Abstract
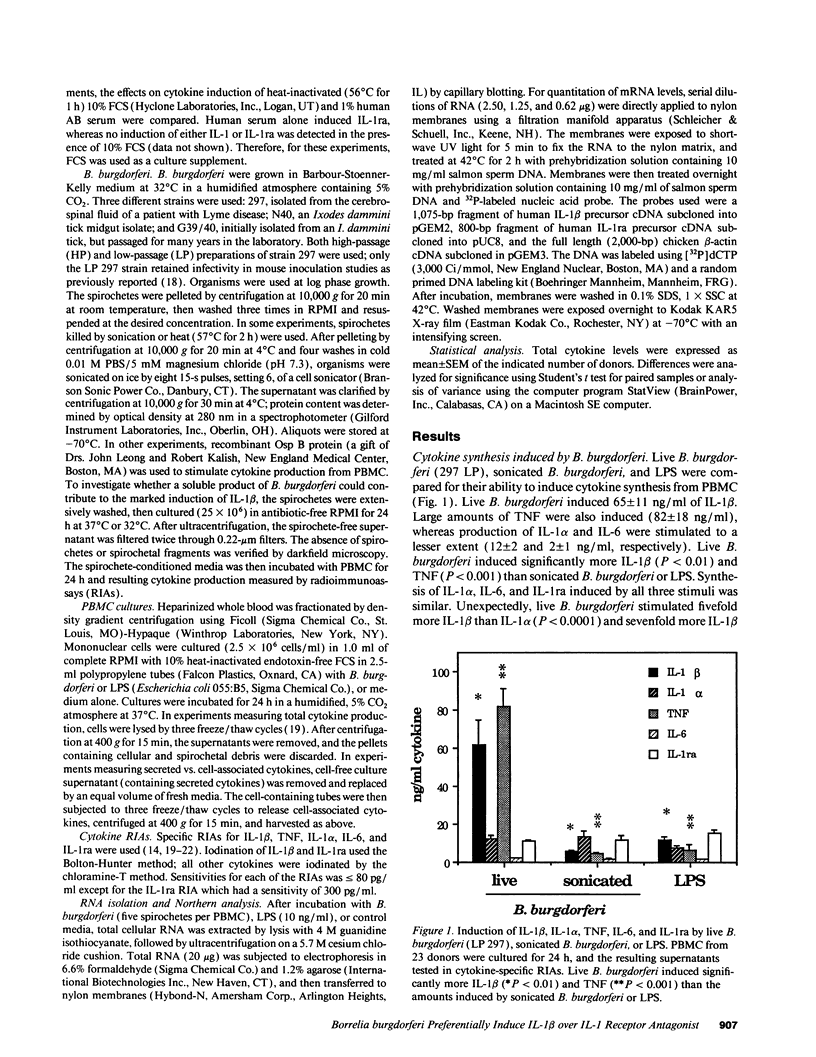
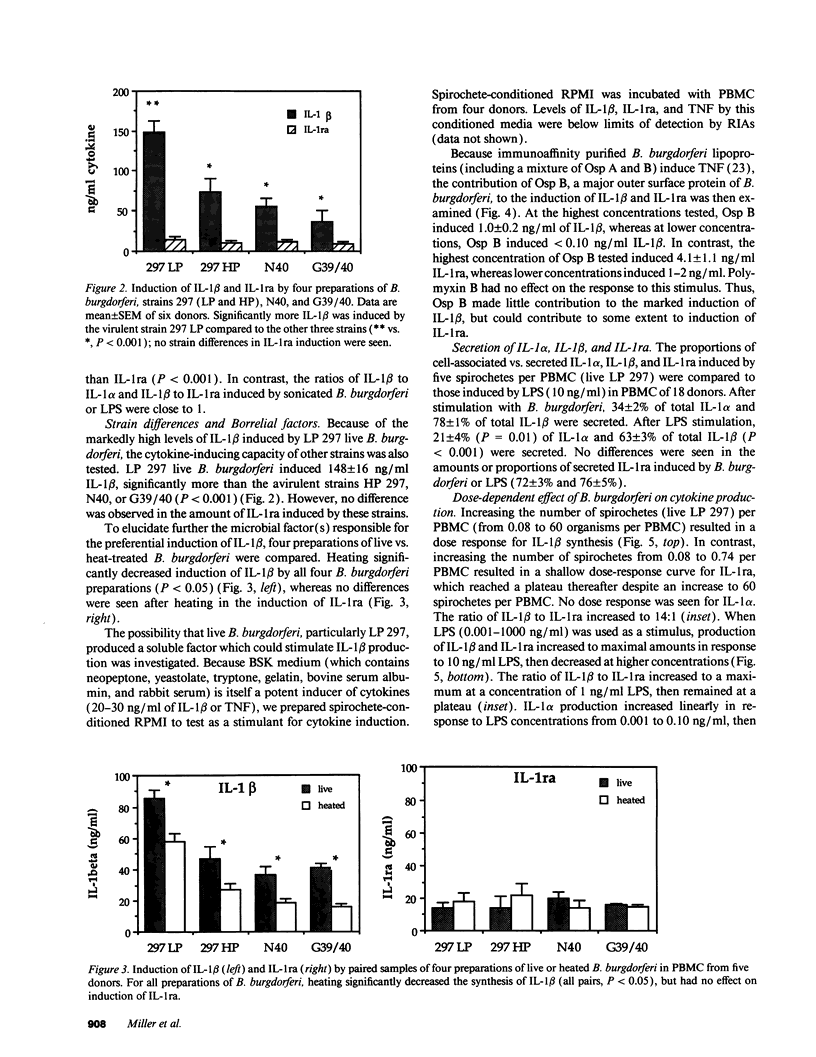
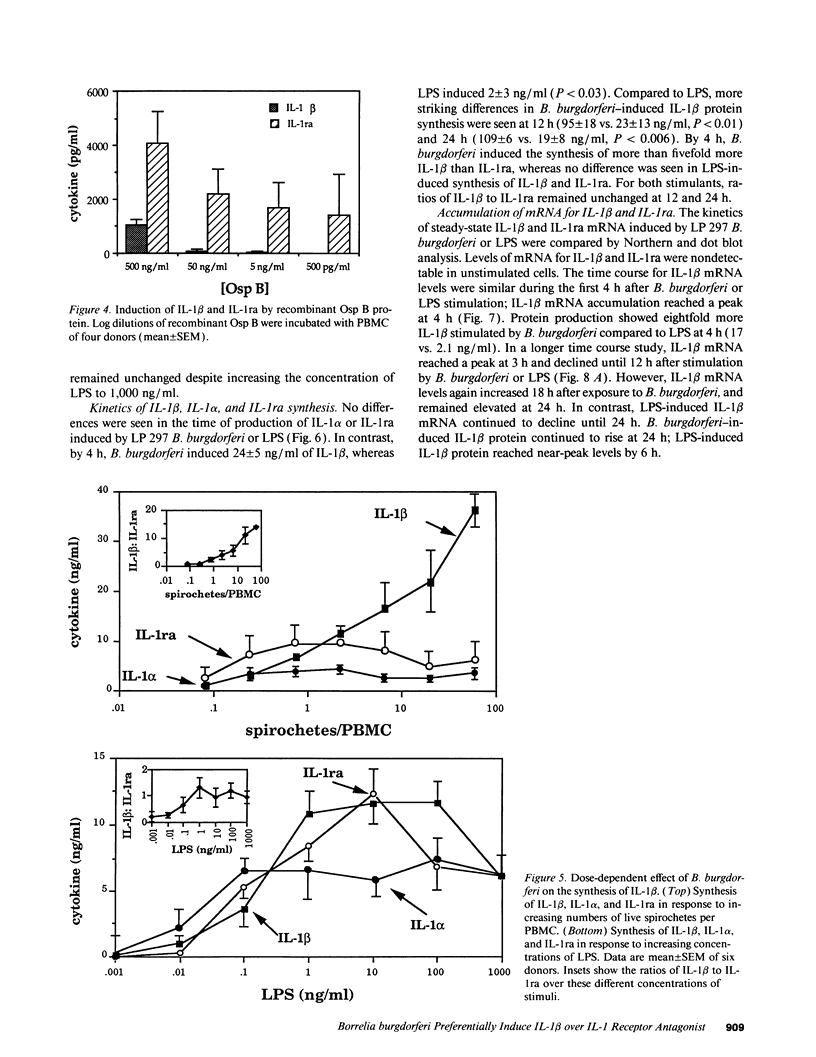
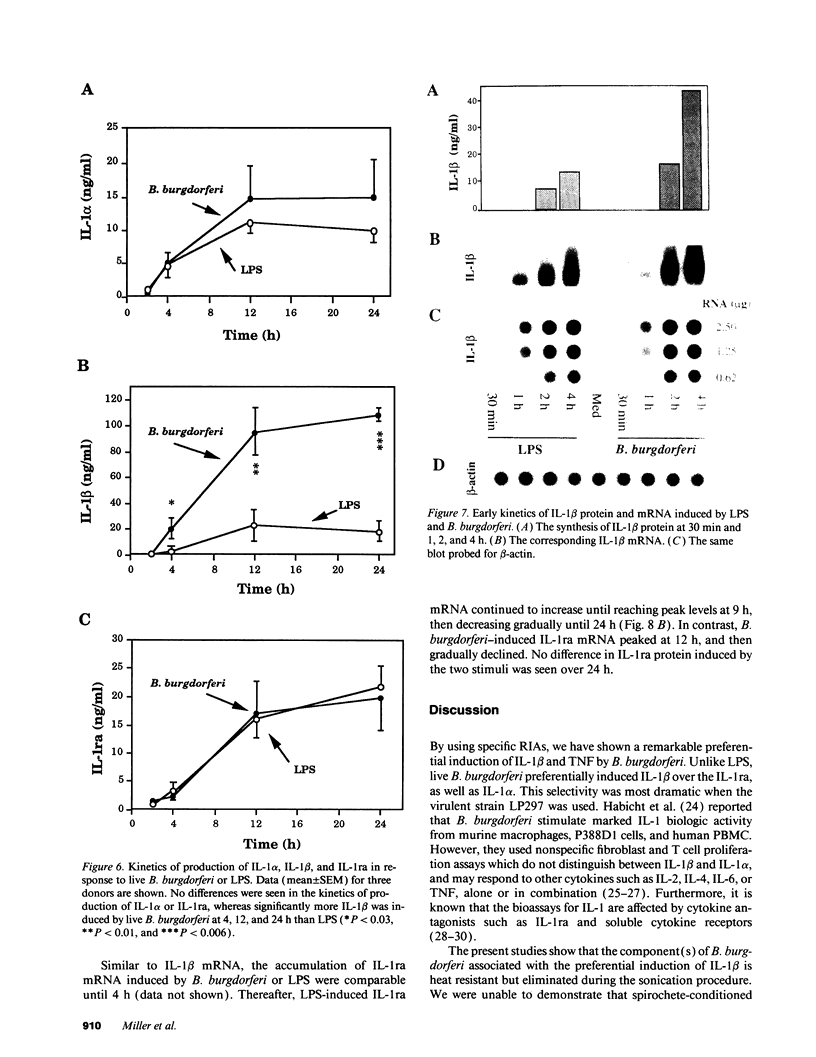
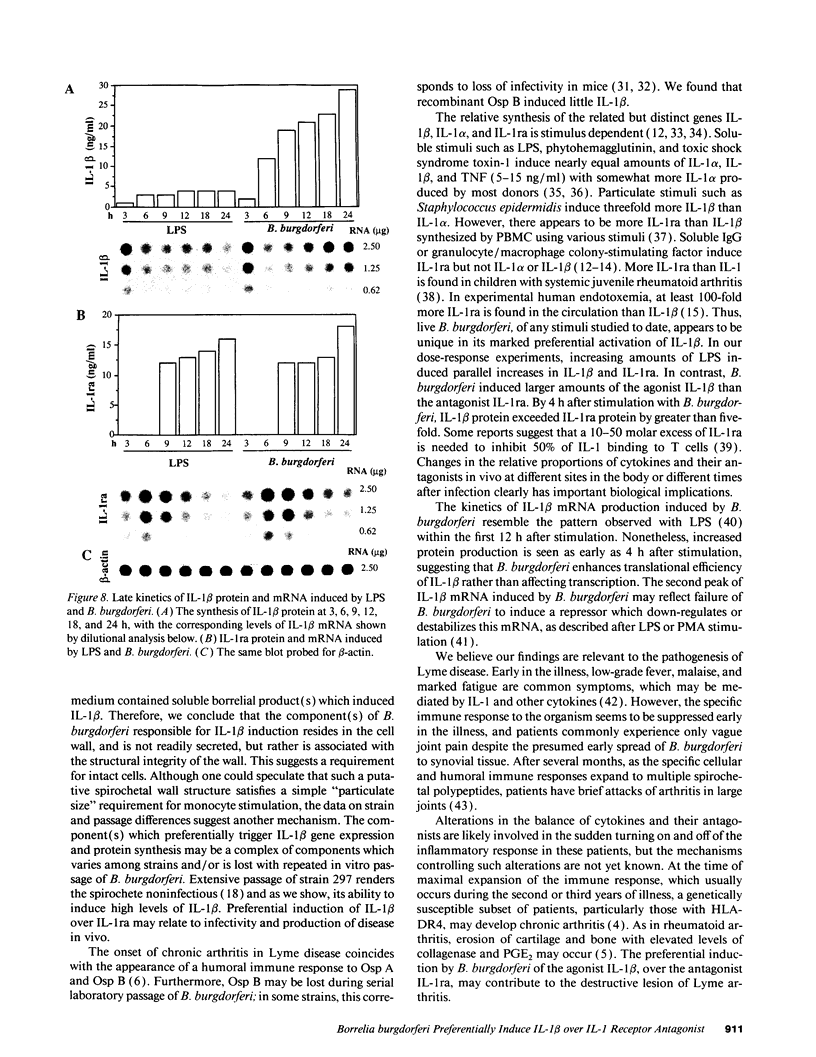
Lyme arthritis is one of the few forms of chronic arthritis in which the cause is known with certainty. Because cytokines are thought to contribute to the pathogenesis of chronic arthritis, we investigated the effect of the Lyme disease spirochete, Borrelia burgdorferi, on the gene expression and synthesis of IL-1 beta and the IL-1 receptor antagonist (IL-1ra) in human peripheral blood mononuclear cells. Live B. burgdorferi induced fivefold more IL-1 beta than IL-1 alpha and sevenfold more IL-1 beta than IL-1ra; LPS or sonicated B. burgdorferi induced similar amounts of all three cytokines. This preferential induction of IL-1 beta was most dramatic in response to a low passage, virulent preparation of B. burgdorferi vs. three high passage avirulent strains. No difference in induction of IL-1ra was seen between these strains. The marked induction of IL-1 beta was partially diminished by heat-treatment and abrogated by sonication; IL-1ra was not affected. This suggested that a membrane component(s) accounted for the preferential induction of IL-1 beta. However, recombinant outer surface protein beta induced little IL-1 beta. By 4 h after stimulation, B. burgdorferi induced sixfold more IL-1 beta protein than LPS. In contrast to LPS-induced IL-1 beta mRNA which reached maximal accumulation after 3 h, B. burgdorferi-induced IL-1 beta mRNA showed biphasic elevations at 3 and 18 h. B. burgdorferi-induced IL-1ra mRNA peaked at 12 h, whereas LPS-induced IL-1ra mRNA peaked at 9 h. IL-1 beta synthesis increased in response to increasing numbers of spirochetes, whereas IL-1ra synthesis did not. The preferential induction by B. burgdorferi of IL-1 beta over IL-1ra is an example of excess agonist over antagonist synthesis induced by a microbial pathogen, and may contribute to the destructive lesion of Lyme arthritis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arend W. P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991 Nov;88(5):1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Thompson R. C., Hannum C. H. An IL-1 inhibitor from human monocytes. Production and characterization of biologic properties. J Immunol. 1989 Sep 15;143(6):1851–1858. [PubMed] [Google Scholar]
- Arend W. P., Smith M. F., Jr, Janson R. W., Joslin F. G. IL-1 receptor antagonist and IL-1 beta production in human monocytes are regulated differently. J Immunol. 1991 Sep 1;147(5):1530–1536. [PubMed] [Google Scholar]
- Arend W. P., Welgus H. G., Thompson R. C., Eisenberg S. P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990 May;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Endres S., Cannon J. G., Ghorbani R., Dempsey R. A., Sisson S. D., Lonnemann G., Van der Meer J. W., Wolff S. M., Dinarello C. A. In vitro production of IL 1 beta, IL 1 alpha, TNF and IL2 in healthy subjects: distribution, effect of cyclooxygenase inhibition and evidence of independent gene regulation. Eur J Immunol. 1989 Dec;19(12):2327–2333. doi: 10.1002/eji.1830191222. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Kelley V. E., Georgilis K., Lonnemann G., van der Meer J. W., Cannon J. G., Rogers T. S., Klempner M. S., Weber P. C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Lonnemann G., van der Meer J. W., Dinarello C. A. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988 Dec;49(3):424–438. doi: 10.1016/0090-1229(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Novick D., Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990 Jan 25;265(3):1531–1536. [PubMed] [Google Scholar]
- Fenton M. J., Clark B. D., Collins K. L., Webb A. C., Rich A., Auron P. E. Transcriptional regulation of the human prointerleukin 1 beta gene. J Immunol. 1987 Jun 1;138(11):3972–3979. [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Clark B. D., Webb A. C., Auron P. E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J Immunol. 1988 Apr 1;140(7):2267–2273. [PubMed] [Google Scholar]
- Georgilis K., Steere A. C., Klempner M. S. Infectivity of Borrelia burgdorferi correlates with resistance to elimination by phagocytic cells. J Infect Dis. 1991 Jan;163(1):150–155. doi: 10.1093/infdis/163.1.150. [DOI] [PubMed] [Google Scholar]
- Granowitz E. V., Clark B. D., Mancilla J., Dinarello C. A. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991 Aug 5;266(22):14147–14150. [PubMed] [Google Scholar]
- Granowitz E. V., Santos A. A., Poutsiaka D. D., Cannon J. G., Wilmore D. W., Wolff S. M., Dinarello C. A. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet. 1991 Dec 7;338(8780):1423–1424. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- Habicht G. S., Beck G., Benach J. L. The role of interleukin-1 in the pathogenesis of Lyme disease. Ann N Y Acad Sci. 1988;539:80–86. doi: 10.1111/j.1749-6632.1988.tb31840.x. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J Infect Dis. 1988 Nov;158(5):1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- Lonnemann G., Endres S., Van der Meer J. W., Cannon J. G., Koch K. M., Dinarello C. A. Differences in the synthesis and kinetics of release of interleukin 1 alpha, interleukin 1 beta and tumor necrosis factor from human mononuclear cells. Eur J Immunol. 1989 Sep;19(9):1531–1536. doi: 10.1002/eji.1830190903. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Bendtzen K., Dinarello C. A., Nerup J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J Immunol. 1987 Dec 15;139(12):4077–4082. [PubMed] [Google Scholar]
- Miller L. C., Dinarello C. A. Biologic activities of interleukin-1 relevant to rheumatic diseases. Pathol Immunopathol Res. 1987;6(1):22–36. doi: 10.1159/000157039. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Nophar Y., Kemper O., Brakebusch C., Englemann H., Zwang R., Aderka D., Holtmann H., Wallach D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990 Oct;9(10):3269–3278. doi: 10.1002/j.1460-2075.1990.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D., Engelmann H., Revel M., Leitner O., Rubinstein M. Monoclonal antibodies to the soluble human IL-6 receptor: affinity purification, ELISA, and inhibition of ligand binding. Hybridoma. 1991 Feb;10(1):137–146. doi: 10.1089/hyb.1991.10.137. [DOI] [PubMed] [Google Scholar]
- Poutsiaka D. D., Clark B. D., Vannier E., Dinarello C. A. Production of interleukin-1 receptor antagonist and interleukin-1 beta by peripheral blood mononuclear cells is differentially regulated. Blood. 1991 Sep 1;78(5):1275–1281. [PubMed] [Google Scholar]
- Prieur A. M., Kaufmann M. T., Griscelli C., Dayer J. M. Specific interleukin-1 inhibitor in serum and urine of children with systemic juvenile chronic arthritis. Lancet. 1987 Nov 28;2(8570):1240–1242. doi: 10.1016/s0140-6736(87)91854-x. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Brandt M. E., Isaacs R. D., Thompson P. A., Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991 Sep 15;147(6):1968–1974. [PubMed] [Google Scholar]
- Schindler R., Dinarello C. A. A method for removing interleukin-1- and tumor necrosis factor-inducing substances from bacterial cultures by ultrafiltration with polysulfone. J Immunol Methods. 1989 Jan 17;116(2):159–165. doi: 10.1016/0022-1759(89)90199-3. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Aarden L., Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989 Dec;53(3):488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Brinckerhoff C. E., Miller D. J., Drinker H., Harris E. D., Jr, Malawista S. E. Elevated levels of collagenase and prostaglandin E2 from synovium associated with erosion of cartilage and bone in a patient with chronic Lyme arthritis. Arthritis Rheum. 1980 May;23(5):591–599. doi: 10.1002/art.1780230511. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Dwyer E., Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990 Jul 26;323(4):219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Gibofsky A., Patarroyo M. E., Winchester R. J., Hardin J. A., Malawista S. E. Chronic Lyme arthritis. Clinical and immunogenetic differentiation from rheumatoid arthritis. Ann Intern Med. 1979 Jun;90(6):896–901. doi: 10.7326/0003-4819-90-6-896. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Schoen R. T., Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987 Nov;107(5):725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Endres S., Lonnemann G., Cannon J. G., Ikejima T., Okusawa S., Gelfand J. A., Dinarello C. A. Concentrations of immunoreactive human tumor necrosis factor alpha produced by human mononuclear cells in vitro. J Leukoc Biol. 1988 Mar;43(3):216–223. doi: 10.1002/jlb.43.3.216. [DOI] [PubMed] [Google Scholar]