Abstract
Laboratory survival experiments have shown that dietary restriction (DR) can increase median and maximum lifespan. This paper provides a meta-analysis of laboratory experiments that have evaluated the effects of DR on lifespan in rats and mice (1934 – present). In rats, DR increased median lifespan by 14 – 45% in half of all experiments, but in mice the effects of DR have been much weaker (4 – 27%). The least favorable effects of DR on lifespan have been observed among inbred rather than non-inbred mouse strains. In fact, some inbred mouse strains do not necessarily live longer with DR, including DBA/2 male mice and several strains from the ILSXISS recombinant inbred panel. Shortening of lifespan with DR has also been observed and confirmed for ILSXISS strain 114. Importantly, all rodent studies may be biased by the effects of laboratory breeding, since one study has shown that median lifespan is not improved by DR in wild-derived mice. These findings suggest that the set of genetic backgrounds studied in rodent DR experiments should be diversified. This will broaden the scope of genotypes studied in aging research, but may also be critical for translation of findings from rodents to historically outbred and genetically heterogeneous primate species.
Keywords: aging, calorie restriction, CR, DBA/2, genetic background, inbred long sleep, inbred short sleep, survivorship, wild-derived mice
1. Introduction
Diet restriction (DR) has been investigated for decades as an intervention that increases the lifespan of model organisms. This effect was initially uncovered in survival experiments performed using laboratory rats (Osborne et al., 1917; McCay et al., 1943), and has since been confirmed in a broad range of experimental settings (Weindruch and Walford, 1988; Turturro et al., 1999). Such findings have laid the foundation for intensive investigations of DR and its associated mechanisms, along with efforts to develop DR mimetic pharmaceuticals and studies for evaluating long-term effects of DR in rhesus monkeys and humans (Ingram et al., 2006; Holloszy and Fontana, 2007; Colman et al., 2009). The conserved character of the DR response and its consistent effects on lifespan has often been emphasized (Cantó and Auwerx, 2009;Anderson and Weindruch, 2010;Omodei and Fontana, 2011; Xiang and He, 2011). To support this idea, abundant evidence is available from survival experiments using laboratory rodents (Weindruch and Walford, 1988; Turturro et al., 1999), and DR protocols have also been shown to increase the lifespan of diverse invertebrate species (Austad, 1989; Partridge et al., 2005; Kaeberlein et al., 2006; Lee et al., 2006). Nevertheless, contrary evidence has been reported. For example, DR protocols have failed to increase lifespan in D. melanogaster (Le Bourg and Minois, 1996; Le Bourg and Minois, 2005; Libert et al., 2007; Lee et al., 2008), the nematode C. remanei (Sutphin and Kaeberlein, 2008), medflies (Carey et al., 2002), rotifers (Kirk, 2001; Weithoff, 2007), butterflies (Beck, 2007; Molleman et al., 2009), the spider L. hasselti (Kasumovic et al., 2009), and houseflies (Cooper et al., 2004). Likewise, despite repeated demonstrations that DR can increase the lifespan of laboratory rodents (Weindruch and Walford, 1988; Turturro et al., 1999), recent studies appear to have identified strains that fail to exhibit increased lifespan in response to DR (Harper et al., 2006a; Liao et al., 2010a; Rikke et al., 2010). These findings have challenged the view of DR as a robust pro-longevity intervention and suggest that connections between DR and basic aging mechanisms may be more complex than is widely believed (Hayflick, 2010).
It has frequently been stated that DR increases the median and/or maximum lifespan of laboratory rodents by 30-50% (e.g., Weindruch and Sohal, 1997; Wanagat et al., 1999; Sonntag et al., 2000; Fontana et al., 2010). This has certainly been observed in some rodent studies (e.g., Goodrick et al., 1982), but the 30-50% approximation may poorly represent the complete spectrum of laboratory outcomes. Indication of this is provided by two studies that evaluated effects of DR on lifespan of mice from the ILSXISS recombinant inbred panel (Liao et al., 2010a; Rikke et al., 2010). Almost none of the inbred strains exhibited the oft-cited 30-50% increase in median or maximum lifespan, and for most strains, DR had either no effect on lifespan or shortened lifespan (Liao et al., 2010a; Rikke et al., 2010). While surprising, this result was not unprecedented. DR protocols have also failed to increase median lifespan of white rats (McCay et al., 1935; McCay et al., 1939), certain inbred mouse strains (e.g., C57BL/6 and DBA/2) (Cheney et al., 1980; Harrison and Archer, 1987; Forster et al., 2003), and laboratory-reared descendants of wild-caught mice (Harper, 2008). These findings have implications for work on DR and its relationship to aging mechanisms. First, rodent strains might be divided into those that respond positively or negatively to DR, which would allow for comparison of DR responses between “positive control” and “negative control” strains (Sohal et al., 2009). This could help identify effects of DR that do and do not co-occur with increased lifespan (Ferguson et al., 2007; Rebrin et al., 2007; Ferguson et al., 2008; Sohal et al., 2009; Hempenstall et al., 2010; Rebrin et al., 2011). Secondly, differential effects of DR among strains demonstrate significant genetic variation underlying responses to low-calorie diet (Rikke et al., 2003; Rikke and Johnson, 2007). This variation can be exploited to identify quantitative trait loci associated with such responses, and indeed, much progress has already been made along these lines using the ILSXISS recombinant inbred strains (Rikke et al., 2004; Rikke et al., 2006; Rikke et al., 2010). Finally, an important long-term objective of many investigators has been to explore the utility of DR in humans (Ingram et al., 2006; Holloszy and Fontana, 2007; Colman et al., 2009). However, humans may also be segregating genetic variants promoting variable responses to DR, some of them leading to improved health, but some of them leading to reduced health.
Nevertheless, studies failing to identify positive effects of DR on rodent lifespan are challenging to interpret against the backdrop of all published data. We know, for instance, that independent studies of the same genotype have in some cases reached conflicting conclusions regarding the efficacy of DR for increasing lifespan (e.g., DBA mice; see Turturro et al. (1999) and Forster et al. (2003)). This shows that failure of DR to increase median and/or maximum lifespan should not necessarily be interpreted in terms of genotype-dependent effects, since negative results may instead be due to study-specific environmental factors or to a “non-optimized” DR protocol. For instance, the abrupt onset of a DR diet shortly after weaning may lead to premature mortality in DR-fed rodents, but this can be circumvented by implementing DR gradually over a period of 3-4 weeks (Weindruch and Walford, 1988). DR-type diets can also lead to unintended malnutrition unless micronutrient supplements are provided to restricted animals (Weindruch et al., 1988; Cerqueira and Kowaltowski, 2010). In addition, inadvertent exposure of experimental animals to pathogens can cause premature death and absence of DR effects that would be detected in better-controlled environments (Miller and Nadon, 2000). Such exposure seems to bias experiments in a direction that favors DR-shortened lifespan, or absence of significant effects on lifespan, perhaps because DR-fed rodents are more susceptible to infection by pathogens or parasites (Gardner, 2005; Kristan, 2008; Ritz et al., 2008; Clinthorne et al., 2010). To address this issue, recent studies have enforced strong barriers to pathogen exposure and some have maintained “specific pathogen free” (SPF) colonies, with regular analysis of sentinel animals and spent bedding to test for known pathogens. These procedures, however, have evolved over time and have been enforced with little consistency.
The main goals of this paper are to evaluate evidence for rodent genotypes that fail to exhibit increased lifespan when provided a DR diet, and to assess the degree to which pro-longevity effects of DR in rodents should be considered genotype-dependent as opposed to universal. The review aggregates laboratory survivorship experiments that have evaluated the effects of DR in rats and mice over the last several decades (1934 – present; 246 experiments in total). These data are analyzed to establish a reference range reflecting the percentage increase in lifespan that has usually been observed with DR, and further analyses are performed to assess evidence for publication bias (Dickersin and Min, 1993). In this context, six rodent strains are closely examined, which are not necessarily representative cases, but for which explanation is warranted due to the failure of one or several studies to demonstrate DR-increased lifespan (McCay's white rats, B6 mice, C3H mice, DBA/2 mice, ILSXISS recombinant inbred mouse strains and wild-derived mice). In each case, evidence for genotype-dependent effects is evaluated against alternative explanations related to experimental factors and DR protocols. The final section of the review states conclusions that can be drawn from these analyses, highlights questions that remain unanswered, and proposes directions for future work.
2. DR protocols have typically increased median lifespan by 14 – 45% in rats and by 4 – 27% in mice (1934 – present) with significant funnel plot asymmetry in rats (excluding ILSXISS strains)
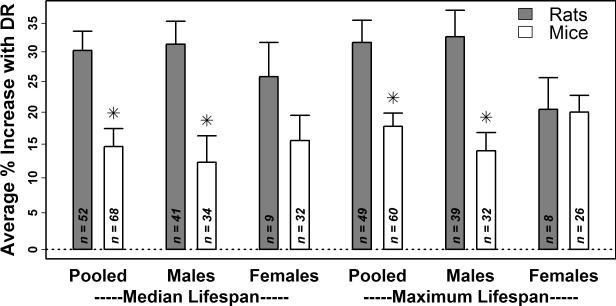
A literature review identified 53 and 72 published survivorship experiments using rats and mice, respectively, where each experiment included a control cohort (ad lib access to food or fixed high-calorie ration) paired with a DR-type cohort (restricted in caloric content) (Supplemental Tables 1 and 2; excluding ILSXISS recombinant inbred mouse strains). In rats, most studies used inbred strains and examined only the male sex. Half of all experiments using rats supported a median lifespan increase between 13.8% and 45.4% with DR, with one quarter of experiments demonstrating weaker effects (< 13.8%), and the remaining quarter demonstrating stronger effects (> 45.4%) (Table 1). DR increased median rat lifespan by 30.4% on average (31.3% in males; 25.8% in females), with an average increase in maximum lifespan of 32.3% (32.6% in males; 20.4% in females) (Table 1).
Table 1. Effects of DR on lifespan in rats and mice (1934 – present; excluding ILSXISS strains).
A literature review identified 53 and 72 laboratory experiments that evaluated the effects of DR-type diets on survival in rats and mice, respectively (Supplemental Tables 1 and 2). Each experiment included one experimental treatment in which rodents were provided a low-calorie diet (e.g., 40% DR) paired with a reference group in which rodents were provided a control diet (e.g., ad lib feeding). For each experiment, the percent change in median and/or maximum lifespan with DR was estimated, based upon the ratio of lifespan estimates from the DR and control treatments (DR / control). In some cases, data provided in published reports did not permit a precise estimate of the DR-induced change in median and/or maximum lifespan. Such experiments were excluded from consideration, which yielded 52 and 68 DR effect estimates for median lifespan in rats and mice, respectively, along with 49 and 60 DR effect estimates for maximum lifespan in rats and mice, respectively. Given all estimates available, the table lists the average % change in median or maximum lifespan among DR-fed rats or mice (calculated with greater weight assigned to studies with larger sample sizes). Additionally, the table lists the middle 50% of estimated DR effects among the n experiments included in each analysis. All ILSXISS recombinant inbred strains were excluded from this analysis (Liao et al., 2010a; Rikke et al., 2010).
| Median Lifespan | Maximum Lifespan | ||||
|---|---|---|---|---|---|
| n | % Change DR (middle 50%) | n | % Change DR (middle 50%) | ||
| Rats | Pooled | 52 | 30.2% (13.8 – 45.4%) | 49 | 31.6% (17.8 – 35.7%) |
| Males | 41 | 31.3% (14.2 – 44.9%) | 39 | 32.6% (18.2 – 39.1%) | |
| Females | 9 | 25.8% (14.6 – 29.2%) | 8 | 20.4% (7.7 – 28.2%) | |
| Mice | Pooled | 68 | 14.6% (4.1 – 27.0%) | 60 | 17.8% (0.7 – 24.5%) |
| Males | 34 | 12.3% (4.1 – 22.8%) | 34 | 14.0% (6.8 – 23.1%) | |
| Females | 32 | 15.5% (0.7 – 34.9%) | 32 | 20.0% (10.1 – 31.3%) | |
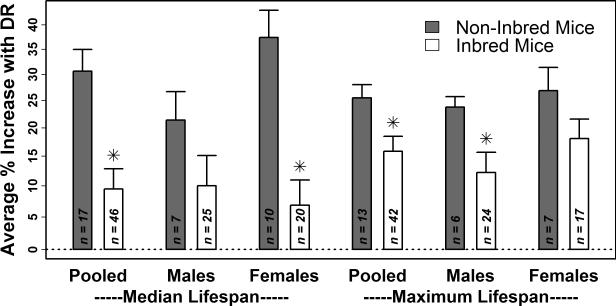
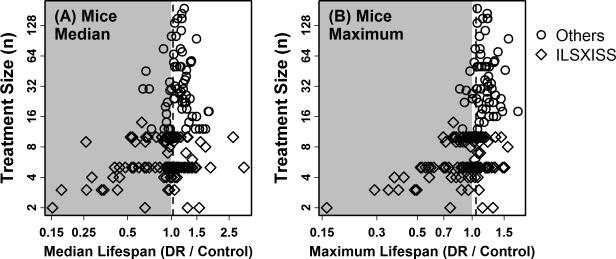
The effects of DR on lifespan were weaker in mice compared to rats (Table 1 and Figure 1). Half of all mouse experiments supported a median lifespan increase between 4.1 and 27.0% with DR, with one quarter of experiments demonstrating weaker effects (< 4.1%), and the remaining quarter of experiments demonstrating stronger effects (> 27.0%) (Table 1). The average increase in median lifespan with DR was 14.6% (12.3% in males and 15.5% in females), while the average increase in maximum lifespan was 17.8% (14.0% in males and 20.0% in females) (Table 1). Compared to rats, the average effect of DR on mouse lifespan was significantly lower with respect to males but not females (P ≤ 0.01 in males, P ≥ 0.21 in females; two-sample t-test; Figure 1). Interestingly, in mice, effects of DR were consistently more favorable among non-inbred genotypes as compared to inbred genotypes (Figure 2). This trend was significant with respect to median and maximum lifespan when experiments from both sexes were pooled (P ≤ 0.02, two-sample t-test), and was also significant with respect to median lifespan for females (P < 0.001, two-sample t-test) and maximum lifespan for males (P = 0.02, two-sample t-test) (Figure 2).
Figure 1. Stronger increases in median and maximum lifespan in DR-fed rats compared to DR-fed mice (1934 – present; excluding ILSXISS strains).
A literature review identified 53 and 72 laboratory experiments that evaluated the effects of DR-type diets on survival in rats and mice, respectively (Supplemental Tables 1 and 2). For each experiment, the percent change in median and maximum lifespan with DR was estimated. The height of each bar corresponds to the average effect of DR on median or maximum lifespan among n experiments for which the necessary information was available (rats, grey bars; mice, white bars). The average effect and associated standard error were calculated by assigning greater weight to studies that used larger sample sizes. The asterisk symbol is used to denote cases in which the average effect of DR on rat lifespan is significantly different from the average effect of DR on mouse lifespan (P < 0.05; two-sample t-test and Wilcoxon rank sum test).
Figure 2. DR has more favorable effects on the lifespan of non-inbred mice compared to inbred mice (1934 – present; excluding ILSXISS strains).
A literature review identified 72 laboratory experiments that evaluated the effects of DR-type diets on survival in mice (Supplemental Table 2). Of these 72 experiments, 24 were performed using non-inbred mouse genotypes, including F1 offspring derived from inbred line crosses, F2 offspring derived from a four-way cross of inbred lines, or wild-derived genetically heterogeneous mice. The height of each bar corresponds to the average effect of DR on median or maximum lifespan among n experiments for which the necessary information was available (non-inbred strains, grey bars; inbred strains, white bars). The average effect and associated standard error were calculated by assigning greater weight to studies that used larger sample sizes. For a given category, asterisk symbols are used to denote cases in which the average effect of DR on the lifespan of inbred strains is significantly different from the average effect of DR on the lifespan of non-inbred strains (P < 0.05; two-sample t-test and Wilcoxon rank sum test).
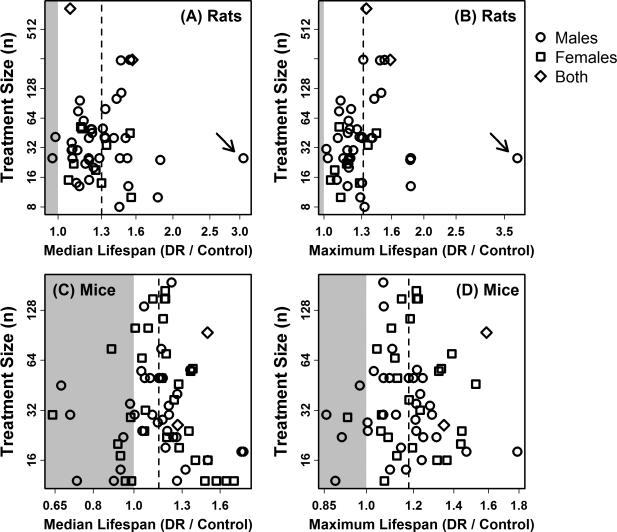
There is recognized bias against publication of non-significant findings in many research domains (Dickersin and Min, 1993). In the present context, two types of bias are plausible. First, the standard bias against small sample size studies may be present, since such studies are less likely to uncover statistically significant effects of DR on lifespan. Secondly, however, bias against large sample size studies with weak effects is conceivable, since larger studies are more expensive to maintain and reliable results are available at intermediate points in an experiment – larger studies may therefore be subject to mid-study tabulation of results and more likely terminated if expected trends fail to develop. To address these possibilities, “funnel plots” were constructed to determine if treatment effect sizes were related to the number of animals used in survivorship experiments (Figure 3). In the absence of bias, treatment effects should be symmetrically distributed about the estimated summary effect, with no relationship between treatment effects and sample sizes used in individual studies (Peters et al., 2006).
Figure 3. Funnel plot analysis of publication bias in studies that have evaluated the effects of DR on rodent lifespan (excluding ILSXISS strains).
A literature review was carried out to identify survivorship experiments that have evaluated effects of DR on lifespan in rodents (Supplemental Tables 1 and 2). A total of 53 rat experiments (A and B) and 72 mouse experiments (C and D) were identified, where each experiment included a control cohort (ad lib access to food or fixed high-calorie ration) paired with a DR cohort (restricted in caloric content). Survivorship experiments using the ILSXISS recombinant inbred strains were excluded from this analysis (Liao et al., 2010a; Rikke et al., 2010). The effect of DR on median or maximum lifespan was estimated in each experiment (horizontal axis; note log2 scale), and this effect was plotted against the size of each experiment (vertical axis). The size of each experiment is defined as min(n1, n2), where n1 is the number of animals assigned to the DR cohort while n2 is the number of animals assigned to the control cohort. Experiments for which DR decreased median or maximum lifespan are plotted within the grey region in each figure. The dotted vertical line denotes the average effect size among all experiments (i.e., average treatment effect weighted by study size). In the absence of publication bias, it is expected that effect sizes from individual experiments will be symmetrically distributed to the left and right of the average effect size (dotted line). In (A) and (B), the arrow denotes an outlying effect size estimate derived from the study of Ross (1961) (Supplemental Table 1). Analyses were performed with and without this outlying effect size estimate (see text).
A visual inspection of funnel plots did not reveal asymmetry with respect to median lifespan estimates in rats (Figure 3A) or with respect to median and maximum lifespan estimates in mice (Figures 3C and 3D). Consistent with this, a weighted least squares regression analysis identified no significant association between effect sizes and sample sizes (P = 0.80, Figure 3A; P = 0.54, Figure 3C; P = 0.92, Figure 3D). However, in rats, effects of DR on maximum lifespan were stronger in those studies that had used larger sample sizes, leading to funnel plot asymmetry, consistent with bias against large sample size studies with weak effects. This trend was not statistically significant (P = 0.41; Figure 3B), although it was marginally significant after removal of one outlying effect size estimate (P = 0.074; see arrow in Figure 3B). Further, if the raw difference in maximum lifespan estimates between restricted and control cohorts was used as the main outcome metric for each study (rather than the log2-transformed DR / control ratio as shown in Figure 3B), the resulting funnel plot was similar in appearance, but the asymmetry was significant once the outlying effect size estimate was removed (P = 0.029; weighted least squares regression) (Peters et al., 2006). This trend is consistent with bias against large studies yielding weak effects of DR on maximum lifespan in rats, although no evidence of such bias was obtained with respect to median lifespan in rats, or with respect to median or maximum lifespan in mice (Figure 3; excluding ILSXISS strains).
3. McCay's white rats
DR has robustly increased lifespan in rats and there were few cases in which a DR-type protocol failed to increase both median and maximum lifespan (Figures 3A and 3B). Some of the weakest effects of DR in rats were reported in a study by Clive McCay and colleagues (McCay et al., 1935), which ironically, has frequently been cited as the earliest demonstration of lifespan extension by DR in laboratory animals. An interesting feature of this work is that experiments were performed using “random-bred” rats that had not been systematically inbred (Park, 2010). Additionally, compared to most present-day rodent colonies, McCay's rats had been maintained in the laboratory for fewer generations.
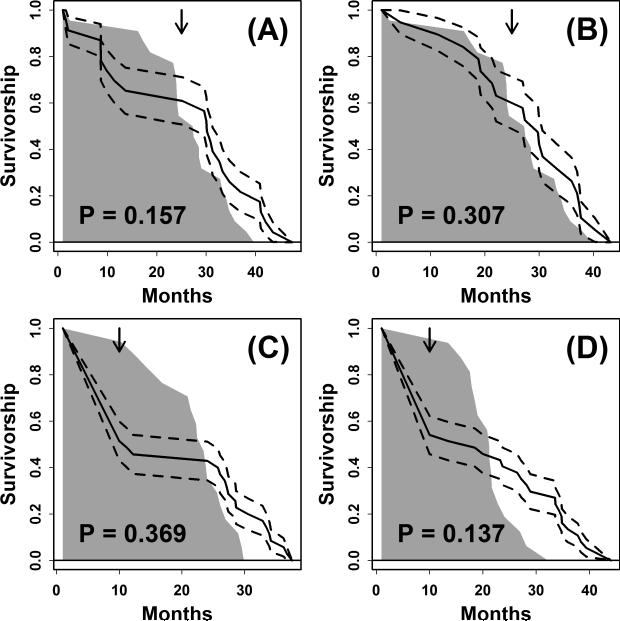
McCay et al. (1935) did demonstrate that DR increased median lifespan of male white rats by more than 50%. However, in concurrent experiments, effects of DR on female lifespan were negligible (Figures 4A and 4B). In one cohort, restricted from the time of weaning, DR increased median lifespan by less than 10% and in fact decreased average female lifespan by 3% (Figure 4A). The estimated effects of DR on overall female survival would not have been significant by contemporary statistical metrics (P ≥ 0.157, log-rank test; Figures 4A and 4B). Even in the absence of statistical arguments, McCay et al. (1935) acknowledged that DR did not increase lifespan of the female rats, and stated that “average life span of the females [from the DR and AL groups] is probably the same”. In these studies, the restricted diet was supplemented with vitamins and minerals (yeast and cod liver oil), and provided approximately 20% fewer calories than was consumed by the ad lib control treatment, although the amount of feed was adjusted during the study to prevent excessive weight loss in restricted rats (McCay et al., 1935; Cerqueira and Kowaltowski, 2010). Additionally, some restricted rats were switched to the control diet very late in the experiment (25 months). Nevertheless, the restricted diet provided sufficiently few calories to stunt growth and decrease body weight of both sexes by 40%.
Figure 4. Effects of DR on survivorship of white rats in early laboratory studies from McCay and colleagues.
Survivorship curves were generated based upon experiments reported by McCay et al. (1935; 1939). Grey regions in (A) – (D) outline the survival curve of AL-fed rats, while the solid line represents the survival curve of DR-fed rats. Dotted lines denote one standard error above and below the estimated survival curve of DR-fed rats. P-values shown in (A) – (D) were generated from a log-rank test of the difference between the AL-fed and DR-fed survival curves. (A) Female rats were restricted in caloric intake at the time of weaning (n = 23 DR-fed; n = 22 AL-fed). At 25 months of age (arrow), half of the restricted rats were placed on the control diet, while the other half remained on the restricted diet. (B) Female rats were restricted in caloric intake two weeks after weaning (n = 19 DR-fed; n = 22 AL-fed). At 25 months of age (arrow), half of the restricted rats were placed on the control diet, while the other half remained on the restricted diet. (C) Male rats were provided a DR diet starting at an early age and maintained on this diet for at least 10 months (n = 35 DR-fed; n = 17 AL-fed). Prior to 10 months of age (arrow), temperature declines in the laboratory caused deaths in approximately half of the DR-fed rats. (D) Female rats were provided a DR diet starting at an early age and maintained on this diet for at least 10 months (n = 37 DR-fed; n = 17 AL-fed). Prior to 10 months of age (arrow), temperature declines in the laboratory caused deaths in approximately half of the DR-fed rats.
A second attempt by McCay and colleagues did not reveal a sex difference, but failed to clearly demonstrate an effect of DR on median survival in white rats of both sexes (McCay et al., 1939) (Figures 4C and 4D). In this study, 33 rats were provided an AL diet and 73 rats were provided a DR diet immediately after weaning and subsequently maintained on this diet for at least 10 months (with vitamin and mineral supplementation). The DR protocol was similar to that used by McCay et al. (1935), except restricted rats were switched to the control diet at 10, 17, 23 or 33 months of age, with an average body weight decline of 20-30% among all DR-fed rats. During the first 10 months of the experiment, however, an unexpected drop in temperature occurred (on two occasions), leading to deaths of 35 of the 73 DR-fed rats - but strikingly - none of the 33 AL-fed rats (Figures 4C and 4D). It was stated that all such deaths were due to low temperature stress, which if correct, would demonstrate dramatically heightened sensitivity of DR-fed rats to this condition. However, DR did increase maximum lifespan and reduced mortality beyond 10 months of age, which led McCay et al. (1939) to conclude that the diet “affords a means of producing very old animals for studying aging”.
McCay and colleagues later reported a convincing demonstration that DR increased median and maximum survivorship in both white rat sexes (McCay et al., 1943). Failure of DR to improve survival in both earlier studies is therefore unlikely specific to the random-bred genetic background evaluated. However, results from McCay et al. (1935) show that, given the same laboratory environment and DR protocol, effects of DR on lifespan can be attenuated in female relative to male rats. This trend appears to have persisted in subsequent studies (Figure 1 and Table 1). Secondly, results from McCay et al. (1939) reveal that, while DR is often effective at improving late-life survival, this effect can be associated with differential sensitivity to certain stress conditions at early ages, such as low temperature or, potentially, pathogen exposure. This principle has also received support in more recent work (Gardner, 2005; Kristan, 2008; Ritz et al., 2008; Clinthorne et al., 2010), and may have been a factor in later studies for which overall effects of DR on median lifespan were weakened by early-life mortality (e.g., Harrison and Archer, 1987; Forster et al., 2003; Harper et al., 2006a).
4. C57BL/6 (B6) Mice
The C57BL/6 (B6) strain has been the most frequently chosen genotype in experiments that have evaluated the effects of DR on lifespan in mice (Supplemental Table 3). B6 mice are prone to obesity and elevated glucose levels, but are also long-lived on a standard diet, with low IGF-1 and moderate body size (Svenson et al., 2007; Yuan et al., 2009). It has been established with certainty that DR protocols can improve the median and maximum lifespan of B6 mice (Turturro et al., 1999). However, there has been heterogeneity in this effect among published studies (Supplemental Table 3). The B6 genotype therefore provides a useful illustration of the range of DR effects that should be expected given repeated evaluations of the same genotype under differing sets of laboratory conditions. Among 22 experiments yielding estimates, effects of DR on median lifespan have ranged from a 32.8% decrease to a 26.8% increase (6.7% increase on average; Supplemental Table 3). Among 21 experiments yielding estimates, effects of DR on maximum lifespan have ranged from a 7% decrease to a 43.8% increase (15.1% increase on average; Supplemental Table 3). These average effects of DR in B6 mice are roughly consistent with those obtained among all inbred mouse genotypes (Figure 2). Notably, among seven experiments that evaluated effects of DR on F1 hybrids generated from crosses between B6 mice and another strain (e.g., B6C3F1 mice), effects of DR have been more consistent and, on average, more favorable (median lifespan: 32% increase; maximum lifespan: 27% increase).
Cheney et al. (1980) reported four experiments in which DR failed to increase median lifespan of B6 mice. This study evaluated survival of B6 mice provided one of three diets (ad lib, 105 calories/week or 60 calories/week). The ad lib diet and 105 calories/week diet appeared similar in terms of their effects on body weight and survival, but as expected, the 60 calorie/week diet consistently decreased body weight of B6 mice. In the first cohort, mice were weaned at 21 days and then provided either 105 calories/week or 60 calories/week (14 ≤ n ≤ 20 per diet per sex). However, the low-calorie diet decreased median lifespan by 7-8% in both sexes. In a second cohort (females only), mice were provided the low or high calorie diet starting at 4 weeks of age (n = 100 females per diet), but the low-calorie diet increased mean lifespan by only 1%. In a third cohort (females only; n = 75 per treatment), the same diets were enforced after 24 days of weaning, but median lifespan was decreased by 12%. Finally, in a fourth cohort, the experiment was performed with female mice provided a restricted diet before weaning (9 mice/litter with every-other-day access to mother) combined with a low-calorie diet post-weaning (60 calories/week), but median lifespan of this group was increased by only 4% relative to controls (5 mice/litter pre-weaning and 105 calories/week post-weaning). In most of these experiments, DR did have a positive effect on maximum lifespan, yielding increases ranging from 4 – 44% (Cheney et al., 1980).
Weak effects of DR on average lifespan of B6 mice were corroborated by the study of Harrison and Archer (1987), which evaluated effects of 33% DR in both B6 and B6CBAF1 male mice (n ≥ 34 per treatment), as well as in leptin-deficient obese males carrying the ob/ob mutation on the B6 background (B6-ob/ob; n = 48 AL mice; n = 53 DR mice). Mice were maintained in barrier cages (3 – 4 mice per cage) in an environmentally controlled room, with filtered air, and tested at least once for presence of 10 standard mouse viruses. DR diets were initiated at an early age (1 month) without stepwise reduction from AL to DR levels. DR increased median lifespan by 21% in B6CBAF1 mice and by 59% in B6-ob/ob mice. However, in B6 males, DR decreased median lifespan by 33%, with approximately one-third of the DR-fed mice dying during the first 400 days, with almost no mortality among AL-fed mice during this same time period. Strikingly, even maximum lifespan was decreased by 2% among the DR-fed mice. An earlier study, by the same laboratory, had also demonstrated weak effects of DR on the survival of B6 females, with median lifespan increased by only 6% under a DR protocol initiated at 1 month of age (Harrison et al., 1984).
At least two factors may explain why some studies have failed to demonstrate DR-increased median and/or maximum lifespan using B6 mice. First, B6 mice may be especially sensitive to the age and pace at which a DR protocol is initiated. Both the studies of Cheney et al. (1980) and Harrison and Archer (1987), for instance, restricted calorie intake of DR cohorts at or before 4 weeks of age, yielding no significant increase in median lifespan. In contrast, when 40% DR was implemented in gradual fashion (over 3 weeks) starting at 14 weeks of age, median lifespan of B6 mice was increased by approximately 20% in a large study sponsored by the National Center for Toxicological Research (NCTR) (Turturro et al., 1999). For B6 mice, therefore, the abrupt onset of DR near the time of weaning may predispose towards early-life mortality despite an overall reduction of neoplastic diseases at later ages (Cheney et al., 1980). A second contributing factor may be the low percentage of fat in basal diets used by Cheney et al. (1980) and Harrison and Archer (1987). Since B6 mice are susceptible to atherosclerosis and obesity, restriction of calories in the context of a high fat basal diet is more likely to improve lifespan. In the NCTR study, for example, 40% DR increased median lifespan of B6 females by 25% given the standard NIH-31 diet, but the same protocol increased median lifespan by 60% when B6 females were provided the high-fat Emory-Morse 911 diet (Turturro et al., 1999).
5. C3H Mice
C3H is a high body weight strain reported to exhibit some features of “metabolic syndrome”, with a tendency for substantial food intake under ad lib conditions and notably high cholesterol and plasma triglycerides (Jiao et al., 1990; Paigen, 1995; Champy et al., 2008). However, both C3H sexes are relatively long-lived with moderate IGF-1 levels (Yuan et al., 2009), and show resistance to further weight gain when provided a high fat diet (Tsukumo et al., 2007), along with attenuated intestinal absorption of cholesterol and only weak atherosclerotic lesion formation (Paigen et al., 1985; Paigen et al., 1990). The effects of DR on median lifespan of C3H mice have not been consistent among independent studies (Lee et al., 1956; Fernandes et al., 1976a; Yoshida et al., 1997).
Fernandes et al. (1976a) evaluated effects of 37% DR in female C3H/Umc mice starting at 1 month of age (n = 17 control mice and n = 18 DR mice). However, the DR diet decreased median survival by 7%, with a median survival time of 13 and 14 months in DR-fed and control mice, respectively (Weindruch and Walford, 1988). Laboratory conditions from this study may have been sub-optimal, since a recent study showed that C3H females can live much longer under AL conditions, with a mean lifespan of 26 months on average (Yuan et al., 2009). Nevertheless, a second study, conducted under specific pathogen free conditions, showed that the effect of 21% DR in male C3H/HeNirMs mice was positive but modest in magnitude (Yoshida et al., 1997). When C3H mice were restricted starting at 6 weeks of age, mean lifespan was increased only by 2% (26.9 vs. 26.3 months; n = 70 DR-fed; n = 165 AL-fed) (Yoshida et al., 1997). A separate group of mice was restricted starting at 10 weeks of age, but mean lifespan was still increased by only 6% (27.8 vs. 26.3 months; n = 135 DR-fed; n = 165 AL-fed) (Yoshida et al., 1997). In contrast to these findings, an early study showed that DR can increase median lifespan by 80% (n = 18 DR-fed; n = 18 AL-fed in each of two studies) (Lee et al., 1956); however, sample sizes in this study were limited and median lifespan of AL-fed mice was only 13-15 months (Lee et al., 1956).
C3H mice may be susceptible to excessive weight loss when provided low-calorie diets (Rikke et al., 2006), and this characteristic could mask other positive effects of DR in this genotype (Fernandes et al., 1976a; Weindruch and Walford, 1988). Along these lines, Yoshida et al. (1997) reported difficulty in developing an appropriate DR protocol for C3H males. Given a protocol corresponding to 32% DR (65 vs. 95 kcal per week), C3H males exhibited “serious emaciation” and early mortality at one year of age (Yoshida et al., 1997). However, if the DR was modulated over time, with the aim of maintaining a constant body weight of about 25 g (approximately 75 kcal per week or 21% DR), then the protocol yielded a weak improvement in median lifespan (Yoshida et al., 1997).
6. DBA Mice
DBA/2 (D2) mice have a moderate lifespan with low IGF-1 levels and small body size (Yuan et al., 2009). D2 mice have been viewed as a “negative control” strain for which DR does not increase median or maximum lifespan (Ferguson et al., 2007; Rebrin et al., 2007; Ferguson et al., 2008; Sohal et al., 2009; Rebrin et al., 2011). This designation remains controversial, however, since four studies have shown that DR decreases D2 lifespan (Silberberg et al., 1962a; Silberberg et al., 1962b; Fernandes et al., 1976b; Forster et al., 2003), while a contrary result has been obtained in two other experiments (Bronson and Lipman, 1991; Turturro et al., 1999).
Silberberg et al. (1962a) and Silberberg et al. (1962b) underfed D2 mice by 40% between 1 and 4 months of age before returning them to an ad lib diet for the remainder of their lives. In females, this brief period of underfeeding decreased mean lifespan by 11.6% and decreased maximum lifespan by 1% (n = 18 restricted mice versus n = 48 AL-fed mice) (Silberberg et al., 1962a). In males, the early-life underfeeding decreased median lifespan by 5.1% and decreased maximum lifespan by 32.7% (n = 18 restricted mice versus n = 47 AL-fed mice) (Silberberg et al., 1962b). This early-life underfeeding (1 – 4 months) also had negative or only weak effects on the lifespan of B6 and A mice, which were concurrently evaluated in the same series of studies (Silberberg et al., 1962a; Silberberg et al., 1962b). However, mortality was increased most among the restricted D2 mice, which led the authors to conclude that “D2 mice were more susceptible to the injurious effect of underfeeding than either A or B6 mice” (Silberberg et al., 1962b).
Fernandes et al. (1976b) evaluated lifespan of male and female DBA/2f mice provided four diets (i) AL diet with 22% protein and 5% fat, (ii) DR diet with 22% protein and 5% fat, (iii) AL diet with 6% protein and 5% fat and (iv) DR diet with 6% protein and 5% fat. The AL diet provided 20 calories per day per mouse, while the DR diet provided 10 calories per day per mouse (i.e., 50% DR). Dietary treatments were initiated at 2 months of age, with n = 10 – 12 mice per sex assigned to each dietary treatment. Mice were maintained under standard housing conditions, but no specific pathogen control and testing safeguards are described in the research report (Fernandes et al., 1976b). For each sex and at both caloric intake levels (AL and DR), the low protein diet increased D2 lifespan. However, for each sex and within each protein level, median lifespan of mice provided the DR diet was shortened relative to AL-fed mice (10% decrease for males with 22% protein; 1% decrease for females with 22% protein; 27% decrease for males with 6% protein; 5% decrease for females with 6% protein). In the same study, DR increased lifespan in (NZB × NZW)F1 hybrid mice (i.e., a positive control group). To explain the DR-shortened lifespan in D2 mice, the authors noted that DR-fed D2 mice seemed “more susceptible to infection, e.g., chronic pneumonia” (Fernandes et al., 1976b).
These findings were corroborated in a larger study reported by Forster et al. (2003), which evaluated effects of 40% DR on lifespan of DBA/2Nnia males provided the NIH-31 diet (20% protein / 3.6% fat; DR initiated at 4 months of age). Importantly, Forster et al. (2003) state that “mouse cages were maintained in positive laminar flow units, and standard serological testing performed on sentinels verified specific pathogen-free conditions for the time period of these studies” (Forster et al., 2003). Consistent with Fernandes et al. (1976b), DR decreased D2 median lifespan by 6% and decreased maximum lifespan by 9%. This result was largely due to strong mortality between 10 – 20 months of age in DR-fed mice but not the AL cohort. Notably, however, Forster et al. (2003) concurrently demonstrated positive effects of DR in B6 and B6D2F1 mice. The DR response of D2 males was therefore unique as compared to these genotypes, given the same protocol and level of pathogen control.
In contrast to these findings, two studies have reported that DR increases median and maximum lifespan of male and female D2 mice (Bronson and Lipman, 1991; Turturro et al., 1999). First, Bronson and Lipman (1991) evaluated effects of 40% DR using the same NIH-31 diet from Forster et al. (2003) (n = 30 mice per sex per diet). However, median lifespan was increased by 56% in females and by 19% in males. Although DR increased lifespan, the investigators noted that “survival curves for both male and female D2 mice did not have the expected square profile seen in most longitudinal rodent experiments...curves for both sexes and both diet groups were quite shallow” (Bronson and Lipman, 1991). In a second study, effects of 40% DR in DBA/2JNia mice (NIH-31 diet) were evaluated as part of the NCTR effort to develop aging biomarkers in rodents (Turturro et al., 1999). Starting at 3.5 months of age, the DR diet was gradually implemented in stepwise fashion over a period of 3 weeks. Mice were maintained in specific pathogen free conditions with strong protections against pathogen exposure (e.g., sterilization of equipment, personnel showered before entering facility and wore sterilized protective gear). DR had weaker effects on D2 survivorship compared to genotypes concurrently evaluated (C57BI/6NNia, B6D2F1 and B6C3F1). Nevertheless, DR increased both median and maximum lifespan in D2 mice of each sex. Finally, it is noteworthy that, in a third study, 40% DR increased average lifespan of D2 females by 63%, although animals from this experiment were short-lived due to treatment with the carcinogen 7,12-dimethylbenz[a]anthracene (Lipman, 2002).
The totality of evidence suggests that effects of DR on lifespan of D2 mice are sensitive to variations among laboratory environments and DR protocols. However, there have been two noteworthy points of consistency among experiments. First, although direction of the DR effect has varied, experiments that have simultaneously evaluated multiple genotypes have each shown that the effect of DR on mean lifespan of D2 males is weaker in comparison to males of other genotypes (Silberberg et al., 1962b; Bronson and Lipman, 1991; Turturro et al., 1999; Forster et al., 2003). However, this is not the case for females (Bronson and Lipman, 1991). D2 males, therefore, appear to provide a strain-sex combination for which positive effects of DR are at least attenuated. Secondly, survival curves generated from D2 mice (AL- or DR-fed) have frequently differed from those of other genotypes, with increased early mortality during an age window between 10-20 months (Bronson and Lipman, 1991; Turturro et al., 1999; Forster et al., 2003). This early mortality may be due to a currently unidentified genetic disease or susceptibility that contributes to early mortality of D2 mice under some laboratory conditions.
Several studies have compared DR responses between D2 and B6 mice, based upon the premise that D2 provides a “negative control” genotype with DR-shortened or DR-insensitive lifespan, while B6 provides a “positive control” strain with DR-increased lifespan (Ferguson et al., 2007; Rebrin et al., 2007; Ferguson et al., 2008; Sohal et al., 2009; Hempenstall et al., 2010; Rebrin et al., 2011). In fact, effects of DR on lifespan have varied considerably and have often been weak for both D2 and B6 genotypes (see above). Nevertheless, two main ideas have emerged from these comparisons. First, it has been noted that body weight of AL-fed B6 mice increases by 20% from 6 to 23 months of age, but remains constant for AL-fed D2 mice (Sohal et al., 2009). D2 mice therefore appear to maintain a more neutral energy balance while B6 mice are disposed towards a positive energy balance. This effect is not due to a difference in food consumption, which is similar in the AL condition for each strain, but instead seems due to higher resting metabolic rate and body temperature in D2 mice (Ferguson et al., 2007; Ferguson et al., 2008; Sohal et al., 2009). Potentially, therefore, DR corrects the positive energy balance in B6 mice, thereby increasing lifespan, while this same effect is deleterious in strains that naturally maintain a more neutral energy balance such as D2 (Sohal et al., 2009). A second possibility is that disparities in glucose homeostasis contribute to differential DR responses of D2 and B6 mice (Hempenstall et al., 2010). When compared to B6 mice, D2 mice are hyperinsulinaemic, insulin resistant, and have better glucose tolerance with AL-feeding or following short-term DR. Since effects of DR on insulin sensitivity and glucose homeostasis are thought to mediate favorable effects of DR (Bartke, 2008), this metabolic profile may be a factor that mitigates positive effects of DR on D2 lifespan.
A final possibility is that D2 mice have weakened ability to withstand even the limited pathogen challenges encountered within the laboratory environment. When compared to B6 and BALB/c mice, D2 mice develop more severe parasite burden following infection with Babesia microti at 2, 6, 12 and 18 months of age (Vannier et al., 2004). Establishment and progression of infection by the tapeworm Echinococcus multilocularis is also more severe in D2 mice compared to the AKR/N, C57BL/10 and B6 strains (Matsumoto et al., 2010). The survival of D2 mice following infection with Bacillus anthracis is decreased relative to eight other commonly used laboratory strains, including B6 and C3H (Welkos et al., 1986). At 18 months of age, vaccination against the intracellular pathogen Brucella abortus was less effective in D2 relative to BALB/c mice (High et al., 2007). Moreover, inflammatory gene expression patterns developing in response to high fat diet are uniquely blunted in D2 mice in comparison to ten other inbred mouse strains (Shockley et al., 2009; Swindell et al., 2010). Collectively, these results suggest that D2 mice are compromised in their capacity for mounting immune responses, which could explain early-life mortality of D2 mice provided either a DR or AL diet (Turturro et al., 1999; Forster et al., 2003). DR diets, in particular, however, may exacerbate such an immunodeficiency to account for DR-decreased lifespan in D2 mice (Gardner, 2005; Kristan, 2008; Ritz et al., 2008; Clinthorne et al., 2010). Although most studies have implemented safeguards against accidental pathogen exposure during survival assays, these standards have been enforced with varying degrees of stringency (Miller and Nadon, 2000; Cerqueira and Kowaltowski, 2010). For instance, the early studies of Silberberg et al. (1962a), Silberberg et al. (1962b) and Fernandes et al. (1976b) housed multiple mice per cage, while in other experiments D2 mice were housed individually with regular testing for at least some mouse pathogens (Bronson and Lipman, 1991; Turturro et al., 1999; Forster et al., 2003).
7. ILSXISS recombinant inbred strains
The Inbred Long Sleep (ILS) and Inbred Short Sleep (ISS) strains were derived from a heterogeneous stock built from crosses among eight mouse strains (A, AKR, BALB/c, C3H/2, C57BL, DBA/2, Is/Bi and RIII) (Williams et al., 2004). The ILS strain was subsequently selected for increased sensitivity to the sedating effects of ethanol, while the ISS strain was selected for decreased sensitivity. Starting in 1996, ILS and ISS strains were reciprocally crossed to generate 125 lines. The 125 lines were then maintained by brother-sister mating for more than twenty generations, with 75 lines (60.0%) surviving until the 33rd generation of inbreeding (Bennett et al., 2006). These surviving lines represent the ILSXISS panel of recombinant inbred strains that have been extensively studied in the context of research on alcohol sensitivity (Williams et al., 2004; Bennett et al., 2006). The ILSXISS panel has also been used to demonstrate significant strain variation with respect to the effects of DR on body weight and temperature (Rikke et al., 2003; Rikke and Johnson, 2007), and to identify quantitative trait loci associated with these metabolic effects of DR (Rikke et al., 2004; Rikke et al., 2006).
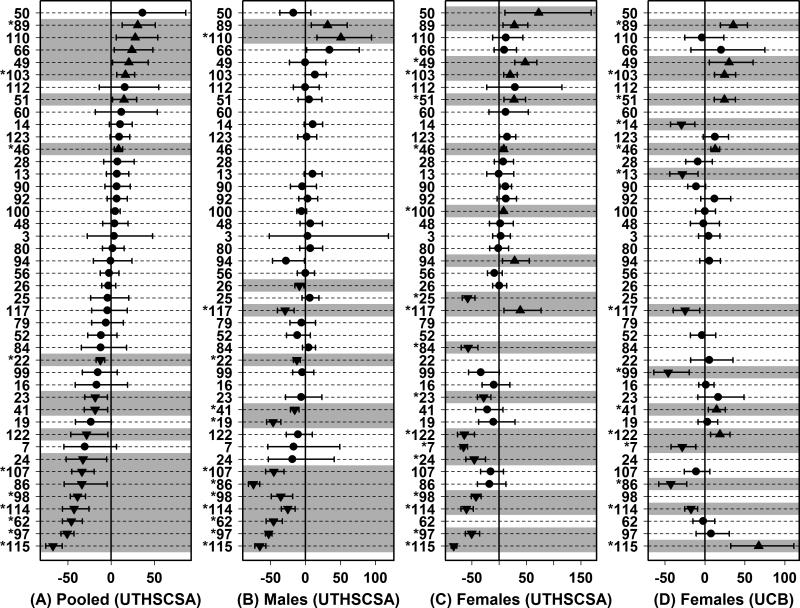
Lifespans of ILSXISS strains under DR and AL conditions have been evaluated in two studies, which were independently conducted at the University of Texas Health Science Center at San Antonio (UTHSCSA) and the University of Colorado at Boulder (UCB) (Liao et al., 2010a; Liao et al., 2010b; Rikke et al., 2010). In the UTHSCSA study, males from 41 strains were evaluated along with females from 39 strains (44 strains for one or both sexes) (Liao et al., 2010a; Liao et al., 2010b). In the UCB study, females from 42 strains were evaluated, including 29 of the 39 strains for which females had been measured at UTHSCSA (Rikke et al., 2010). In both studies, DR mice were provided a diet 40% less in caloric content relative to the AL caloric intake estimated for each strain on a weekly basis, although DR diets were initiated at a later age at UTHSCSA (2 – 5 months) relative to UCB (1 month). In both studies and for each sex, an unexpected pattern emerged, whereby DR decreased lifespan for 20 – 25% of strains, increased lifespan for only 5 – 25% of strains, and had no significant effect on lifespan for the majority of ILSXISS strains (P < 0.05 by t-test) (Liao et al., 2010a; Rikke et al., 2010). A positive control strain, known to respond favorably to DR, was not concurrently evaluated. In Figure 5, survivorship data from both studies are re-analyzed using an accelerated failure time (AFT) model, which provides 95% confidence intervals for the effect of DR on lifespan in each strain (Swindell, 2009). This analysis demonstrates significant heterogeneity among strains with respect to the estimated effect of DR on lifespan (i.e., non-overlap of 95% confidence intervals), and shows that these effects remain significant in 10 – 15 strains per study even following FDR correction for multiple hypothesis testing (Figure 5).
Figure 5. Accelerated failure time model-based estimates of DR effects on survivorship in 44 ILSXISS strains.
Effects of DR on survivorship across ILSXISS recombinant inbred mouse strains were evaluated based upon survivorship experiments performed at the University of Texas Health Science Center at San Antonio (UTHSCSA) or University of Colorado at Boulder (UCB) (Liao et al., 2010a; Rikke et al., 2010). For each strain, the percent increase in survivorship due to DR was estimated by fitting an accelerated failure time model to survival data (Swindell, 2009). Calculations were performed using UTHSCSA data pooled from both sexes (A), UTHSCSA data from males only (B), UTHSCSA data from females only (C) or UCB female data (D). Filled symbols represent the estimated percent increase in survivorship due to DR and error bars span the 95% confidence interval associated with this estimate. Shaded regions denote significant effects of DR (P < 0.05), including significantly elevated survivorship due to DR (up-triangles) or significantly decreased survivorship due to DR (down-triangles). Strain labels in the left margin have an asterisk symbol (*) if the estimated effect of DR on lifespan remained significant following FDR adjustment of p-values for multiple testing among all ILSXISS strains.
It seems difficult to understate the potential importance of these findings to our broader understanding of DR and its impacts on aging in the laboratory mouse. In prior decades (1934 – present), effects of DR on lifespan had been evaluated in 27 unique genotypes (Supplemental Table 2). In combination, the studies of Liao et al. (2010a) and Rikke et al. (2010) have evaluated 54 new genotypes, thus tripling the number of genotypes for which the effects of DR on mouse lifespan have been examined. Among previous experiments (1934 – present), DR increased median and maximum lifespan in mice by 15% and 18% on average, respectively (Table 1). However, if these average effects are re-calculated with inclusion of the ILSXISS results, the average increase in median lifespan with DR is only 2.9% (2.9% in males; 2.0% in females; n = 189 experiments; Table 2), while the average increase in maximum lifespan with DR is just 5.2% (3.7% in males; 5.0% in females; n = 181 experiments; Table 2). Furthermore, consideration of the ILSXISS results leads to significant funnel plot asymmetry (see Figure 6; median lifespan: P = 4.4 × 10-5; maximum lifespan: P = 2.7 × 10-10) (Peters et al., 2006). The pattern is similar to that observed for maximum lifespan in rats (Figure 3B) and is consistent with bias against large sample size studies failing to demonstrate positive effects of DR. This could reflect a disproportionate tendency to terminate (more expensive) large sample size studies for which expected trends have not emerged mid-way through experiments. Alternatively, since the pattern is driven by ILSXISS strains and is absent without them (Figures 3C and 3D), the trend may not be due to bias, but instead the true mean effect of DR in ILSXISS strains could differ from that of other mouse genotypes. This would be the case, for instance, if ILSXISS strains are “genotypic outliers”, which have become fixed for unique allelic variants absent from other mouse strains.
Table 2. Effects of DR on lifespan in mice (1934 – present; including ILSXISS strains).
The mouse analysis presented in Table 1 was repeated, except recently published results from ILSXISS recombinant inbred strains were included in calculations (Liao et al., 2010a; Rikke et al., 2010). Combined with all previous studies (1934 – present), this yielded a total of 193 experiments in which the effects of DR on mouse lifespan had been evaluated (Supplemental Table 2). This total includes 79 experiments carried out at the University of Texas Health Science Center at San Antonio (UTHSCSA), which evaluated the effects of 40% DR in males or females from different ILSXISS strains (Liao et al., 2010a). Additionally, the total includes 42 experiments conducted at the University of Colorado at Boulder (UCB), which evaluated the effects of DR in females from different ILSXISS strains (Rikke et al., 2010). In some cases, it was not possible to estimate precisely the DR-induced change in median and/or maximum lifespan using information for published research reports. These cases were thus excluded from consideration, and estimated effects of DR on median lifespan and maximum lifespan were calculated from 189 and 181 experiments, respectively. The average effects of DR listed in the table were calculated as described in the Table 1 caption, with greater weight assigned to experiments that had used larger sample sizes.
| Median Lifespan | Maximum Lifespan | ||||
|---|---|---|---|---|---|
| n | % Change DR (middle 50%) | n | % Change DR (middle 50%) | ||
| Mice | Pooled | 189 | 2.9% (-8.0 – 20.0%) | 181 | 5.2% (-7.3 – 18.5%) |
| Males | 74 | 2.9% (-8.0 – 18.8%) | 72 | 3.7% (-11.5 – 16.5%) | |
| Females | 113 | 2.0% (-8.3 – 19.1%) | 107 | 5.0% (-7.0 – 18.5%) | |
Figure 6. Funnel plot analysis of publication bias in studies that have evaluated the effects of DR on mouse lifespan (including ILSXISS strains).
The funnel plot analyses shown in Figures 3C and 3D were repeated using survival data from 72 mouse experiments in combination with 121 experiments that had evaluated ILSXISS recombinant inbred mouse strains (193 experiments total; Supplemental Table 2) (Liao et al., 2010a; Rikke et al., 2010). Experiments for which DR decreased median or maximum lifespan are plotted within the grey region in each figure. The dotted vertical line denotes the average effect size among all experiments (i.e., average treatment effect weighted by study size). In the absence of publication bias, it is expected that effect sizes from individual experiments will be symmetrically distributed to the left and right of the average effect size (dotted line).
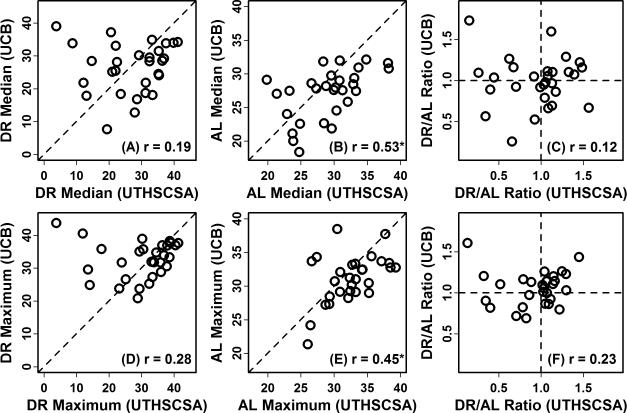
Previous experiments have usually evaluated 20 – 40 mice per dietary treatment in order to assess the effects of DR on lifespan (Supplemental Table 2; Figure 6). However, sample sizes were much lower in studies of ILSXISS mice conducted at UTHSCSA and UCB, with only 5 - 15 mice evaluated for each strain-sex-diet combination (Figure 6). If the effects of DR on female lifespan are compared between the two study sites, there is a trend suggesting that cases of DR-increased lifespan are better replicated than cases of DR-decreased lifespan (see Figures 5C and 5D). Among 8 strains for which DR significantly increased lifespan at UTHSCSA, the effect was confirmed in 5 strains at UCB (P < 0.05; AFT model). However, among 6 strains for which DR significantly decreased lifespan at UTHSCSA (P < 0.05; AFT model), the effect was confirmed in only two cases at UCB (strains 7 and 114), while in two other such cases DR increased lifespan at UCB (strains 115 and 122). Across all strains, female lifespan estimates under the AL condition were significantly correlated between study sites (Spearman r ≥ 0.45; Figure 7). However, under the DR condition, estimates of median and maximum lifespan were not significantly correlated (Spearman r ≤ 0.28), and consequently, the estimated effects of DR on female lifespan were not significantly correlated between sites (Spearman r ≤ 0.23; Figure 7).
Figure 7. Female ILSXISS lifespan estimates are correlated between independent studies under the AL condition but not the DR condition.
Female lifespan of the ILSXISS recombinant inbred mouse strains was measured at University of Texas Health Science Center at San Antonio (UTHSCSA) and independently measured at the University of Colorado (UCB) (Liao et al., 2010a; Rikke et al., 2010). Figures (A) and (B) show the association between median lifespan estimates from the two studies. In part (C), a comparison is made between ratios (DR / AL) of the median lifespan estimates derived from each study. Figures (D) and (E) show the association between maximum lifespan estimates from the two studies, where maximum lifespan is calculated by averaging the two longest survival times for each strain and each study. In part (F), a comparison is made between ratios (DR / AL) of the maximum lifespan estimates derived from each study. The Spearman rank correlation is shown in the lower-right of each figure, and an asterisk symbol is used to denote correlation estimates that are significant (P < 0.05).
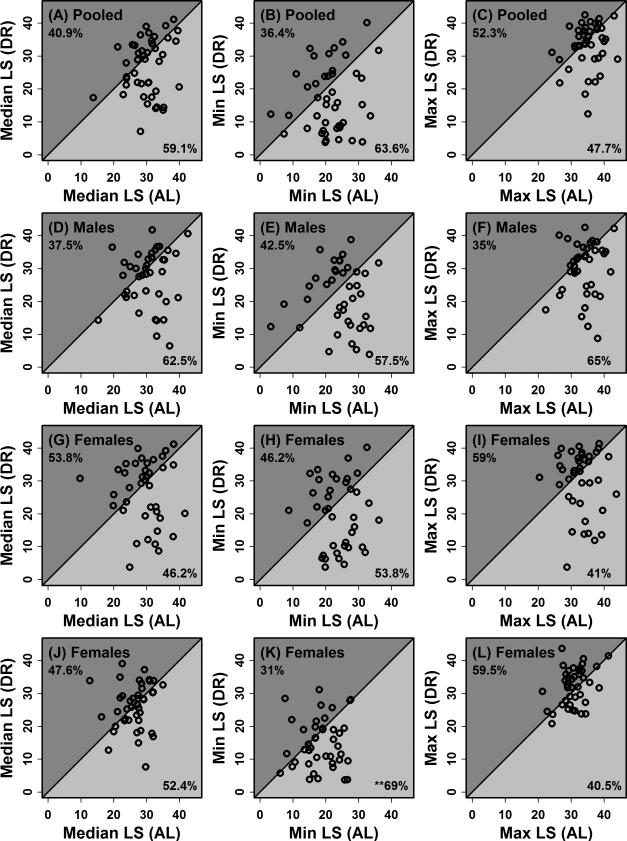
In studies that have failed to demonstrate a positive effect of DR on median lifespan, it has often been possible to discern a trend towards improved late-life survivorship (McCay et al., 1935; McCay et al., 1939). Figure 8 plots DR versus AL lifespan, based upon median survival times of all mice in each treatment (Figure 8; left column), the average of the two shortest survival times per treatment (Figure 8; middle column) and the average of the two longest survival times per treatment (Figure 8; right column). In most cases, effects of DR on lifespan were similar regardless of whether only early or only late survival times are considered (Figure 8). However, in the UCB study, there was some indication that DR increased early mortality and decreased later mortality (Figures 8K and 8L). In 29 of 42 strains evaluated (69%), the average of the two shortest survival times per dietary treatment was lower in DR-fed mice compared to AL-fed mice (P = 0.02; sign test; Figure 8K). In 25 of the 42 strains evaluated (59%), the average of the two longest survival times per dietary treatment was greater in DR-fed mice compared to AL-fed mice (P = 0.28; sign test; Figure 8L). It will be valuable to repeat survivorship assays for selected strains with larger sample sizes per dietary treatment (e.g., n ≥ 50), which would better allow age-specific effects of DR on survival to be estimated. One possibility, for instance, would be to concurrently evaluate the effects of DR on the survival of three strains – one with DR-shortened lifespan (e.g., Strain 114), one with DR-lengthened lifespan (e.g., Strain 89), and one strain for which DR appears to have no effect on lifespan (e.g., Strain 48).
Figure 8. Effects of DR on median, early and late survival in ILSXISS recombinant inbred mouse strains.
Effects of DR on median, early and late survivorship were evaluated across ILSXISS strains based upon experiments performed at University of Texas Health Science Center at San Antonio (A – I) and University of Colorado at Boulder (J – L) (Liao et al., 2010a; Rikke et al., 2010). In the left column (A, D, G and J), the median lifespan is plotted for each strain under the AL and DR conditions. In the middle column (B, E, H and K), the lowest two survival times for each strain were averaged and plotted for the AL and DR conditions. In the right column (C, F, I and L), the highest two survival times for each strain were averaged and plotted for the AL and DR conditions. In each plot, the percentage of strains with a larger survival estimate in the DR condition (i.e., above-diagonal points) is indicated in the upper left (dark grey region). The percentage of strains with a larger survival estimate in the AL condition (i.e., below-diagonal points) is indicated in the lower right (light grey region). In one case, survival estimates were significantly more likely to be lower under the DR condition in comparison to the AL condition (see Figure 8K; P = 0.02; sign test with n = 42 strains).
A follow-up study has indicated that some variation in the effects of DR among ILSXISS strains can be explained by body fat maintenance as reflected by changes in body composition under DR conditions (Liao et al., 2011). Among all strains, the effect of DR on lifespan (DR – AL lifespan) was negatively correlated with its effect on body fat at 15 – 17 months of age (AL – DR body fat), such that lifespan tended to decline most in strains exhibiting the strongest late-life declines in body fat with DR. Strains losing the most body fat with DR thus appeared most susceptible to lifespan-shortening, which may contradict the idea of fat loss as a factor mediating pro-longevity effects of DR (Liao et al., 2011). However, as acknowledged by Liao et al. (2011), shifts in body fat mass with DR were evaluated only at 15 – 17 months age. At this late age, apparent cause and effect may be reversed, with DR-shortening of lifespan in fact precipitating decreased fat mass in DR-fed ILSXISS strains. In several strains, median lifespan of DR-fed mice was less than 15 months, or only slightly greater (Figure 6). At 15 months, therefore, disease processes contributing to mortality may have been driving “terminal weight loss” in DR-fed mice, similar to that described in senescent rats prior to death (Black et al., 2003), as well as in frail humans at increased risk of near-term mortality (Fried et al., 2001). Loss of body fat with DR at 15 – 17 months, therefore, may only be an indicator of disease progression and impending death among the sickest DR-fed strains, reflecting what can already be discerned on the basis of mortality patterns. Given this interpretation, the correlation between body fat decline (AL – DR fat) and effect of DR on lifespan (DR – AL lifespan) may become more negative for body fat measurements recorded at increasingly later ages, while at an early age, the correlation may be absent or even reversed in direction. These predictions can be tested in further studies, which should clarify the role of fat maintenance in the effects of DR on lifespan across the panel of ILSXISS recombinant inbred strains.
The demonstrated effects of DR on lifespan across ILSXISS strains challenge the view of DR as a robust pro-longevity intervention. To some degree, these effects appear out-of-step with previous decades of experimentation (Figure 6), and an important direction for future work will be to understand the significance of this observation. On the one hand, ILSXISS strains may be representative of many more mouse genotypes for which effects of DR on lifespan have not yet been studied. However, the genetic heritage of ILSXISS strains is also unique. The strains were derived from an ancestral population that had undergone differential selection for ethanol sensitivity, which might have eliminated alleles with indirect effects on DR responses (Bennett et al., 2006). In Drosophila, for instance, direct laboratory selection for sensitivity to ethanol has indirect effects on other traits, such as body weight and reproductive capacity (Cohan and Hoffmann, 1986; Hoffman and Cohan, 1987). Whether aberrant DR responses are an indirect effect of selection on ethanol sensitivity in mice is unknown, but the possibility cannot be altogether dismissed. Additionally, following selection for ethanol sensitivity, ILSXISS strains were inbred at a fast rate for more than 30 generations (i.e., sib mating) (Bennett et al., 2006). As with other inbred strains, this enforced period of close inbreeding is expected to promote randomized fixation of deleterious recessive alleles within lines, yielding the fixation of deleterious allele combinations, some of which might drive idiosyncratic late-life genetic diseases (Swindell and Bouzat, 2006a). This process appears to reduce the likelihood of observing favorable responses to DR (Figure 2), and may also have engendered a late-life disease spectrum in ILSXISS strains that is unique relative to other mouse genotypes. These possibilities can be explored by further post-mortem pathology studies of ILSXISS mice, as well as by evaluating effects of DR on the lifespan of F1 hybrids generated from crosses between ILSXISS strains.
8. Laboratory-reared descendants of wild-caught mice not adapted to laboratory conditions
Studies discussed above had been performed with rodent strains maintained for many generations under laboratory conditions. Laboratory environments, however, can decrease lifespan and promote age-related genetic illness by at least three evolutionary mechanisms. First, laboratory breeding directly selects for early reproduction, which can lead to fixation of alleles that increase early-life fitness, but which have deleterious effects on late-life fitness and lifespan (i.e., antagonistic pleiotropy) (Hughes and Charlesworth, 1994; Reed and Bryant, 2000). Second, relative to wild conditions, laboratory environments further diminish the “force” of natural selection with increased age, and in the absence of such selection, spontaneous mutations affecting late-age characteristics accumulate, contributing to genetic drift within inbred lineages over time and leading to degradation of health at older ages and decreased lifespan (i.e., mutation accumulation) (Promislow and Tatar, 1998; Gong et al., 2006; Joyner-Matos et al., 2009). Third, laboratory breeding almost always involves maintenance of populations at low effective population sizes, which in the absence of strong selection can lead to fixation of deleterious recessive alleles, idiosyncratic genetic illness, and shortened lifespan (i.e., inbreeding depression) (Vermeulen and Bijlsma, 2004; Swindell and Bouzat, 2006a). In combination, all these effects render it difficult to gauge whether positive effects of DR in laboratory-bred animals reflect general mechanisms applicable outside the laboratory, or whether DR is only attenuating one of the genetic consequences of laboratory maintenance.
Studies of wild-derived mice, recently introduced into the laboratory, have identified distinct phenotypic features relative to standard lab mice, including increased or at least similar lifespan (Miller et al., 2002; Harper et al., 2006a; Harper, 2008), reduced serum IGF-1 (Harper et al., 2006b), increased food consumption (Austad and Kristan, 2003), decreased fat mass (Harper et al., 2005), hyperglycemia (Harper et al., 2005), slower maturation and smaller litter sizes (Bronson, 1984) and elevated glucocorticoid levels with heightened hypothalamic-pituitary-adrenal activity (Harper et al., 2006b) (see Harper (2008) for review). All of these characteristics may render the DR response of laboratory-adapted mice aberrant in comparison to wild-derived mice. Such effects of laboratory maintenance may even have been noticed during early experiments performed by McCay et al. (1935), who stated that “the tendency of white rats to attain a greater body weight then they did 20 years ago has been observed”. Likewise, detailed investigations of long-term laboratory data are consistent with genetic decay fostering an accumulation of age-related illness over time in lab mice. For example, over 30 generations from 1971 to 1981, assays of F344/N rats identified a temporal trend towards faster growth, elevated body weight, increased frequency of leukemia and several types of tumors (anterior pituitary, thyroid C-cell, adrenal pheochromocytomas and mammary tumors), and progressive declines in average lifespan (Rao et al., 1990). Likewise, in B6C3F1 mice, during the period from 1981 to 1990, successive laboratory assays revealed a temporal trend towards increasing body weight at 12 months of age and increasing frequency of liver tumors (Turturro et al., 1996).
The effects of DR have seldom been evaluated in wild-derived mice that have not yet been adapted to the laboratory environment. In one study, however, wild mice were trapped in and around buildings near University of Idaho (Moscow, Idaho, USA), and subsequently bred in the laboratory to avoid close mating between related mice and attendant inbreeding depression (Harper et al., 2006a). The number of founders used to establish the laboratory population was not reported; however, a larger founder size would lessen sampling effects to impart greater generality to the findings. The DR diet reduced calories by 40% relative to the ad lib control treatment (n = 39 AL and n = 35 DR mice), but led to a non-significant 2% decrease in mean lifespan, despite a lower percentage of mice with tumors at necropsy (12% versus 58.3%) (Harper et al., 2006a). There was further indication that DR shifted the shape of the mortality curve towards increased early-life mortality and decreased late-life mortality, with maximum lifespan increased 14% in the DR group. A limitation of this study is that a positive control cohort was not included, and thus it was not definitively demonstrated that the DR protocol and laboratory conditions recapitulate those known to foster positive DR effects with other genetic backgrounds. Additionally, early-life mortality was increased within the DR group, which potentially, could be due to infectious disease since the study was not specific pathogen free; however, only 2 of 49 necropsy results uncovered evidence for death related to bacterial infection (Harper et al., 2006a).
These findings suggest that DR is less effective for increasing the (mean) lifespan of wild-derived mice in the laboratory as compared to other mouse strains that are adapted to laboratory conditions. This result seems contrary to the trend shown in Figure 2, which shows more favorable effects of DR in non-inbred mice as compared to inbred mice. This pattern is most likely due to the effects of inbreeding depression on mortality rates, and in particular, may represent a case of inbreeding-by-environment interaction, whereby the severity of inbreeding depression is increased under the more “stressful” DR condition relative to the less “stressful” AL condition (Swindell and Bouzat, 2006b; Fox and Reed, 2011). The conundrum, however, is that wild-derived mice are non-inbred and genetically diverse. It is therefore surprising to observe weak effects of DR on lifespan, since inbreeding depression should be absent, and effects of DR on lifespan should resemble those of other non-inbred mouse strains – but this is not the case.
One explanation is that positive effects of DR in both inbred and non-inbred strains have been inflated by laboratory breeding, since both classes of genotypes have undergone adaptation to laboratory environments. Decades of laboratory breeding, for instance, may favor genetic variants that predispose mice towards higher energy consumption when fed ad lib, since the excessive energy intake could support faster growth and earlier reproduction, yielding greater reproductive success under lab breeding protocols (Austad, 2001; Austad and Kristan, 2003; Martin et al., 2010). This would yield laboratory-adapted genotypes that consume more calories with ad lib feeding compared to the ancestral populations. Evidence of this was provided by the study of Austad and Kristan (2003), which showed that wild-derived Mus musculus consumed approximately 45 kJ of energy per day when provided an ad lib diet in the laboratory (n = 2 estimates). However, laboratory-adapted strains consumed, on average, 33% more energy per day (59.9 kJ) when provided an ad lib lab diet (n = 12 estimates; range: 42 – 75 kJ/day). When energy consumption estimates were adjusted for total body mass, which is lower in wild-derived mice, the consumption difference narrowed, but mass-adjusted energy consumption estimates remained 11% lower among wild-derived mice (P = 0.038; see Figure 2 from Austad and Kristan (2003)). This suggests that, in laboratory-adapted mice, standard DR treatments may simply nullify the unhealthy effects of lab-adaptation on energy intake, leading to increased lifespan as observed in many studies (Martin et al., 2010). Wild-derived mice, however, are inclined to consume fewer calories in the laboratory environment, and 40% DR relative to their AL intake may amount to a near starvation diet, yielding smaller effects on longevity overall and potentially increased early-life mortality, despite the absence of inbreeding depression.
The confounding influence of laboratory adaptation on DR responses could be of even greater importance in Drosophila and C. elegans. With their brief generation time, some strains have been maintained in the laboratory for thousands of generations. Over such time scales, spontaneously arising mutations would have an even stronger impact on late-life genetic disease, in combination with inbreeding depression and positive selection for early-life fitness (Shabalina et al., 1997; Promislow and Tatar, 1998; Vassilieva et al., 2000; Gong et al., 2006). In flies, effects of laboratory adaptation on the DR response have not been investigated; however, it is known that laboratory adaptation selects for increased pre-adult development time, increased early fecundity, decreased late fecundity and curtailed lifespan (Sgrò and Partridge, 2000; Swindell and Bouzat, 2006a). For C. elegans, an advantage is that inbreeding depression is less likely to occur when a wild-derived strain is initially cultured in the laboratory, since the species is hermaphroditic and mostly self-fertilizing in the wild (Johnson and Hutchinson, 1993). In principle, moreover, C. elegans cultures can be frozen to prevent mutation accumulation under relaxed selection in the lab; although, since its establishment in 1956, the N2 strain was continually passaged in axenic for many years prior to freezing by any laboratory (Nicholas et al., 1959; Johnson and Hutchinson, 1993), and mutation accumulation does appear to have diminished lifespan for at least some N2 sub-lineages (Gems and Riddle, 2000).
One study has evaluated the effects of DR (by bacterial deprivation) on the C. elegans N2 strain, five “wild-derived” strains (JU262, MY2, PS2025, PX176 and PX178), and females of C. remanei strains (EM464 and SB146) (Sutphin and Kaeberlein, 2008). Interestingly, the N2 strain, all five of the wild-derived strains, and the EM464 C. remanei strain (females) did exhibit increased lifespan with bacterial deprivation; in contrast, the SB146 C. remanei strain (females) did not exhibit significantly increased mean lifespan with bacterial deprivation (although maximum lifespan was increased). In these experiments, wild-derived strains were not directly isolated from the wild, but had been passaged in the laboratory for an unknown, though presumably limited, number of generations (Sutphin and Kaeberlein, 2008). The exact number of generations, however, could impact results from such a study, since most genetic variation within the wild-derived isolate may be depleted (non-randomly) during the initial generations of laboratory cultivation. Additionally, it is unclear whether other C. elegans DR protocols would have similar effects on the lifespan of wild-derived strains. Potentially, for instance, laboratory adaptation could involve modulation of the insulin-FOXO pathway. This may not influence DR responses to bacterial deprivation, which is independent of insulin-FOXO, but may influence DR responses to other forms of DR that are insulin-FOXO dependent (e.g., dilution of peptone and serial dilution of the bacterial food source) (Greer and Brunet, 2009).
9. Conclusions and Future Directions
Dietary restriction (DR) has been advanced as an intervention that reliably increases median and maximum lifespan in rodents, and it is believed that these effects are mediated by conserved pathways closely tied to basic aging mechanisms (Weindruch and Walford, 1988; Cantó and Auwerx, 2009; Anderson and Weindruch, 2010; Omodei and Fontana, 2011; Xiang and He, 2011). In rats, the literature is dominated by studies of the male sex only, but the middle 50% of experiments support a 14 – 45% increase in median lifespan with DR (Table 1). In mice, however, effects of DR have been less robust, with nearly all studies reporting weak or negative effects of DR on lifespan having used mice rather than rats (Fernandes et al., 1976a; 1976b; Cheney et al., 1980; Harrison and Archer, 1987; Forster et al., 2003; Harper et al., 2006a; Liao et al., 2010a; Rikke et al., 2010). The middle 50% of mouse studies support a 4 – 27% lengthening of median lifespan with DR, although this range drops considerably if results from ILSXISS strains are considered (8% decrease – 20% increase in median lifespan; Table 2) (Liao et al., 2010a; Rikke et al., 2010). Notably, in both rats and mice, most studies have evaluated inbred genotypes, which may have been a factor mitigating favorable effects of DR in many experiments (Figure 2). These results show that our understanding of DR and its effects, both in terms of its magnitude and consistency, still remain a developing picture. An implication of these findings is that connections between DR and basic aging mechanisms may lack a universal character, and that greater attention to species- and genotype-specific effects will be valuable for developing a nuanced model of how DR impacts aging and longevity.
This review identified a number of rodent experiments in which DR failed to improve median and/or maximum lifespan, but it is challenging to discern genotype-dependent effects from these cases. B6 mice provide an illustrative example. There were 21 experiments that yielded estimates for the effect of DR on median lifespan, but study outcomes have ranged from a 32% lifespan decrease to a 27% increase (Supplemental Table 3). It is likely that any other genotype would exhibit a similar range of DR responses if repeatedly evaluated to the same degree, suggesting that confirmation of results through independent experiments should be viewed as an important criterion in weighing evidence for genotype-dependent effects of DR on lifespan. Given this standard, three observations appear to be well-supported. First, in DBA/2 males, any positive effects of DR on lifespan are at least attenuated relative to males of other genotypes (Silberberg et al. 1962b; Bronson and Lipman, 1991; Turturro et al., 1999; Forster et al., 2003). Second, the mouse ILSXISS strain 114 provides an example of a genotype for which lifespan is negatively affected by 40% DR, since a significant lifespan decrease has been observed in all trials, including separate studies conducted at UTHSCSA and UCB (Liao et al., 2010a; Rikke et al., 2010) (Figure 5). Third, there are 15 other ILSXISS strains for which significant and positive effects of DR on lifespan have not been observed in each of four experiments (see Figure 5), including both sexes and studies conducted at UTHSCSA and UCB (Liao et al., 2010a; Rikke et al., 2010). It is interesting to note that each of these is an inbred rather than non-inbred mouse genotype. At present, no non-inbred genotype has been identified that, in multiple studies, has failed to exhibit greater longevity with a DR diet (but see Harper et al., 2006a).
There also remain several backgrounds for which DR has failed to improve lifespan in a single experiment, or for which the effects of DR on lifespan differ among published studies (e.g., white rats, C3H mice, B6 mice and wild-derived mice) (McCay et al., 1935; Cheney et al., 1980; Harrison and Archer, 1987). In these cases, it is either already established, or cannot yet be ruled out, that effects of DR are protocol-dependent, and will be sensitive to the age at which DR is initiated, whether calories are restricted gradually over 3-4 weeks or immediately upon weaning, composition of the basal diet, pathogen controls, individual versus group housing, litter sizes of experimental and control animals, or potentially other factors not often controlled in survivorship studies. B6 mice, for instance, are susceptible to atherosclerosis, and positive effects of DR appear strongest when the basal diet is high in fat (Turturro et al., 1999). C3H mice, on the other hand, may represent a strain with poor “fuel efficiency” and limited ability to convert food resources into growth and body mass (Rikke et al., 2010), leading to negative energy balance with low-calorie diets (Sohal et al., 2009), thus rendering the strain prone to shortened lifespan under 40% DR protocols (Fernandes et al., 1976a; Yoshida et al., 1997). Potentially, however, a less intense protocol of 20% DR enforced 1-2 months after weaning could bolster positive effects of DR in C3H mice. This may also be the case with respect to DBA/2 and wild- derived mice, as well as some ILSXISS strains, for which effects of DR on lifespan have only been evaluated using 40% DR protocols.
It is often expected that DR will increase lifespan by 30 – 50% in rodents, but this appears more realistic for rats rather than mice (Table 1). In rats, the effects of DR have been more consistent, with few studies having obtained negative results or only weak effects of DR (Figures 1 and 3). These robust DR responses seem surprising, particularly since nearly all studies have evaluated inbred genotypes (Supplemental Data File 1). Compared to mice, however, effects of DR have been evaluated in only a small number of rat genotypes. Among all DR studies, for instance, only 11 rat genotypes have been evaluated, with 36 of 53 experiments having used Fisher 344, Sprague Dawley, or Wistar rats (Supplemental Table 1). In contrast, among mouse DR studies, 27 distinct genotypes have been evaluated in addition to 54 ILSXISS strains (Supplemental Table 2). In mice, therefore, the broader range of genotypes could provide a more representative estimate of the effects of DR on lifespan, and potentially, the rat-mouse disparity will shift if and when more rat genotypes are studied in future work. It is also possible that metabolic differences exist between rats and mice, such that rats should be expected to respond more favorably to DR. Under ad lib conditions, for instance, the Fisher 344, Sprague Dawley, and Wistar rats appear more susceptible to obesity as compared to many mouse strains (Weindruch, 1996). Potentially, therefore, the standard rat genotypes (e.g., Fisher 344, Sprague Dawley and Wistar) may better resemble those mouse strains with a “fuel efficient” metabolism, which are best able to maintain positive energy balance and more likely to exhibit favorable responses to DR (Sohal et al., 2009; Rikke et al., 2010). Alternatively, such differences may be related to the larger size of rats relative to cage dimensions, which would limit activity levels and thus contribute to obesity with ad lib feeding (Weindruch, 1996).
Unfortunately, nearly all rodent studies that have evaluated the effects of DR on lifespan are complicated by the consequences of laboratory breeding. On the one hand, inbred strains are less vigorous due to inbreeding depression and/or mutation accumulation, and this appears to weaken positive effects of DR on lifespan (Figure 2). This issue is addressed by the use of F1 or F2 hybrid strains, but results from non-inbred hybrids are still complicated by the effects of laboratory adaptation, as suggested by the failure of DR to improve mean lifespan in wild-derived mice (Harper et al., 2006a). To avoid all possible artifacts, therefore, including the effects of inbreeding depression, mutation accumulation and laboratory adaptation, the most promising direction for future work is greater focus on wild-derived mice recently introduced into the laboratory (Austand and Kristan, 2003; Harper et al., 2006a; Harper, 2008), as well as wild-derived rats (for which effects of DR on lifespan are currently unknown). The main disadvantage of wild-derived rodent colonies is difficulty associated with their establishment and their lack of genetic stability (Harper, 2008). For this reason, wild-derived inbred strains could provide a more feasible option for some laboratories, or better still, F1 hybrids formed by the crossing of wild-derived inbred strains. One interesting example is the WSB/EiJ strain, which has been inbred in the laboratory for more than 80 generations, but is among the longest-lived of all inbred strains with extremely low IGF-1 levels (Yuan et al., 2009). Compared to standard lab strains, wild-derived WSB mice also have a healthy lean metabolic phenotype with low body fat, striking resistance to obesity, low fasting insulin and glucose levels, and high insulin sensitivity and glucose tolerance (Lee et al., 2011).
This line of investigation will have implications for the translation of findings from rodents to primate species, including humans. Given the diversity of rodent genotypes available to the research community, some genotypes may provide more suitable models for primate species, while others may prove to be less appropriate. IGF-1, for instance, is a key endocrine factor that regulates aging trajectory and lifespan in rodents, which is also suppressed by DR in at least some rodent strains (Breese et al., 1991; Dunn et al., 1997; Sonntag et al., 1999). However, in both humans and rhesus monkeys, long-term DR may not attenuate IGF-1 levels as observed in rodents (Ramsey et al., 2000; Fontana et al., 2008). Given that circulating IGF-1 levels differ 2-to 3-fold across 31 inbred mouse strains (Yuan et al., 2009), some strains may better recapitulate the primate IGF-1 response to DR. In a similar way, certain genetic backgrounds may provide better models for understanding how DR influences inflammation, body composition or cardiovascular function in humans and monkeys. Potentially, such backgrounds will be identified by further exploring the genetic and phenotypic diversity across rodent strains. This can lead to stronger rodent models of the human DR response, which can in turn improve translation of findings from rodents to the historically outbred and genetically heterogeneous primate species.
Supplementary Material
Supplemental Table 1. Summary survivorship statistics for rat laboratory experiments comparing DR-fed to control-fed cohorts (1935 – present). This table lists summary statistics and references for rat survivorship experiments considered in Figures 1 – 3.
Supplemental Table 2. Summary survivorship statistics for mouse laboratory experiments comparing DR-fed to control-fed cohorts (1934 – present). This table lists summary statistics and references for rat survivorship experiments considered in Figures 1, 2, 3 and 6.
Supplemental Table 3. Summary survivorship statistics for 26 experiments that have evaluated the effects of DR in C57BL/6 mice (1961 – present). This table lists summary statistics and references for each experiment in which C57BL/6 mice have been evaluated. Most experiments included in this table are also included in Supplemental Table 2. Details on DR protocols followed in each experiment are provided as footnotes.
Supplemental Table 4. Summary survivorship statistics for 11 experiments that have evaluated the effects of DR in DBA/2 mice (1962 – present). This table lists summary statistics and references for each experiment in which DBA/2 mice have been evaluated. Most experiments included in this table are also included in Supplemental Table 2. Details on DR protocols followed in each experiment are provided as footnotes.
Research Highlights.
▶ Meta-analysis: DR typically increases lifespan by 14 – 45% in rats and 4 – 27% in mice.
▶ Inbred mice have shown less favorable responses to DR than non-inbred mice.
▶ DBA/2 males and ILSXISS strain 114: DR has weak or negative effects on lifespan.
▶ Wild-derived mice: Limited DR response suggests a role of laboratory breeding.
▶ Translation to primates may require expanding the set of well-studied rodent genotypes.
Acknowledgements
This work was supported by NIH grant R01-DK088718-01. The author thanks Dr. Brad Rikke (University of Colorado) and Dr. Tom Johnson (University of Colorado) for providing the raw survival time data used to construct Figure 5. Additionally, this manuscript benefited from the constructive critique and suggestions of five anonymous reviewers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab. 2010;21:134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp. Gerontol. 1989;24:83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Austad SN. Does caloric restriction in the laboratory simply prevent overfeeding and return house mice to their natural level of food intake? Sci. Aging Knowledge Environ. 2001:pe3. doi: 10.1126/sageke.2001.6.pe3. 2001. [DOI] [PubMed] [Google Scholar]
- Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–207. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Bartke A. Insulin and aging. Cell Cycle. 2008;7:3338–3343. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- Beck J. The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia. 2007;151:741–747. doi: 10.1007/s00442-006-0613-y. [DOI] [PubMed] [Google Scholar]
- Bennett B, Carosone-Link P, Zahniser NR, Johnson TE. Confirmation and fine mapping of ethanol sensitivity quantitative trait loci, and candidate gene testing in the LXS recombinant inbred mice. J. Pharmacol. Exp. Ther. 2006;319:299–307. doi: 10.1124/jpet.106.103572. [DOI] [PubMed] [Google Scholar]
- Black BJ, Jr., McMahan CA, Masoro EJ, Ikeno Y, Katz MS. Senescent terminal weight loss in the male F344 rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R336–R342. doi: 10.1152/ajpregu.00640.2001. [DOI] [PubMed] [Google Scholar]
- Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J. Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Energy allocation and reproductive development in wild and domestic house mice. Biol. Reprod. 1984;31:83–88. doi: 10.1095/biolreprod31.1.83. [DOI] [PubMed] [Google Scholar]
- Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth. Dev. Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Harshman L, Zhang Y, Müller HG, Partridge L, Wang JL. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Cerqueira FM, Kowaltowski AJ. Commonly adopted caloric restriction protocols often involve malnutrition. Ageing Res. Rev. 2010;9:424–430. doi: 10.1016/j.arr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm. Genome. 2008;19:318–331. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- Cheney KE, Liu RK, Smith GS, Leung RE, Mickey MR, Walford RL. Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp. Gerontol. 1980;15:237–258. doi: 10.1016/0531-5565(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Clinthorne JF, Adams DJ, Fenton JI, Ritz BW, Gardner EM. Short-term re-feeding of previously energy-restricted C57BL/6 male mice restores body weight and body fat and attenuates the decline in natural killer cell function after primary influenza infection. J. Nutr. 2010;140:1495–1501. doi: 10.3945/jn.110.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan FM, Hoffmann AA. Genetic divergence under uniform selection. II. Different responses to selection for knockdown resistance to ethanol among Drosophila melanogaster populations and their replicate lines. Genetics. 1986;114:145–164. doi: 10.1093/genetics/114.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Min YI. Publication bias: the problem that won't go away. Ann. N. Y. Acad. Sci. 1993;703:135–146. doi: 10.1111/j.1749-6632.1993.tb26343.x. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp. Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech. Ageing Dev. 2007;128:539–545. doi: 10.1016/j.mad.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature. 1976a;263:504–507. doi: 10.1038/263504b0. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc. Natl. Acad. Sci. U S A. 1976b;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CW, Reed DH. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution. 2011;65:246–258. doi: 10.1111/j.1558-5646.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:688–694. doi: 10.1093/gerona/60.6.688. [DOI] [PubMed] [Google Scholar]
- Gems D, Riddle DL. Defining wild-type life span in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B215–B219. doi: 10.1093/gerona/55.5.b215. [DOI] [PubMed] [Google Scholar]
- Gong Y, Thompson JN, Jr., Woodruff RC. Effect of deleterious mutations on life span in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1246–1252. doi: 10.1093/gerona/61.12.1246. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–241. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM. Wild-derived mouse stocks: an underappreciated tool for aging research. Age (Dordr) 2008;30:135–145. doi: 10.1007/s11357-008-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Durkee SJ, Dysko RC, Austad SN, Miller RA. Genetic modulation of hormone levels and life span in hybrids between laboratory and wild-derived mice. J. Gerontol. A Biol. Sci. Med. Sci. 2006b;61:1019–1029. doi: 10.1093/gerona/61.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Durkee SJ, Smith-Wheelock M, Miller RA. Hyperglycemia, impaired glucose tolerance and elevated glycated hemoglobin levels in a long-lived mouse stock. Exp. Gerontol. 2005;40:303–314. doi: 10.1016/j.exger.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006a;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr. 1987;117:376–382. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc. Natl. Acad. Sci. U S A. 1984;81:1835–1838. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Dietary restriction: theory fails to satiate. Science. 2010;329:1014–1015. doi: 10.1126/science.329.5995.1014. [DOI] [PubMed] [Google Scholar]
- Hempenstall S, Picchio L, Mitchell SE, Speakman JR, Selman C. The impact of acute caloric restriction on the metabolic phenotype in male C57BL/6 and DBA/2 mice. Mech. Ageing Dev. 2010;131:111–118. doi: 10.1016/j.mad.2009.12.008. [DOI] [PubMed] [Google Scholar]
- High KP, Prasad R, Marion CR, Schurig GG, Boyle SM, Sriranganathan N. Outcome and immune responses after Brucella abortus infection in young adult and aged mice. Biogerontology. 2007;8:583–593. doi: 10.1007/s10522-007-9106-6. [DOI] [PubMed] [Google Scholar]
- Hoffman AA, Cohan FM. Genetic divergence under uniform selection. III. Selection for knockdown resistance to ethanol in Drosophila pseudoobscura populations and their replicate lines. Heredity. 1987;58:425–433. doi: 10.1038/hdy.1987.71. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp. Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KA, Charlesworth B. A genetic analysis of senescence in Drosophila. Nature. 1994;367:64–66. doi: 10.1038/367064a0. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Jiao S, Cole TG, Kitchens RT, Pfleger B, Schonfeld G. Genetic heterogeneity of lipoproteins in inbred strains of mice: analysis by gel-permeation chromatography. Metabolism. 1990;39:155–160. doi: 10.1016/0026-0495(90)90069-o. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Hutchinson EW. Absence of strong heterosis for life span and other life history traits in Caenorhabditis elegans. Genetics. 1993;134:465–474. doi: 10.1093/genetics/134.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner-Matos J, Upadhyay A, Salomon MP, Grigaltchik V, Baer CF. Genetic (Co)variation for life span in rhabditid nematodes: role of mutation, selection, and history. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1134–1145. doi: 10.1093/gerona/glp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Kasumovic MM, Brooks RC, Andrade MC. Body condition but not dietary restriction prolongs lifespan in a semelparous capital breeder. Biol. Lett. 2009;5:636–638. doi: 10.1098/rsbl.2009.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KL. Dietary restriction and aging: comparative tests of evolutionary hypotheses. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B123–B129. doi: 10.1093/gerona/56.3.b123. [DOI] [PubMed] [Google Scholar]
- Kristan DM. Calorie restriction and susceptibility to intact pathogens. Age (Dordr) 2008;30:147–156. doi: 10.1007/s11357-008-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E, Minois N. Failure to confirm increased longevity in Drosophila melanogaster submitted to a food restriction procedure. J. Gerontol. A Biol. Sci. Med. Sci. 1996;51:B280–B283. doi: 10.1093/gerona/51a.4.b280. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Minois N. Does dietary restriction really increase longevity in Drosophila melanogaster? Ageing Res. Rev. 2005;4:409–421. doi: 10.1016/j.arr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Lee KT, Karunakaran S, Ho MM, Clee SM. PWD/PhJ and WSB/EiJ Mice Are Resistant to Diet-Induced Obesity But Have Abnormal Insulin Secretion. Endocrinology. 2011;152:3005–3017. doi: 10.1210/en.2011-0060. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Visscher MB, King JT. Life span and causes of death in inbred mice in relation to diet. J. Gerontol. 1956;11:364–371. doi: 10.1093/geronj/11.4.364. [DOI] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, Wolkow CA, de Cabo R, Ingram DK, Zou S. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010a;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. No evidence that competition for food underlies lifespan shortening by dietary restriction in multiply housed mice: response to commentary. Aging Cell. 2010b;9:450–452. [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi: 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lipman RD. Effect of calorie restriction on mortality kinetics in inbred strains of mice following 7,12-dimethylbenz[a]anthracene treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B153–B157. doi: 10.1093/gerona/57.4.b153. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc. Natl. Acad. Sci. U S A. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Kouguchi H, Oku Y, Yagi K. Primary alveolar echinococcosis: course of larval development and antibody responses in intermediate host rodents with different genetic backgrounds after oral infection with eggs of Echinococcus multilocularis. Parasitol. Int. 2010;59:435–444. doi: 10.1016/j.parint.2010.06.003. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life and upon the ulimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McCay CM, Maynard LA, Sperling G, Barnes LL. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. J. Nutr. 1939;18:1–13. doi: 10.1111/j.1753-4887.1975.tb05227.x. [DOI] [PubMed] [Google Scholar]
- McCay CM, Sperling G, Barnes LL. Growth, Ageing, chronic diseases and life span in rats. Arch. Biochem. Biophys. 1943;2:469. [Google Scholar]
- Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Exp. Biol. Med. (Maywood) 2002;227:500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Boggs CL, Carey JR, Arlet ME. Does dietary restriction reduce life span in male fruit-feeding butterflies? Exp. Gerontol. 2009;44:601–606. doi: 10.1016/j.exger.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas WL, Dougherty EC, Hansen EL. Axenic cultivation of Caenorhabditis briggsae (Nematoda: Rhabditidae) with chemically undefined supplements: comparative studies with related nematodes. Ann. N.Y. Acad. Sci. 1959;77:218–236. [Google Scholar]
- Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585:1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB, Ferry EL. The effect of retardation of growth upon the breeding period and the duration of life of rats. Science. 1917;45:294–295. doi: 10.1126/science.45.1160.294. [DOI] [PubMed] [Google Scholar]
- Paigen B. Genetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am. J. Clin. Nutr. 1995;62:458S–462S. doi: 10.1093/ajcn/62.2.458S. [DOI] [PubMed] [Google Scholar]
- Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990;10:316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Park H-W. Longevity, aging and caloric restriction: Clive Maine McCay and the construction of a multidisciplinary research program. Hist. Stud. Nat. Sci. 2010;40:79–124. doi: 10.1525/hsns.2010.40.1.79. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech. Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- Promislow DE, Tatar M. Mutation and senescence: where genetics and demography meet. Genetica. 1998;102-103:299–314. [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp. Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Rao GN, Haseman JK, Grumbein S, Crawford DD, Eustis SL. Growth, body weight, survival, and tumor trends in F344/N rats during an eleven-year period. Toxicol. Pathol. 1990;18:61–70. doi: 10.1177/019262339001800109. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain. Res. 2007;1127:10–18. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Association between life-span extension by caloric restriction and thiol redox state in two different strains of mice. Free Radic. Biol. Med. 2011;51:225–233. doi: 10.1016/j.freeradbiomed.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DH, Bryant EH. The evolution of senescence under curtailed life span in laboratory populations of Musca domestica (the housefly). Heredity. 2000;85:115–121. doi: 10.1046/j.1365-2540.2000.00737.x. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Battaglia ME, Allison DB, Johnson TE. Murine weight loss exhibits significant genetic variation during dietary restriction. Physiol. Genomics. 2006;27:122–130. doi: 10.1152/physiolgenomics.00068.2006. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Johnson TE. Physiological genetics of dietary restriction: uncoupling the body temperature and body weight responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1522–R1527. doi: 10.1152/ajpregu.00215.2007. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Liao CY, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp. Gerontol. 2010;45:691–701. doi: 10.1016/j.exger.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke BA, Yerg JE, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Strain variation in the response of body temperature to dietary restriction. Mech. Ageing Dev. 2003;124:663–678. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Yerg JE, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Quantitative trait Loci specifying the response of body temperature to dietary restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:118–125. doi: 10.1093/gerona/59.2.b118. [DOI] [PubMed] [Google Scholar]
- Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J. Nutr. 2008;138:2269–2275. doi: 10.3945/jn.108.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MH. Length of life and nutrition in the rat. J. Nutr. 1961;75:197–210. doi: 10.1093/jn/75.2.197. [DOI] [PubMed] [Google Scholar]
- Sgrò CM, Partridge L. Evolutionary responses of the life history of wild-caught Drosophila melanogaster to two standard methods of laboratory culture. Am. Nat. 2000;156:341–353. [Google Scholar]
- Shabalina SA, Yampolsky L. Yu, Kondrashov AS. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Natl. Acad. Sci. U S A. 1997;94:13034–13039. doi: 10.1073/pnas.94.24.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley KR, Witmer D, Burgess-Herbert SL, Paigen B, Churchill GA. Effects of atherogenic diet on hepatic gene expression across mouse strains. Physiol. Genomics. 2009;39:172–182. doi: 10.1152/physiolgenomics.90350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg R, Jarrett SR, Silberberg M. Longevity of female mice kept on various dietary regimens during growth. J. Gerontol. 1962a;17:239–244. doi: 10.1093/geronj/17.3.239. [DOI] [PubMed] [Google Scholar]
- Silberberg R, Silberberg M, Jarrett SR. Effects of diet during growth. Studies in male mice of various strains. Pathol. Microbiol. (Basel) 1962b;25:56–66. [PubMed] [Google Scholar]
- Sohal RS, Ferguson M, Sohal BH, Forster MJ. Life span extension in mice by food restriction depends on an energy imbalance. J. Nutr. 2009;139:533–539. doi: 10.3945/jn.108.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J. Anat. 2000;197:575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin GL, Kaeberlein M. Dietary restriction by bacterial deprivation increases life span in wild-derived nematodes. Exp. Gerontol. 2008;43:130–135. doi: 10.1016/j.exger.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J. Appl. Physiol. 2007;102:2369–2378. doi: 10.1152/japplphysiol.01077.2006. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Accelerated failure time models provide a useful statistical framework for aging research. Exp. Gerontol. 2009;44:190–200. doi: 10.1016/j.exger.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Bouzat JL. Inbreeding depression and male survivorship in Drosophila: implications for senescence theory. Genetics. 2006a;172:317–327. doi: 10.1534/genetics.105.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Bouzat JL. Selection and inbreeding depression: effects of inbreeding rate and inbreeding environment. Evolution. 2006b;60:1014–1022. [PubMed] [Google Scholar]
- Swindell WR, Johnston A, Gudjonsson JE. Transcriptional profiles of leukocyte populations provide a tool for interpreting gene expression patterns associated with high fat diet in mice. PLoS ONE. 2010;5:e11861. doi: 10.1371/journal.pone.0011861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- Turturro A, Duffy P, Hart R, Allaben WT. Rationale for the use of dietary control in toxicity studies--B6C3F1 mouse. Toxicol. Pathol. 1996;24:769–775. doi: 10.1177/019262339602400621. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Vannier E, Borggraefe I, Telford SR, 3rd, Menon S, Brauns T, Spielman A, Gelfand JA, Wortis HH. Age-associated decline in resistance to Babesia microti is genetically determined. J. Infect. Dis. 2004;189:1721–1728. doi: 10.1086/382965. [DOI] [PubMed] [Google Scholar]
- Vassilieva LL, Hook AM, Lynch M. The fitness effects of spontaneous mutations in Caenorhabditis elegans. Evolution. 2000;54:1234–1246. doi: 10.1111/j.0014-3820.2000.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Vermeulen CJ, Bijlsma R. Changes in mortality patterns and temperature dependence of lifespan in Drosophila melanogaster caused by inbreeding. Heredity. 2004;92:275–281. doi: 10.1038/sj.hdy.6800412. [DOI] [PubMed] [Google Scholar]
- Wanagat J, Allison DB, Weindruch R. Caloric intake and aging: mechanisms in rodents and a study in nonhuman primates. Toxicol. Sci. 1999;52:35–40. doi: 10.1093/toxsci/52.2.35. [DOI] [PubMed] [Google Scholar]
- Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol. Pathol. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The retardation of aging and disease by dietary restriction. Charles C. Thomas; Springfield, Illinois: 1988. [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Bennett B, Lu L, Gu J, DeFries JC, Carosone-Link PJ, Rikke BA, Belknap JK, Johnson TE. Genetic structure of the LXS panel of recombinant inbred mouse strains: a powerful resource for complex trait analysis. Mamm. Genome. 2004;15:637–647. doi: 10.1007/s00335-004-2380-6. [DOI] [PubMed] [Google Scholar]
- Xiang L, He G. Caloric restriction and antiaging effects. Ann. Nutr. Metab. 2011;58:42–48. doi: 10.1159/000323748. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Inoue T, Nojima K, Hirabayashi Y, Sado T. Calorie restriction reduces the incidence of myeloid leukemia induced by a single whole-body radiation in C3H/He mice. Proc. Natl. Acad. Sci. U S A. 1997;94:2615–2619. doi: 10.1073/pnas.94.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Summary survivorship statistics for rat laboratory experiments comparing DR-fed to control-fed cohorts (1935 – present). This table lists summary statistics and references for rat survivorship experiments considered in Figures 1 – 3.
Supplemental Table 2. Summary survivorship statistics for mouse laboratory experiments comparing DR-fed to control-fed cohorts (1934 – present). This table lists summary statistics and references for rat survivorship experiments considered in Figures 1, 2, 3 and 6.
Supplemental Table 3. Summary survivorship statistics for 26 experiments that have evaluated the effects of DR in C57BL/6 mice (1961 – present). This table lists summary statistics and references for each experiment in which C57BL/6 mice have been evaluated. Most experiments included in this table are also included in Supplemental Table 2. Details on DR protocols followed in each experiment are provided as footnotes.
Supplemental Table 4. Summary survivorship statistics for 11 experiments that have evaluated the effects of DR in DBA/2 mice (1962 – present). This table lists summary statistics and references for each experiment in which DBA/2 mice have been evaluated. Most experiments included in this table are also included in Supplemental Table 2. Details on DR protocols followed in each experiment are provided as footnotes.