Abstract
Patients with Lyme borreliosis (LB) usually develop a vigorous T cell response against the causative pathogen Borrelia burgdorferi, but little is known about the antigens recognized in the cellular response. Therefore, T cell reactivities against whole bacteria, recombinant 31-kD (outer surface protein A, [OspA]), and 41-kD proteins (flagellin) from B. burgdorferi were studied in patients with LB, non-LB patients, and healthy donors. In parallel, specific antibodies were determined by Western blot analysis. Virtually all patients with LB exhibited marked cellular responses to whole B. burgdorferi, which were significantly elevated compared with the control groups in both early and late disease stages. However, analyses using the purified antigens OspA and flagellin revealed considerable heterogeneity in the cellular reactivities among individuals as well as variations during the course of infection. T cell responses to OspA were significantly increased in patients with early LB compared with both control groups whereas in late-stage disease responses only exceeded those of non-LB patients and were not different from normal donors. Cellular immune reactivities to flagellin were significantly higher only in early LB compared with both control groups. Reciprocally, several control subjects demonstrated marked cellular responses to OspA and flagellin, suggesting that reactions to these proteins may not always be related to LB. T cell reactivity did not correlate well with the presence of specific antibodies. Almost all seropositive patients in both early and late stage LB had serum antibodies against flagellin, but antibodies to OspA were detectable only in a subset of late LB sera. These data demonstrate the complexity of the humoral and the cellular immune responses to components of B. burgdorferi.
Full text
PDF

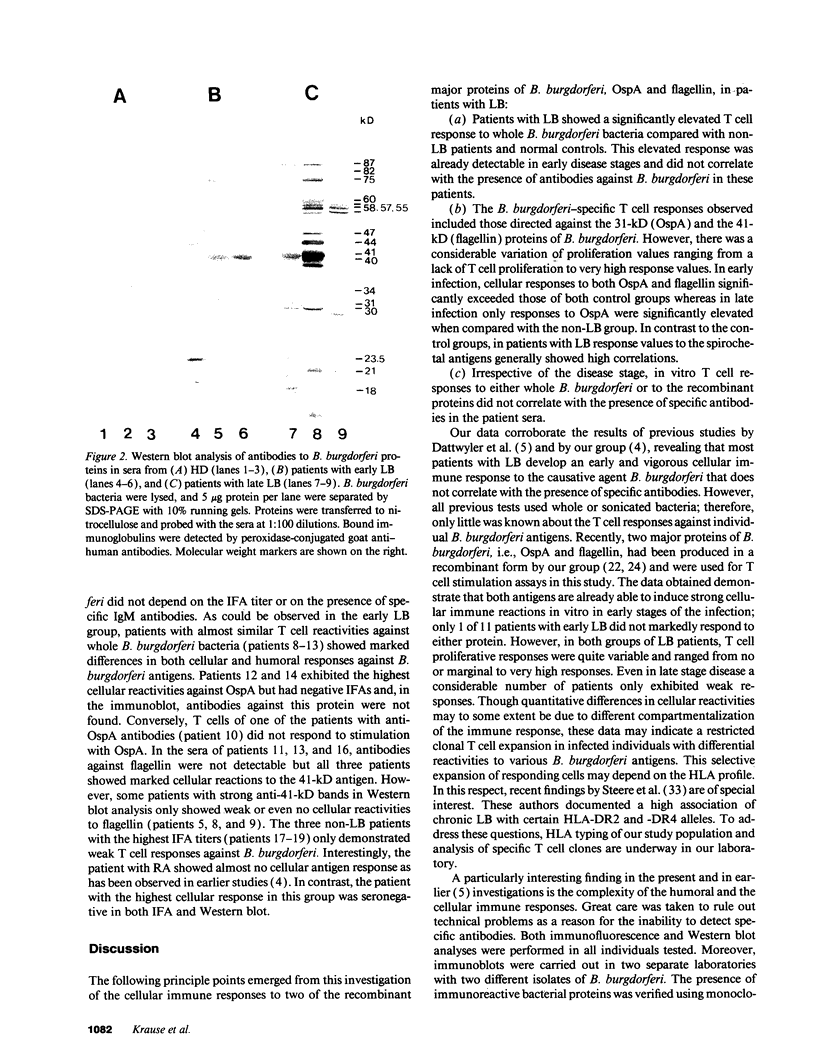
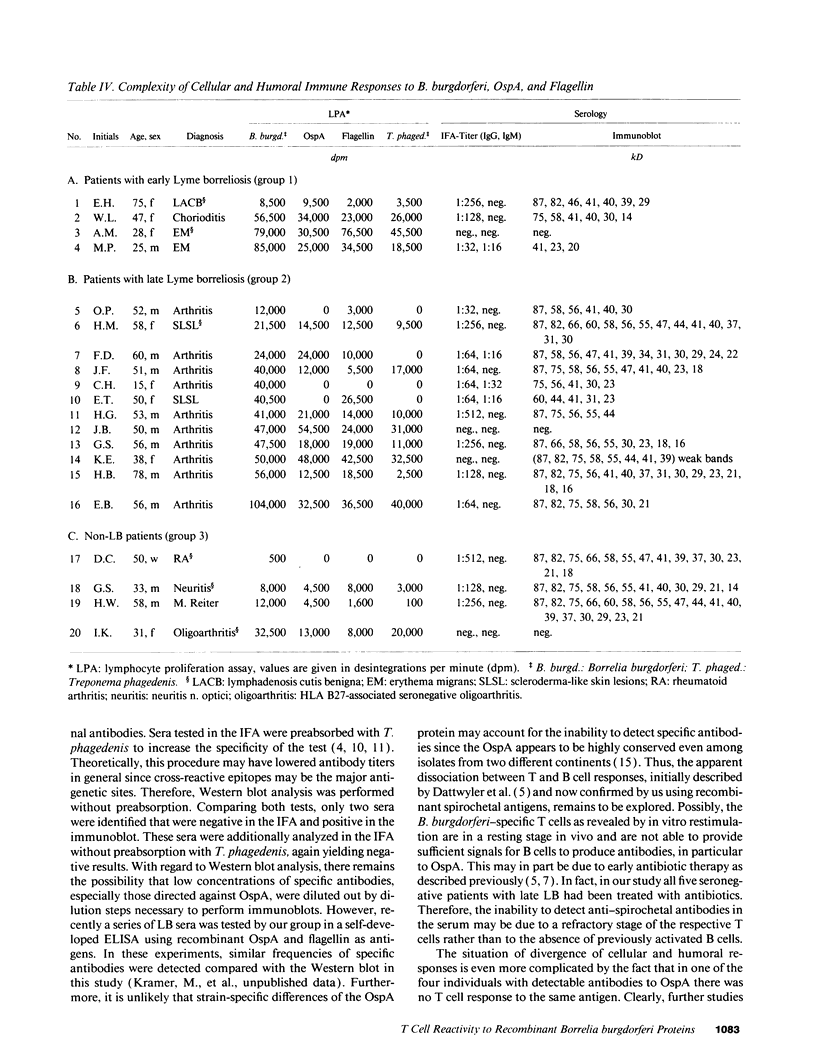

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattwyler R. J., Thomas J. A., Benach J. L., Golightly M. G. Cellular immune response in Lyme disease: the response to mitogens, live Borrelia burgdorferi, NK cell function and lymphocyte subsets. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):151–159. doi: 10.1016/s0176-6724(86)80118-3. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Halperin J. J., Luft B. J., Thomas J., Golightly M. G. Specific immune responses in Lyme borreliosis. Characterization of T cell and B cell responses to Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:93–102. doi: 10.1111/j.1749-6632.1988.tb31842.x. [DOI] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Luft B. J., Halperin J. J., Thomas J., Golightly M. G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988 Dec 1;319(22):1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Gassmann G. S., Kramer M., Göbel U. B., Wallich R. Nucleotide sequence of a gene encoding the Borrelia burgdorferi flagellin. Nucleic Acids Res. 1989 May 11;17(9):3590–3590. doi: 10.1093/nar/17.9.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
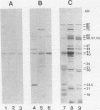
- Grellner W., Erbguth F., Brade V. Serodiagnostik bei Lyme-Borreliose: Antikörpertiter und -spezifität im IFT und Western-Blot. Immun Infekt. 1989 Dec;17(6):189–194. [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A., Brade V., Schoerner C., Solbach W., Kalden J. R., Burmester G. R. T cell proliferation induced by Borrelia burgdorferi in patients with Lyme borreliosis. Autologous serum required for optimum stimulation. Arthritis Rheum. 1991 Apr;34(4):393–402. doi: 10.1002/art.1780340404. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Jiang W., Munoz P., Dattwyler R. J., Gorevic P. D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect Immun. 1989 Nov;57(11):3637–3645. doi: 10.1128/iai.57.11.3637-3645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Miller J. N., Anderson J. F., Riviere G. R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990 Jun;28(6):1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Kramer M. D., Eckerskorn C., Museteanu C., Müller-Hermelink H. K., Wallich R. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991 Jul;164(1):123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Dwyer E., Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990 Jul 26;323(4):219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Wallich R., Moter S. E., Simon M. M., Ebnet K., Heiberger A., Kramer M. D. The Borrelia burgdorferi flagellum-associated 41-kilodalton antigen (flagellin): molecular cloning, expression, and amplification of the gene. Infect Immun. 1990 Jun;58(6):1711–1719. doi: 10.1128/iai.58.6.1711-1719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Schaible U. E., Simon M. M., Heiberger A., Kramer M. D. Cloning and sequencing of the gene encoding the outer surface protein A (OspA) of a European Borrelia burgdorferi isolate. Nucleic Acids Res. 1989 Nov 11;17(21):8864–8864. doi: 10.1093/nar/17.21.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Gueye W., Herzer P., Weber K. Immunochemische Analyse der Immunantwort bei Spätmanifestationen der Lyme Borreliose. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;267(4):549–558. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Zöller L., Burkard S., Schäfer H. Validity of western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991 Jan;29(1):174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]