Abstract
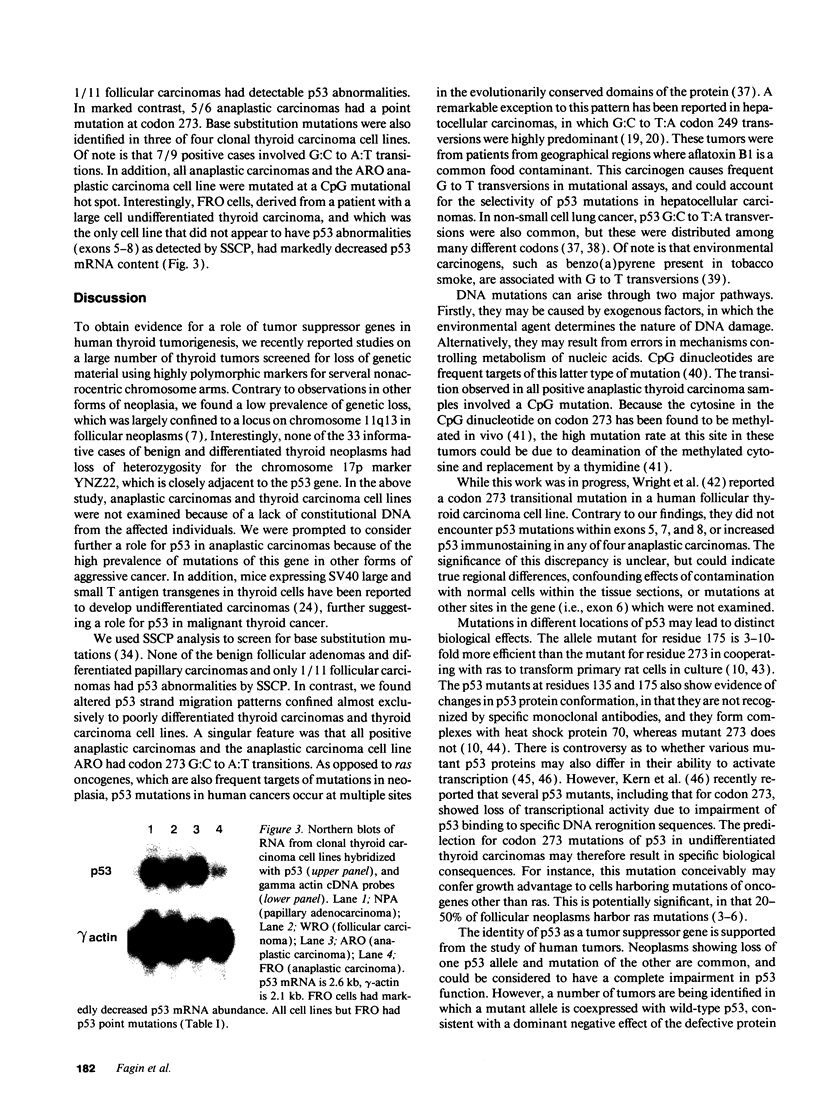
The development and progression of thyroid tumors is signaled by phenotype-specific mutations of genes involved in growth control. Molecular events associated with undifferentiated thyroid cancer are not known. We examined normal, benign, and malignant thyroid tissue for structural abnormalities of the p53 tumor suppressor gene. Mutations were detected by single-strand conformation polymorphisms of PCR-amplified DNA, using primers bracketing the known hot spots on either exons 5, 6, 7, or 8. The prevalence of mutations was as follows: normal thyroid 0/6; follicular adenomas 0/31; papillary carcinomas 0/37; medullary carcinomas 0/2; follicular carcinomas 1/11; anaplastic carcinomas 5/6; thyroid carcinoma cell lines 3/4. Positive cases were confirmed by direct sequencing of the PCR products. All five anaplastic carcinoma tissues and the anaplastic carcinoma cell line ARO had G:C to A:T transitions leading to an Arg to His substitution at codon 273. In both tumors and cell lines, examples of heterozygous and homozygous p53 mutations were identified. The only thyroid carcinoma cell line in which p53 mutations were not detected in exons 5-8 had markedly decreased p53 mRNA levels, suggesting the presence of a structural abnormality of either p53 itself or of some factor controlling its expression. The presence of p53 mutations almost exclusively in poorly differentiated thyroid tumors and thyroid cancer cell lines suggests that inactivation of p53 may confer these neoplasms with aggressive properties, and further loss of differentiated function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991 Apr 4;350(6317):429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- Chiba I., Takahashi T., Nau M. M., D'Amico D., Curiel D. T., Mitsudomi T., Buchhagen D. L., Carbone D., Piantadosi S., Koga H. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Davidoff A. M., Kerns B. J., Iglehart J. D., Marks J. R. Maintenance of p53 alterations throughout breast cancer progression. Cancer Res. 1991 May 15;51(10):2605–2610. [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estour B., Van Herle A. J., Juillard G. J., Totanes T. L., Sparkes R. S., Giuliano A. E., Klandorf H. Characterization of a human follicular thyroid carcinoma cell line (UCLA RO 82 W-1). Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(3):167–174. doi: 10.1007/BF02899078. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gaidano G., Ballerini P., Gong J. Z., Inghirami G., Neri A., Newcomb E. W., Magrath I. T., Knowles D. M., Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M., Santoro M., Berlingieri M. T., Melillo R. M., Donghi R., Bongarzone I., Pierotti M. A., Della Porta G., Fusco A., Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990 Feb 23;60(4):557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M. A., Hay I. D., Bartelt D. H., Jr, Ritland S. R., Dahl R. J., Grant C. S., Jenkins R. B. Cytogenetic and molecular genetic studies of follicular and papillary thyroid cancers. J Clin Invest. 1991 Nov;88(5):1596–1604. doi: 10.1172/JCI115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hollstein M. C., Metcalf R. A., Welsh J. A., Montesano R., Harris C. C. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9958–9961. doi: 10.1073/pnas.87.24.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Ledent C., Dumont J., Vassart G., Parmentier M. Thyroid adenocarcinomas secondary to tissue-specific expression of simian virus-40 large T-antigen in transgenic mice. Endocrinology. 1991 Sep;129(3):1391–1401. doi: 10.1210/endo-129-3-1391. [DOI] [PubMed] [Google Scholar]
- Lemoine N. R., Mayall E. S., Wyllie F. S., Williams E. D., Goyns M., Stringer B., Wynford-Thomas D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989 Feb;4(2):159–164. [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Marks J. R., Davidoff A. M., Kerns B. J., Humphrey P. A., Pence J. C., Dodge R. K., Clarke-Pearson D. L., Iglehart J. D., Bast R. C., Jr, Berchuck A. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991 Jun 1;51(11):2979–2984. [PubMed] [Google Scholar]
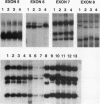
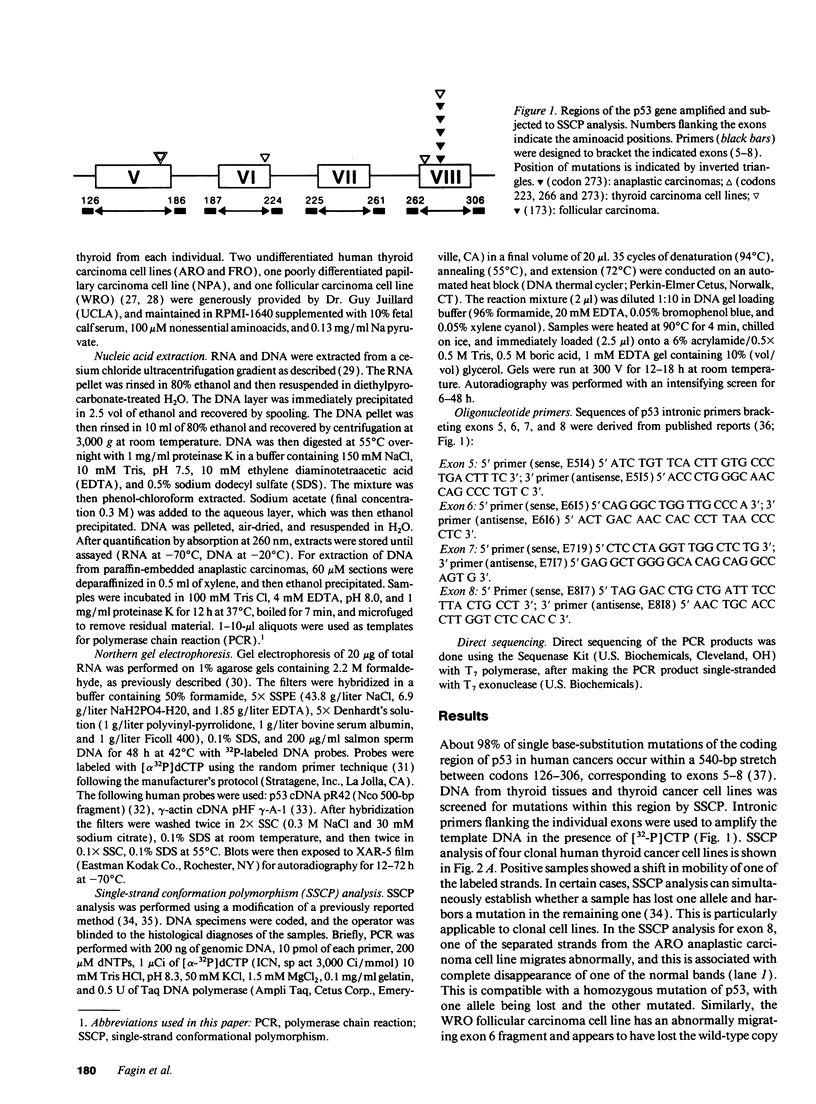
- Matsuo K., Tang S. H., Fagin J. A. Allelotype of human thyroid tumors: loss of chromosome 11q13 sequences in follicular neoplasms. Mol Endocrinol. 1991 Dec;5(12):1873–1879. doi: 10.1210/mend-5-12-1873. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. p53 mutations: gains or losses? J Cell Biochem. 1991 Jan;45(1):22–29. doi: 10.1002/jcb.240450108. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Hayashi K., Sekiya T. Detection of aberrations of the p53 alleles and the gene transcript in human tumor cell lines by single-strand conformation polymorphism analysis. Cancer Res. 1991 Jul 1;51(13):3356–3361. [PubMed] [Google Scholar]
- Namba H., Gutman R. A., Matsuo K., Alvarez A., Fagin J. A. H-ras protooncogene mutations in human thyroid neoplasms. J Clin Endocrinol Metab. 1990 Jul;71(1):223–229. doi: 10.1210/jcem-71-1-223. [DOI] [PubMed] [Google Scholar]
- Namba H., Matsuo K., Fagin J. A. Clonal composition of benign and malignant human thyroid tumors. J Clin Invest. 1990 Jul;86(1):120–125. doi: 10.1172/JCI114673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba H., Rubin S. A., Fagin J. A. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990 Oct;4(10):1474–1479. doi: 10.1210/mend-4-10-1474. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Von Eschenbach A., Tsai Y. C., Jones P., Summerhayes I., Marshall F., Paul M., Green P., Hamilton S. R., Frost P. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991 May 3;252(5006):706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Slingerland J. M., Minden M. D., Benchimol S. Mutation of the p53 gene in human acute myelogenous leukemia. Blood. 1991 Apr 1;77(7):1500–1507. [PubMed] [Google Scholar]
- Suarez H. G., du Villard J. A., Severino M., Caillou B., Schlumberger M., Tubiana M., Parmentier C., Monier R. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990 Apr;5(4):565–570. [PubMed] [Google Scholar]
- Takahashi T., D'Amico D., Chiba I., Buchhagen D. L., Minna J. D. Identification of intronic point mutations as an alternative mechanism for p53 inactivation in lung cancer. J Clin Invest. 1990 Jul;86(1):363–369. doi: 10.1172/JCI114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Wright P. A., Lemoine N. R., Goretzki P. E., Wyllie F. S., Bond J., Hughes C., Röher H. D., Williams E. D., Wynford-Thomas D. Mutation of the p53 gene in a differentiated human thyroid carcinoma cell line, but not in primary thyroid tumours. Oncogene. 1991 Sep;6(9):1693–1697. [PubMed] [Google Scholar]