Abstract
PURPOSE
To evaluate the repeatability of Fourier-domain optical coherence tomography (OCT) pachymetric mapping in patients with corneal opacities, and to assess the reliability of Fourier-domain OCT with 830 nm wavelength as a pachymetric measurement tool in opaque corneas.
METHODS
A Fourier-domain OCT system was used to map the corneal thickness of patients with corneal scars or dystrophy. A retrospective study of a consecutive series was conducted. The repeatability was measured using pooled standard deviation of repeated measurements. A slit-scanning tomography device provided pachymetric mapping for comparison.
RESULTS
Seventeen eyes of 12 patients with corneal scars (7 trauma and 3 post-infection) or dystrophy (2 Reiss Buckler and 5 granular dystrophy) were included. The posterior corneal boundary was detectable in all cases. The average corneal thickness measured by OCT was 536 ± 89 μm in central 2 mm area, 553 ± 76 μm in pericentral 2–5 mm area, and 508 ± 93 μm for the minimum corneal thickness. The slit-scanning tomography central corneal thickness, 433 ± 111 μm, was significantly lower than OCT readings (mean difference −91.1 ± 33.3 μm, p = 0.002). Repeatability of the OCT measurements was 2.1 μm centrally and 1.2 μm pericentrally.
CONCLUSION
Pachymetric mapping with Fourier-domain OCT was highly repeatable. Fourier-domain OCT is a reliable pachymetric tool in opaque corneas. In comparison, corneal thickness measured by the slit-scanning tomography is significantly thinner than those measured by the Fourier-domain OCT in the presence of corneal opacities.
Keywords: Fourier-domain optical coherence tomography, pachymetry map, corneal opacities, repeatability, corneal scar, corneal dystrophy
Introduction
A wide range of treatment options are available for corneas with opacities, including phototherapeutic keratectomy, lamellar keratoplasty, and penetrating keratoplasty. Surgical decisions greatly depend on accurate pachymetric measurement as well as accurate determination of opacity depth. Ultrasound pachymetry has been the standard method for clinical assessment of corneal thickness.1, 2 Optical pachymetry,3 confocal microscopy,4 and optical low coherence reflectometry,5, 6 are other options for localized, single point corneal thickness measurements.
More recent methods such as the slit-scanning tomography,7 Scheimpflug photography,7 and very-high frequency ultrasound imaging8 provide corneal pachymetric mapping with several advantages over spot measurements. The very-high frequency ultrasound imaging requires immersion which makes it less practical for routine and postoperative use. The slit-scanning corneal topography/tomography system (Orbscan II; Bausch & Lomb, Inc., Rochester, NY, USA) is one of the most popular methods to obtain pachymetry maps for refractive procedures. However, the slit-scanning technology tends to underestimate corneal thickness in the presence of corneal opacities due to its limited resolution.9, 10
Optical coherence tomography (OCT) is a non-contact imaging technique based on principles of low-coherence interferometry.11 Because OCT has high depth resolution, it is able to accurately detect corneal boundaries and measure corneal thickness even in opacified corneas. Our previous investigation using a time-domain OCT operating at 1310 nm wavelength showed that OCT and ultrasound pachymetry agreed closely in the measurement of opacified corneas, while Orbscan II grossly underestimated corneal thickness.9
Fourier-domain technology is used in more recent generations of ophthalmic OCT products. Fourier-domain OCT systems are capable of providing 10–100 times faster scan speeds than time-domain OCT systems. In this study, we used a Fourier-domain OCT system working at 830 nm wavelength. In addition to the higher scan speed, it provides better depth resolution than previous anterior-segment time-domain OCT instruments operating at 1310 nm wavelength.12,13 The higher speed and resolution could potentially improve precision in pachymetric mapping by reducing motion error and increasing the precision of boundary detection. However, the shorter wavelength may limit its ability to penetrate through the corneal opacities. The purpose of this study was to assess the reliability and repeatability of Fourier-domain OCT pachymetric mapping in opaque corneas. To our knowledge this is the first such study to assess repeatability in corneas with opacities.
Material and methods
Subjects
Cases with corneal dystrophy (granular dystrophy and Reis Buckler dystrophy) and corneal scars (traumatic, postoperative, and post-infection cases) were included. We retrospectively reviewed consecutive corneal scar and dystrophy cases presented at the Cornea Services of Doheny Eye Institute, Los Angeles, CA, USA, between February 2007 and January 2010. Corrected distance visual acuity (CDVA) was recorded for each eye. This study followed the tenets of the Declaration of Helsinki, was in accordance with the Health Insurance Portability and Accountability Act of 1996, and was approved by the Institutional Review Board of the University of Southern California.
OCT Imaging
A Fourier-domain OCT system (RTVue, software version 4.0, Optovue Inc., Fremont, CA, USA) with a long corneal adaptor module (CAM) lens was used. The system worked at 830 nm wavelength with a scan speed of 26,000 axial scans per second. The depth resolution of this system was 5 μm in tissue. The scan width was 6 mm and the transverse resolution was 15 μm (focused spot size).
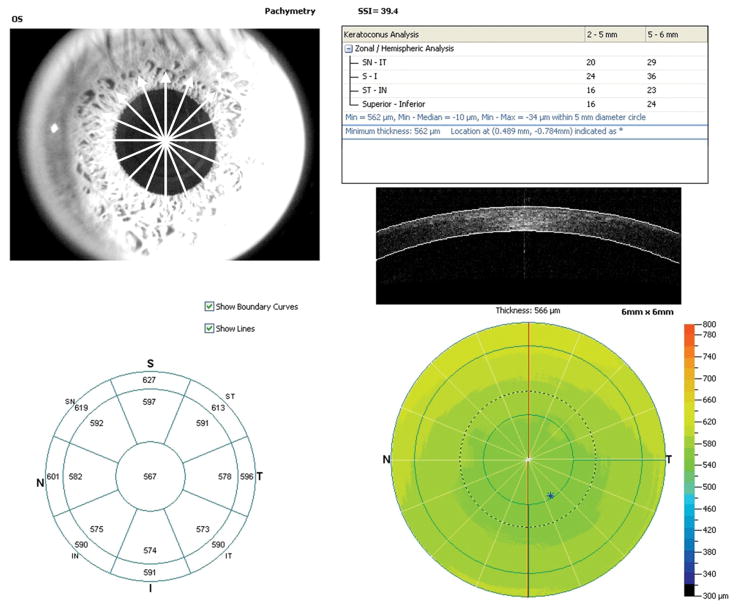
The “Pachymetry” scan pattern was used to map the cornea. The pattern consisted of 8 high-definition meridional scans (1024 axial scans per meridian) acquired in only 0.32 seconds (Figure 1). The average pachymetry of the central 2 mm area was recorded as the central corneal thickness (CCT) for OCT measurements. The sector average pachymetry of superior, superotemporal, temporal, inferotemporal, inferior, inferonasal, nasal, and superonasal octants from 2 to 5 mm diameter were averaged as pericentral corneal thicknesses. The minimum corneal thickness inside the 5 mm diameter was also recorded. Each eye was scanned at least 2 times within a single visit. Subjects were repositioned after each OCT scan. One of the two landmarks, the corneal vertex or the pupil, was used to center the corneal mapping scans.
Figure 1.
Fourier-domain optical coherence tomography pachymetry map printout. Octants: ST, superotemporal; T, temporal, IT, inferotemporal, I, inferior, IN, inferonasal, N, nasal, SN, superonasal
RTVue OCT software automatically identified anterior and posterior corneal boundaries and calculated the pachymetry map. Visual inspection was performed with boundaries overlaid with the corneal image to verify the correctness of boundary detection.
Slit-scanning topography/tomography
Slit-scanning topography/tomography (Orbscan II, Bausch & Lomb, Rochester, NY, USA) was used to measure the cornea. An acoustic equivalent correction factor of 0.92 was used according to manufacturer’s recommendations. The central 2-mm corneal thickness was obtained from the Orbscan II pachymetry maps for comparison.
Statistics
Pooled standard deviations were calculated to evaluate the repeatability of the measurements. For each eye, we calculated the standard deviation from the repeated measurements. Since there were only two measurements on most of the eyes, the standard deviation was simply the positive difference between the two measurements. The measurement difference from each eye was then pooled together to obtain the pooled standard deviation as the repeatability of the measurements.
To compare the corneal thickness measured by OCT and slit-scanning tomography, we used the generalized estimating function14 to account for the correlation between the two eyes from the same patient. Descriptive statistics, including means and standard deviations (SD) and all statistical analyses were done using SAS 9.2 (SAS Institute, Cary, NC, USA). P-values < 0.05 were considered statistically significant.
Results
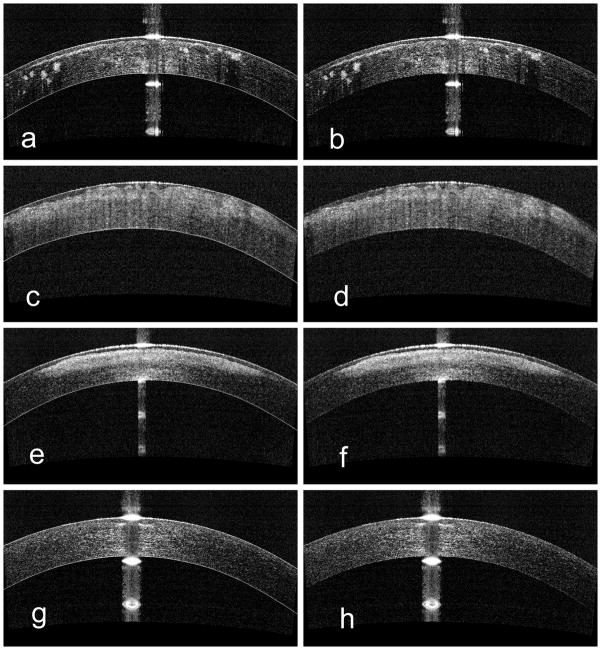
Seventeen eyes of 12 subjects were included in this study. There were 9 males and 3 females, and the age was 57.8 ± 14 years (range 23 to 72 years). Ten eyes had corneal scars (7 trauma, 3 post-infection), and 7 eyes had corneal dystrophy (2 Reis-Buckler, 5 granular dystrophy). The average LogMar CDVA was 0.33±0.17, equivalent to 20/42.9 Snellen acuity. Opacity depth and density varied clinically from moderately dense (Figure 2b scattered, 2d diffuse), very dense and deep (Figure 2f), to faint and superficial (Figure 2h small central scar). Anterior, posterior, central, and peripheral opacities were all included in the series. The location and density of the scars did not affect the accuracy of corneal boundary detection and pachymetry measurement. Visualization of the posterior corneal border through the corneal opacity was possible in all cases, including the case of a dense central scar 245 μm deep (Figure 2f). For each eye, visual inspection verified accurate computer boundary segmentation (Figure 2). The software identified the Descemet’s membrane layer in all cases, even in dense scars (Figure 2b, f).
Figure 2.
Optical coherence tomography images with (left panel: a, c, e, and g) and without (right panel: b, d, f, h) corneal boundaries overlaid on the cornea. Top: granular dystrophy; Middle upper: Reiss Buckler dystrophy; Middle lower: dense scar; Bottom: superficial scar. Computer boundary segmentation was accurate in all cases.
The OCT pachymetry scan was centered on the corneal vertex in 13 eyes and on the pupil in 4 eyes. The repeatability of the OCT pachymetric mapping (Table 1) was 2.1 μm centrally (0–2 mm) and 1.2 μm pericentrally (2–5 mm).
Table 1.
Repeatability of the OCT pachymetric mapping
Optical Coherence Tomography Pachymetry Statistics by Region.
| Central 2 mm | 2–5 mm diameter | Minimum1 | ||||
|---|---|---|---|---|---|---|
| Thickness2 (μm) | Repeatability | Thickness2 (μm) | Repeatability | Thickness2 (μm) | Repeatability | |
| All Cases | 536 ± 89 | 2.1 | 553 ± 76 | 1.2 | 508 ± 93 | 1.9 |
| Scar Group | 560 ± 68 | 1.2 | 574 ± 62 | 1.0 | 542±61 | 2.3 |
| Dystrophy Group | 523±98 | 2.6 | 542 ± 84 | 1.3 | 489 ± 104 | 1.4 |
Minimum thickness was measured within the 5 mm diameter;
Values are mean ± SD.
The central corneal thickness was 536.2 ± 88.6 μm by OCT and 433.1 ± 110.9 μm by slit-scanning tomography. The difference, 91.1 ± 33.3 μm (range −138.9 to 334.2 μm), was statistically significant (P=0.002). One case, following pterygium surgery, had a peripheral corneal opacity and a clear central cornea. In that particular case, the OCT mean CCT was 508.3 μm, whereas for slit-scanning tomography it was 450 μm, a difference of 58.3 μm. Focal measurements of corneal thickness, opacity depth, and epithelium thickness could be made using computer calipers in all cases (Figure 3).
Figure 3.
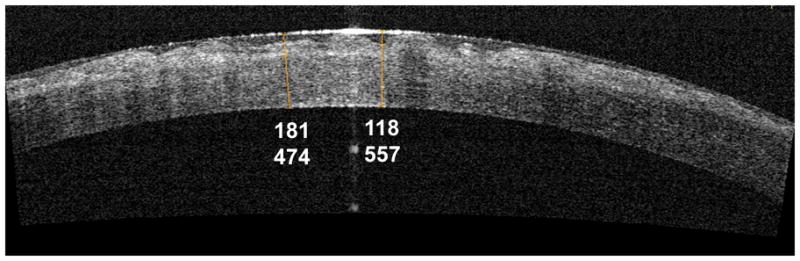
Use of computer calipers to measure corneal thicknesses. Computer calipers were used to measure epithelial thickness (118 μm), opacity depth (from the anterior epithelial surface to the posterior edge of the opacity, 181 μm), and corneal thickness (474 and 557 μm) in this optical coherence tomography image of a cornea with Reiss Buckler dystrophy.
Discussion
Accurate pachymetric measurement in an eye with a corneal opacity is challenging and of great importance in guiding treatment or retreatment in refractive surgeries. Overestimation or underestimation in corneal thickness could be misleading in selecting the treatment type as well as deciding the depth of treatment.
In a previous study, Khurana et al., compared corneal thickness measured by time-domain OCT, slit-scanning tomography, and ultrasound pachymetry in eyes with central opacities.9 They found that OCT central cornea thickness measurements were statistically equivalent to ultrasound pachymetry, whereas slit-scanning tomography measurements were significantly less than ultrasound pachymetry (difference, −132.7 μm). The OCT and ultrasound pachymetry results were obtained for all eyes whereas slit-scanning tomography was unable to provide readings in 17% of the cases due to irregular corneal surfaces.9 This was consistent with other studies showing that slit-scanning tomography underestimates pachymetry in the presence of corneal haze.15, 16 Scattering from corneal haze and stromal opacities interferes with the identification of the posterior corneal boundary because of the limited resolution of slit scanning. In contrast, the higher resolution of OCT allows corneal boundaries to be defined clearly and makes OCT measurements more robust than slit-scanning tomography results. In this current study, we also found that the CCT measured by Orbscan II was significantly thinner, 91.1 μm, than that by Fourier-domain OCT.
We expected the 830 nm Fourier-domain OCT technology to provide more precise pachymetry due to higher resolution and speed compared to previous 1310 nm time-domain OCT. The time-domain OCT used in a number of previous studies had 17 μm axial resolution in tissue and a speed of 200 to 2000 Hz axial scan rate.9,17 The Fourier-domain OCT system used in this study provides 5 μm axial resolution and 26,000 Hz axial scan rate. The 3 times finer resolution should sharpen the precision of boundary delineation, and the 13–130 times faster speed should reduce error due to eye movement during scan acquisition. Li et al.17 reported CCT repeatability in clear corneas of 4.9 μm and 5.8 μm with Visante OCT (2000 Hz) and slit-lamp–adapted optical coherence tomography (200 Hz), respectively. Our recently published study showed that the Fourier-domain OCT provided 1.3 – 1.7 μm repeatability in normal eyes.18 The scan speed is particularly important in measuring the eye with corneal opacities. In the presence of corneal surface irregularity, a shift in OCT image due to eye movement may introduce more error in the pachymetric map than in the presence of smooth corneal surfaces. In the current study, we found that pachymetry mapping repeatability was between 1.0 to 2.6 μm, which is comparable to the results obtained in clear cornea with Fourier-domain OCT, and superior to the precision of time-domain OCT in clear corneas. This shows that the speed of the current Fourier-domain OCT system is sufficient for accurate production of the pachymetry map in the presence of irregularities and opacities caused by scars and dystrophy.
The Fourier-domain OCT in this study used 830 nm wavelength light. Because 830 nm light is scattered more in tissue than 1310 nm light, we anticipated a potential problem with delineation of the posterior corneal boundary in cases with denser opacities. In fact, this problem did not occur. In all cases of this study, the corneal boundaries were clearly visualized by human graders and accurately identified by the computer software. This included a dense 50% depth anterior stromal scar (Fig. 2f) and a case with CDVA as low as 20/80. This suggests that 830 nm light penetration is not a major problem for OCT imaging in corneal scars and dystrophies.
In terms of scan pattern centration, our recently published study showed that pupil centration could provide slightly better pachymetric repeatability than the vertex centration.18 In this study, only 4 eyes were scanned with pupil centration. Therefore, we were not able to provide a comparison. However, both centration methods yielded repeatable pachymetry maps.
In summary, Fourier-domain OCT was able to provide highly repeatable pachymetric maps in corneas with opacities. The anterior and posterior corneal boundaries identified by RTVue software appeared to be correct according to the visual inspection with boundaries overlaid with the corneal image. There was no significant shadowing problem due to blocking of the OCT beam despite relatively dense opacities in some cases. In comparison, corneal thickness measured by the slit-scanning tomography is significantly thinner than those measured by the Fourier-domain OCT in the presence of corneal opacities.
Acknowledgments
Financial Support: NIH grant R01EY018184, research grant from Optovue, Inc. (Freemont, CA, USA), Charles C. Manger III, MD Chair in Corneal Laser Surgery endowment
Footnotes
Proprietary interests: David Huang received stock options, patent royalty, speaker royalty and travel support from Optovue, Inc. (Fremont, CA). David Huang and Yan Li received research grant support from Optovue. David Huang receives a royalty from an optical coherence tomography-related patent licensed to Carl Zeiss Meditec, Inc. (Dublin, CA, USA) by the Massachusetts Institute of Technology. Other authors have no proprietary interest in the topic of this manuscript.
References
- 1.Christensen A, Narvaez J, Zimmerman G. Comparison of central corneal thickness measurements by ultrasound pachymetry, konan noncontact optical pachymetry, and orbscan pachymetry. Cornea. 2008;27:862–865. doi: 10.1097/ICO.0b013e31816ed532. [DOI] [PubMed] [Google Scholar]
- 2.Schneider M, Borgulya G, Seres A, Nagy Z, Nemeth J. Central corneal thickness measurements with optical coherence tomography and ultrasound pachymetry in healthy subjects and in patients after photorefractive keratectomy. Eur J Ophthalmol. 2009;19:180–187. doi: 10.1177/112067210901900202. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Fonn D, Simpson TL, Jones L. Relation between optical coherence tomography and optical pachymetry measurements of corneal swelling induced by hypoxia. Am J Ophthalmol. 2002;134(1):93–98. doi: 10.1016/s0002-9394(02)01517-9. [DOI] [PubMed] [Google Scholar]
- 4.McLaren JW, Nau CB, Erie JC, Bourne WM. Corneal thickness measurement by confocal microscopy, ultrasound, and scanning slit methods. Am J Ophthalmol. 2004;137:1011–1020. doi: 10.1016/j.ajo.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 5.Bohnke M, Masters B, Walti R, et al. Precision and reproducibility of measurements of human corneal thickness with rapid optical low-coherence reflectometry (OLCR) Journal of Biomedical Optics. 1999;4:152–156. doi: 10.1117/1.429901. [DOI] [PubMed] [Google Scholar]
- 6.Huang D, Wang J, Lin CP, Puliafito CA, Fujimoto JG. Micron-resolution ranging of cornea anterior chamber by optical reflectometry. Lasers Surg Med. 1991;11:419–425. doi: 10.1002/lsm.1900110506. [DOI] [PubMed] [Google Scholar]
- 7.Ho T, Cheng AC, Rao SK, Lau S, Leung CK, Lam DS. Central corneal thickness measurements using Orbscan II, Visante, ultrasound, and Pentacam pachymetry after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2007;33:1177–1182. doi: 10.1016/j.jcrs.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Reinstein DZ, Silverman RH, Raevsky T, Simoni GJ, Lloyd HO, Najafi DJ, Rondeau MJ, Coleman DJ. Arc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16:414–430. doi: 10.3928/1081-597X-20000701-04. [DOI] [PubMed] [Google Scholar]
- 9.Khurana RN, Li Y, Tang M, Lai MM, Huang D. High-speed optical coherence tomography of corneal opacities. Ophthalmology. 2007;114:1278–1285. doi: 10.1016/j.ophtha.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Prisant O, Calderon N, Chastang P, Gatinel D, Hoang-Xuan T. Reliability of pachymetric measurements using orbscan after excimer refractive surgery. Ophthalmology. 2003;110:511–515. doi: 10.1016/S0161-6420(02)01298-8. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11:2183–2189. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 13.Huang D, Izatt J, Yasuno Y, Boer Jd. Future direction of anterior segment optical coherence tomography. In: Steinert R, Huang D, editors. Anterior Segment Optical Coherence Tomography. Thorofare, NJ: SLACK; 2008. pp. 165–173. [Google Scholar]
- 14.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 15.Boscia F, La Tegola MG, Alessio G, Sborgia C. Accuracy of Orbscan optical pachymetry in corneas with haze. J Cataract Refract Surg. 2002;28:253–258. doi: 10.1016/s0886-3350(01)01162-2. [DOI] [PubMed] [Google Scholar]
- 16.Fakhry MA, Artola A, Belda JI, Ayala MJ, Alio JL. Comparison of corneal pachymetry using ultrasound and Orbscan II. J Cataract Refract Surg. 2002;28:248–252. doi: 10.1016/s0886-3350(01)01277-9. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Leung CK, Wong L, et al. Comparative study of central corneal thickness measurement with slit-lamp optical coherence tomography and visante optical coherence tomography. Ophthalmology. 2008;115:796–801. e792. doi: 10.1016/j.ophtha.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Tang M, Zhang X, Salaroli CH, Ramos JL, Huang D. Pachymetric mapping with Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2010;36:826–831. doi: 10.1016/j.jcrs.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]