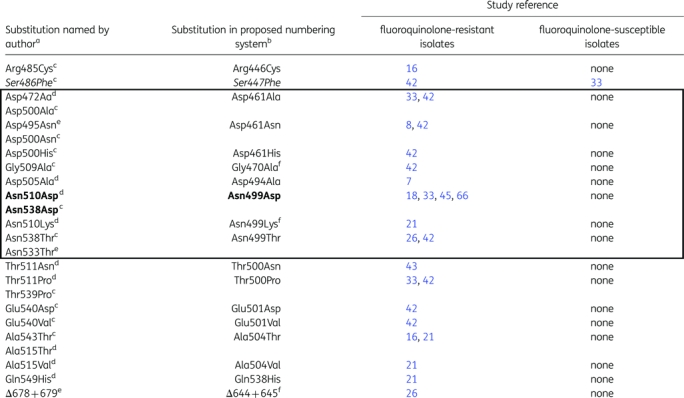 |
The QRDR is enclosed in the bold box.
Substitutions not demonstrated to confer fluoroquinolone resistance in M. tuberculosis are in italics.67
Substitutions demonstrated to confer fluoroquinolone resistance in M. tuberculosis are in bold.18,64
aSee Table 1 for corresponding M. tuberculosis numbering systems.
bSubstitutions are standardized to P0C5C5|1-675,34 where the QRDR ranges from codon 461 to codon 499.
dZhou et al.44
Table 5.
Multiple substitutions in GyrA, GyrB or both GyrA and GyrB reported in fluoroquinolone-resistant and -susceptible clinical M. tuberculosis isolates
| Study reference |
||
|---|---|---|
| Substitutiona | fluoroquinolone-resistant isolatesb | fluoroquinolone-susceptible isolates |
| Multiple substitutions in GyrA | ||
| Ala74Serc + Asp94Gly | 46, 47 | none |
| Thr80Ala + Ala90Glu | 33 | none |
| Thr80Ala + Ala90Glyc | none | 18, 26, 33 |
| Thr80Ala + Ala90Glyc + Asp94Gly | 33 | none |
| Gly88Ala + Ala90Val | 42 | none |
| Gly88Ala + Asp94Tyr | 9 | none |
| Ala90Val + Asp94Ala | 23, 42, 43 | none |
| Ala90Val + Pro102His | 20, 54 | none |
| Ala90Val + Ser91Pro | 17, 46, 47 | none |
| Ala90Val + Asp94Asn | 26, 46, 47 | none |
| Ala90Val + Asp94Gly | 19, 21, 33, 42, 46, 47 | none |
| Ser91Pro + Asp94Gly | 17, 19 | none |
| Ser91Pro + Asp94Gly + Asp94Ala | 42 | none |
| Asp94Ala + Asp94Tyr | 19 | none |
| Asp94Asn + Asp94Gly | 19, 21 | none |
| Asp94Asn + Asp94Gly + Asp94Tyr | 21 | none |
| Asp94Gly + Asp94Ala | 42 | none |
| Multiple substitutions in GyrB | ||
| Asp461His + Gly470Alac | 42 | |
| Multiple substitutions in GyrA and GyrB | ||
| Ala90Val + Asp461Ala | 42 | none |
| Ala90Val + Asn499Thr | 42 | none |
| Ala90Val + Asp94Ala + Asn499Thr | 42 | none |
| Ala90Val + Ser91Pro + Asp94Gly + Asp94Ala + Asn499Thr | 42 | none |
| Ala90Val + Thr500Pro | 42 | none |
| Asp94Ala + Asp461Asn | 42 | none |
| Asp94Gly + Asn499Lysc | 21 | none |
| Asp94Gly + Asn499Thr | 42 | none |
| Asp94Asn + Ala504Val | 21 | none |
| Asp94His + Δ644 + 645c | 26 | none |
Mutations in gyrA
Of the 26 GyrA mutations, 21 (81%) were inside the QRDR and 5 (19%) were outside the QRDR, including 1 (4%) that was in the putative promoter (Table 3). Of the 1220 fluoroquinolone-resistant isolates sequenced, 780 (64%) had mutations inside the QRDR of GyrA and 6 (0.5%) had mutations outside of the QRDR of GyrA (Table 3). Substitutions at codon 94 were the most prevalent; they were reported in 37% of the fluoroquinolone-resistant strains. Various substitutions were reported at codon 94 in GyrA: aspartic acid (Asp) was replaced by alanine (Ala), asparagine (Asn), glycine (Gly), histidine (His), phenylalanine (Phe), tyrosine (Tyr) or valine (Val). The Asp94Gly substitution in GyrA occurred most frequently; it was reported in 234 (19%) resistant isolates. Overall, mutations at codons 90, 91 or 94 were reported in 654 (54%) of the 1220 fluoroquinolone-resistant isolates, with substitutions at codons 90 and 91 occurring in 13% and 4% of the isolates, respectively.
Of the 1262 fluoroquinolone-susceptible isolates studied for GyrA, 14 (1%) had mutations inside the QRDR of GyrA and 2 had a substitution in the putative promoter of GyrA26 (Table 3).
Mutations in gyrB
Of the 18 GyrB mutations, 8 (44%) were inside the QRDR, 9 (50%) were outside the QRDR and 1 (6%) was a deletion also outside the QRDR (Table 4). Although all 42 studies sequenced the GyrA QRDR, only 14 studies sequenced GyrB (mostly the QRDR) (Table 2). Of the 534 resistant isolates studied for gyrB, 17 (3%) harboured mutations in the QRDR and 13 (2%) harboured mutations outside of the QRDR. Ten (2%) isolates had a substitution at codon 499; Asn499Asp (in the proposed numbering system, 2002; Table 1) was reported in 4 (0.7%) of the 10 resistant isolates. In one case, deletion of codons 644 and 645 in GyrB was reported in a fluoroquinolone-resistant strain.26 Among the 377 susceptible strains, there was one substitution reported in GyrB (Ser447Phe in the proposed numbering system; Table 1) outside the QRDR. Codon mutations and associated MICs are presented in Table 6.
Table 6.
Number of M. tuberculosis isolates with specific codon mutations and the fluoroquinolone MICs for these isolates
| Codona | Number of isolates with this mutation | OFX MIC range (mg/L) | MXF MIC range (mg/L) | Study reference |
|---|---|---|---|---|
| Single mutations in gyrA | ||||
| putative promoter | 2 | 1 | ≤0.125 | 26 |
| 8 | 1 | 0.5 | ≤0.125 | 26 |
| 68 | 1 | <2 | 24 | |
| 70 | 2 | 1 | 43b | |
| 80 | 7 | 0.2–>4 | ≤0.125–1 | 18, 25, 26, 40b |
| 88a | 11 | 2–16 | 2 | 8, 17, 21,c33,c48,c54, 60,b64 |
| 89 | 2 | >2–4 | 2 | 26, 48c |
| 90a | 167 | 0.5–20 | 0.25–4 | 3,b6, 8, 9, 10,c11,c12, 16, 17, 18, 19,c20, 21, 23, 24, 26, 27, 31, 33, 42, 43,b46, 47, 48, 53, 54, 55, 61,b62, 63,b,c65, 66c |
| 91a | 44 | 1–64 | 1–2 | 3,b6, 8, 10,c16, 17, 19,c20, 22, 24, 27, 31, 42, 43,b46, 47, 48,c54, 65 |
| 92 | 1 | 4 | 11b | |
| 94a | 447 | 0.5–64 | 0.5–8 | 3,b6, 8, 9, 10, 12, 16, 17, 19, 20, 21,c22, 23, 24, 25,c26, 27, 31, 33,c38, 39, 41, 42, 43,b46, 47, 48,c53, 54, 55, 61,b62, 63,b65,c66c |
| 102 | 1 | ND | 20 | |
| 109 | 1 | 2 | 24 | |
| 126 | 4 | 4.8–8 | 20, 54 | |
| Single mutations in gyrB | ||||
| 446 | 1 | ND | 16 | |
| 447 | 2 | ND | 33, 42 | |
| 461 | 3 | >2–4 | 8, 33,c42 | |
| 494 | 1 | >32 | 7 | |
| 499 | 5 | 1–8 | 0.25–4 | 18, 26, 33,c45, 66 |
| 500 | 2 | 1–>2 | 33,c43b | |
| 501 | 2 | 12 | 42 | |
| 504a | 3 | >2 | 16, 21c | |
| 538 | 1 | >2 | 21c | |
| Multiple mutations in gyrA | ||||
| 74 + 94 | 21 | 2–32 | 46, 47 | |
| 80 + 90 | 7 | <0.25–>2 | <0.12–0.25 | 18, 26, 33 |
| 80 + 90 + 94 | 1 | >2 | 33c | |
| 88 + 90 | 1 | ND | 42 | |
| 88 + 94 | 1 | >2 | 9c | |
| 90 + 91 | 11 | 2–20 | 17, 46, 47 | |
| 90 + 94a | 48 | 12–32 | >8 | 19,c21,c23, 26, 33c42,c43,b46, 47 |
| 90 + 102 | 3 | 4–4.8 | 20, 54 | |
| 91 + 94 | 2 | >2 | 17, 19 | |
| 91 + 94 + 94 | 1 | ND | 42 | |
| 94 + 94a | 6 | >2–12 | 19,c21,c42 | |
| 94 + 94 + 94a | 1 | >2 | 21c | |
| Multiple mutations in gyrB | ||||
| 461 + 470a | 1 | ND | 42 | |
| Multiple mutations in gyrA and gyrB | ||||
| 90 + 461a | 1 | 8 | 42 | |
| 90 + 499a | 1 | ND | 42 | |
| 90 + 94 + 499a | 1 | ND | 42 | |
| 90 + 91 + 94 + 94 + 499a | 1 | ND | 42 | |
| 90 + 500 | 1 | >12 | 42 | |
| 94 + 461a | 1 | ND | 42 | |
| 94 + 499a | 2 | >2 | 21,c42 | |
| 94 + 504 | 1 | >2 | 21c | |
| 94 + Δ644 + 645 | 1 | 4 | 2 | 26 |
CIP, ciprofloxacin; LVX, levofloxacin; MXF, moxifloxacin; ND, not determined; OFX, ofloxacin.
aCodon in which heterogeneity was reported.
bStudies in which MIC was measured for levofloxacin and/or ciprofloxacin. In Takiff et al.,3 Bozeman et al.,61 Xu et al.63 and Soudani et al.,11 MIC was measured only for ciprofloxacin; in Perlman et al.60 and Yin and Yu,43 MIC was measured only for levofloxacin.
cStudies in which MIC is not specified for every distinct mutation.
Multiple mutations and heteroresistance
Several studies reported multiple mutations in gyrA, gyrB or in both gyrA and gyrB (Table 5), with up to five mutations in the same strain.42 Double mutations in gyrA have often been described as two mutations in the same gyrA allele and have been associated with high-level resistance.36 They may result from a two-step selection of fluoroquinolone-resistant mutants.18 The combination of Ala90Val with Asp94Gly is frequently associated with high-level resistance and was reported in 34 (3%) of the fluoroquinolone-resistant isolates.19,21,33,42,46,47 The combination of Thr80Ala with Ala90Gly, which was demonstrated to confer fluoroquinolone susceptibility18 was the most frequent double mutation reported among fluoroquinolone-susceptible isolates [6 (0.5%) isolates] (Tables 5–7).
Table 7.
GyrA and GyrB substitutions demonstrated and not demonstrated to confer fluoroquinolone resistance in M. tuberculosis
| Gyrase subunit alteration |
IC50 (mg/L) |
|||
|---|---|---|---|---|
| GyrA | GyrB | OFX | MXF | Study reference |
| WT | WT | 2–10 | 1–2 | 18, 37 |
| Thr80Ala | WT | 5 | 1 | 18 |
| Ala90Gly | WT | 10 | 2 | 18 |
| Thr80Ala + Ala90Gly | WT | 2.5 | 0.5 | 18 |
| Glu21Gln + Ser95Thr + Gly668Asp + Ala74Ser | WT | 16 | 14 | 37 |
| Gly88Ala | WT | 40 | 10 | 64 |
| Gly88Cys | WT | 50 | 35 | 64 |
| Ala90Val | WT | 100 | 35 | 18 |
| Asp94Gly | WT | 350 | 50 | 18 |
| Asp94His | WT | 800 | 90 | 18 |
| Ala90Val + Asp94Gly | WT | >1600 | >160 | 18 |
| WT | Asn499Asp | 120 | 35 | 18 |
IC50, 50% inhibitory concentration (measured by inhibition of 50% of DNA supercoiling); MXF, moxifloxacin; OFX, ofloxacin; WT, wild-type.
Substitutions not demonstrated to confer fluoroquinolone resistance in M. tuberculosis are in italics.
Substitutions demonstrated to confer fluoroquinolone resistance in M. tuberculosis are in bold.
In several studies, sequencing showed heterogeneous peaks in up to 31.2% of the isolates.19,42,48 Such heteroresistance refers to the concomitant presence of susceptible and resistant bacilli in the same clinical specimen or the presence of several clones, each harbouring a distinct mutation.49 It probably results from a strain splitting into two or more bacterial clones or less likely from infection by two different strains.50
Mutations that conferred fluoroquinolone resistance (rather than simply being present in fluoroquinolone-resistant M. tuberculosis isolates) were identified based on biochemical studies demonstrating that the altered DNA gyrase subunit was resistant to fluoroquinolone inhibition. Table 7 lists the mutations that have been demonstrated to confer fluoroquinolone resistance.
Discussion
Because M. tuberculosis is a slow-growing bacterium, the detection of drug resistance by traditional methods requires 5–12weeks. Several studies have demonstrated that molecular tests facilitate rapid diagnosis of resistance in M. tuberculosis, including MDR-TB and XDR-TB, which can occur 2–3 months faster than with conventional susceptibility testing.51 To maximize the sensitivity and specificity of molecular testing for fluoroquinolone resistance in M. tuberculosis, it is critical to have comprehensive knowledge of the fluoroquinolone resistance mutations.
Because the primary mechanism of fluoroquinolone resistance in M. tuberculosis occurs via modification of DNA gyrase, it is important to (i) summarize all mutations described in DNA gyrase genes of M. tuberculosis and (ii) clarify the role of these mutations in fluoroquinolone resistance.
Our review differs from the compilation of mutations in tbdream,35 in that our review includes all mutations reported outside the QRDR of gyrA and gyrB (including heterogeneity at codons), addresses the four different gyrB numbering systems, provides the prevalence of common resistance mutations, notes which mutations have been demonstrated to confer resistance and provides the range of MICs reported for mutations at each codon.
We needed first to clarify the discrepancies of the GyrB numbering system to be able to differentiate novel from previously described mutations. We propose that the GyrB sequence that was re-annotated in 200234 should be used as the reference sequence (Table 1 and Figure S3).
Having clarified the numbering system of GyrB, we reviewed all the mutations described in the DNA gyrase genes of clinical M. tuberculosis isolates.
Mutations at GyrA codons 90, 91 or 94 were most common,3,6–8,38,52 and seen in 654 (54%) resistant isolates. Although a majority of fluoroquinolone-resistant M. tuberculosis isolates had mutations in these codons, a substantial proportion did not. This suggests that the sensitivity of line-probe assays or other molecular diagnostic tests that focus on these codons could have low sensitivity. However, the isolates in this systematic review, although representing all fluoroquinolone-resistant M. tuberculosis isolates reported to date, and encompassing multiple countries of the world (e.g. China, France, India, Russia, the USA, Uzbekistan and Vietnam), may differ from population-based studies.
Based on our review, the most widely used molecular method to detect fluoroquinolone resistance in M. tuberculosis was PCR and DNA sequencing (Table 2). Other, more rapid testing methods included multiplex allele-specific PCR (MAS-PCR),53 PCR single-stranded conformation polymorphism/multiplex PCR amplimer conformation (PCR-SSCP/MPAC),54 pyrosequencing,55 denaturing HPLC (DHPLC),47 non-radioactive PCR-SSCP,3,8,41 locked nucleic acid probe real-time PCR (LNA-PCR)48 and GenoType® MTBDRsl (Hain Lifescience) line probe.19,31,33 These rapid genotypic assays detect fluoroquinolone resistance in isolated cultures of M. tuberculosis,28 and less frequently, directly in respiratory specimens.51
Knowledge of all mutations that are involved in fluoroquinolone resistance will help in the design of more sensitive and specific rapid molecular tests. We based our assessment of specific mutations that confer fluoroquinolone resistance on biochemical studies demonstrating that the altered DNA gyrase subunit was resistant to fluoroquinolone inhibition (Table 7). Discrepancies between the results of drug susceptibility testing and biochemical studies can exist since clinical isolates may harbour additional mutations or resistance mechanisms. There are several fluoroquinolone resistance mechanisms in bacteria besides mutations in the gyrA and gyrB genes. These include mutations in the parC and parE genes, enhancement of efflux pumps and plasmid-mediated mechanisms such as qnr genes encoding pentapeptide repeat proteins, aac(6′)-Ib-cr encoding a variant aminoglycoside acetyltransferase and oqxAB and qepA encoding efflux pumps.5 For example, biochemical studies on mutant DNA gyrases have demonstrated that the GyrA mutations Thr80Ala and Ala90Gly, and GyrB mutation Ser447Phe do not confer resistance, but these mutations were found in resistant isolates. This may have been because the isolates were misclassified or resistance was conferred by another mechanism (Tables 3, 4 and 7).18,25,33,42 On the other hand, it is highly surprising to observe that some strains carrying mutations that have previously been demonstrated to confer fluoroquinolone resistance are susceptible to fluoroquinolones (Tables 3 and 7).17,54 Such discrepancies may be due to misclassification of the strain as susceptible, an error in the molecular detection of resistance or the presence of a mixture of strains. For example, in a specimen that harbours mutations leading to GyrA Ala90Val, Asp94Ala and wild-type alleles, results of phenotypic susceptibility testing may have reflected only the wild-type strain.
It is likely that all substitutions observed at codon 94 in GyrA confer fluoroquinolone resistance, even if biochemical tests have not been performed for all substitutions described at this codon. Similarly, it is likely that substitutions at codon 91, reported in fluoroquinolone-resistant strains in 19 studies, are responsible for fluoroquinolone resistance in M. tuberculosis (Tables 3 and 6). However, less frequently described mutations such as His70Arg, Asp89Asn and Ile92Met in GyrA and most GyrB mutations (except Ser447Phe and Asn499Asp previously studied) deserve biochemical studies to determine whether or not they confer fluoroquinolone resistance. It is especially important since it has been shown that some mutations may confer high levels of resistance or hypersusceptibility depending on the amino acid substitution.18 Indeed, it has been previously demonstrated that when alanine at codon 90 of GyrA is replaced by valine, the fluoroquinolone affinity of the resulting enzyme decreases compared with the wild-type, resulting in resistance. However, when it is replaced by glycine the fluoroquinolone affinity of the resulting enzyme increases, resulting in susceptibility (Table 7).18
Although the majority of the described mutations occur in the QRDR of GyrA and GyrB (Tables 3–5), some substitutions were described outside of the QRDR. Further studies are necessary to characterize the significance and relevance of these mutations, especially in GyrB, where they are seen more frequently. The GyrB QRDR may need to be redefined if it is determined that substitutions at codons 500–504 (in the proposed numbering system, 2002; Table 1) are implicated in fluoroquinolone resistance.
In several studies, gyrA or gyrB mutations were revealed as heteropeaks, which are a combination of either wild-type and mutated alleles, or different mutated alleles.19,21,42,46 Heteropeaks in fluoroquinolone-resistant M. tuberculosis have been described in detail previously.48 In contrast to most bacteria, M. tuberculosis acquisition of drug resistance does not occur as a result of horizontal transfer of resistance-bearing genetic elements.56 Selection of drug resistance during treatment of TB results in a mixture of subpopulations of bacilli, some of which bear different genetic mutations that account for the drug resistance, whereas others are drug susceptible. Consequently, the simultaneous presence of drug-resistant and drug-susceptible bacilli, also designated as heteroresistance, may be found in sputum.56 These heteroresistant isolates are distinct from isolates with multiple mutations. In fact, fluoroquinolone MICs for isolates with multiple mutations are much higher than those for strains harbouring one mutation, even if presented as a polyclonal population (Table 6). In the presence of anti-TB drugs, drug-resistant bacilli would be expected to outgrow drug-susceptible bacilli over time.
New WHO guidelines include the use of moxifloxacin for the treatment of XDR-TB patients.57 Although there is cross-resistance within the fluoroquinolone group, moxifloxacin MICs are usually lower than those of other fluoroquinolones (Table 6),18,20,58 making it a potentially effective drug to treat ofloxacin-resistant TB. The WHO recommendation is supported by a few clinical observations,59 and also by experiments performed in murine models.58
There were some limitations of this review. First, research studies published in languages other than English were not included. Given that some mutations have been reported more frequently in certain populations, we may have excluded mutations associated with fluoroquinolone resistance simply by excluding non-English studies. Second, genotyping lineage information was only available for 10 of the 42 studies reviewed; this information would have been useful in differentiating the polymorphisms predominant in a specific lineage. Third, MEDLINE was the only database searched. Although it contains more than 20 million citations, other databases may contain publications that would have met the inclusion criteria for this study.
This extensive review of mutations described in the gyrA and gyrB genes in conjunction with the proposed consensus numbering system will facilitate the identification of novel mutations in M. tuberculosis isolates. New molecular testing methods may benefit from this information to enhance detection of fluoroquinolone-resistant M. tuberculosis.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (R01 AI 063200, K24 AI 65298, T32 AI 07474) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) research grant 2010.
Transparency declarations
Conflicts of interest: none to declare.
The study sponsors did not contribute to the design, conduct, interpretation or writing of the article for this project.
Author contributions
F. M., A. W. K. and A. A. performed the literature review. F. M. wrote the first draft of the article. T. R. S. designed the study and provided substantial input in interpreting the data and writing the article. A. B., Y. F. van der H. and C. M. assisted with the literature review and cross-referencing of information. E. C. and A. A. provided substantial input in interpreting the data and writing the article.
Supplementary data
Acknowledgements
We thank Wladimir Sougakoff and Stéphanie Petrella for helpful discussions regarding sequence alignments and corresponding numbering systems between E. coli and M. tuberculosis GyrA and GyrB. We also thank Alix Pantel for her help in searching studies reporting gyrA and gyrB mutations.
References
- 1.American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1–77. [PubMed] [Google Scholar]
- 2.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 3.Takiff HE, Salazar L, Guerrero C, et al. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob Agents Chemother. 1994;38:773–80. doi: 10.1128/aac.38.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mdluli K, Ma Z. Mycobacterium tuberculosis DNA gyrase as a target for drug discovery. Infect Disord Drug Targets. 2007;7:159–68. doi: 10.2174/187152607781001763. [DOI] [PubMed] [Google Scholar]
- 5.Hooper D, Rubinstein E. Quinolone Antimicrobial Agents. Washington, DC: ASM Press; 2008. [Google Scholar]
- 6.Cheng AF, Yew WW, Chan EW, et al. Multiplex PCR amplimer conformation analysis for rapid detection of gyrA mutations in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2004;48:596–601. doi: 10.1128/AAC.48.2.596-601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AS, Tang LL, Lim IH, et al. Characterization of pyrazinamide and ofloxacin resistance among drug resistant Mycobacterium tuberculosis isolates from Singapore. Int J Infect Dis. 2002;6:48–51. doi: 10.1016/s1201-9712(02)90136-0. [DOI] [PubMed] [Google Scholar]
- 8.Pitaksajjakul P, Wongwit W, Punprasit W, et al. Mutations in the gyrA and gyrB genes of fluoroquinolone-resistant Mycobacterium tuberculosis from TB patients in Thailand. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 4):228–37. [PubMed] [Google Scholar]
- 9.Huang TS, Kunin CM, Shin-Jung Lee S, et al. Trends in fluoroquinolone resistance of Mycobacterium tuberculosis complex in a Taiwanese medical centre: 1995–2003. J Antimicrob Chemother. 2005;56:1058–62. doi: 10.1093/jac/dki353. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi N, Shamim M, Hussain S, et al. Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in North India. Antimicrob Agents Chemother. 2002;46:443–50. doi: 10.1128/AAC.46.2.443-450.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soudani A, Hadjfredj S, Zribi M, et al. First report of molecular characterization of fluoroquinolone-resistant Mycobacterium tuberculosis isolates from a Tunisian hospital. Clin Microbiol Infect. 2010;16:1454–7. doi: 10.1111/j.1469-0691.2009.03087.x. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan EA, Kreiswirth BN, Palumbo L, et al. Emergence of fluoroquinolone-resistant tuberculosis in New York City. Lancet. 1995;345:1148–50. doi: 10.1016/s0140-6736(95)90980-x. [DOI] [PubMed] [Google Scholar]
- 13.Hegde SS, Vetting MW, Roderick SL, et al. A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science. 2005;308:1480–3. doi: 10.1126/science.1110699. [DOI] [PubMed] [Google Scholar]
- 14.Pasca MR, Guglierame P, Arcesi F, et al. Rv2686c-Rv2687c-Rv2688c, an ABC fluoroquinolone efflux pump in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2004;48:3175–8. doi: 10.1128/AAC.48.8.3175-3178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takiff HE, Cimino M, Musso MC, et al. Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc Natl Acad Sci USA. 1996;93:362–6. doi: 10.1073/pnas.93.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerriegel S, Cox HS, Zarkua N, et al. Sequence analyses of just four genes to detect extensively drug-resistant Mycobacterium tuberculosis strains in multidrug-resistant tuberculosis patients undergoing treatment. Antimicrob Agents Chemother. 2009;53:3353–6. doi: 10.1128/AAC.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonova OV, Gryadunov DA, Lapa SA, et al. Detection of mutations in Mycobacterium tuberculosis genome determining resistance to fluoroquinolones by hybridization on biological microchips. Bull Exp Biol Med. 2008;145:108–13. doi: 10.1007/s10517-008-0034-5. [DOI] [PubMed] [Google Scholar]
- 18.Aubry A, Veziris N, Cambau E, et al. Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob Agents Chemother. 2006;50:104–12. doi: 10.1128/AAC.50.1.104-112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillemann D, Rusch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2009;47:1767–72. doi: 10.1128/JCM.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kam KM, Yip CW, Cheung TL, et al. Stepwise decrease in moxifloxacin susceptibility amongst clinical isolates of multidrug-resistant Mycobacterium tuberculosis: correlation with ofloxacin susceptibility. Microb Drug Resist. 2006;12:7–11. doi: 10.1089/mdr.2006.12.7. [DOI] [PubMed] [Google Scholar]
- 21.Mokrousov I, Otten T, Manicheva O, et al. Molecular characterization of ofloxacin-resistant Mycobacterium tuberculosis strains from Russia. Antimicrob Agents Chemother. 2008;52:2937–9. doi: 10.1128/AAC.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perdigao J, Macedo R, Malaquias A, et al. Genetic analysis of extensively drug-resistant Mycobacterium tuberculosis strains in Lisbon, Portugal. J Antimicrob Chemother. 2010;65:224–7. doi: 10.1093/jac/dkp452. [DOI] [PubMed] [Google Scholar]
- 23.Sekiguchi J, Miyoshi-Akiyama T, Augustynowicz-Kopec E, et al. Detection of multidrug resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2007;45:179–92. doi: 10.1128/JCM.00750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sulochana S, Narayanan S, Paramasivan CN, et al. Analysis of fluoroquinolone resistance in clinical isolates of Mycobacterium tuberculosis from India. J Chemother. 2007;19:166–71. doi: 10.1179/joc.2007.19.2.166. [DOI] [PubMed] [Google Scholar]
- 25.Umubyeyi AN, Rigouts L, Shamputa IC, et al. Limited fluoroquinolone resistance among Mycobacterium tuberculosis isolates from Rwanda: results of a national survey. J Antimicrob Chemother. 2007;59:1031–3. doi: 10.1093/jac/dkm038. [DOI] [PubMed] [Google Scholar]
- 26.Von Groll A, Martin A, Jureen P, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob Agents Chemother. 2009;53:4498–500. doi: 10.1128/AAC.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams KJ, Chan R, Piddock LJ. gyrA of ofloxacin-resistant clinical isolates of Mycobacterium tuberculosis from Hong Kong. J Antimicrob Chemother. 1996;37:1032–4. doi: 10.1093/jac/37.5.1032. [DOI] [PubMed] [Google Scholar]
- 28.Chang KC, Yew WW, Chan RC. Rapid assays for fluoroquinolone resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2010;65:1551–61. doi: 10.1093/jac/dkq202. [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Espinal MA, Abe C, et al. Is second-line anti-tuberculosis drug susceptibility testing reliable? Int J Tuberc Lung Dis. 2004;8:1157–8. [PubMed] [Google Scholar]
- 30.WHO. Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. Geneva: WHO; 2010. http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. (13 June 2011, date last accessed) [Google Scholar]
- 31.Giannoni F, Iona E, Sementilli F, et al. Evaluation of a new line probe assay for rapid identification of gyrA mutations in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005;49:2928–33. doi: 10.1128/AAC.49.7.2928-2933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiet VS, Lan NT, An DD, et al. Evaluation of the MTBDRsl test for detection of second-line-drug resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2010;48:2934–9. doi: 10.1128/JCM.00201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brossier F, Veziris N, Aubry A, et al. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:1683–9. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camus JC, Pryor MJ, Medigue C, et al. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148:2967–73. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- 35.Sandgren A, Strong M, Muthukrishnan P, et al. Tuberculosis drug resistance mutation database. PLoS Med. 2009;6:e2. doi: 10.1371/journal.pmed.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocagoz T, Hackbarth CJ, Unsal I, et al. Gyrase mutations in laboratory-selected, fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob Agents Chemother. 1996;40:1768–74. doi: 10.1128/aac.40.8.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau RW, Ho PL, Kao RY, et al. Molecular characterization of fluoroquinolone resistance in Mycobacterium tuberculosis: functional analysis of gyrA mutation at position 74. Antimicrob Agents Chemother. 2010;55:608–14. doi: 10.1128/AAC.00920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alangaden GJ, Manavathu EK, Vakulenko SB, et al. Characterization of fluoroquinolone-resistant mutant strains of Mycobacterium tuberculosis selected in the laboratory and isolated from patients. Antimicrob Agents Chemother. 1995;39:1700–3. doi: 10.1128/aac.39.8.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cambau E, Sougakoff W, Besson M, et al. Selection of a gyrA mutant of Mycobacterium tuberculosis resistant to fluoroquinolones during treatment with ofloxacin. J Infect Dis. 1994;170:479–83. doi: 10.1093/infdis/170.2.479. [DOI] [PubMed] [Google Scholar]
- 40.Mitarai S. Mycobacterium tuberculosis and gyrA variation in Zambia. Trop Med Health. 2005;33:91–4. [Google Scholar]
- 41.Sougakoff W, Lemaitre N, Cambau E, et al. Nonradioactive single-strand conformation polymorphism analysis for detection of fluoroquinolone resistance in mycobacteria. Eur J Clin Microbiol Infect Dis. 1997;16:395–8. doi: 10.1007/BF01726372. [DOI] [PubMed] [Google Scholar]
- 42.Duong DA, Nguyen TH, Nguyen TN, et al. Beijing genotype of Mycobacterium tuberculosis is significantly associated with high-level fluoroquinolone resistance in Vietnam. Antimicrob Agents Chemother. 2009;53:4835–9. doi: 10.1128/AAC.00541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin X, Yu Z. Mutation characterization of gyrA and gyrB genes in levofloxacin-resistant Mycobacterium tuberculosis clinical isolates from Guangdong Province in China. J Infect. 2010;61:150–4. doi: 10.1016/j.jinf.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Dong Y, Zhao X, et al. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J Infect Dis. 2000;182:517–25. doi: 10.1086/315708. [DOI] [PubMed] [Google Scholar]
- 45.Veziris N, Martin C, Brossier F, et al. Treatment failure in a case of extensively drug-resistant tuberculosis associated with selection of a GyrB mutant causing fluoroquinolone resistance. Eur J Clin Microbiol Infect Dis. 2007;26:423–5. doi: 10.1007/s10096-007-0298-0. [DOI] [PubMed] [Google Scholar]
- 46.Shi R, Zhang J, Li C, et al. Emergence of ofloxacin resistance in Mycobacterium tuberculosis clinical isolates from China as determined by gyrA mutation analysis using denaturing high-pressure liquid chromatography and DNA sequencing. J Clin Microbiol. 2006;44:4566–8. doi: 10.1128/JCM.01916-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Z, Zhang J, Zhang X, et al. Comparison of gyrA gene mutations between laboratory-selected ofloxacin-resistant Mycobacterium tuberculosis strains and clinical isolates. Int J Antimicrob Agents. 2008;31:115–21. doi: 10.1016/j.ijantimicag.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 48.van Doorn HR, An DD, de Jong MD, et al. Fluoroquinolone resistance detection in Mycobacterium tuberculosis with locked nucleic acid probe real-time PCR. Int J Tuberc Lung Dis. 2008;12:736–42. [PubMed] [Google Scholar]
- 49.Rinder H, Mieskes KT, Loscher T. Heteroresistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2001;5:339–45. [PubMed] [Google Scholar]
- 50.Hofmann-Thiel S, van Ingen J, Feldmann K, et al. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur Respir J. 2009;33:368–74. doi: 10.1183/09031936.00089808. [DOI] [PubMed] [Google Scholar]
- 51.Lau RW, Ho PL, Kao RY, et al. Rapid diagnosis of multidrug-resistant smear-positive pulmonary tuberculosis. Int J Antimicrob Agents. 2010;35:202–3. doi: 10.1016/j.ijantimicag.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13:1320–30. [PubMed] [Google Scholar]
- 53.Evans J, Segal H. Novel multiplex allele-specific PCR assays for the detection of resistance to second-line drugs in Mycobacterium tuberculosis. J Antimicrob Chemother. 2010;65:897–900. doi: 10.1093/jac/dkq047. [DOI] [PubMed] [Google Scholar]
- 54.Chan RC, Hui M, Chan EW, et al. Genetic and phenotypic characterization of drug-resistant Mycobacterium tuberculosis isolates in Hong Kong. J Antimicrob Chemother. 2007;59:866–73. doi: 10.1093/jac/dkm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bravo LT, Tuohy MJ, Ang C, et al. Pyrosequencing for rapid detection of Mycobacterium tuberculosis resistance to rifampin, isoniazid, and fluoroquinolones. J Clin Microbiol. 2009;47:3985–90. doi: 10.1128/JCM.01229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Post FA, Willcox PA, Mathema B, et al. Genetic polymorphism in Mycobacterium tuberculosis isolates from patients with chronic multidrug-resistant tuberculosis. J Infect Dis. 2004;190:99–106. doi: 10.1086/421501. [DOI] [PubMed] [Google Scholar]
- 57.WHO. Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. Geneva: WHO; 2008. http://apps.who.int/bookorders/anglais/detart1.jsp?sesslan=&codlan=&codcol=15&codcch=2663. (13 June 2011, date last accessed) [Google Scholar]
- 58.Poissy J, Aubry A, Fernandez C, et al. Should moxifloxacin be used for the treatment of extensively drug-resistant tuberculosis? An answer from a murine model. Antimicrob Agents Chemother. 2010;54:4765–71. doi: 10.1128/AAC.00968-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feasey NA, Pond M, Coleman D, et al. Moxifloxacin and pyrazinamide susceptibility testing in a complex case of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2011;15:417–20. [PubMed] [Google Scholar]
- 60.Perlman DC, El Sadr WM, Heifets LB, et al. Susceptibility to levofloxacin of Mycobacterium tuberculosis isolates from patients with HIV-related tuberculosis and characterization of a strain with levofloxacin monoresistance. Community Programs for Clinical Research on AIDS 019 and the AIDS Clinical Trials Group 222 Protocol Team. AIDS. 1997;11:1473–8. doi: 10.1097/00002030-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Bozeman L, Burman W, Metchock B, et al. Fluoroquinolone susceptibility among Mycobacterium tuberculosis isolates from the United States and Canada. Clin Infect Dis. 2005;40:386–91. doi: 10.1086/427292. [DOI] [PubMed] [Google Scholar]
- 62.Sun Z, Chao Y, Zhang X, et al. Characterization of extensively drug-resistant Mycobacterium tuberculosis clinical isolates in China. J Clin Microbiol. 2008;46:4075–7. doi: 10.1128/JCM.00822-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu C, Kreiswirth BN, Sreevatsan S, et al. Fluoroquinolone resistance associated with specific gyrase mutations in clinical isolates of multidrug-resistant Mycobacterium tuberculosis. J Infect Dis. 1996;174:1127–30. doi: 10.1093/infdis/174.5.1127. [DOI] [PubMed] [Google Scholar]
- 64.Matrat S, Veziris N, Mayer C, et al. Functional analysis of DNA gyrase mutant enzymes carrying mutations at position 88 in the A subunit found in clinical strains of Mycobacterium tuberculosis resistant to fluoroquinolones. Antimicrob Agents Chemother. 2006;50:4170–3. doi: 10.1128/AAC.00944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siddiqi N, Shamim M, Jain NK, et al. Molecular genetic analysis of multi-drug resistance in Indian isolates of Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 1998;93:589–94. doi: 10.1590/s0074-02761998000500006. [DOI] [PubMed] [Google Scholar]
- 66.Wang JY, Lee LN, Lai HC, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates: associated genetic mutations and relationship to antimicrobial exposure. J Antimicrob Chemother. 2007;59:860–5. doi: 10.1093/jac/dkm061. [DOI] [PubMed] [Google Scholar]
- 67.Pantel A, Brossier F, Bastian S. Mutations in the GyrB subunit of clinical Mycobacterium tuberculosis strains: consequences on resistance to fluoroquinolones. Abstracts of the Fiftieth Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010; Washington, DC, USA: American Society for Microbiology; Abstract C1-085. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


