Abstract
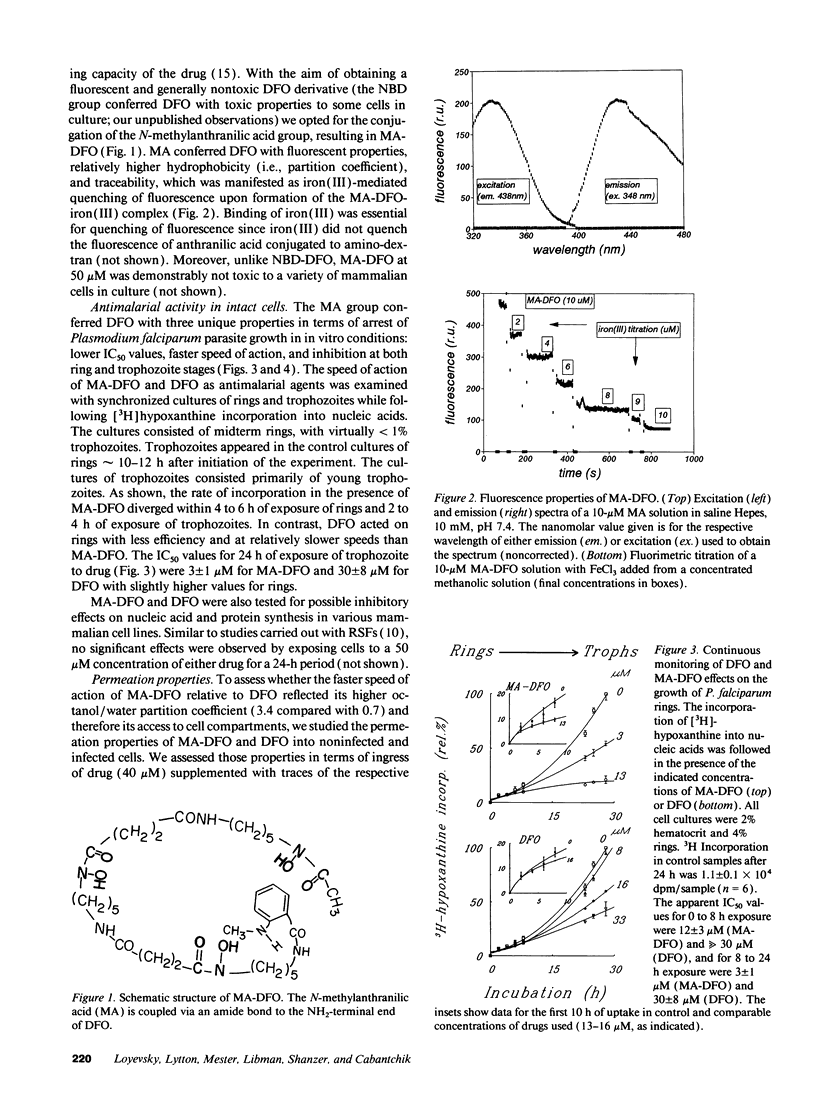
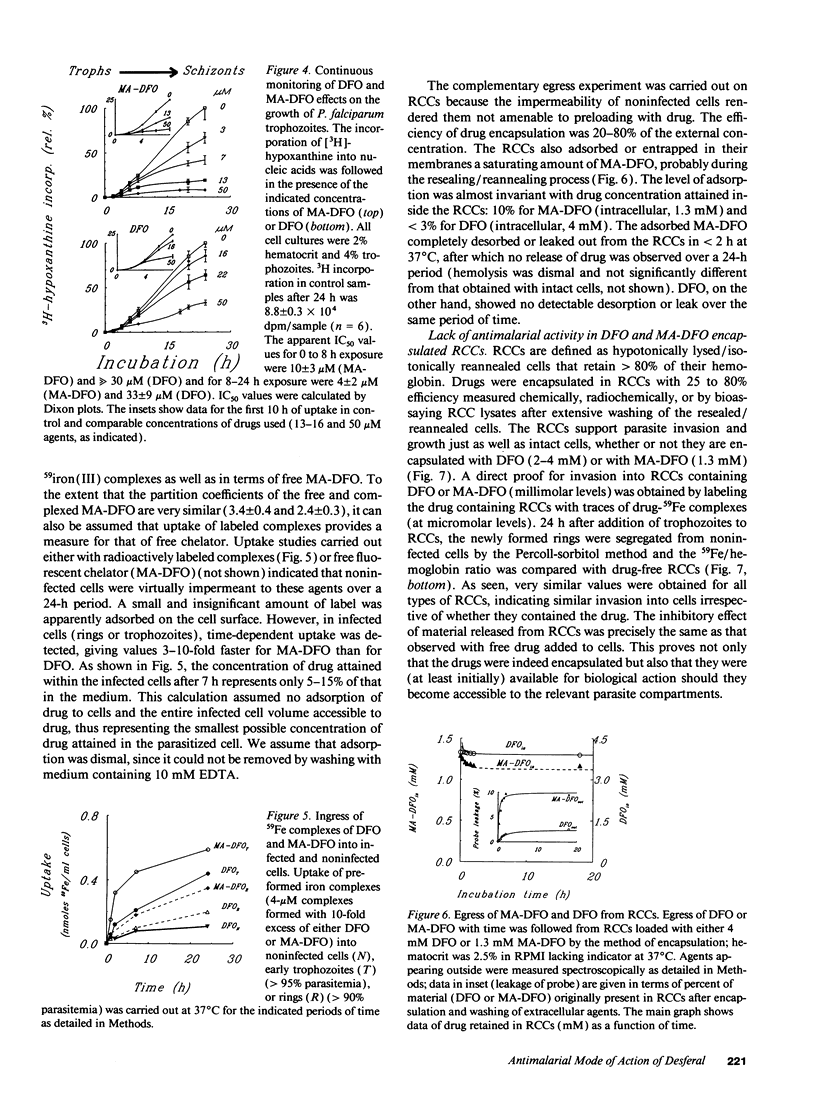
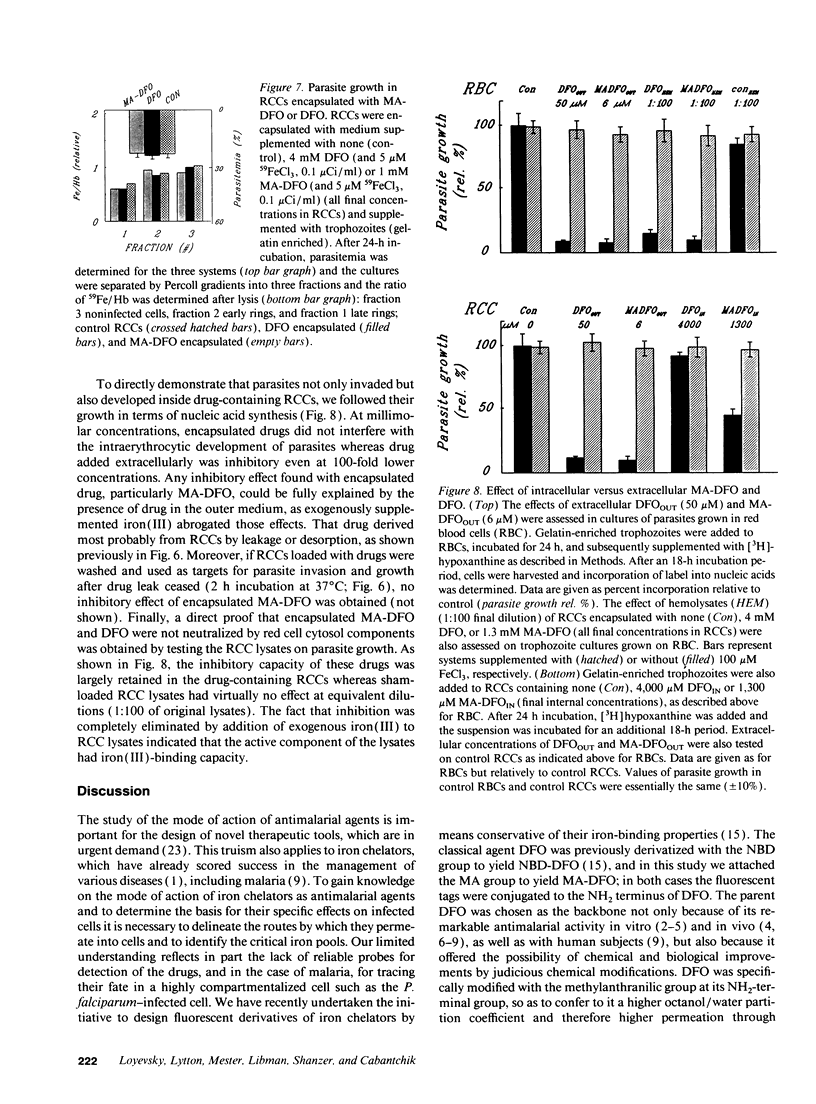
We designed the N-methylanthranilic-desferrioxamine (MA-DFO) as a fluorescent iron (III) chelator with improved membrane permeation properties. Upon binding of iron (III), MA-DFO fluorescence is quenched, thus allowing traceability of drug-iron (III) interactions. MA-DFO is well tolerated by mammalian cells in culture. Its antimalarial activity is pronounced: IC50 values on in vitro (24-h) growth of Plasmodium falciparum were 3 +/- 1 microM for MA-DFO compared with 30 +/- 8 for DFO. The onset of growth inhibition of rings or trophozoites occurs 2-4 h after exposure to 13 microM MA-DFO. This effect is commensurate with MA-DFO permeation into infected cells. In a 24-h exposure to MA-DFO or DFO, trophozoites take up either compound to approximately 10% of the external concentration, rings to 5%, and noninfected cells to < 1%. Red cells encapsulated with millimolar concentrations of DFO or MA-DFO fully support parasite invasion and growth. We conclude that extracellular MA-DFO and DFO gain selective access into parasites by bypassing the host. The rate-limiting step is permeation through the parasite membrane, which MA-DFO accomplishes faster than DFO, in accordance with its higher hydrophobicity. These views are consistent with the proposed duct, which apparently provides parasitized cells with a window to the external medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson C. T., Bayne M. T., Gordeuk V. R., Brittenham G. M., Aikawa M. Stage-specific ultrastructural effects of desferrioxamine on Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1991 Nov;45(5):593–601. doi: 10.4269/ajtmh.1991.45.593. [DOI] [PubMed] [Google Scholar]
- Burns E. R., Pollack S. P. falciparum infected erythrocytes are capable of endocytosis. In Vitro Cell Dev Biol. 1988 May;24(5):481–486. doi: 10.1007/BF02628503. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I. Properties of permeation pathways induced in the human red cell membrane by malaria parasites. Blood Cells. 1990;16(2-3):421–432. [PubMed] [Google Scholar]
- Cabantchik Z. L. Altered membrane transport of malaria-infected erythrocytes: a possible pharmacologic target. Blood. 1989 Oct;74(5):1464–1471. [PubMed] [Google Scholar]
- Fritsch G., Jung A. 14C-desferrioxamine B: uptake into erythrocytes infected with Plasmodium falciparum. Z Parasitenkd. 1986;72(6):709–713. doi: 10.1007/BF00925092. [DOI] [PubMed] [Google Scholar]
- Fritsch G., Treumer J., Spira D. T., Jung A. Plasmodium vinckei: suppression of mouse infections with desferrioxamine B. Exp Parasitol. 1985 Oct;60(2):171–174. doi: 10.1016/0014-4894(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Hershko C., Peto T. E. Deferoxamine inhibition of malaria is independent of host iron status. J Exp Med. 1988 Jul 1;168(1):375–387. doi: 10.1084/jem.168.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko C., Weatherall D. J. Iron-chelating therapy. Crit Rev Clin Lab Sci. 1988;26(4):303–345. doi: 10.3109/10408368809105894. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Kutner S., Breuer W. V., Ginsburg H., Aley S. B., Cabantchik Z. I. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J Cell Physiol. 1985 Dec;125(3):521–527. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- Lederman H. M., Cohen A., Lee J. W., Freedman M. H., Gelfand E. W. Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood. 1984 Sep;64(3):748–753. [PubMed] [Google Scholar]
- Lytton S. D., Cabantchik Z. I., Libman J., Shanzer A. Reversed siderophores as antimalarial agents. II. Selective scavenging of Fe(III) from parasitized erythrocytes by a fluorescent derivative of desferal. Mol Pharmacol. 1991 Oct;40(4):584–590. [PubMed] [Google Scholar]
- Lytton S. D., Mester B., Libman J., Shanzer A., Cabantchik Z. I. Monitoring of iron(III) removal from biological sources using a fluorescent siderophore. Anal Biochem. 1992 Sep;205(2):326–333. doi: 10.1016/0003-2697(92)90443-b. [DOI] [PubMed] [Google Scholar]
- Peto T. E., Hershko C. Iron and infection. Baillieres Clin Haematol. 1989 Apr;2(2):435–458. doi: 10.1016/s0950-3536(89)80026-5. [DOI] [PubMed] [Google Scholar]
- Peto T. E., Thompson J. L. A reappraisal of the effects of iron and desferrioxamine on the growth of Plasmodium falciparum 'in vitro': the unimportance of serum iron. Br J Haematol. 1986 Jun;63(2):273–280. doi: 10.1111/j.1365-2141.1986.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Pollack S., Rossan R. N., Davidson D. E., Escajadillo A. Desferrioxamine suppresses Plasmodium falciparum in Aotus monkeys. Proc Soc Exp Biol Med. 1987 Feb;184(2):162–164. doi: 10.3181/00379727-184-42461. [DOI] [PubMed] [Google Scholar]
- Pouvelle B., Spiegel R., Hsiao L., Howard R. J., Morris R. L., Thomas A. P., Taraschi T. F. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature. 1991 Sep 5;353(6339):73–75. doi: 10.1038/353073a0. [DOI] [PubMed] [Google Scholar]
- Raventos-Suarez C., Pollack S., Nagel R. L. Plasmodium falciparum: inhibition of in vitro growth by desferrioxamine. Am J Trop Med Hyg. 1982 Sep;31(5):919–922. doi: 10.4269/ajtmh.1982.31.919. [DOI] [PubMed] [Google Scholar]
- Rivadeneira E. M., Wasserman M., Espinal C. T. Separation and concentration of schizonts of Plasmodium falciparum by Percoll gradients. J Protozool. 1983 May;30(2):367–370. doi: 10.1111/j.1550-7408.1983.tb02932.x. [DOI] [PubMed] [Google Scholar]
- Scott M. D., Ranz A., Kuypers F. A., Lubin B. H., Meshnick S. R. Parasite uptake of desferroxamine: a prerequisite for antimalarial activity. Br J Haematol. 1990 Aug;75(4):598–602. doi: 10.1111/j.1365-2141.1990.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Shanzer A., Libman J., Lytton S. D., Glickstein H., Cabantchik Z. I. Reversed siderophores act as antimalarial agents. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6585–6589. doi: 10.1073/pnas.88.15.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W., Zidovetzki R. A parasitophorous duct in Plasmodium-infected red blood cells. Parasitol Today. 1992 Jan;8(1):2–3. doi: 10.1016/0169-4758(92)90294-c. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., Pouvelle B., Howard R. J. A parasitophorous duct in Plasmodium-infected red blood cells - reply. Parasitol Today. 1992 Jan;8(1):17–17. doi: 10.1016/0169-4758(92)90304-k. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Traore O., Carnevale P., Kaptue-Noche L., M'Bede J., Desfontaine M., Elion J., Labie D., Nagel R. L. Preliminary report on the use of desferrioxamine in the treatment of Plasmodium falciparum malaria. Am J Hematol. 1991 Jul;37(3):206–208. doi: 10.1002/ajh.2830370316. [DOI] [PubMed] [Google Scholar]
- Whitehead S., Peto T. E. Stage-dependent effect of deferoxamine on growth of Plasmodium falciparum in vitro. Blood. 1990 Sep 15;76(6):1250–1255. [PubMed] [Google Scholar]
- Yayon A., Timberg R., Friedman S., Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J Protozool. 1984 Aug;31(3):367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Yinnon A. M., Theanacho E. N., Grady R. W., Spira D. T., Hershko C. Antimalarial effect of HBED and other phenolic and catecholic iron chelators. Blood. 1989 Nov 1;74(6):2166–2171. [PubMed] [Google Scholar]


