Abstract
The exposure of the human population to environmental contaminants is recognized as a significant contributing factor for the development of Parkinson’s disease (PD) and other forms of parkinsonism. While pesticides have repeatedly been identified as risk factors for PD, these compounds represent only a subset of environmental toxicants that we are exposed to on a regular basis. Thus, non-pesticide contaminants, such as metals, solvents, and other organohalogen compounds have also been implicated in the clinical and pathological manifestations of these movement disorders and it is these non-pesticide compounds that are the subject of this review. As toxic exposures to these classes of compounds can result in a spectrum of PD or PD-related disorders, it is imperative to appreciate shared clinico-pathological characteristics or mechanisms of action of these compounds in order to further delineate the resultant disorders as well as identify improved preventive strategies or therapeutic interventions.
Introduction
Parkinson disease (PD) is a progressive neurodegenerative movement disorder that affects 1% of people over the age of 55, increasing to 5% by 85 years of age. Although the average age of onset is 70 years old, a significant number of patients (~4%) will develop early-onset PD, which can occur before they are 50 years old (Farrer, 2006). Clinically, PD is predominantly defined as a movement disorder, characterized by an alteration in the ability to initiate and maintain normal movement, manifesting as slowness of movement, a resting tremor, and postural instability. These symptoms are a direct result of the loss of dopamine-producing neurons located in the substantia nigra pars compacta (SNpc) of the midbrain and a concomitant reduction of the neurotransmitter dopamine and dopaminergic terminals in the caudate and putamen of the striatum (Fahn, 2003). These deficits can be readily assessed in the awake patient through the use of several imaging techniques, including positron emission tomography (PET) and single photon emission computer tomography (SPECT), which both incorporate the use of ligands that are specific for dopaminergic transporters or receptors. Importantly, many of these motor symptoms can be alleviated, albeit not completely or permanently, through dopamine replacement therapies, such as L-DOPA (Fahn et al., 2004).
In addition to motor abnormalities, PD patients can also present with a suite of non-motor problems that accompany or may even precede the motor issues and can range from gastrointestinal and cognitive deficits to olfactory and sleep disturbances. While damage to the nigrostriatal dopamine system underlies the motor perturbations, many of the peripheral alterations are thought to arise from damage to other neurotransmitter systems, including loss of noradrenergic neurons and projections from the locus coeruleus as well as cholinergic deficits in the nucleus basalis of Meynert (Fahn, 2003).
It should be kept in mind that PD is only a singular syndrome that is a part of a much larger clinico-pathological definition of movement disorders, defined as parkinsonism (Tuite and Krawczewski, 2007). Parkinsonism encompasses multiple different movement disorders that all seem to share a similar tetrad of movement deficits, including rigidity, tremor, slowness of movement, and postural instability, although these may be present in different combinations. The etiology of these deficits is extremely varied, ranging from multiple systems atrophy and progressive supranuclear palsy to drug- or toxicant-induced syndromes, such as carbon monoxide and manganese. Furthermore, the cause of parkinsonism will be further defined by the presence or absence of pathological signs and symptoms. For instance, while PD shows a discrete loss of dopaminergic neurons in the SNpc and a favorable response to dopamine replacement therapy, other conditions such as parkinsonism as a result of manganese toxicity do not (Guilarte, 2010). It can be appreciated that this spectrum of parkinsonian clinico-pathological presentations can significantly complicate the differential diagnosis of PD and other parkinsonian disorders.
Although PD is primarily viewed as a disease of aging the signs and symptoms of PD can be accelerated through a genetic predisposition to the disease or exposure to an environmental risk factor (Farrer, 2006). To date, mutations to several genes have been identified as genetic risk factors for the disease, yet these genetic alterations are only able to account for 5–10% of the cases of PD. This would suggest that there are exogenous or environmental factors that influence the risk of development of PD, that either work independently or in conjunction with genetic predisposition to facilitate the onset of the disease (Gao and Hong, 2011).
Indeed, work over the last several decades has provided extensive support for the idea that exposure to different environmental factors could be a significant risk factor for the development of PD (Wirdefeldt et al., 2011). The first indication that an exogenous insult could be responsible came in the form of I.V. drug users who had injected a synthetic meperidine compound that was contaminated with the neurotoxic species, 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), causing an acute onset of an irreversible parkinsonian state that resembled idiopathic PD (Langston et al., 1983, Markey et al., 1984). MPTP is rapidly taken up into the brain and quickly converted to its neurotoxic metabolite, MPP+ by astrocytes. It is then extruded from the astrocytes by the organic cation transporter 3 (Cui et al., 2009). Once in the extracellular space MPP+ is shuttled through the dopamine transporter located on the presynaptic terminal of dopamine neurons and is transported into the mitochondria where it inhibits mitochondrial respiration, resulting in the death of dopaminergic neurons and their projections in the SNpc and striatum, respectively. The role of an environmental factor or factors was further bolstered through a series of epidemiological studies that examined the incidence of PD in mono- and dizygotic twins (Tanner et al., 1999). Through this work it was uncovered that the incidence of PD in each of these groups was virtually identical suggesting the heritability of PD was low. More importantly, it provided evidence that an exogenous factor was significantly influencing the relative risk of PD.
While multiple studies have provided extensive support for an environmental role in the etiopathogenesis of PD, the identification of certain settings or toxicants has remained relatively elusive. However, in the last several years a significant effort has been made to identify particular toxicants or classes of toxicants, which may promote the development of the disorder. While exposure to pesticides and other agricultural products has received a considerable amount of attention and has been demonstrated to be a substantial contributor to the incidence of PD, they represent only a single class of environmental toxicants to which the human population is routinely exposed (Priyadarshi et al., 2000, Ascherio et al., 2006). The reader is referred to other reviews on the subject (Dick et al., 2007, Hatcher et al., 2008). In addition to agricultural products, industrial contaminants are beginning to receive recognition as potential risk factors for the development of PD (Steenland et al., 2006, Goldman, 2010, Seegal et al., 2010). Industrial toxicants are a broad and diverse class of compounds that are utilized in the manufacture and production of various commercial and household products, ranging from the use of carbon disulfide in the vulcanization of rubber to brominated flame retardants in the insulation of electrical components in a computer. As with pesticides, the human population is routinely exposed to industrial toxicants, either through an occupational setting or via contaminated food or their presence in everyday household products.
In this review we will direct our focus to relevant clinical and research findings, that provide support for the role of industrial toxicants in the etiology of PD and other parkinsonian disorders. Our decisions for and discussion of a particular compound was based upon its adherence to one or more criteria: 1). Has this compound been associated with PD in the human population? 2). Does exposure to this compound produce a movement disorder associated with PD or parkinsonism in the human population? 3). Does the compound target similar mechanisms and produce a similar pathology as that seen in PD?
Organohalogen Industrial Contaminants
Organohalogenated compounds (OHC) are a class of carbon-containing chemicals with varying degrees of halogen substitution on carbon atoms. The most prevalent halogen substitutions are comprised of chlorine, bromine, and fluorine to produce organochlorine, organobromine, and organofluorine compounds, respectively. The use of these compounds in an industrial setting is varied and can range from electrical insulating coatings, flame-retardant oils and in adhesives and plastics. The same properties that have made these compounds so attractive to industrial use, including being highly stable and resistant to degradation, have also made them extremely dangerous to the environment and the human population. Although there are several different compounds that are classified as being organohalogens, we will focus our discussion on the two that have received the most attention given their effects on the brain and as a risk factor for PD.
Polychlorinated Biphenyls
Polychlorinated biphenyls (PCBs) were first introduced into industrial and commercial use around 1930 and are composed of biphenyl rings with varying degrees and positions of chlorine substitutions present on each ring, allowing for the production of 209 different conformations or congeners. In the United States PCBs were predominantly manufactured as mixtures of various congeners and sold under the trade name of Aroclor. Of these mixtures, Aroclor 1242, 1254 and 1260, which contain 42%, 54% and 60% chlorine, respectively, were the major mixtures produced. Because of their thermal stability, PCBs have been widely used as coolants and lubricants in transformers and capacitors, in hydraulic fluids, as well as other electrical equipment (Erickson, 1986). Although the manufacturing and use of PCBs was discontinued in 1977 in the United States, their physiochemical properties, allow them to persist and bioaccumulate in the environment, increasing the risk for human exposure (Safe, 1993, Kamrin et al., 1994).
One danger of PCBs is their high degree of lipophilicity, allowing them to preferentially deposit in lipid-rich regions of the body, such as adipose tissue and the brain. This characteristic of PCBs coupled with the difficulty in degrading or metabolizing and eliminating them from the body, allows them to accumulate within the body and create a toxic setting of constant exposure to these compounds over several years. This type of exposure is of serious concern as PCBs have been shown to be detrimental to the central nervous system (Faroon et al., 2001). Both epidemiological as well as laboratory studies have routinely demonstrated PCB exposure to be a key mediator of several neurological deficits including alterations in cognitive function and motor development. Whether the exposure was in utero or later in adulthood was inconsequential as alterations in these neurobehavioral parameters were observed. In addition to these neurobehavioral deficits, exposure to PCBs has been associated with the incidence of PD. The brain has an extremely high concentration of lipids and PCBs are found to deposit ubiquitously within the brain. Indeed, Corrigan et al., (1998, 2000) identified significant levels of total PCBs and several of their major congeners in human brain. Interestingly, when stratified between patients with PD and pathological controls the total concentration of PCB as well as the concentrations of specific PCB congeners were found to be elevated in the PD specimens. Furthermore, a recent epidemiological study demonstrated an increased incidence of PD in females who had been occupationally exposed to PCBs while working in an electrical capacitor plant (Steenland et al., 2006). The relevance of gender in this finding is unclear.
More recently, the potential cellular and molecular pathways and mechanisms responsible for the association between PCB exposure and PD are being uncovered and have identified several neuronal targets, including alterations in calcium homeostasis (Kodavanti et al., 1998), generation of oxidative stress, and disruption to neurotransmitter systems (Fonnum et al., 2006, Lyng et al., 2007, Lyng and Seegal, 2008, Lee et al., 2011). Of these, alterations to dopamine neurotransmission have been the most extensively studied, both in in vitro and in vivo model systems. Of these studies, the consensus finding is that exposure to PCBs results in a reduction of dopamine, either in dopaminergic cells lines or slices as well as from the nigrostriatal system of mice, rats, and nonhuman primates (Seegal et al., 1986, Seegal et al., 1989, Seegal et al., 1990, Seegal et al., 1991, Shain et al., 1991, Seegal et al., 1994, Seegal et al., 1998, Lee and Opanashuk, 2004, Richardson and Miller, 2004). The mechanisms leading to the reduction in dopamine levels are varied as exposure has been demonstrated to inhibit tyrosine hydroxylase and aromatic acid decarboylase, which are both enzymes involved in the synthesis of dopamine. Additionally, PCBs have been shown to alter the expression and function of the plasmalemmal dopamine transporter (DAT) as well as the vesicular monoamine transporter (VMAT2) to sequester dopamine (Bemis and Seegal, 2004, Richardson and Miller, 2004, Caudle et al., 2006, Fonnum et al., 2006). Interestingly, a recent SPECT imaging study of capacitor workers who had high occupational exposures to PCBs revealed a reduction in DAT density in the striatum of patients diagnosed with PD compared with control, similar to the results demonstrated by Caudle et al., (2006) in mice.
The implication of alterations to dopamine handling via disruption of DAT and VMAT2 is still under investigation. It is important to point out that deficits in the ability of VMAT2 to sequester cytosolic dopamine can result in an increase in oxidative stress in the dopamine neuron and subsequent loss of dopamine in the striatum, degeneration of dopamine neurons in the SNpc as well as behavioral alterations reminiscent of those found in PD (Caudle et al., 2007, Taylor et al., 2009). It has been postulated that this dopaminergic degeneration is a result of the accumulation of dopamine in the cytosol and its subsequent oxidation to neurotoxic species and the formation of reactive oxygen and nitrogen species (Caudle et al., 2008).
Polybrominated Diphenyl Ethers
Polybrominated diphenyl ethers (PBDE) are a class of brominated compounds chemically similar to PCBs, composed of varying degrees and positions of bromine substitutions to give 209 different structures or congeners. As PCB manufacture and use were being phased-out in the late 1970’s due to their public health concerns, PBDEs were quickly introduced to replace them. Similar to PCBs, PBDEs are additive flame retardants primarily used in the manufacturing and insulation of electronic equipment. In addition, they are also found in polyurethane foam used in carpeting, furniture cushions and home insulation (Darnerud et al., 2001, de Wit, 2002). The major commercial PBDE products produced were marketed as one of three mixtures, known as pentabrominated BDE, octabrominated BDE, and decabrominated BDE. Although the manufacture of penta and octa BDEs has been discontinued since 2004, Deca BDEs are the most extensively produced and widely used PBDEs. The additive binding of these compounds, rather than the chemical incorporation into the products allows PDBEs to migrate out of the plastic and enter the environment. Furthermore, like PCBs their lipophilicity, resistance to degradation, and the ability to biomagnify make PBDEs extremely persistent in the environment (Norstrom et al., 2002). However, in contrast to PCBs and other organochlorines, whose environmental levels have been decreasing, levels of PBDEs have significantly increased, raising concern over the safety of PBDEs and their potential adverse health effects. Like PCBs, the predominant route of human exposure to PBDEs is through the ingestion of contaminated food as well as occupational exposure (Alaee et al., 2001, Luross et al., 2002, Norstrom et al., 2002). Exposure to PBDEs, whether through diet or occupational exposure has resulted in a considerable rise in PBDE body burden in the United States population, especially in serum and breast milk (Sjodin et al., 2004).
While data concerning the health effects of PBDEs on the human population is lacking, several studies have characterized the toxicological outcomes of exposure to PBDEs in in vitro and in vivo systems. Interestingly, the neuronal components disrupted are strikingly similar to those altered following exposure to PCBs. Indeed, several in vitro studies have demonstrated the ability of PBDEs and other brominated flame retardant compounds to elicit an increase in oxidative stress (Madia et al., 2004, Reistad et al., 2005, Reistad et al., 2006, Reistad et al., 2007), disruption of the calcium signaling pathway, (Kodavanti and Derr-Yellin, 2002, Kodavanti and Ward, 2005, Reistad et al., 2005), as well as inhibition of DAT and VMAT2 function at low micromolar concentrations. Furthermore, as seen with PCBs, these effects appear to be specific for the dopamine system, as PBDEs demonstrated low inhibitory potential for other neurotransmitter systems. As with PCBs, this specificity for the dopamine system and VMAT2, in particular raises concerns about the neurotoxic potential of these compounds to the nigrostriatal dopamine system, as mentioned above. Further studies will need to be conducted in order to parcel out the cumulative effects of PBDEs on the dopamine system and their possible role in PD.
Metals
Metals have been utilized in biological processes since the beginning of cellular life on Earth as humans require a plethora of metals for enzymes that make use of iron, copper, magnesium, manganese, zinc, selenium, cobalt and molybdenum. The utility of these metals in biology stem from their catalytic properties as they vastly increase the rates of enzyme reactions due to their facility in transferring electrons. In addition to their biological use, metals have been used for over 7,000 years in commerce and industry. As a result, metals are recognized as some of the earliest toxicants in history, with reports of metal toxicity by Hippocrates around 370 BC. Since then, the use and potential for human exposure to metals has evolved as metals are being used in new and diverse ways, including as reactive metal surfaces of nanoparticles. Thus, it is imperative to understand the toxicological processes involved in metal toxicity and the implications of their exposure to the human population.
While the toxic effects of metal exposure on the human body has been well documented, it has only been in the last 20–30 years that the neurotoxicological outcomes of metal exposure have been more appreciated. Indeed, the brain appears to be exquisitely vulnerable to metal toxicity as metals target various aspects of the neuron, including the generation of oxidative stress and disruption of neurotransmission. These effects are especially detrimental when they occur in the basal ganglia. As with other environmental factors, metal exposure has been suggested to be a risk factor for the development of Parkinson disease and other parkinsonian-related movement disorders. This section will present recent findings concerning the potential role and mechanisms of action of metal toxicity in Parkinson disease and parkinsonism.
Iron
Iron (Fe) is the most abundant element on earth and an essential metal for life as it is used extensively by proteins involved in the electron transport chain and oxygen transport. Normal exposure to iron occurs through our diet, especially meat, poultry, and fish, as well as iron supplementation. Further iron exposure can occur through occupational exposures, predominantly from metal fumes or metal dust, as would be generated during welding and in iron and steel production. Iron that has entered the body is absorbed across cell membranes by the divalent metal tranpsorter 1 (DMT1) before it is bound by proteins, such as transferrin, which transports iron into tissues, and ferritin (H-and L-ferritin), which serves as a storage depot for free iron. These mechanisms are important for the cell as they function to regulate and maintain iron homeostasis, as an increase in free iron is highly susceptible to free radical generation via the Fenton reaction, which catalyzes the conversion of hydrogen peroxide to the highly reactive hydroxyl radical. These hydroxyl radicals readily react with multiple components of the cell, including DNA, membrane lipids, and protein, leading to their dysfunction. Within the dopamine neurons in the substantia nigra pars compacta, a readily available supply of hydrogen peroxide is present as this is a normal byproduct of the production and metabolism of dopamine within the neuron, suggesting that a decrement in iron homeostasis could facilitate the further production of reactive species that could subsequently damage various aspects of the neuron.
The oxidative stress caused by free iron can directly lead to neuronal damage and neurotoxicity, as evidenced in two genetic forms of iron mishandling. Friedrich’s ataxia in which the iron-regulating gene, frataxin is silenced, results in cytosolic iron accumulation and motor deficits. In addition, neurodegeneration with brain iron accumulation 1 (NBIA1), also called pantothenate kinase-associated neurodegeneration (PKAN), leads to iron buildup in the basal ganglia and subsequent death of neurons. This neuronal death leads to parkinsonian motor symptoms as well as dementia and dystonia. In addition to these two disorders, several epidemiological studies have determined a significant positive correlation between plasma levels of iron and the incidence of PD. Related to these findings, an increased accumulation of iron has been found in the SNpc of PD patients compared with controls (Sofic et al., 1988). While the cause for this accumulation is not clear, studies have demonstrated an increase in the expression of the lactoferrin receptors and the DMT1, on dopaminergic neurons in the SNpc of patients with PD, which could facilitate the transport of iron into the dopamine neurons. In a similar study, Friedman et al (2011) found a reduction in the amount of L-ferritin, which functions to sequester and store free cytosolic iron, in PD patients. Taken in concert, an increase in iron transport mechanisms as well as a reduction in the ability of the dopamine neurons to store free iron could provide an advantageous environment for the generation of oxidative stress.
The deleterious effects of iron mishandling have also been demonstrated in animal models of PD. Exposure of neonatal mice to levels of iron routinely found in baby formula resulted in an age-dependent reduction in TH+ neurons in the SNpc as well as a loss of dopamine in the striatum of aged animals (Kaur et al., 2007). Conversely, treatment of mice with an iron chelator attenuated the parkinsonian pathology induced by MPTP. Similarly, overexpression of H-ferritin in nigral neurons rendered them resistant to the dopaminergic effects of MPTP (Kaur et al., 2003). Finally, unilateral injection of FeCl3 into the SNpc of adult rats resulted in a significant reduction in striatal dopamine as well as other dopaminergic markers and a concomitant deficit in dopamine-related behaviors (Sengstock et al., 1994, Junxia et al., 2003). While a significant amount of attention has been paid to the potential role of iron-mediated generation of oxidative stress as a causative factor in damage to the nigrostriatal dopamine system, several studies have focused on the ability of iron, as well as other metals to facilitate the fibrillization and aggregation of the PD-related protein, alpha-synuclein (Uversky et al., 2001). It is this aggregated and fibrillar conformation that is considered to the predominant toxic form of alpha-synuclein and involved in the degenerative process of dopaminergic neurons. While iron in and of itself is believed to contribute to the change in conformation of alpha-synuclein, additional studies have demonstrated the ability of oxidative species to also participate in the formation of neurotoxic alpha-synuclein species (Giasson et al., 2000).
Copper
Copper (Cu) is another essential metal that has numerous biological roles in the body, including formation of red blood cells, iron transport, mitochondrial respiration, as well as being an integral part of key enzymes, such as superoxide dismutase and dopamine β-hydroxylase (Harris, 2000). However, like iron, copper is highly reactive and can undergo the Fenton reaction to generate hydroxyl radicals from hydrogen peroxide, which can have detrimental effects on many facets of normal neuronal function. Thus, a tight homeostatic control of copper needs to be in place. Exposure to copper primarily occurs via ingestion of copper-containing food and beverages in addition to occupational exposure through welding fumes and metal smelting and mining. Following exposure, copper is absorbed from the gut by the copper membrane transporter 1 (CMT1) and is predominantly found bound to ceruloplasmin in the blood before it is transported into cells (Hellman and Gitlin, 2002).
The role of altered copper homeostasis in neurological disorders is well documented, especially as it relates to Wilson’s disease. Wilson’s disease is a genetic disorder that results in the accumulation of copper in tissues and damages specific regions of the brain, most notably the basal ganglia nuclei and substantia nigra. This accumulation appears to cause a form of parkinsonism characterized by movement deficits that are moderately responsive to L-DOPA, as well as pathological changes in pre- and postsynaptic dopamine markers in the striatum (Barbeau and Friesen, 1970, Hitoshi et al., 1991, Oder et al., 1993, Barthel et al., 2003). In addition to Wilson’s disease, there is some evidence that provides support for a role of copper exposure and PD pathology. Previous epidemiological studies by Gorell et al., (Gorell et al., 1997, 1999a) have suggested an association between copper exposure and PD, showing an almost 2.5-fold increase in risk for PD following a 20 year occupational exposure to copper. More recently, further evidence for copper exposure as a risk factor for PD has been demonstrated following intranigral injection of copper into rats. Investigators noted a significant reduction in several indices of dopaminergic neurodegeneration, including reduced striatal dopamine and loss of tyrosine hydroxylase projections and neurons in the striatum and substantia nigra, respectively (Yu et al., 2008).
The mechanisms of action that underlie copper-induced neuropathology are still unclear. However, given the propensity for the accumulation of free copper to generate highly neurotoxic reactive species, it can be speculated that oxidative stress plays a substantial part in mediating the neurodegenerative process. As mentioned above, the effects of oxidative stress on neuronal function are promiscuous, causing damage to multiple aspects of the cell. A major target of oxidative damage appears to be the mitochondria (Rossi et al., 2004). Indeed, recent reports have suggested that mitochondria are preferentially damaged following exposure to copper (Paris et al., 2009). Alteration to mitochondria function has long been appreciated as a potential pathogenic cascade in PD, most notably, through the aberrant generation of oxidative species following damage (Schapira, 2008). Additionally, like iron, copper has been shown to accelerate the oligomerization of alpha-synuclein monomers into neurotoxic fibrils, which can impair mitochondrial function (Uversky et al., 2001). Whether or not this interaction underlies dopaminergic loss is still unclear.
Manganese
Metals, especially transition metals, play an essential role in the normal functioning of numerous biological processes, particularly as cofactors necessary for enzyme function. For instance, manganese is an essential cofactor for several enzymes, including superoxide dismutase (SOD), and plays a role in the synthesis and metabolism of neurotransmitters (Schroeder et al., 1966, Hurley et al., 1984, Golub et al., 2005). In contrast, as with other metals, manganese can have detrimental effects on this system through its accumulation and generation of reactive species among other mechanisms. Although manganese exposure can occur through several different forms, including ingestion of food, exposure to manganese-containing fuel additives, the predominant route of exposure is via occupational inhalation of manganese fumes in an industrial setting, most notably from volatilization of manganese containing substrates used during welding (Huang et al., 1989, Hudnell, 1999). Manganese is also present in flux agents, such as electrodes as well as being added to consumables containing between 2–15% manganese (Villaume, 1979, Burgess, 1995). Inhalation of particulate manganese is able to bypass the blood-brain barrier (BBB) where it is taken up directly by presynaptic nerve endings in the olfactory bulb. However, these results have mostly been obtained through a rodent model of exposure, thus the relevance of this pathway is unclear in humans, given the interspecies difference in the olfactory system and brain between rodents and humans (Brenneman et al., 2000, Aschner et al., 2005). After being taken up, manganese is retrogradely transported to the cell body where it can be released into the interstitial space (Tjalve and Henriksson, 1999, Vitarella et al., 2000, Fechter et al., 2002, Normandin et al., 2004). Exposure to high levels of airborne manganese has been associated with several neurologic symptoms, including reduced neurobehavioral performance, neuropsychological impairment, and disruption of motor function (Huang et al., 1993, Gibbs et al., 1999). However, the most significant consequence of manganese toxicity appears to be impaired motor function, which culminates in a disorder referred to as manganism. Thus, welding and manganese have received considerable attention due to the presence of motor symptoms that are shared with many clinical features of PD.
The mechanisms by which manganese mediates manganism are not wholly understood. Manganese, when in excess, can inhibit mitochondrial function, reduce glutathione levels, increase NMDA-mediated neurotoxicity and alter calcium homeostasis, all which culminate in cellular dysfunction (Maynard and Cotzias, 1955, Brouillet et al., 1993, Gavin et al., 1999). Furthermore, manganese is a potent mediator of pro-oxidant activities, through its production of reactive species, particularly superoxides, peroxide, and hydroxyl radical (Graham et al., 1978, Cohen, 1984). In addition, it can catalyze the oxidation of dopamine to reactive quinones (Graham, 1978, Halliwell and Gutteridge, 1984). Significant evidence has been collected demonstrating the toxicity of these oxidative species in dopaminergic neurodegeneration (Jenner, 2003). The controversy related to the contribution of manganese to PD revolves around two main issues.
First, epidemiologically (reviewed by Santamaria et al., 2007 (Santamaria et al., 2007)), while several reports have identified occupational exposure to manganese to significantly increase the risk of PD (Gorell et al., 1999a, Gorell et al., 1999b), others have failed to establish such an association (Semchuk et al., 1993, Seidler et al., 1996, Marsh and Gula, 2006). The fundamental problems appear to relate to the fact that some of the studies have relied heavily upon self-reported neurological symptoms, lack of exposure data, small sample size, and inadequate control groups. These factors make it difficult to reach a substantial conclusion as to the influence of welding and/or manganese on the development of neurological deficits.
The clinical and pathological evidence also raises doubts on the contribution of manganese to the risk of PD. The pathology observed with PD is largely attributable to the progressive degeneration of dopamine-containing neurons in the SNpc and loss of the dopaminergic projections to the striatum (Ehringer and Hornykiewicz, 1960, Albin et al., 1989, Crossman, 1989, DeLong, 1990, Fahn, 2003). Another important pathological feature of PD is the presence of eosinophilic, cytoplasmic inclusions of fibrillar, misfolded proteins, including α-synuclein, termed Lewy bodies and Lewy neurites, located in the perikarya and cellular processes, respectively, of remaining neurons in the SNpc and other brain regions (Braak et al., 1995, Forno, 1996). In contrast, symptoms related to manganism are attributed primarily to damage to neurons in the globus pallidus (internal and external segments), while largely sparing the caudate, putamen, and SNpc. Furthermore, the lack of Lewy body inclusions differentiates the pathology of manganism from PD, in addition to magnetic resonance imaging (MRI) detection of manganese accumulation in the pallidum following exposure, compared to a normal MRI in PD. However, while most report the preservation of dopaminergic regions involved in PD pathology (Shinotoh et al., 1995, Olanow et al., 1996, Pal et al., 1999, Olanow, 2004), others suggest a mild damage to these regions as well as a general alteration in their function in manganism (Suzuki et al., 1975, Eriksson et al., 1992, Kim et al., 2002, Wright et al., 2004, Chen et al., 2006, Guilarte et al., 2006).
Similarly, laboratory studies using nonhuman primates largely confirm the almost exclusive role of the globus pallidus in Mn pathology, while leaving the dopaminergic inputs relatively untouched. In general, a lack of dopaminergic pathology, as evidenced by no change in DAT expression, striatal dopamine levels, some reductions in dopamine receptor expression, and nonresponse to L-DOPA treatment following an exposure paradigm that closely mimics that seen in humans. Taken together, while manganese exposure is widely studied in relation to PD, many believe that manganism is a separate entity as it predominantly involves the globus pallidus rather than the SNpc, and the seemingly overlapping clinical signs may just be a result of the involvement of basal ganglia dysfunction and damage to common output pathways in both disorders (Dobson et al., 2004).
Lead
Lead is a non-essential metal that can replace other metals in biological processes to the detriment of an organism. The predominant route of exposure within the human population is through ingestion of contaminated food products. However, higher than normal exposures to lead can also occur via lead-based paint, lead in dust, lead contaminated drinking water, and lead in the air from combustion of leaded fuels (Manton et al., 2005). Although the environmental levels of lead have been drastically reduced over the last several decades, lead exposure still represents a significant health concern, especially for infants and young children who are still progressing through critical periods of neurodevelopment.
Exposure to lead can manifest in several different neurological deficits, depending on amount of exposure as well as age of the subject, with children demonstrating a substantially more drastic neurotoxicological response compared with adults (Goyer, 1996). In general, neuropathological symptoms include severe edema and loss of neurons in the central nervous system as well as demyelination and axonal degeneration in the periphery, which manifests as a motor-based peripheral neuropathy and ataxia. In addition to these motor abnormalities, recent epidemiological studies have indicated a potential association between exposure to lead and an increased risk of PD, suggesting a 2–3-fold increase in risk for PD following lead exposure (Coon et al., 2006, Weisskopf et al., 2010). Animal studies have also demonstrated a significant alteration in the function of the nigrostriatal dopamine system following exposure to lead, including a reduction in the number of dopamine neurons in the SNpc as well as a reduction in the firing rate of dopaminergic neurons, which is independent of neuronal loss (Scortegagna and Hanbauer, 1997, Tavakoli-Nezhad et al., 2001).
The precise mechanisms of action leading to these effects remain to be determined and could arise from several different avenues, as lead-mediated neuronal dysfunction is somewhat promiscuous, affecting multiple aspects of neuronal function (Gmerek et al., 1981, Lasley and Gilbert, 1996, Ferguson et al., 2000). For example, lead can disrupt calcium handling of the neuron by inhibiting uptake of calcium through calcium channels in the presynaptic terminal as well as the mitochondria. In addition, lead can dissipate the proton gradient across synaptic vesicles, possibly leading to decreased DA sequestration into vesicles (Borisova et al., 2011). Indeed, recent work from our lab has clearly demonstrated the detrimental effects mishandling of cytosolic dopamine by synaptic vesicles can have on the nigrostriatal dopamine system, including reduction in dopamine neurons in the SNpc and loss of dopamine in the striatum, as well as formation of neurotoxic reactive species (Caudle et al., 2007).
Mercury
Mercury exists in several different forms or species. These forms include inorganic, such as mercury chloride, elemental mercury, or organic mercury, such as methylmercury. Of these species, elemental mercury and methylmercury are the most toxicologically important as they elicit the most detrimental effects following human exposure. Exposure to these compounds usually occurs in an occupational setting, as is the case with inhalation of vaporized mercury, while exposure to methylmercury predominantly occurs through ingestion of contaminated fish. Elemental mercury can be vaporized leading to an increased incidence of inhalational exposure to mercury, especially in an occupational setting. Mercury has a very high affinity for sulfur and will readily bind to thiol containing molecules, such as cysteine. The binding of methylmercury to cysteine allows it to be transported across the blood brain barrier by the neutral amino acid transporter (Yin et al., 2008).
The neurological effects of mercury exposure have been well documented. Identification of mercury exposure mediating neurological alterations was in the 1800’s in the milliner’s trade where hat makers routinely inhaled vaporized mercuric nitrate, which was used to cure the felt of hats. Those who had become intoxicated exhibited signs of movement disorders, including tremors and polyneuropathy. Widespread intoxication by methylmercury has also occurred as with the mass contamination of Minimata Bay, Japan for several decades in the mid 1900’s. Consumption of contaminated fish, especially by women who were pregnant resulted in severe neurodevelopmental deficits, including incapacitating alterations in movement and cognition. Adult exposure was similarly debilitating, causing ataxia, tremor, and sensory deficits, most likely the result of cerebral edema and atrophy. While these data provide a very clear indication that mercury intoxication causes significant motor symptoms, to date, an association between mercury exposure and PD has not been established (Gorell et al., 1999a). However, a significant amount of data suggests that exposure to mercury can have substantial impact on the normal functioning of the dopamine nigrostriatal dopamine system. Most notably, Lin et al., (2011) recently reported a significant reduction in DAT function in the striatum of workers occupationally exposed to mercury vapor. This dysfunction in DAT was similarly demonstrated in vitro with a reduction in dopamine uptake in synaptosomes treated with mercury (Hare et al., 1990, Dreiem et al., 2009). In addition, mercury exposure reduced neurites of dopaminergic neurons isolated from the ventral mesencephalon and caused a reduction of striatal dopamine in mice exposed to methylmercury (Gotz et al., 2002, Bourdineaud et al., 2011). The mechanisms of action related to these deficits are not clearly defined, however, methylmercury has been shown to be involved in the generation of oxidative stress through various routes, including acceleration of lipid peroxidation, mitochondrial damage, and stimulate superoxide production (Yee and Choi, 1996).
Nanoparticles
Nanoparticles can be grouped into two distinct, yet similar categories, based upon their origin. Combustible nanoparticles are those, which arise from environmental sources, such as diesel exhaust or welding fumes. On the hand, engineered or manufactured nanoparticles refer to compounds that are synthesized such as titanium oxide, zinc oxide, or carbon nanotubes, among others (Oberdorster et al., 2005). Our discussion will be focused on engineered nanoparticles as these are seeing an emerging utility in various biological and biomedical avenues, such as cosmetics, adjuncts for medical imaging, vehicles for drug delivery, and biosensors. More specifically, they are currently being used as therapeutic avenues in several neurodegenerative disease, including PD (Huang et al., 2009, Modi et al., 2009). While these capabilities have provided new and exciting opportunities for their use their physiochemical properties have increased concern for the potential health effects that increased exposure to nanoparticles may have. Nanoparticles vary in size from 1–100 nm and can be covered with metallic coatings, ranging from titanium (Ti), aluminum (Al), iron (Fe), manganese (Mn), copper, (Cu), and gold (Au), among others (Win-Shwe and Fujimaki, 2011). Thus, given our previous discussion of the neurotoxic effects of metals, especially in terms of oxidative stress, nanoparticles represent a new risk category for metals exposure. Furthermore, their small size introduces new problems with how the body handles absorption, distribution and excretion of these materials. Indeed, nanoparticles in the blood can gain access to the brain through the blood brain barrier. And once inside the brain they can interact with neurons and glia, affecting their function and expression.
Unfortunately, manufactured nanoparticle technology is too new to have identified cohorts of exposed workers with health problems and specific brain disorders, thus there is no definitive proof that exposure to engineered nanoparticles increases the risk of neurodegenerative diseases such as PD (Oberdorster 2009). However, recent laboratory studies have suggested that exposure to engineered nanoparticles can have deleterious effects on the dopamine system. Several in vitro studies using PC12 cells have demonstrated reductions in dopamine, increases in oxidative stress, as well as alterations to dopamine- and PD associated genes, including tyrosine hydroxylase, alpha-synuclein, and parkin (Hussain et al., 2006, Wang et al., 2009). Similarly, in vivo exposures have also demonstrated reductions in striatal dopamine, in addition to increased oxidative stress and neuroinflammation following exposure to metal nanoparticles (Hu et al., 2010, Wu et al., 2011). While the precise cause for these changes is unclear, increase in oxidative stress may be a major contributor.
Solvents
Solvents represent a broad range of chemicals with the common utility of dissolving one substance into another. The most common solvents in use today include trichloroethylene (TCE), toluene, acetone, hexane, carbon disulfide, which all serve multiple purposes in industrial and home uses. The human population is exposed to solvents through numerous different routes. The most prevalent route is occupational exposure via inhalation or dermal exposure. However, non-occupational exposure can also occur through ingestion of contaminated water as well as inhalation exposure, both following improper waste disposal or accidental release of solvents. In general, solvents are lipophilic, allowing them to be amenable to quick and easy absorption into the body and to target organs following exposure. Over the years, several neurological deficits have been associated with solvent exposure. In general, these could be classified as exhibiting movement disorders, such as tremor as well as other motor deficits. However, it is unclear whether these symptoms represent PD or a parkinsonian-related disorder. Although many epidemiological studies have attempted to elucidate an association between solvent exposure and PD, a solid link has yet to be determined.
Trichloroethylene
Trichloroethylene is a chlorinated hydrocarbon that has proved to be very versatile in its uses and applications, including its early use as an anesthetic giving its properties as a central nervous system depressant. However, TCE has a broader use as a solvent in many different settings, including the rubber industry, adhesive formulations, dyeing and finishing operations, printing inks, paints, lacquers, varnishes, adhesives, and paint strippers. It is applied prior to plating, anodizing, and painting. While TCE use has been greatly reduced in most of the aforementioned applications, it is still commonly used in the degreasing of metals and as an intermediate for hydrofluorocarbon production. In addition to a high occupational exposure the human population are also routinely exposed to TCE via contact or consumption of contaminated water, food or contact with consumer goods containing TCE.
The overall toxicity of TCE appears to result from its ability to disrupt cellular membrane integrity, formation of oxidative free radicals resulting in lipid peroxidation, as well as disruption of calcium transport. This membrane disruption leads to demeylination with TCE exposure having been shown to result in loss of sensory nerve function (Huber, 1969, Feldman et al., 1970, Feldman et al., 1985). The main metabolites of TCE are trichloroethanol, thrichlorothanol-glucuronide and trichloroacetic acid (TCA), the former excreted rapidly in the urine and latter appearing slower, suggesting TCA to be a major source of TCE toxicity (Cole et al., 1975, Yoshida et al., 1996). With growing reports of TCE contamination of drinking water, understanding the possible long-term effects of TCE exposure has become more important. There has been increasing interest into the link between chronic exposure to TCE and Parkinson’s disease and a few case reports and a population based study have provided more evidence to suggest that a potential association between long term exposure to TCE and PD pathology (Bringmann et al., 1992, Guehl et al., 1999, Kochen et al., 2003, Gash et al., 2008, Goldman, 2010). Most recently a study of twins discordant for PD identified exposure to TCE as having a 6-fold increased risk for PD (Goldman et al., 2011). These results are largely supported by the experimental literature through the use of animal models of TCE exposure. Two reports in the last few years have demonstrated significant damage to the nigrostriatal dopamine system in rats following TCE administration. Following up on case study data, Gash et al., (2008) found that TCE exposure resulted in a loss of dopaminergic neurons in the SNpc, as well as a concomitant reduction in dopamine in the striatum and midbrain. While the exact mechanisms of action of TCE on these neurons is not know, they were able to show that TCE inhibits mitochondrial complex I, as seen with other parkinsonian mimetics, such as MPTP and rotenone. Another study demonstrated similar findings of loss of dopamine neurons in the SNpc, again in rats exposed to TCE, in addition to other pathological hallmarks of PD, such as accumulation of alpha-synuclein and an increase in reactive oxygen species (Liu et al., 2010). They were also able to show that the effects of TCE were preferential to the dopamine neurons in the SNpc as no loss of GABAergic or cholinergic neurons was observed in this area and the dopaminergic neurons of the ventral tegmental area were similarly spared, as seen in PD.
Methanol
The primary use of methanol is as a fuel additive and as a precursor in the production of plastics, formaldehyde, acetic acid and explosives. Methanol is also an additive in paint strippers, aerosol spray paints and car windshield washer products. Methanol exposure can occur via inhalation of methanol vapors, dermal exposure to aqueous solutions containing methanol, however, the most common route of methanol intoxication involves the deliberate or accidental ingestion. Like n-hexane, the most toxic compound is not methanol, rather metabolic intermediates of methanol, specifically formic acid, give rise to the toxic nature of methanol exposure. Methanol is oxidized by alcohol dehydrogenase in the liver to yield formaldehyde which is then rapidly oxidized into formic acid (Teng et al., 2001). Symptoms of methanol poisoning resemble that of ethanol intoxication, such as lethargy, confusion, headache, nausea, ataxia and vision impairment. Following the initial symptoms, neurological deficits can occur that can be classified as parkinsonian or dystonic with reports of lesions to the basal ganglia, putamen and other white matter regions (McLean et al., 1980, Ley and Gali, 1983, Verslegers et al., 1988, Carcaba et al., 2002, Finkelstein and Vardi, 2002, Reddy et al., 2007). In these cases, the appearance of motor deficits is slow to present and may occur weeks after initial exposure. The rigidity and bradykinesia may be partially relieved with levodopa treatment but the white matter lesions may extend beyond the basal ganglia (Reddy et al., 2010).
Conclusion
Toxic exposure to non-pesticide compounds, such as organohalogens, metals, and solvents have received strong support as risk factors for PD and other parkinsonian disorders. Unfortunately, making a clear delineation as to the contribution of a class of compounds or a specific compound to a particular suite of pathological and clinical symptoms remains to be achieved. While several compounds and classes of compounds may appear to share similar features in their neurotoxicity we simply do not have enough relevant data to make that assessment. The reasons for this are varied and could be due to lack of a large enough cohort in the human population or a clinical heterogeneity within the cohort, a lack of a systematic molecular and behavioral appraisal of damage to the nigrostriatal and other relevant neural circuits following exposure. It also must be considered that these compounds, in and of themselves, do not explicitly cause PD or parkinsonism. Rather, they serve to facilitate the expression of the disorder through their interaction with other underlying genetic or environmental factors. And without these factors in place, the neurotoxicity of these exposures is less pronounced, making it more difficult to identify a particular compound as the singular causative factor in the disorder.
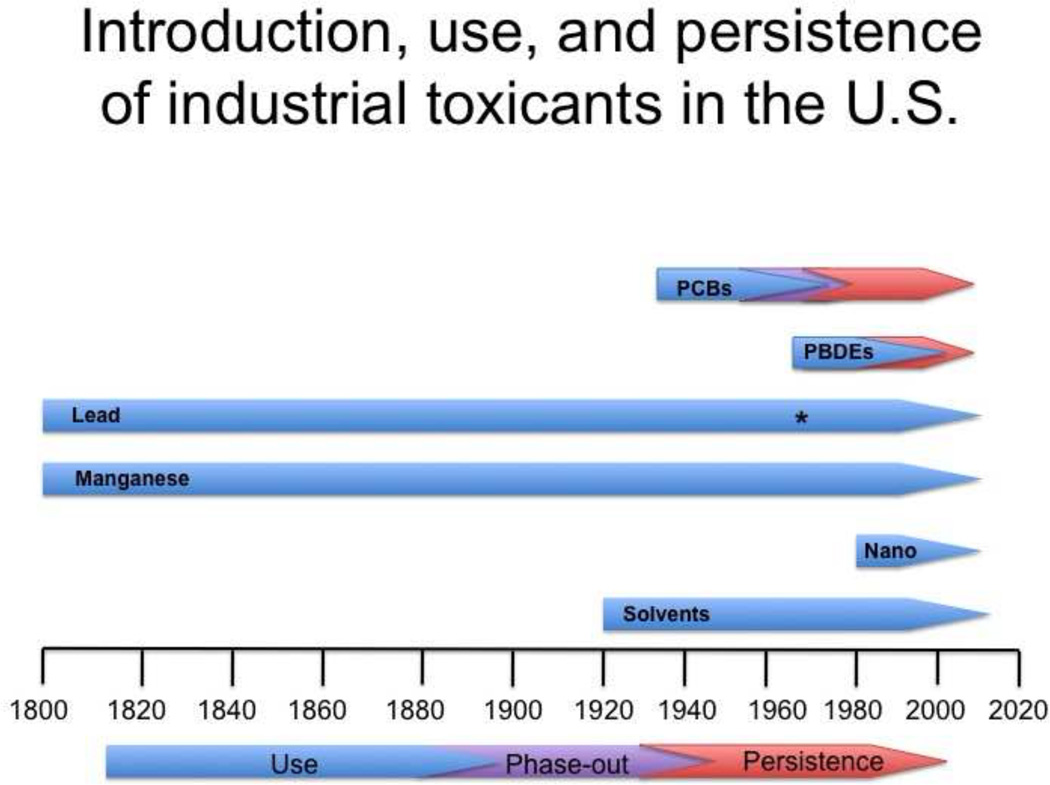
Figure 1.
Introduction and use of various industrial toxicants in the U.S. The commercial introduction and use of several widely used industrial contaminants are shown for the last two centuries, beginning around the Industrial Revolution. While some of these compounds, such as PCBs have had their use phased out, others such as many solvents have only had their use restricted, yet are still in high use in the U.S. In contrast, most metals are still widely used and have seen little or no restriction placed on their use. An exception to this is lead, which saw a removal from use as a gasoline additive in the 1970’s (denoted by an asterisk), resulting in a drastic reduction in the blood lead levels of the human population.
Table 1.
Association between exposure to industrial toxicants and Parkinson’s disease and potential mechanisms of pathology.
| Chemical | Strength of Association | Mechanisms of action | |
|---|---|---|---|
| Organohalogens | Human | Animal | |
| PCBs | ++ | ++ | Oxidative stress, dopamine homeostasis, calcium homeostasis |
| PBDEs | 0 | + | Oxidative stress, dopamine homeostasis, calcium homeostasis |
| Metals | |||
| Iron | + | ++ | Oxidative stress, alpha-synuclein fibrillization |
| Copper | + | + | Oxidative stress, alpha-synuclein fibrillization, mitochondrial dysfunction |
| Manganese | + | + | Oxidative stress, mitochondrial dysfunction, calcium homeostasis |
| Lead | + | + | Dopamine homeostasis, calcium homeostasis |
| Mercury | 0 | + | Oxidative stress, dopamine homeostasis, mitochondrial dysfunction |
| Nanoparticles | 0 | + | Oxidative stress, neuroinflammation |
| Solvent | |||
| TCE | ++ | ++ | Oxidative stress, mitochondria dysfunction, calcium homeostasis, alpha-synuclein accumulation |
strong association, i.e. multiple reports.
some association, i.e. individual reports, small sample size. 0 = no association or insufficient evidence.
There is evidence for all of these compounds to cause some level of parkinsonism in either humans or animal models. Additionally, the major pathogenic mechanisms of action, as suggested by the literature, have been provided.
Acknowledgements
This work was supported by grants from the National Institutes of Health 1P01ES016731, 5T32 ES 012870, 1P50NS071669, and 4R00ES017477.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaee M, Sergeant DB, Ikonomou MG, Luross JM. A gas chromatography/high-resolution mass spectrometry (GC/HRMS) method for determination of polybrominated diphenyl ethers in fish. Chemosphere. 2001;44:1489–1495. doi: 10.1016/s0045-6535(00)00311-8. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MG, O'Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Critical reviews in toxicology. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Barbeau A, Friesen H. Treatment of Wilson's disease with L-dopa after failure with penicillamine. Lancet. 1970;1:1180–1181. doi: 10.1016/s0140-6736(70)91259-6. [DOI] [PubMed] [Google Scholar]
- Barthel H, Hermann W, Kluge R, Hesse S, Collingridge DR, Wagner A, Sabri O. Concordant pre- and postsynaptic deficits of dopaminergic neurotransmission in neurologic Wilson disease. AJNR Am J Neuroradiol. 2003;24:234–238. [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Borisova T, Krisanova N, Sivko R, Kasatkina L, Borysov A, Griffin S, Wireman M. Presynaptic malfunction: the neurotoxic effects of cadmium and lead on the proton gradient of synaptic vesicles and glutamate transport. Neurochem Int. 2011;59:272–279. doi: 10.1016/j.neuint.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Bourdineaud JP, Fujimura M, Laclau M, Sawada M, Yasutake A. Deleterious effects in mice of fish-associated methylmercury contained in a diet mimicking the Western populations' average fish consumption. Environ Int. 2011;37:303–313. doi: 10.1016/j.envint.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, Schultz C, de Vos RA, Jansen EN. Nigral and extranigral pathology in Parkinson's disease. J Neural Transm. 1995 Suppl 46:15–31. [PubMed] [Google Scholar]
- Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, Dorman DC. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicology and applied pharmacology. 2000;169:238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Friedrich H, Feineis D. Trichloroharmanes as potential endogenously formed inducers of Morbus Parkinson: synthesis, analytics, and first in vivo-investigations. J Neural Transm. 1992 Suppl 38:15–26. [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Experimental neurology. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Burgess W. Welding. In: Burgess W, editor. Recognition of health hazards in industry: A review of materials and processes. New York: John Wiley & Sons; 1995. pp. 167–204. [Google Scholar]
- Carcaba V, Garcia Amorin Z, Rodriguez Junquera R, Velasco L. Parkinsonism and putaminal lesion from methanol intoxication. An Med Interna. 2002;19:438–439. [PubMed] [Google Scholar]
- Caudle WM, Colebrooke RE, Emson PC, Miller GW. Altered vesicular dopamine storage in Parkinson's disease: a premature demise. Trends Neurosci. 2008;31:303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–236. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cohen G. Oxy-radical toxicity in catecholamine neurons. Neurotoxicology. 1984;5:77–82. [PubMed] [Google Scholar]
- Cole WJ, Mitchell RG, Salamonsen RF. Isolation, characterization and quantitation of chloral hydrate as a transient metabolite of trichloroethylene in man using electron capture gas chromatography and mass fragmentography. Journal of Pharmacy and Pharmacology. 1975;27:167–171. doi: 10.1111/j.2042-7158.1975.tb09431.x. [DOI] [PubMed] [Google Scholar]
- Coon S, Stark A, Peterson E, Gloi A, Kortsha G, Pounds J, Chettle D, Gorell J. Whole-body lifetime occupational lead exposure and risk of Parkinson's disease. Environ Health Perspect. 2006;114:1872–1876. doi: 10.1289/ehp.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson's disease. Exp Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Crossman AR. Neural mechanisms in disorders of movement. Comp Biochem Physiol A. 1989;93:141–149. doi: 10.1016/0300-9629(89)90201-6. [DOI] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109 Suppl 1:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A. Environmental risk factors for Parkinson's disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese Neurotoxicity. Annals of the New York Academy of Sciences. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Shan M, Okoniewski RJ, Sanchez-Morrissey S, Seegal RF. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicol Teratol. 2009;31:312–317. doi: 10.1016/j.ntt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Erickson BD. Analytical Chemistry of PCBs. Boston: Butterworth; 1986. [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, Bjurling P, Langstrom B, Hedstrom KG, Heilbronn E. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Archives of toxicology. 1992;66:403–407. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Faroon O, Jones D, de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol Ind Health. 2001;16:305–333. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Johnson DL, Lynch RA. The relationship of particle size to olfactory nerve uptake of a non-soluble form of manganese into brain. Neurotoxicology. 2002;23:177–183. doi: 10.1016/s0161-813x(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Feldman RG, Mayer R, Taub A. Evidence for peripheral neurotoxic effect of trichloroethylene. Neurology. 1970;20:599–606. doi: 10.1212/wnl.20.6.599. [DOI] [PubMed] [Google Scholar]
- Feldman RG, White RF, Currie JN, Travers PH, Lessell S. Long-term follow-up after single toxic exposure to trichloroethylene. American Journal of Industrial Medicine. 1985;8:119–126. doi: 10.1002/ajim.4700080206. [DOI] [PubMed] [Google Scholar]
- Ferguson C, Kern M, Audesirk G. Nanomolar concentrations of inorganic lead increase Ca2+ efflux and decrease intracellular free Ca2+ ion concentrations in cultured rat hippocampal neurons by a calmodulin-dependent mechanism. Neurotoxicology. 2000;21:365–378. [PubMed] [Google Scholar]
- Finkelstein Y, Vardi J. Progressive parkinsonism in a young experimental physicist following long-term exposure to methanol. Neurotoxicology. 2002;23:521–525. doi: 10.1016/s0161-813x(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J Toxicol Environ Health A. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Friedman A, Arosio P, Finazzi D, Koziorowski D, Galazka-Friedman J. Ferritin as an important player in neurodegeneration. Parkinsonism Relat Disord. 2011;17:423–430. doi: 10.1016/j.parkreldis.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20:445–453. [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gibbs JP, Crump KS, Houck DP, Warren PA, Mosley WS. Focused medical surveillance: a search for subclinical movement disorders in a cohort of U.S. workers exposed to low levels of manganese dust. Neurotoxicology. 1999;20:299–313. [PubMed] [Google Scholar]
- Gmerek DE, McCafferty MR, O'Neill KJ, Melamed BR, O'Neill JJ. Effect of inorganic lead on rat brain mitochondrial respiration and energy production. J Neurochem. 1981;36:1109–1113. doi: 10.1111/j.1471-4159.1981.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Goldman SM. Trichloroethylene and Parkinson's disease: dissolving the puzzle. Expert Rev Neurother. 2010;10:835–837. doi: 10.1586/ern.10.61. [DOI] [PubMed] [Google Scholar]
- Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS, Comyns K, Korell M, Chade AR, Kasten M, Priestley B, Chou KL, Fernandez HH, Cambi F, Langston JW, Tanner CM. Solvent exposures and parkinson disease risk in twins. Ann Neurol. 2011 doi: 10.1002/ana.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicology and teratology. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology. 1999a;20:239–247. [PubMed] [Google Scholar]
- Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson's disease. Neuroepidemiology. 1999b;18:303–308. doi: 10.1159/000026225. [DOI] [PubMed] [Google Scholar]
- Gotz ME, Koutsilieri E, Riederer P, Ceccatelli S, Dare E. Methylmercury induces neurite degeneration in primary culture of mouse dopaminergic mesencephalic cells. J Neural Transm. 2002;109:597–605. doi: 10.1007/s007020200049. [DOI] [PubMed] [Google Scholar]
- Goyer RA. Results of lead research: prenatal exposure and neurological consequences. Environ Health Perspect. 1996;104:1050–1054. doi: 10.1289/ehp.961041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Molecular pharmacology. 1978;14:633–643. [PubMed] [Google Scholar]
- Graham DG, Tiffany SM, Bell WR, Jr, Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Molecular pharmacology. 1978;14:644–653. [PubMed] [Google Scholar]
- Guehl D, Bezard E, Dovero S, Boraud T, Bioulac B, Gross C. Trichloroethylene and parkinsonism: a human and experimental observation. Eur J Neurol. 1999;6:609–611. doi: 10.1046/j.1468-1331.1999.650609.x. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Experimental neurology. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of iron in oxygen radical reactions. Methods Enzymol. 1984;105:47–56. doi: 10.1016/s0076-6879(84)05007-2. [DOI] [PubMed] [Google Scholar]
- Hare MF, Rezazadeh SM, Cooper GP, Minnema DJ, Michaelson IA. Effects of inorganic mercury on [3H]dopamine release and calcium homeostasis in rat striatal synaptosomes. Toxicol Appl Pharmacol. 1990;102:316–330. doi: 10.1016/0041-008x(90)90030-x. [DOI] [PubMed] [Google Scholar]
- Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson's disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Iwata M, Yoshikawa K. Mid-brain pathology of Wilson's disease: MRI analysis of three cases. J Neurol Neurosurg Psychiatry. 1991;54:624–626. doi: 10.1136/jnnp.54.7.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Gong X, Duan Y, Li N, Che Y, Cui Y, Zhou M, Liu C, Wang H, Hong F. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31:8043–8050. doi: 10.1016/j.biomaterials.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB. Chronic manganese intoxication. Archives of neurology. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W, Calne DB. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- Huang R, Han L, Li J, Ren F, Ke W, Jiang C, Pei Y. Neuroprotection in a 6-hydroxydopamine-lesioned Parkinson model using lactoferrin-modified nanoparticles. J Gene Med. 2009;11:754–763. doi: 10.1002/jgm.1361. [DOI] [PubMed] [Google Scholar]
- Huber F. Clinical aspects and neuropathology of trichloroethylene poisoning. Z Unfallmed Berufskr. 1969;62:226–267. [PubMed] [Google Scholar]
- Hudnell HK. Effects from environmental Mn exposures: a review of the evidence from non-occupational exposure studies. Neurotoxicology. 1999;20:379–397. [PubMed] [Google Scholar]
- Hurley LS, Keen CL, Baly DL. Manganese deficiency and toxicity: effects on carbohydrate metabolism in the rat. Neurotoxicology. 1984;5:97–104. [PubMed] [Google Scholar]
- Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, Schlager JJ. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol Sci. 2006;92:456–463. doi: 10.1093/toxsci/kfl020. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Annals of neurology. 2006;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. discussion S36-28. [DOI] [PubMed] [Google Scholar]
- Junxia X, Hong J, Wenfang C, Ming Q. Dopamine release rather than content in the caudate putamen is associated with behavioral changes in the iron rat model of Parkinson's disease. Exp Neurol. 2003;182:483–489. doi: 10.1016/s0014-4886(03)00123-7. [DOI] [PubMed] [Google Scholar]
- Kamrin MA, Fischer LJ, Suk WA, Fouts JR, Pellizzari E, Thornton K. Assessment of human exposure to chemicals from Superfund sites. Environ Health Perspect. 1994;102 Suppl 1:221–228. doi: 10.1289/ehp.94102s1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging. 2007;28:907–913. doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord. 2002;17:568–575. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- Kochen W, Kohlmuller D, De Biasi P, Ramsay R. The endogeneous formation of highly chlorinated tetrahydro-beta-carbolines as a possible causative mechanism in idiopathic Parkinson's disease. Adv Exp Med Biol. 2003;527:253–263. doi: 10.1007/978-1-4615-0135-0_29. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Derr-Yellin EC. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol Sci. 2002;68:451–457. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Derr-Yellin EC, Mundy WR, Shafer TJ, Herr DW, Barone S, Choksi NY, MacPhail RC, Tilson HA. Repeated exposure of adult rats to Aroclor 1254 causes brain region-specific changes in intracellular Ca2+ buffering and protein kinase C activity in the absence of changes in tyrosine hydroxylase. Toxicol Appl Pharmacol. 1998;153:186–198. doi: 10.1006/taap.1998.8533. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Presynaptic glutamatergic function in dentate gyrus in vivo is diminished by chronic exposure to inorganic lead. Brain Res. 1996;736:125–134. doi: 10.1016/0006-8993(96)00666-x. [DOI] [PubMed] [Google Scholar]
- Lee DW, Notter SA, Thiruchelvam M, Dever DP, Fitzpatrick R, Kostyniak PJ, Cory-Slechta DA, Opanashuk LA. Subchronic Polychorinated Biphenyl (Aroclor 1254) Exposure Produces Oxidative Damage and Neuronal Death of Ventral Midbrain Dopaminergic Systems. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Ley CO, Gali FG. Parkinsonian syndrome after methanol intoxication. Eur Neurol. 1983;22:405–409. doi: 10.1159/000115593. [DOI] [PubMed] [Google Scholar]
- Lin CY, Liou SH, Hsiech CM, Ku MC, Tsai SY. Dose-response relationship between cumulative mercury exposure index and specific uptake ratio in the striatum on Tc-99m TRODAT SPECT. Clin Nucl Med. 2011;36:689–693. doi: 10.1097/RLU.0b013e3181e9fa93. [DOI] [PubMed] [Google Scholar]
- Liu M, Choi D-Y, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim H-C, Gash DM, Bing G. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. Journal of Neurochemistry. 2010;112:773–783. doi: 10.1111/j.1471-4159.2009.06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luross JM, Alaee M, Sergeant DB, Cannon CM, Whittle DM, Solomon KR, Muir DC. Spatial distribution of polybrominated diphenyl ethers and polybrominated biphenyls in lake trout from the Laurentian Great Lakes. Chemosphere. 2002;46:665–672. doi: 10.1016/s0045-6535(01)00230-2. [DOI] [PubMed] [Google Scholar]
- Lyng GD, Seegal RF. Polychlorinated biphenyl-induced oxidative stress in organotypic co-cultures: experimental dopamine depletion prevents reductions in GABA. Neurotoxicology. 2008;29:301–308. doi: 10.1016/j.neuro.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng GD, Snyder-Keller A, Seegal RF. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol Sci. 2007;97:128–139. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicology letters. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Manton WI, Angle CR, Krogstrand KL. Origin of lead in the United States diet. Environ Sci Technol. 2005;39:8995–9000. doi: 10.1021/es051145e. [DOI] [PubMed] [Google Scholar]
- Markey SP, Johannessen JN, Chiueh CC, Burns RS, Herkenham MA. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984;311:464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Marsh GM, Gula MJ. Employment as a welder and Parkinson disease among heavy equipment manufacturing workers. J Occup Environ Med. 2006;48:1031–1046. doi: 10.1097/01.jom.0000232547.74802.d8. [DOI] [PubMed] [Google Scholar]
- Maynard LS, Cotzias GC. The partition of manganese among organs and intracellular organelles of the rat. The Journal of biological chemistry. 1955;214:489–495. [PubMed] [Google Scholar]
- McLean DR, Jacobs H, Mielke BW. Methanol poisoning: a clinical and pathological study. Ann Neurol. 1980;8:161–167. doi: 10.1002/ana.410080206. [DOI] [PubMed] [Google Scholar]
- Modi G, Pillay V, Choonara YE, Ndesendo VM, du Toit LC, Naidoo D. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog Neurobiol. 2009;88:272–285. doi: 10.1016/j.pneurobio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Normandin L, Ann Beaupre L, Salehi F, St -Pierre A, Kennedy G, Mergler D, Butterworth RF, Philippe S, Zayed J. Manganese distribution in the brain and neurobehavioral changes following inhalation exposure of rats to three chemical forms of manganese. Neurotoxicology. 2004;25:433–441. doi: 10.1016/j.neuro.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Norstrom RJ, Simon M, Moisey J, Wakeford B, Weseloh DV. Geographical distribution (2000) and temporal trends (1981–2000) of brominated diphenyl ethers in Great Lakes hewing gull eggs. Environ Sci Technol. 2002;36:4783–4789. doi: 10.1021/es025831e. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oder W, Prayer L, Grimm G, Spatt J, Ferenci P, Kollegger H, Schneider B, Gangl A, Deecke L. Wilson's disease: evidence of subgroups derived from clinical findings and brain lesions. Neurology. 1993;43:120–124. doi: 10.1212/wnl.43.1_part_1.120. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann N Y Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, Beal MF, Calne DB, Perl DP. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–498. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Paris I, Perez-Pastene C, Couve E, Caviedes P, Ledoux S, Segura-Aguilar J. Copper dopamine complex induces mitochondrial autophagy preceding caspase-independent apoptotic cell death. J Biol Chem. 2009;284:13306–13315. doi: 10.1074/jbc.M900323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21:435–440. [PubMed] [Google Scholar]
- Reddy NJ, Lewis LD, Gardner TB, Osterling W, Eskey CJ, Nierenberg DW. Two cases of rapid onset Parkinson's syndrome following toxic ingestion of ethylene glycol and methanol. Clin Pharmacol Ther. 2007;81:114–121. doi: 10.1038/sj.clpt.6100013. [DOI] [PubMed] [Google Scholar]
- Reddy NJ, Sudini M, Lewis LD. Delayed neurological sequelae from ethylene glycol, diethylene glycol and methanol poisonings. Clin Toxicol (Phila) 2010;48:967–973. doi: 10.3109/15563650.2010.532803. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Archives of toxicology. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol Sci. 2007;96:268–278. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Rossi L, Lombardo MF, Ciriolo MR, Rotilio G. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res. 2004;29:493–504. doi: 10.1023/b:nere.0000014820.99232.8a. [DOI] [PubMed] [Google Scholar]
- Safe S. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ Health Perspect. 1993;100:259–268. doi: 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. State-of-the-science review: Does manganese exposure during welding pose a neurological risk? Journal of toxicology and environmental health. 2007;10:417–465. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Schroeder HA, Balassa JJ, Tipton IH. Essential trace metals in man: manganese. A study in homeostasis. Journal of chronic diseases. 1966;19:545–571. doi: 10.1016/0021-9681(66)90094-4. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Hanbauer I. The effect of lead exposure and serum deprivation on mesencephalic primary cultures. Neurotoxicology. 1997;18:331–339. [PubMed] [Google Scholar]
- Seegal RF, Brosch K, Bush B, Ritz M, Shain W. Effects of Aroclor 1254 on dopamine and norepinephrine concentrations in pheochromocytoma (PC-12) cells. Neurotoxicology. 1989;10:757–764. [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Bush B. Polychlorinated biphenyls produce regional alterations of dopamine metabolism in rat brain. Toxicol Lett. 1986;30:197–202. doi: 10.1016/0378-4274(86)90103-7. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Sub-chronic exposure of the adult rat to Aroclor 1254 yields regionally-specific changes in central dopaminergic function. Neurotoxicology. 1991;12:55–65. [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Decreases in dopamine concentrations in adult, non-human primate brain persist following removal from polychlorinated biphenyls. Toxicology. 1994;86:71–87. doi: 10.1016/0300-483x(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Shain W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol Appl Pharmacol. 1990;106:136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Marek KL, Seibyl JP, Jennings DL, Molho ES, Higgins DS, Factor SA, Fitzgerald EF, Hills EA, Korrick SA, Wolff MS, Haase RF, Todd AC, Parsons P, McCaffrey RJ. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis. 2010;38:219–225. doi: 10.1016/j.nbd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Pappas BA, Park GA. Neurochemical effects of consumption of Great Lakes salmon by rats. Regul Toxicol Pharmacol. 1998;27:S68–S75. doi: 10.1006/rtph.1997.1192. [DOI] [PubMed] [Google Scholar]
- Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, Oertel WH, Ulm G, Schneider E. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology. 1996;46:1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson's disease: a test of the multifactorial etiologic hypothesis. Neurology. 1993;43:1173–1180. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- Sengstock GJ, Olanow CW, Dunn AJ, Barone S, Jr, Arendash GW. Progressive changes in striatal dopaminergic markers, nigral volume, and rotational behavior following iron infusion into the rat substantia nigra. Exp Neurol. 1994;130:82–94. doi: 10.1006/exnr.1994.1187. [DOI] [PubMed] [Google Scholar]
- Shain W, Bush B, Seegal R. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol Appl Pharmacol. 1991;111:33–42. doi: 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, Perl DP, Olanow W, Calne DB. MRI and PET studies of manganese-intoxicated monkeys. Neurology. 1995;45:1199–1204. doi: 10.1212/wnl.45.6.1199. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, 3rd, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG., Jr Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT, 2nd, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Suzuki Y, Nishiyama K, Fujii N. Study of subacute toxicity of manganese dioxide in monkeys. The Tokushima journal of experimental medicine. 1975;22:5–10. [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Tavakoli-Nezhad M, Barron AJ, Pitts DK. Postnatal inorganic lead exposure decreases the number of spontaneously active midbrain dopamine neurons in the rat. Neurotoxicology. 2001;22:259–269. doi: 10.1016/s0161-813x(01)00010-9. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW. Nonmotor symptoms of Parkinson's disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;29:8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, O'Brien PJ. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chemico-Biological Interactions. 2001;130–132:285–296. doi: 10.1016/s0009-2797(00)00272-6. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- Tuite PJ, Krawczewski K. Parkinsonism: a review-of-systems approach to diagnosis. Semin Neurol. 2007;27:113–122. doi: 10.1055/s-2007-971174. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Verslegers W, Van den Kerchove M, Crols R, De Potter W, Appel B, Lowenthal A. Methanol intoxication. Parkinsonism and decreased Met-enkephalin levels dueto putaminal necrosis. Acta Neurol Belg. 1988;88:163–171. [PubMed] [Google Scholar]
- Villaume J, Wasti K, Liss-Sutter D, Hsaio S. Welding Society. Miami, FL: 1979. Effects of welding on health; pp. 1–121. [Google Scholar]
- Vitarella D, Wong BA, Moss OR, Dorman DC. Pharmacokinetics of inhaled manganese phosphate in male Sprague-Dawley rats following subacute (14-day) exposure. Toxicology and applied pharmacology. 2000;163:279–285. doi: 10.1006/taap.1999.8874. [DOI] [PubMed] [Google Scholar]
- Wang J, Rahman MF, Duhart HM, Newport GD, Patterson TA, Murdock RC, Hussain SM, Schlager JJ, Ali SF. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology. 2009;30:926–933. doi: 10.1016/j.neuro.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Weuve J, Nie H, Saint-Hilaire MH, Sudarsky L, Simon DK, Hersh B, Schwartz J, Wright RO, Hu H. Association of cumulative lead exposure with Parkinson's disease. Environ Health Perspect. 2010;118:1609–1613. doi: 10.1289/ehp.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J Mol Sci. 2011;12:6267–6280. doi: 10.3390/ijms12096267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26 Suppl 1:S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Wright AK, Atherton JF, Norrie L, Arbuthnott GW. Death of dopaminergic neurones in the rat substantia nigra can be induced by damage to globus pallidus. The European journal of neuroscience. 2004;20:1737–1744. doi: 10.1111/j.1460-9568.2004.03636.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang C, Sun J, Xue Y. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano. 2011;5:4476–4489. doi: 10.1021/nn103530b. [DOI] [PubMed] [Google Scholar]
- Yee S, Choi BH. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 1996;17:17–26. [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem. 2008;107:1083–1090. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Fukabori S, Hara K, Yuasa H, Nakaaki K, Yamamura Y, Yoshida K. Concentrations of trichloroethylene and its metabolites in blood and urine after acute poisoning by ingestion. Human & Experimental Toxicology. 1996;15:254–258. doi: 10.1177/096032719601500312. [DOI] [PubMed] [Google Scholar]
- Yu WR, Jiang H, Wang J, Xie JX. Copper (Cu2+) induces degeneration of dopaminergic neurons in the nigrostriatal system of rats. Neurosci Bull. 2008;24:73–78. doi: 10.1007/s12264-008-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]