Abstract
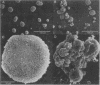
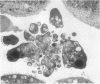
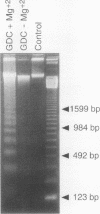
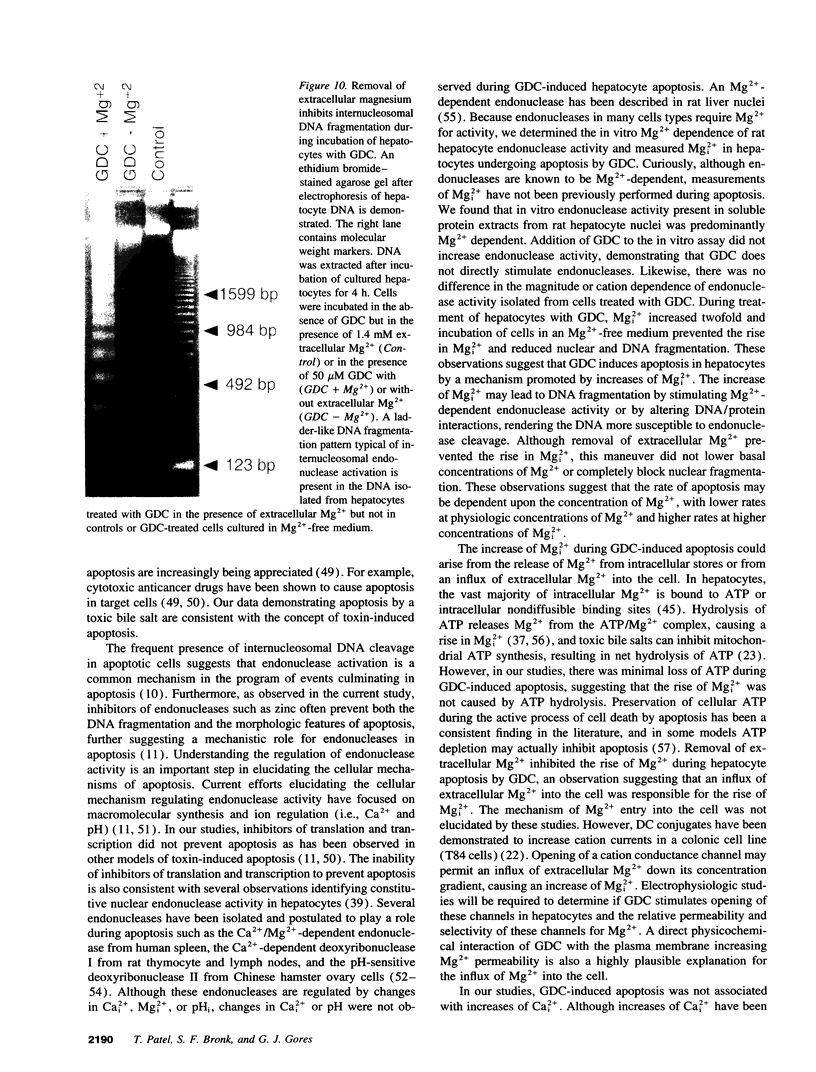
Retention of bile salts by the hepatocyte contributes to liver injury during cholestasis. Although cell injury can occur by one of two mechanisms, necrosis versus apoptosis, information is lacking regarding apoptosis as a mechanism of cell death by bile salts. Our aim was to determine if the bile salt glycodeoxycholate (GDC) induces apoptosis in rat hepatocytes. Morphologic assessment included electron microscopy and quantitation of nuclear fragmentation by fluorescent microscopy. Biochemical studies included measurements of DNA fragmentation, in vitro endonuclease activity, cytosolic free Ca2+ (Cai2+), and cytosolic free Mg2+ (Mgi2+). Morphologic studies demonstrated typical features of apoptosis in GDC (50 microM) treated cells. The "ladder pattern" of DNA fragmentation was also present in DNA obtained from GDC-treated cells. In vitro endonuclease activity was 2.5-fold greater with Mg2+ than Ca2+. Although basal Cai2+ values did not change after addition of GDC, Mgi2+ increased twofold. Incubation of cells in an Mg(2+)-free medium prevented the rise in Mgi2+ and reduced nuclear and DNA fragmentation. In conclusion, GDC induces apoptosis in hepatocytes by a mechanism promoted by increases of Mgi2+ with stimulation of Mg(2+)-dependent endonucleases. These data suggest for the first time that changes of Mgi2+ may participate in the program of cellular events culminating in apoptosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer M. S., Engelking L. R., Nolan K., Sullivan D., Zimniak P., Lester R. Hepatotoxic bile acids increase cytosolic Ca++ activity of isolated rat hepatocytes. Hepatology. 1988 Jul-Aug;8(4):887–891. doi: 10.1002/hep.1840080430. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Engelking L. R., Nolan K., Sullivan D., Zimniak P., Lester R. Hepatotoxic bile acids increase cytosolic Ca++ activity of isolated rat hepatocytes. Hepatology. 1988 Jul-Aug;8(4):887–891. doi: 10.1002/hep.1840080430. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Attili A. F., Angelico M., Cantafora A., Alvaro D., Capocaccia L. Bile acid-induced liver toxicity: relation to the hydrophobic-hydrophilic balance of bile acids. Med Hypotheses. 1986 Jan;19(1):57–69. doi: 10.1016/0306-9877(86)90137-4. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem Biophys Res Commun. 1992 Jul 31;186(2):782–789. doi: 10.1016/0006-291x(92)90814-2. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Eastman A. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys. 1993 Jan;300(1):440–450. doi: 10.1006/abbi.1993.1060. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Perotti M., Taddei F., Mirabelli F., Finardi G., Nicotera P., Orrenius S. Tumor necrosis factor alpha induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation. Cancer Res. 1992 Mar 1;52(5):1342–1346. [PubMed] [Google Scholar]
- Beuers U., Spengler U., Zwiebel F. M., Pauletzki J., Fischer S., Paumgartner G. Effect of ursodeoxycholic acid on the kinetics of the major hydrophobic bile acids in health and in chronic cholestatic liver disease. Hepatology. 1992 Apr;15(4):603–608. doi: 10.1002/hep.1840150409. [DOI] [PubMed] [Google Scholar]
- Columbano A., Ledda-Columbano G. M., Coni P. P., Faa G., Liguori C., Santa Cruz G., Pani P. Occurrence of cell death (apoptosis) during the involution of liver hyperplasia. Lab Invest. 1985 Jun;52(6):670–675. [PubMed] [Google Scholar]
- Compton M. M. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992 Sep;11(2):105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Corkey B. E., Duszynski J., Rich T. L., Matschinsky B., Williamson J. R. Regulation of free and bound magnesium in rat hepatocytes and isolated mitochondria. J Biol Chem. 1986 Feb 25;261(6):2567–2574. [PubMed] [Google Scholar]
- Crosignani A., Podda M., Battezzati P. M., Bertolini E., Zuin M., Watson D., Setchell K. D. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991 Dec;14(6):1000–1007. [PubMed] [Google Scholar]
- Devor D. C., Sekar M. C., Frizzell R. A., Duffey M. E. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84) J Clin Invest. 1993 Nov;92(5):2173–2181. doi: 10.1172/JCI116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Bronk S. F., Gores G. J. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992 Jun;102(6):2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- Evans D. L., Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res. 1993 May 1;53(9):2133–2139. [PubMed] [Google Scholar]
- Gerschenson L. E., Rotello R. J. Apoptosis: a different type of cell death. FASEB J. 1992 Apr;6(7):2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greim H., Trülzsch D., Czygan P., Rudick J., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology. 1972 Nov;63(5):846–850. [PubMed] [Google Scholar]
- Greim H., Trülzsch D., Roboz J., Dressler K., Czygan P., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972 Nov;63(5):837–845. [PubMed] [Google Scholar]
- Groskreutz J. L., Bronk S. F., Gores G. J. Ruthenium red delays the onset of cell death during oxidative stress of rat hepatocytes. Gastroenterology. 1992 Mar;102(3):1030–1038. doi: 10.1016/0016-5085(92)90193-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harman A. W., Nieminen A. L., Lemasters J. J., Herman B. Cytosolic free magnesium, ATP and blebbing during chemical hypoxia in cultured rat hepatocytes. Biochem Biophys Res Commun. 1990 Jul 31;170(2):477–483. doi: 10.1016/0006-291x(90)92116-h. [DOI] [PubMed] [Google Scholar]
- Heuman D. M. Hepatoprotective properties of ursodeoxycholic acid. Gastroenterology. 1993 Jun;104(6):1865–1870. doi: 10.1016/0016-5085(93)90672-y. [DOI] [PubMed] [Google Scholar]
- Heuman D. M., Mills A. S., McCall J., Hylemon P. B., Pandak W. M., Vlahcevic Z. R. Conjugates of ursodeoxycholate protect against cholestasis and hepatocellular necrosis caused by more hydrophobic bile salts. In vivo studies in the rat. Gastroenterology. 1991 Jan;100(1):203–211. doi: 10.1016/0016-5085(91)90602-h. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Hibino Y., Yoneda T., Sugano N. Purification and properties of a magnesium-dependent endodeoxyribonuclease endogenous to rat-liver nuclei. Biochim Biophys Acta. 1988 Sep 7;950(3):313–320. doi: 10.1016/0167-4781(88)90127-3. [DOI] [PubMed] [Google Scholar]
- Kandell R. L., Bernstein C. Bile salt/acid induction of DNA damage in bacterial and mammalian cells: implications for colon cancer. Nutr Cancer. 1991;16(3-4):227–238. doi: 10.1080/01635589109514161. [DOI] [PubMed] [Google Scholar]
- Kimura T. [Cytotoxicity of bile acids on cultured cells (author's transl)]. Nihon Shokakibyo Gakkai Zasshi. 1980 Feb;77(2):185–194. [PubMed] [Google Scholar]
- Lennon S. V., Kilfeather S. A., Hallett M. B., Campbell A. K., Cotter T. G. Elevations in cytosolic free Ca2+ are not required to trigger apoptosis in human leukaemia cells. Clin Exp Immunol. 1992 Mar;87(3):465–471. doi: 10.1111/j.1365-2249.1992.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Hagenbuch B., Stieger B., Meier P. J. Parallel decrease of Na(+)-taurocholate cotransport and its encoding mRNA in primary cultures of rat hepatocytes. Hepatology. 1993 Nov;18(5):1162–1166. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Wyllie A. H., Orrenius S. Stimulation of endogenous endonuclease activity in hepatocytes exposed to oxidative stress. Toxicol Lett. 1988 Aug;42(2):123–130. doi: 10.1016/0378-4274(88)90069-0. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. J., Butler W. H. Apoptosis--the mechanism of cell death in dimethylnitrosamine-induced hepatotoxicity. J Pathol. 1989 Jul;158(3):253–260. doi: 10.1002/path.1711580314. [DOI] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S., Kanz M. F., Chieco P., Moslen M. T. 1,1-Dichloroethylene: an apoptotic hepatotoxin? Environ Health Perspect. 1984 Aug;57:313–320. doi: 10.1289/ehp.8457313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. M., Carson D. A. Ca2+/Mg(2+)-dependent endonuclease from human spleen: purification, properties, and role in apoptosis. Biochemistry. 1993 Sep 7;32(35):9129–9136. doi: 10.1021/bi00086a018. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Roda A., Cappelleri G., Aldini R., Roda E., Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J Lipid Res. 1982 Mar;23(3):490–495. [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993 Apr;14(2):133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- Schwartzman R. A., Cidlowski J. A. Mechanism of tissue-specific induction of internucleosomal deoxyribonucleic acid cleavage activity and apoptosis by glucocorticoids. Endocrinology. 1993 Aug;133(2):591–599. doi: 10.1210/endo.133.2.8393769. [DOI] [PubMed] [Google Scholar]
- Schölmerich J., Becher M. S., Schmidt K., Schubert R., Kremer B., Feldhaus S., Gerok W. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties--studies on isolated hepatocytes and lipid membrane vesicles. Hepatology. 1984 Jul-Aug;4(4):661–666. doi: 10.1002/hep.1840040416. [DOI] [PubMed] [Google Scholar]
- Spivey J. R., Bronk S. F., Gores G. J. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993 Jul;92(1):17–24. doi: 10.1172/JCI116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Thakkar N. S., Potten C. S. Inhibition of doxorubicin-induced apoptosis in vivo by 2-deoxy-D-glucose. Cancer Res. 1993 May 1;53(9):2057–2060. [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol. 1992 Apr;4(2):227–232. doi: 10.1016/0955-0674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Van der Meer R., Termont D. S., De Vries H. T. Differential effects of calcium ions and calcium phosphate on cytotoxicity of bile acids. Am J Physiol. 1991 Jan;260(1 Pt 1):G142–G147. doi: 10.1152/ajpgi.1991.260.1.G142. [DOI] [PubMed] [Google Scholar]
- Whyte M. K., Hardwick S. J., Meagher L. C., Savill J. S., Haslett C. Transient elevations of cytosolic free calcium retard subsequent apoptosis in neutrophils in vitro. J Clin Invest. 1993 Jul;92(1):446–455. doi: 10.1172/JCI116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yonemura K., Maeda H. A new assay method for DNase by fluorescence polarization and fluorescence intensity using DNA-ethidium bromide complex as a sensitive substrate. J Biochem. 1982 Oct;92(4):1297–1303. doi: 10.1093/oxfordjournals.jbchem.a134048. [DOI] [PubMed] [Google Scholar]
- Zimniak P., Little J. M., Radominska A., Oelberg D. G., Anwer M. S., Lester R. Taurine-conjugated bile acids act as Ca2+ ionophores. Biochemistry. 1991 Sep 3;30(35):8598–8604. doi: 10.1021/bi00099a015. [DOI] [PubMed] [Google Scholar]