Abstract
Fibrils of type I collagen in the skin are exposed to ultraviolet (UV) light and there have been claims that collagen photo-degradation leads to wrinkles and may contribute to skin cancers. To understand the effects of UV radiation on collagen, type I collagen solutions were exposed to the UV-C wavelength of 254 nm for defined lengths of time at 4°C. Circular dichroism (CD) experiments show that irradiation of collagen leads to high loss of triple helical content with a new lower thermal stability peak and SDS-gel electrophoresis indicates breakdown of collagen chains. To better define the effects of UV radiation on the collagen triple-helix, the studies were extended to peptides which model the collagen sequence and conformation. CD studies showed irradiation for days led to lower magnitudes of the triple-helix maximum at 225 nm and lower thermal stabilities for two peptides containing multiple Gly-Pro-Hyp triplets. In contrast, the highest radiation exposure led to little change in the Tm values of (Gly-Pro-Pro)10 and (Ala-Hyp-Gly)10, although (Gly-Pro-Pro)10 did show a significant decrease in triple helix intensity. Mass spectroscopy indicated preferential cleavage sites within the peptides, and identification of some of the most susceptible sites of cleavage. The effect of radiation on these well defined peptides gives insight into the sequence and conformational specificity of photo-degradation of collagen.
INTRODUCTION
Ultraviolet (UV) irradiation is considered to be one of the most hazardous external factors, resulting in physical and chemical alterations in living organisms. There are three main UV wavelength regions, UV-A (400-315 nm), UV-B (315-280 nm) and UV-C (280-160 nm), and the highest energy region, UV-C, causes the most biological damage. As a result of UV irradiation, free radicals accumulate in cells and the extracellular matrix, causing oxidative damage of macromolecules.1,2 Skin represents the primary target of UV radiation. Fibrils of type I collagen, the most abundant component of human connective tissue (skin, bone, tendons) are always under UV stress. Collagen photo-degradation has been implicated in skin wrinkles and it is possible that it could play a role in skin cancers.3,4 There are several reports suggesting the degradation of collagen by UV radiation5,6 may involve aromatic amino acids and polypeptide chain scission. 7,8 UV irradiation appears to accelerate the collagen damage found in normal aging.
The native collagen structure is characterized by the presence of a characteristic triple-helix conformation, formed when three polyproline II like chains are supercoiled about a common axis.9,10 The close packing of the three chains generates a constraint that Gly be every third residue in the amino acid sequence, (Gly-Xaa-Yaa)n. This structure is stabilized by a high content of imino acids, proline (Pro) and the post-translationally modified hydroxyproline (Hyp). Inter-chain hydrogen bonds and water mediated hydrogen bonding also play an important role in stability.10 Type I collagen, the major collagen in skin, is a heterotrimer composed of two α1 and one α2 chains, with a triple-helix domain of (Gly-Xaa-Yaa)338, MW~ 300 KD. In type I collagen, about 30% of the triplets have a Pro in the Xaa position and 30% have a Hyp in the Yaa position, with the very stabilizing Gly-Pro-Hyp constituting ~10% of all tripeptide sequences.
There are many studies from different laboratories characterizing the collagen oxidative damage as a result of UV radiation.8, 11–20 The most widespread hypothesis is that collagen peptide bonds are cleaved randomly by UV without disrupting the triple helical structure.8 It has been suggested that the bonds between nitrogen and α-carbon of the polypeptide chains are preferentially cleaved.11 SDS-PAGE has been used to visualize degradation/cleavage of collagen chains8,18 and FTIR was used to monitor conformational changes in collagen after UV radiation.17,18,21
The amino acid sequence along the length of the collagen molecule may influence susceptibility to UV damage both because of their different chemical structures and also because they may lead to conformational variations. Although collagen molecules adopt a triple-helix conformation, variations in imino acid content along the sequence can lead to modulation of the super-helix twist, where imino acid poor regions may be less tightly wound and less stable.22 Collagen model peptides offer an approach to investigating the effects of amino acid composition, sequence, conformation, and stability on UV degradation. Peptides with Gly as every third residue and a high content of Pro and Hyp will self-associate to form stable triple-helices which can serve as models for collagen.23–26 The peptide size and solubility are advantageous and have allowed successful investigations with various physico-chemical techniques25,26 as well as high resolution structures by X-ray crystallography and NMR spectroscopy.27,28 Peptides with many different sequences have been studied, including repeating tripeptides e.g. (Pro-Hyp-Gly)10 or peptides that contain biologically interesting collagen sequences flanked by Pro-Hyp-Gly triplets.26
To better understand the effects of UV radiation on collagen, model collagen peptides and type I collagen solutions were exposed to the UV-C wavelength of 254 nm for defined lengths of time (4, 17 and 66 hrs) at 4°C and the effect of UV on triple-helix stability and structure was studied. The consequences for primary structure, secondary structure, conformation and stability of model collagen peptides were investigated using circular dichroism spectroscopy and mass spectrometry. The effect of radiation on these well defined peptides gives insight into the mechanism of photo-degradation of collagen.
RESULTS
Circular Dichroism Studies on the Effect of UV radiation on Collagen
CD spectra in the far-UV region (250-190 nm) were recorded after exposure of soluble rat tail tendon collagen for different lengths of time to UV-C radiation (254 nm; 4 to 66 hrs). All spectra showed a maximum near 222 nm, which is characteristic of the triple-helix, and there was a significant decrease in the Mean Residue Ellipiticity at 222 nm (MRE222) after the longest 66 hrs exposure (Table I).
Table I.
Mean residue ellipticity (MRE, deg.cm2.dmol−1) of collagen and collagen-like peptides at respective CD maxima before and after UV irradiation
| Sample | 0h | 4h | 17h | 66h | % reduction in MRE without UV irradiation and UV irradiation up to 66hrs* |
|---|---|---|---|---|---|
| Collagen | 4050 | 3965 | 3985 | 2900 | 28.4% |
| (PPG)10 | 2365 | 2283 | 2098 | 1650 | 30.2% |
| (POG)10 | 4966 | 4868 | 4784 | 3793 | 23.6% |
| GPKGPE | 4006 | 3918 | 3786 | 2580 | 35.6% |
| (AOG)10 | 2679 | 2789 | 2457 | 2423 | 9.5% |
| GLY- | 2144 | 1954 | 1968 | 1411 | 34.2% |
-
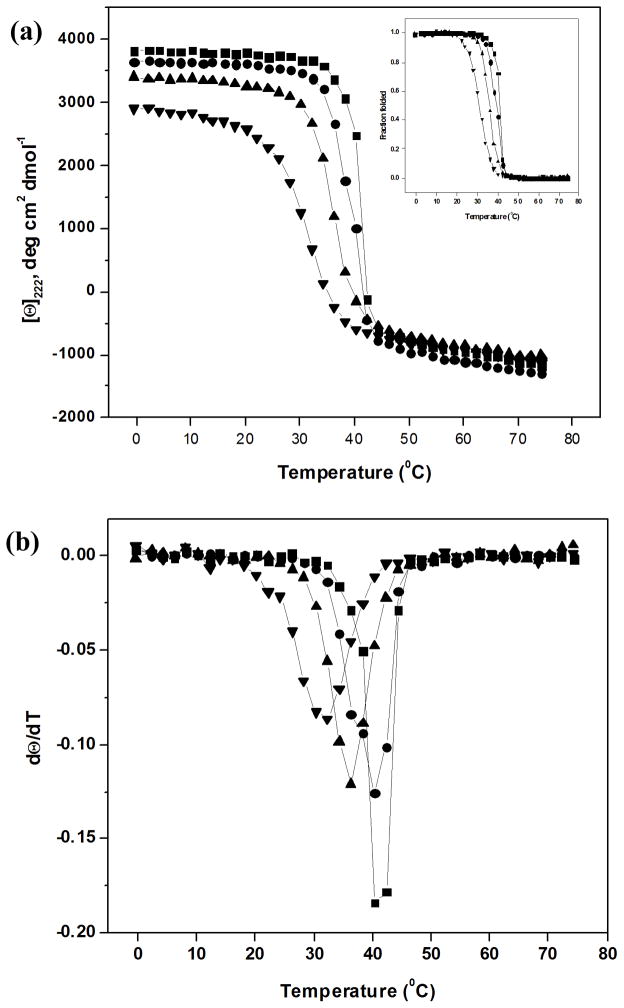
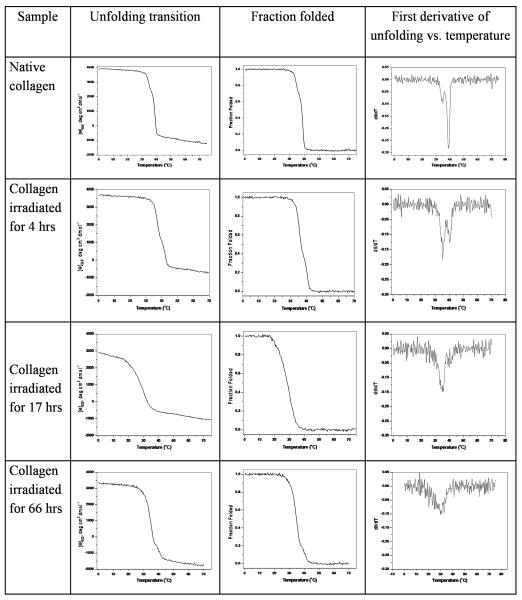
The melting temperature, reflecting the triple-helix to disordered monomer transition, was determined by monitoring the MRE222 vs. temperature at two different heating rates, 1°C/min and 0.1°C/min (Figure 1, Table II). For the control collagen solution, a single sharp thermal transition was observed for the faster heating rate, with Tm = 41°C (Figure 1), while a biphasic curve with a sharp thermal transition at 39°C and a small transition at ~ 35°C was seen for the slower heating rate. The small difference between the position of the major transition at the two heating rates (Tm = 41°C for 1°C/min and Tm = 39°C for 0.1°C/min) suggests that the CD melt is not completely at equilibrium, consistent with previous reports29, but that it is close to equilibrium. Exposure to UV radiation (4 hrs) decreases the magnitude of the transition at ~40°C, and increases the magnitude of the 35°C transition for the slower heating rate, while the CD melt at the faster heating rate also shows a decreased magnitude for the original 41°C transition and the beginning of a second less stable species with Tm = 36°C. For both heating rates, increasing the length of exposure time to 17 hrs eliminates the original 40°C transition, and leads to only a broad thermal transition near 35°C; a 66 hr irradiation shows only a single broad transition around 30°C.
FIGURE 1.
(a) Circular dichroism (CD) melting transitions at heating rate of 1°C/min of Type I collagen after UV-C irradiation for different lengths of time; ■– 0 hr control, ● – 4hr UV, ▲ – 17hr UV, ▼ – 66hr UV; the insert shows the melting curves represented as fraction folding vs. temperature and (b) the first derivatives of fraction folded shown in (a)
Table II.
CD Melting temperature (Tm, °C; heating rate 1°C/min) of collagen and collagen-like peptides before and after UV irradiation
| Collagen Peptide | Control* | 4h UV | 17h UV | 66h UV |
|---|---|---|---|---|
| Collagen | 41.1 (39.4, 35) | 39.3, 36.0 (38.7, 35) | 36.0 (35) | 31.3 (31) |
| (PPG)10 | 38.9 | 38.6 | 38.5 | 38.3 |
| (POG)10 | 64.9 | 64.5 | 63.6 | 60.4 |
| GPKGPE | 36.9 | 36.7 | 36.3 | 35.6 |
| (AOG)10 | 29.6 | 29.7 | 29.3 | 31.0 |
| GLY- | - | - | - | - |
- before UV irradiation;
Values indicated in parenthesis are Tm of collagen at heating rate 0.1°C/min
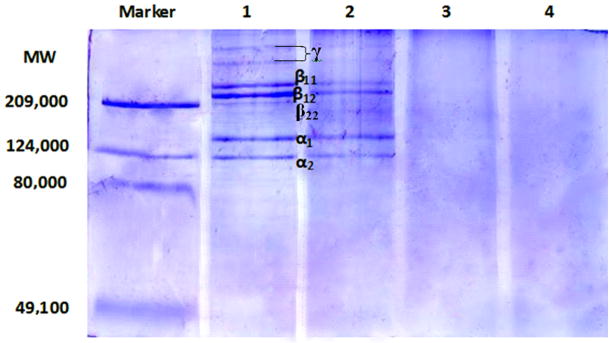
To investigate whether increasing UV radiation is leading to chain scission, SDS-PAGE was run on all rat tail tendon collagen samples (Figure 3). After UV irradiation for 4 hrs, gel electrophoresis showed significant reduction in the intensity of the bands for α1 and α2 chains, as well as the β11, β12 and β22 bands and higher bands (γ). Further irradiation of the collagen solution for 17 and 66 hrs led to complete disappearance of the bands, indicating UV induced cleavage left no intact α-chains. The absence of any discrete lower molecular weight bands is consistent with random scission, and/or cleavage to very small peptides.
FIGURE 3.
SDS gel electrophoresis of collagen solutions exposed to UV-C for different lengths of time. lanes from left to right: Marker, with molecular weights of the standards shown on left of gel; native collagen – 1; collagen irradiated for 4 hrs – 2; collagen irradiated for 17 hrs – 3; and collagen irradiated for 66 hrs - 4. The positions of the collagen components β11, β12, α1 and α2 are shown
Effect of UV irradiation on Collagen model peptides
To better define the effects of UV radiation on the collagen triple-helix, the studies were extended to model peptides which have defined differences in amino acid sequence, Pro/Hyp content, length, thermal stability, and conformation. The peptide (Pro-Hyp-Gly)10, abbreviated as (POG)10, forms a stable triple-helix structure (Tm=64.9°C), and its crystal structure has been solved.30,31 (Pro-Pro-Gly)10, with all proline imino acid residues and no Hyp, forms a less stable triple-helix (Tm=38.9°C), while the (Ala-Hyp-Gly)10 peptide, denoted as (AOG)10, appears to form only a small amount of triple-helix with a stability of Tm ~29.6°C, using a heating rate of 1°C/min for all of these CD melts. The peptide Gly-, with the deletion of one glycine in the middle of (POG)10, does not form any triple-helix structure and serves as a control for conformational features.32 Peptide GPKGPE, containing a Gly-Pro-Lys-Gly-Pro-Glu sequence surrounded by 3 triplets of Gly-Pro-Hyp on each side, has a melting temperature around 36.9°C. All peptides were exposed to UV-C irradiation for 0, 4, 17 and 66 hrs in PBS, and conformation, stability and intactness of their polypeptide chains were examined.
CD experiments on Collagen Peptides
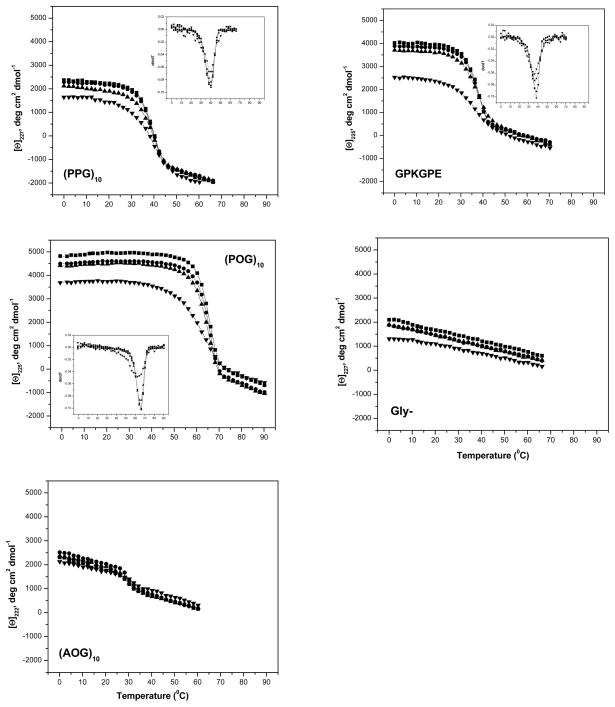
The peptides (POG)10 and GPKGPE have been previously reported to adopt a stable triple-helical conformation at low temperature and have a high content of Pro-Hyp-Gly tripeptides in their sequence (10 and 6 POG triplets, respectively), while (PPG)10 lacks Hyp, (AOG)10 lack Pro, and Gly- is not triple-helical. UV irradiation exposures of 4 and 17 hrs led to small decreases in MRE, while 66 hrs exposure led a very significant drop in magnitude for all of the peptides except for (AOG)10, which has its maximum at 219 nm (Table I, Figure 4). Irradiation of all of the triple-helical peptides for 4 and 17 hrs showed little effect on their thermal stability, but a small, significant decrease and significant broadening of the thermal transition was seen after 66 hrs for (POG)10 and GPKGPE, while the extended irradiation did not affect the Tm values for (PPG)10 and (AOG)10 (Table II).
FIGURE 4.
CD melting curves (heating rate of 1°C/min) of unfolding of various collagen peptides at varied time intervals of UV-C irradiation; the first derivative of fraction folded is shown as insert; ■ – 0hr UV control; ● – 4hr UV; ▲ – 17hr UV; ▼ – 66hr UV.
The Gly- peptide, which does not form a triple-helix because of the interruption in the repeating Gly-X-Y pattern, has a CD maximum at 225 nm accordingly which must reflect its polyproline II nature, but does not show any thermal transition. Irradiation of Gly- peptide with UV for 66 hrs showed a 34.2% decrease in ellipticity with reference to the control values (2144 deg.cm2.dmol−1).
MALDI-TOF experiments on UV Irradiated Collagen Peptides
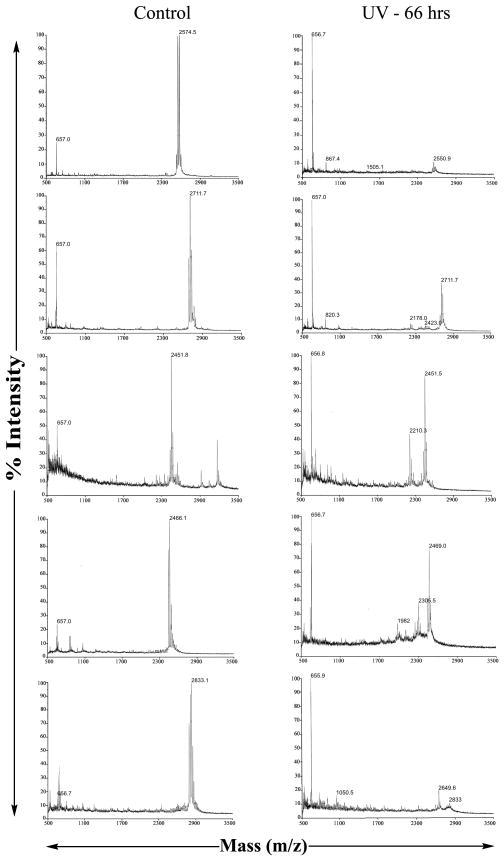
Mass spectroscopy was applied to detect chain degradation and to define sites of polypeptide cleavage in collagen model peptides after UV irradiation. The mass spectroscopy data of peptides (PPG)10, (POG)10, (AOG)10, GPKGPE and Gly- showed the main peaks at 2574, 2712, 2451, 2466 and 2833 respectively corresponding to the predicted molecular weight or with additional weight corresponding to the bound Na or Cl ions as the peptides were prepared in PBS solution. Irradiation for 4 and 17 hrs did not lead to any changes, while 66 hrs showed a significant increase in lower molecular weight fragments which could be identified for some peptides.
Molecular ion peak of (PPG)10 almost disappeared after 66 hrs of UV irradiation (Figure 5a). The mass spectroscopy data of peptide (POG)10 show the intensity of the main peak at 2711.7 (with Na ion) (Figure 5b), corresponding to the predicted peptide molecular weight was reduced significantly after 66 hrs of irradiation. (POG)10 appears to be somewhat more resistant to degradation than (PPG)10. The (AOG)10 peptide, with an original mass of 2451 shows a new peak at 2210.3 after UV irradiation which corresponds to deletion of one AOG triplet either at the C- or N-terminal (Figure 5c). Exposure of peptide GPKGPE for 66 hrs to UV irradiation led to a new peak at a smaller mass, 2305.5, corresponding to the cleavage between Gly and Tyr (Figure 5d). The mass spectra of the Gly- peptide shows almost total loss of the original peak near 2797, with a new small peak at 2649 corresponding to the deletion of a Tyr and other smaller peaks correspond to the deletion of POG (Figure 5e).
FIGURE 5.
MALDI-TOF of native collagen peptides and collagen peptides irradiated with UV-C for 66 hrs; a) (PPG)10; b) (POG)10; c) (AOG)10; d) GPKGPE; e) Gly-.
DISCUSSION
Previous DSC studies on the effect of UV radiation on the properties of the collagen molecule indicated a new molecular species with stability considerably lower than native collagen, which was identified as an intermediate state in the degradation of collagen.8 Here, circular dichroism spectroscopic studies on collagen solutions exposed to UV irradiation support and extend these earlier observations. The CD thermal transitions of collagen, which have been shown to be non-equilibrium events29, depend on the rate of heating, with the faster heating rate leading to a 2°C higher Tm value. Using a consistent fast heating rate of 1°C/min, exposure to radiation leads to the appearance of a second lower stability transition near 36°C, which becomes predominant after 17 hrs exposure. After 66 hours exposure only a broad transition near 31.3°C remains. A much slower heating rate leads to a biphasic transition even for the control rat tail tendon collagen, consistent with the observations of Hayashi (1979)12 suggesting that long exposure to UV radiation (222 nm) from the CD instrument during an overnight melt leads to scission of bonds in the collagen chains and this less stable species. CD melts performed at the slower heating rate after UV exposure showed an increased magnitude of the lower stability transition in the biphasic curves seen after 4 hrs exposure, while the results after longer exposures were the same as seen at the faster heating rate. It appears that the low heating rate experiments represent the additive results of damage inflicted on collagen by the UV-C exposure at 254nm for defined time periods, together with the ~7hr exposure to the 222nm UV light during the CD run. In contrast, the faster heating rate, with 222nm exposure for less than 1 hour, is likely to reflect only the collagen damage caused by UV-C.
SDS-PAGE experiments confirm UV exposure leads to scission of collagen polypeptide chains, with band intensity corresponding to α and β chains reduced after 4 hrs and gone by 17 hrs, confirming previous results.8 The lower stability thermal transitions observed for the 4, 17 and 66 hrs exposure times suggests that a triple-helical structure, but with reduced stability, can be formed when every collagen chains is fragmented. The stability of triple-helices formed from fragmented chains appears to decrease with increasing UV-C exposure and increasing scission, from 36°C to 31°C. Strong inter-chain interactions within the triple-helix, including van der Waals forces and hydrogen bonding, are likely to maintain the conformation over extended regions, even though there are no longer intact ~1000 residues collagen α-chains. There is no transition observed below 30°C, suggesting a stable triple-helix cannot be maintained after some threshold of cleavage is reached. This threshold could be related to some minimum size of the polypeptides chains, or related to the number of chains within a triple-helix with cleavage sites, or to their location along the triple-helix. But clearly a number of intermediates or damaged triple-helical species of lower stability are possible before sufficient number of bonds are cleaved and prevent triple-helix formation.
UV irradiation of model collagen peptides for 66 hrs, but not for shorter time periods, generally decreased the magnitude of the characteristic triple-helix 225 nm maximum, and in some cases led to a significant reduction in melting temperature. The drop in thermal stability for the peptides (ΔTm ~ 0–4.5°C) was substantially less than seen for collagen after 66 hrs exposure (ΔTm ~ 10°C). Mass spectroscopy indicated there was significant scission of peptide bonds in these peptides, but some intact peptide was present even after 66 hrs exposure, in contrast to the absence of any intact collagen α-chains after 17 hrs exposure. This is consistent with the larger target size of collagen compared to peptides which are 1/30 of its length. The absence of biphasic transitions or significnatly lower thermal transition for irradiated collagen peptides, such as those seen for irradiated collagen, suggests these relatively short peptides (30 residue chain length) may lack the ability to exist in trimer form after scission in any site, in contrast to the ~1000 residue collagen chain where a single cleavage may still allow a stable triple-helix. Broadening of thermal transitions was observed for collagen peptides irradiated with UV for 66 hrs in some cases, which might result from partial cleavage of a small number of residues near the chain termini, as suggested by mass spectrometry data. The loss of triple-helix CD signal could also reflect cleavage of the peptide chains into smaller fragments, leaving a smaller number of intact peptides capable of forming triple-helices.
Comparison of different peptides suggests an influence of imino acid content on UV mediated chain scission. The (PPG)10 peptide shows more UV damage than (POG)10 by mass spectrometry (Figure 5) and a larger decrease in mean residue ellipticity (Table I), suggesting that proline residues may be more susceptible to UV damage than hydroxyproline. CD and mass spectroscopy observations of peptide (AOG)10 showed minimal changes due to radiation, less than any other peptide, again supporting the susceptibility of Pro and importance of Gly-Pro-Pro and Gly-Pro-Hyp sequences for UV degradation. Thus, although the UV damage to collagen peptides is non-specific, there appears to be a high preference for the cleavage between Gly and Pro (X position) peptide bonds, which could be the most targeted site prone for UV damage in collagen.
UV radiation of collagen
CD studies show irradiation of collagen leads to the appearance of a lower stability molecular species, in addition to the native molecule, and the intensity of the less stable species grows with increasing exposure. SDS-PAGE results indicate radiation leads to extensive peptide bond cleavage, with no intact α or β chains visible after 17 hrs exposure. The new lower stability collagen species is likely to contain cleaved peptide bonds at one or more sites within each polypeptide chain.
UV studies of model collagen peptides
CD studies show that UV exposure leads to a decreased magnitude of the characteristic triple-helix peak, and a broadening of the thermal transitions, with a small decrease in stability. Mass spectrometry on the peptides shows that irradiation fragmented the chains, which is likely to cause the observed changes in conformation and stability. Some preferential cleavage sites were observed and may give insight into the collagen cleavage sites.
MATERIALS AND METHODS
Collagen
Rat tail tendon collagen was selected for investigation. Acid soluble rat tail tendon (RTT) type I collagen was isolated according to the method described by Chandrakasan et al33. The procedure included acetic acid extraction and salting out with NaCl. The purity of collagen preparation was confirmed by SDS-polyacrylamide gel electrophoresis.
Peptides
Collagen-like peptides (POG)10 and (PPG)10 were obtained from Peptides International (Louisville, Kentucky) and peptides GPKGPE, Gly- and (AOG)10 were synthesized by the Tufts University Core Facility (Boston, MA). The sequence of each peptide is given below:
(PPG)10: H-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Pro-Gly-OH; MW - 2530.83;
(POG)10: H-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-OH; MW – 2690.83;
(AOG)10: H-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-Ala-Hyp-Gly-OH; MW – 2431.
GPKGPE: NH2-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Lys-Gly-Pro-Glu-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Tyr-COOH; MW - 2420;
GLY-: H-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Tyr-OH; MW - 2797.0044;
Peptides were used after purification on a reverse-phase high pressure liquid chromatography system (Shimadzu) with a C-18 column. Purity of all peptides was ensured by mass spectrometry using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF). All experimental studies used peptides at a concentration of 1 mg/mL in 20 mM PBS (10 mM NaH2PO4, 10 mM Na2HPO4, 150 mM NaCl) at pH 7.
UV radiation
Collagen and collagen peptide solutions were irradiated using UV-C (high intensity 254 nm) light from Ultra-violet products (UVP) from Upland, California (Model R-52 Grid Lamp), the intensity of which was 0.1 J/cm2min at 15.24 cm. The solutions were taken in a quartz cuvette, which was placed centrally in the beam. The UV radiation experiments were carried at 4°C and at varied time intervals viz., 4, 17 and 66 hrs.
Determination of melting temperature by CD
The denaturation of the collagen (0.6 mg/mL) and collagen peptides were monitored using an Aviv model 62DS CD spectrophotometer with a Peltier thermoelectric temperature controller attached for temperatures between 0 to 80°C, 1 mm cuvette. The Tm values for collagen peptides with and without UV irradiation were determined at a heating rate of 1°C/min, faster than our standard procedure,34 to minimize damage from exposure to UV light during the CD melt.12 In the case of collagen, melting experiments were carried out both at a rate of 1°C/min and 0.1°C/min. From experiments repeated on independently prepared samples, the error of determination of the Tm is ±0.5°C.
SDS-PAGE
A solution (0.6 mg/ml) of Type I collagen was irradiated with a UV lamp for different periods of time (0, 4, 17 and 66 hrs). Each sample was loaded onto a 10 % polyacrylamide gel for SDS-PAGE by the method of Laemmli.35
Mass spectroscopy
The fragmentation of the collagen peptide after UV irradiation was monitored using a Voyager DE-PRO MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight) Mass spectrometer, Applied Biosystems. The samples were prepared using alpha-cyano-4-hydroxy-cinnamic acid matrix from Sigma chemicals.
FIGURE 2.
CD melting curves of type I collagen after varied time intervals of UV-C irradiation, at a heating rate of 0.1°C/min, showing the unfolding transitions (MRE222 vs. temperature), their representation as fraction folding vs. temperature, and the first derivative of fraction folded
Acknowledgments
This project has been made possible by financial support from the Shota Rustaveli National Science Foundation (Grant # 2–2/06). All ideas expressed herewith are those of the author, and may not represent the opinion of the Foundation itself. The research was made possible in part by Award # NNS-11-07 of the U.S. Civilian Research & Development Foundation (CRDF), the Georgia National Science Foundation (GNSF) and the Georgian Research and Development Foundation (GRDF) to K. J. This research was supported by NIH grant GM60048 (BB). We wish to thank Dr. Richard Lehman for use of the UV-C lamp, Dr. Ayumi Yoshizumi for help with SDS-PAGE experiments and Dr. Karunakar Kar for help with Mass spectroscopy.
References
- 1.Herrling Th, Jung K, Fuchs J. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2006;63:840–845. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Davies KJA, Pryor WA. Free Radical Biology & Medcine. 2005;39:1263. doi: 10.1016/j.freeradbiomed.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Pugliese P. Physiology of the skin II. Allured Publishing Corporation; 2001. pp. 109–137. [Google Scholar]
- 4.Jackson R. Can Fam Physician. 2001;47:1236–1243. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimori E. FEBS Lett. 1988;235:98–102. doi: 10.1016/0014-5793(88)81241-9. [DOI] [PubMed] [Google Scholar]
- 6.Sionkowska A. Polym Deg Stab. 2000;68:147–151. [Google Scholar]
- 7.Kato Y, Uchida K, Kawakishi S. Photochem Photobiol. 1994;59:343–349. doi: 10.1111/j.1751-1097.1994.tb05045.x. [DOI] [PubMed] [Google Scholar]
- 8.Miles CA, Sionkowska A, Hulin SL, Sims TJ, Avery NC, Bailey AJ. J Biol Chem. 2000;275(42):33014–33020. doi: 10.1074/jbc.M002346200. [DOI] [PubMed] [Google Scholar]
- 9.Rich A, Crick FH. Nature. 1955;176:915–916. doi: 10.1038/176915a0. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran GN. In: Treatise on collagen. Ramachandran GN, editor. Academic Press; New York, US: 1967. pp. 103–183. [Google Scholar]
- 11.Miyata T, Sohde T, Rubin AL, Stenzel KH. Biochimica et Biophisica Acta. 1971;229:672–680. doi: 10.1016/0005-2795(71)90283-2. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Curran-Patel S, Prockop DJ. Biochemistry. 1979;18:4182–4187. doi: 10.1021/bi00586a022. [DOI] [PubMed] [Google Scholar]
- 13.Fujimor E. Eur J Biochem. 1985;152:299–306. doi: 10.1111/j.1432-1033.1985.tb09198.x. [DOI] [PubMed] [Google Scholar]
- 14.Metreveli N, Namicheishvili L, Jariashvili K, Dgebuadze M, Chikvaidze E, Mrevlishvili G. Biofizika. 2006;51:39–43. [PubMed] [Google Scholar]
- 15.Metreveli N, Namicheishvili L, Jariashvili K, Mrevlishvili G, Sionkowska A. International Journal of Photoenergy. 2006:Article ID 76830, 1–4. [Google Scholar]
- 16.Metreveli N, Namicheishvili L, Jariashvili K, Dgebuadze M, Chikvaidze E, Sionkowska A. Journal of Photochemistry and Photobiology B: biology. 2008;93:61–65. doi: 10.1016/j.jphotobiol.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Metreveli N, Jariashvili K, Namicheishvili L, Svintradze D, Chikvaidze E, Sionkowska A, Skopinska J. Ecotoxicology and Environmental Safety. 2010;73:448–455. doi: 10.1016/j.ecoenv.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Rabotyagova OS, Cebe P, Kaplan DL. Materials Science and Engineering: C. 2008;28:1420–1429. doi: 10.1016/j.msec.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato Y, Uchida K, Kawakishi ShJ. Agric Food Chem. 1992;40:373–379. [Google Scholar]
- 20.Sionkowska A, Kaminska A. Journal of Photochemistry and Photobiology, A: chemistry. 1998;120:207–210. [Google Scholar]
- 21.Kaminska A, Sionkowska A. Polym Degradation and Stability. 1996;51:19–26. [Google Scholar]
- 22.Kramer RZ, Bella J, Mayville P, Brodsky B, Berman HM. Nat Struct Biol. 1999;6:454–457. doi: 10.1038/8259. [DOI] [PubMed] [Google Scholar]
- 23.Persikov AV, Xu Y, Brodsky B. Protein Science. 2004;13:893–902. doi: 10.1110/ps.03501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kar K, Amin P, Bryan MA, Persikov AV, Mohs A, Wang YH, Brodsky B. J Biol Chem. 2006;281(44):33283–33290. doi: 10.1074/jbc.M605747200. [DOI] [PubMed] [Google Scholar]
- 25.Bryan MA, Brauner JW, Anderle G, Flach CR, Brodsky B, Mendelsohn R. J Am Chem Soc. 2007;129(25):7877–7884. doi: 10.1021/ja071154i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodsky B, Thiagarajan G, Madhan B, Kar K. Biopolymers. 2008;89(5):345–353. doi: 10.1002/bip.20958. [DOI] [PubMed] [Google Scholar]
- 27.Madhan B, Xiao J, Thiagarajan G, Baum J, Brodsky B. J Am Chem Soc. 2008;130(41):13520–13521. doi: 10.1021/ja805496v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bella J, Eaton M, Brodsky B, Berman HM. Science. 1994;266(5182):75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 29.Leikina E, Mertts MV, Kuznetsova N, Leikin S. PNAS. 2002;99:1314–1318. doi: 10.1073/pnas.032307099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berisio R, Vitagliano L, Mazzarella L, Zagari A. Biopolymers. 2001;56:8–13. doi: 10.1002/1097-0282(2000)56:1<8::AID-BIP1037>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Okuyama K, Hongo C, Fukushima R, Wu G, Narita H, Noguchi K, Tanaka Y, Nishino N. Biopolymers. 2004;76:367–377. doi: 10.1002/bip.20107. [DOI] [PubMed] [Google Scholar]
- 32.Long CG, Braswell E, Zhu D, Apigo J, Baum J, Brodsky B. Biochemistry. 1993;32:11688–11695. doi: 10.1021/bi00094a027. [DOI] [PubMed] [Google Scholar]
- 33.Chandrakasan G, Torchia DA, Piez KA. J Biol Chem. 1976;251:60–62. [PubMed] [Google Scholar]
- 34.Persikov AV, Xu Y, Brodsky B. Protein Sci. 2004;13:893–902. doi: 10.1110/ps.03501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]