Abstract
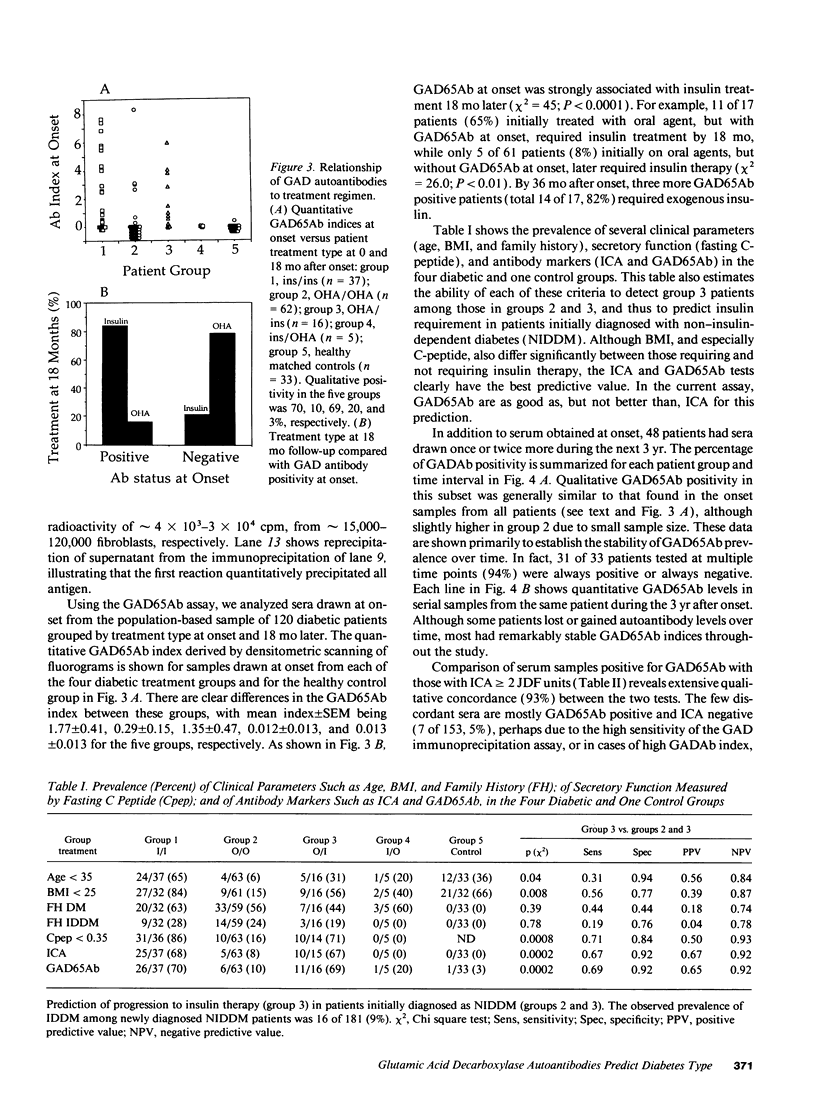
At and before onset, most insulin-dependent diabetics (IDDM) have islet GAD65 autoantibodies (GAD65Ab). Since IDDM also occurs in older patients where non-insulin-dependent diabetes is common, we studied GAD65Ab at onset to classify diabetes type. Our quantitative immunoprecipitation assay uses recombinant human islet GAD65 stably expressed in hamster fibroblasts. Electrophoretic mobility was identical to native islet GAD65. Like native antigen, recombinant GAD65 migrated as two bands during electrophoresis, but converted to one under stronger reduction. Immunoprecipitation was linear with respect to antibody or antigen concentration. In 120 population-based diabetic patients of all ages grouped by treatment at onset and after 18 mo, GAD65Ab were present in 70% on insulin (n = 37), 10% on oral agent (n = 62, P < 0.0001), 69% changing from oral agent to insulin (n = 16, P < 0.001), and 1 of 33 controls. 65% with GAD65Ab, versus 8% without, changed from oral agent to insulin (P < 0.01). The GAD65Ab quantitative index was remarkably stable, and only 2 of 32 patients changed antibody status during follow-up. Concordance between GAD65Ab and islet cell antibodies was 93%. Quantitative correlation was approximate but significant. This highly sensitive, quantitative, high capacity assay for GAD65Ab reveals treatment requirements better than clinical criteria, perhaps guiding immunomodulatory therapy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Pollare T., Lithell H. Different aetiologies of type 2 (non-insulin-dependent) diabetes mellitus in obese and non-obese subjects. Diabetologia. 1991 Jul;34(7):483–487. doi: 10.1007/BF00403284. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K. Autoantibodies in nonobese diabetic mice immunoprecipitate 64,000-Mr islet antigen. Diabetes. 1988 Nov;37(11):1587–1590. doi: 10.2337/diab.37.11.1587. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K., Scharp D. W., Lacy P. E., Riley W. J. 64,000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990 Jun 9;335(8702):1357–1360. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Dyrberg T., Lernmark A. Autoantibodies to a 64-kilodalton islet cell protein precede the onset of spontaneous diabetes in the BB rat. Science. 1984 Jun 22;224(4655):1348–1350. doi: 10.1126/science.6374896. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Landin M., Kristensen J. K., Srikanta S., Bruining G. J., Mandrup-Poulsen T., de Beaufort C., Soeldner J. S., Eisenbarth G., Lindgren F. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest. 1987 Mar;79(3):926–934. doi: 10.1172/JCI112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bu D. F., Erlander M. G., Hitz B. C., Tillakaratne N. J., Kaufman D. L., Wagner-McPherson C. B., Evans G. A., Tobin A. J. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S. J., Mulvihill E., Rao D., Kumar A. A., Lioubin P., Heipel M., Sprecher C., Halfpap L., Prunkard D., Gambee J. Expression of recombinant human plasminogen in mammalian cells is augmented by suppression of plasmin activity. J Biol Chem. 1991 Aug 15;266(23):15286–15292. [PubMed] [Google Scholar]
- Bärmeier H., McCulloch D. K., Neifing J. L., Warnock G., Rajotte R. V., Palmer J. P., Lernmark A. Risk for developing type 1 (insulin-dependent) diabetes mellitus and the presence of islet 64K antibodies. Diabetologia. 1991 Oct;34(10):727–733. doi: 10.1007/BF00401518. [DOI] [PubMed] [Google Scholar]
- Christgau S., Schierbeck H., Aanstoot H. J., Aagaard L., Begley K., Kofod H., Hejnaes K., Baekkeskov S. Pancreatic beta cells express two autoantigenic forms of glutamic acid decarboxylase, a 65-kDa hydrophilic form and a 64-kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem. 1991 Nov 5;266(31):21257–21264. [PubMed] [Google Scholar]
- Christie M. R., Daneman D., Champagne P., Delovitch T. L. Persistence of serum antibodies to 64,000-Mr islet cell protein after onset of type I diabetes. Diabetes. 1990 Jun;39(6):653–656. doi: 10.2337/diab.39.6.653. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Vohra G., Champagne P., Daneman D., Delovitch T. L. Distinct antibody specificities to a 64-kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med. 1990 Sep 1;172(3):789–794. doi: 10.1084/jem.172.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M., Landin-Olsson M., Sundkvist G., Dahlquist G., Lernmark A., Baekkeskov S. Antibodies to a Mr-64,000 islet cell protein in Swedish children with newly diagnosed type 1 (insulin-dependent) diabetes. Diabetologia. 1988 Aug;31(8):597–602. doi: 10.1007/BF00264766. [DOI] [PubMed] [Google Scholar]
- Dahlquist G., Blom L., Tuvemo T., Nyström L., Sandström A., Wall S. The Swedish childhood diabetes study--results from a nine year case register and a one year case-referent study indicating that type 1 (insulin-dependent) diabetes mellitus is associated with both type 2 (non-insulin-dependent) diabetes mellitus and autoimmune disorders. Diabetologia. 1989 Jan;32(1):2–6. doi: 10.1007/BF00265396. [DOI] [PubMed] [Google Scholar]
- Erlander M. G., Tobin A. J. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991 Mar;16(3):215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Gianani R., Pugliese A., Bonner-Weir S., Shiffrin A. J., Soeldner J. S., Erlich H., Awdeh Z., Alper C. A., Jackson R. A., Eisenbarth G. S. Prognostically significant heterogeneity of cytoplasmic islet cell antibodies in relatives of patients with type I diabetes. Diabetes. 1992 Mar;41(3):347–353. doi: 10.2337/diab.41.3.347. [DOI] [PubMed] [Google Scholar]
- Gillard B. K., Thomas J. W., Nell L. J., Marcus D. M. Antibodies against ganglioside GT3 in the sera of patients with type I diabetes mellitus. J Immunol. 1989 Jun 1;142(11):3826–3832. [PubMed] [Google Scholar]
- Gottlieb D. I., Chang Y. C., Schwob J. E. Monoclonal antibodies to glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8808–8812. doi: 10.1073/pnas.83.22.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen A. E., Hagopian W. A., Grubin C. E., Dube S., Disteche C. M., Adler D. A., Bärmeier H., Mathewes S., Grant F. J., Foster D. Cloning and primary structure of a human islet isoform of glutamic acid decarboxylase from chromosome 10. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8337–8341. doi: 10.1073/pnas.88.19.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karounos D. G., Nell L. J., Thomas J. W. Autoantibodies present at onset of type I diabetes recognize multiple islet cell antigens. Autoimmunity. 1990;6(1-2):79–91. doi: 10.3109/08916939008993372. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., Erlander M. G., Clare-Salzler M., Atkinson M. A., Maclaren N. K., Tobin A. J. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992 Jan;89(1):283–292. doi: 10.1172/JCI115573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Itoh T., Kosaka K., Sato K., Tsuji K. Time course of islet cell antibodies and beta-cell function in non-insulin-dependent stage of type I diabetes. Diabetes. 1987 Apr;36(4):510–517. doi: 10.2337/diab.36.4.510. [DOI] [PubMed] [Google Scholar]
- Landin-Olsson M., Nilsson K. O., Lernmark A., Sundkvist G. Islet cell antibodies and fasting C-peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologia. 1990 Sep;33(9):561–568. doi: 10.1007/BF00404145. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Baekkeskov S. Islet cell antibodies-theoretical and practical implications. Diabetologia. 1981 Nov;21(5):431–435. doi: 10.1007/BF00257781. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Molenaar J. L., van Beers W. A., Yamaguchi Y., Nagataki S., Ludvigsson J., Maclaren N. K. The Fourth International Serum Exchange Workshop to standardize cytoplasmic islet cell antibodies. The Immunology and Diabetes Workshops and Participating Laboratories. Diabetologia. 1991 Jul;34(7):534–535. doi: 10.1007/BF00403293. [DOI] [PubMed] [Google Scholar]
- Madsbad S., Krarup T., McNair P., Christiansen C., Faber O. K., Transbøl I., Binder C. Practical clinical value of the C-peptide response to glucagon stimulation in the choice of treatment in diabetes mellitus. Acta Med Scand. 1981;210(3):153–156. doi: 10.1111/j.0954-6820.1981.tb09793.x. [DOI] [PubMed] [Google Scholar]
- Michelsen B. K., Petersen J. S., Boel E., Møldrup A., Dyrberg T., Madsen O. D. Cloning, characterization, and autoimmune recognition of rat islet glutamic acid decarboxylase in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8754–8758. doi: 10.1073/pnas.88.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Kobayashi T., Sugimoto T., Murase T., Itoh T., Kosaka K. Predictive value of insulin autoantibodies for further progression of beta cell dysfunction in non-insulin-dependent diabetics. Diabetes Res. 1988 Nov;9(3):105–109. [PubMed] [Google Scholar]
- Nyström L., Ostman J., Wall S., Wibell L. Mortality of all incident cases of diabetes mellitus in Sweden diagnosed 1983-1987 at age 15-34 years. Diabetes Incidence Study in Sweden (DISS) Group. Diabet Med. 1992 Jun;9(5):422–427. doi: 10.1111/j.1464-5491.1992.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. The biosociology of pancreatic B cells. Diabetologia. 1987 May;30(5):277–291. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991 Feb;40(2):166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- Rewers M. The changing face of the epidemiology of insulin-dependent diabetes mellitus (IDDM): research designs and models of disease causation. Ann Med. 1991 Oct;23(4):419–426. doi: 10.3109/07853899109148085. [DOI] [PubMed] [Google Scholar]
- Talavera A., Basilico C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J Cell Physiol. 1977 Sep;92(3):425–436. doi: 10.1002/jcp.1040920310. [DOI] [PubMed] [Google Scholar]




