Abstract
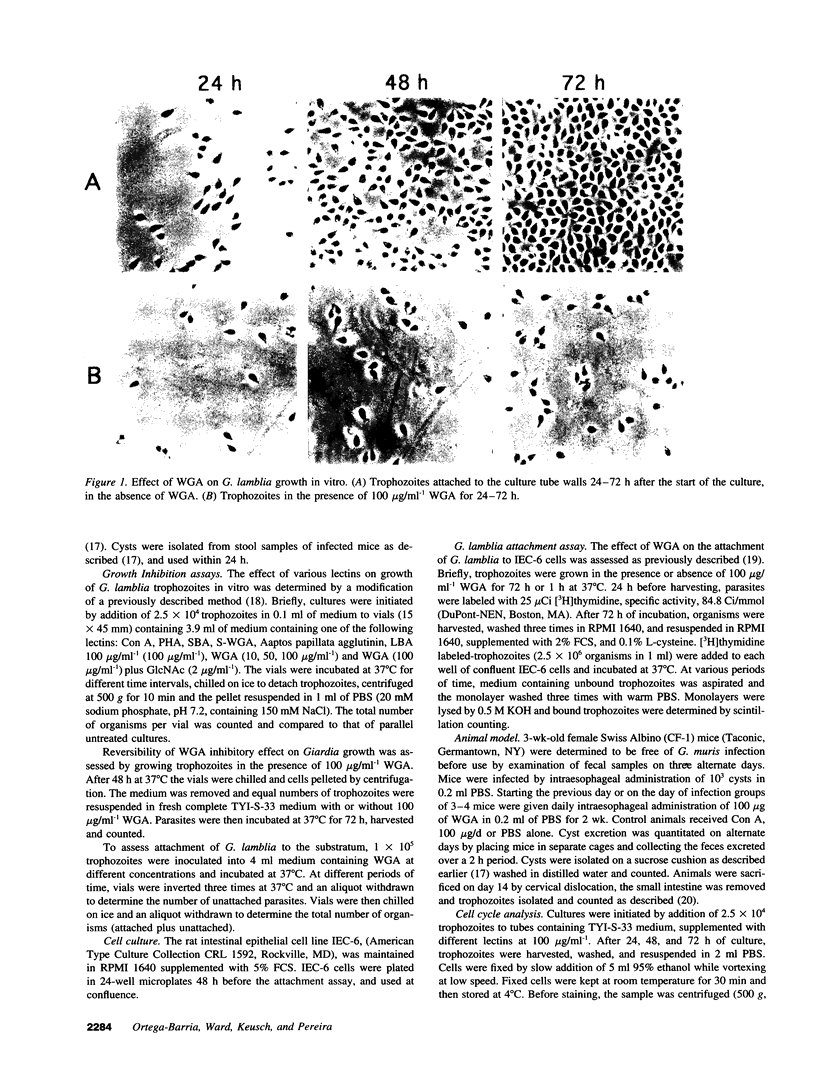
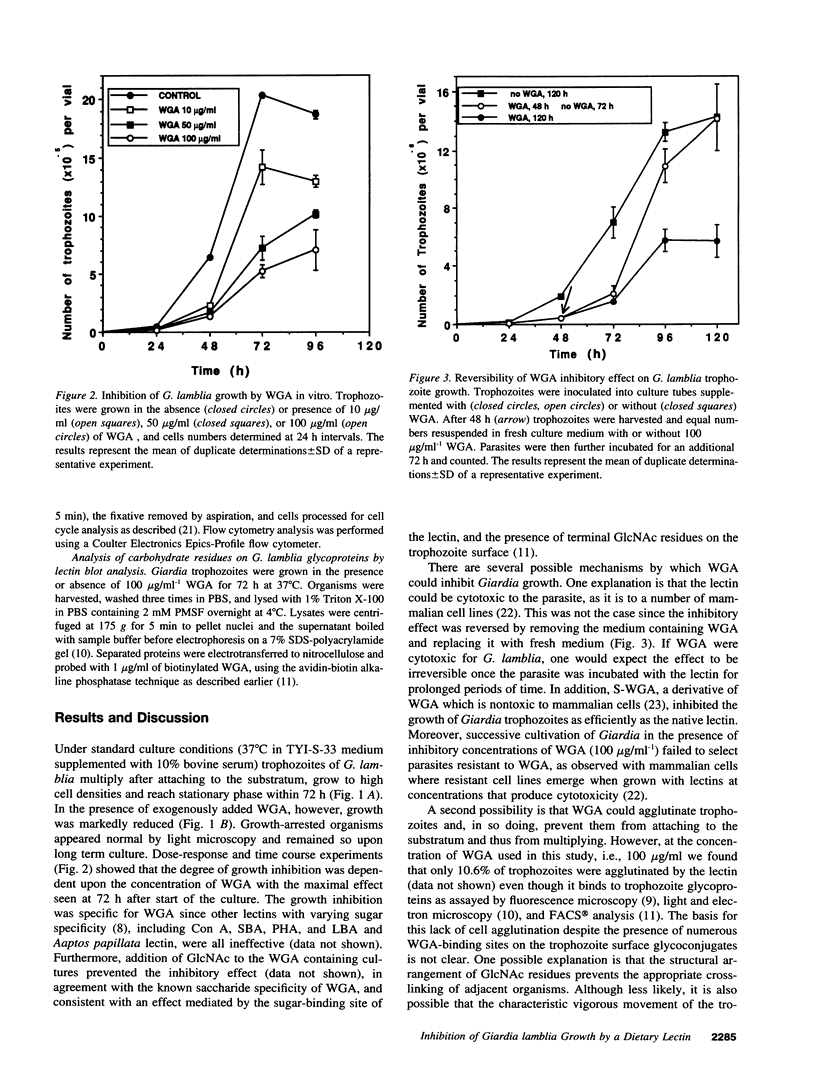
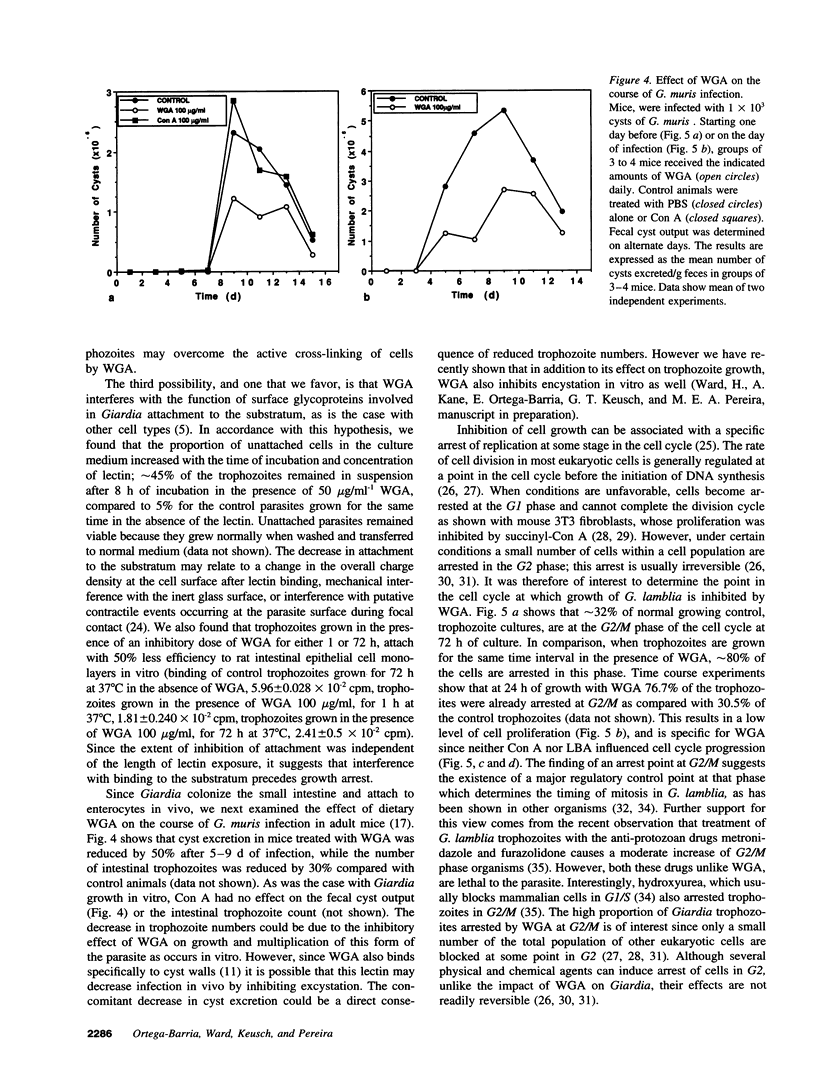
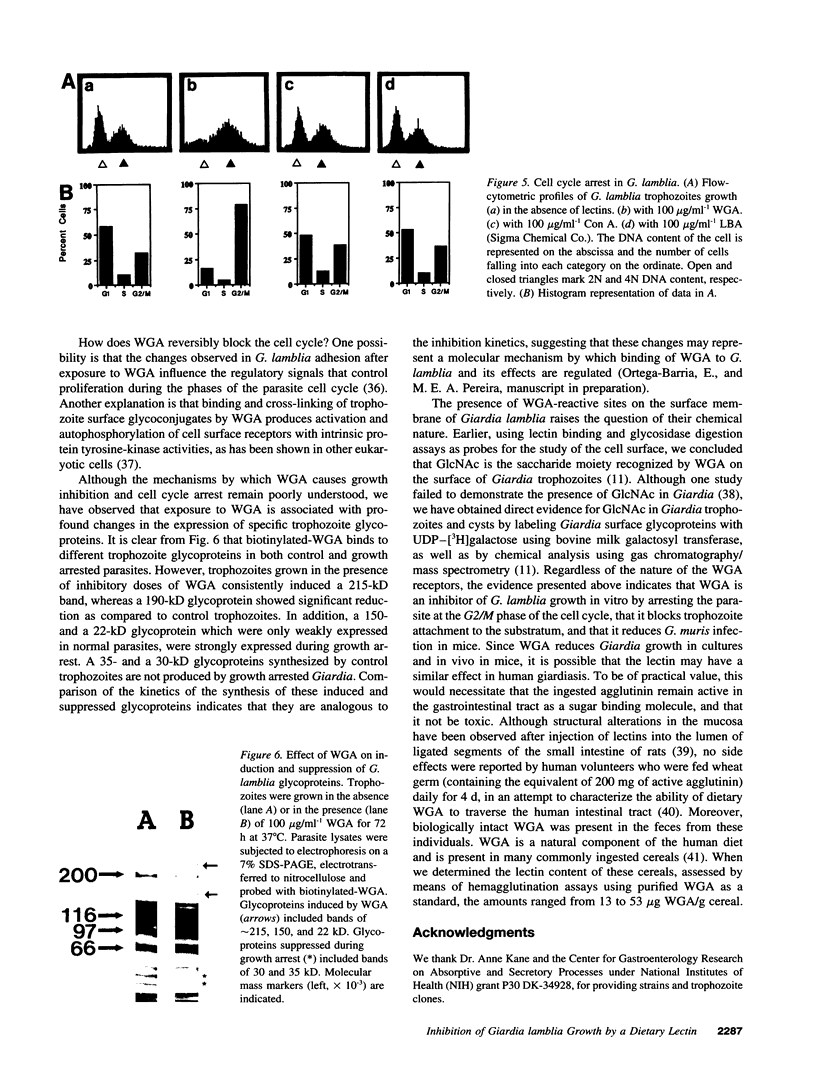
Giardia lamblia, a cause of diarrheal disease throughout the world, is a protozoan parasite that thrives in the small intestine. It is shown here that wheat germ agglutinin (WGA), a naturally occurring lectin widely consumed in normal human diets, reversibly inhibits the growth of G. lamblia trophozoites in vitro, and reduces infection by G. muris in the adult mouse model of giardiasis. The inhibitory effect was dose related, not associated with cytotoxicity and reversed by N-acetyl-D-glucosamine in accordance with the known specificity of the lectin and in agreement with the presence of GlcNAc residues on the surface membrane of G. lamblia trophozoites. Cell cycle analysis revealed that parasites grown in the presence of WGA are arrested in the G2/M phase, providing an explanation for the lectin-induced inhibition of cell proliferation. Comparison of electrophoretic profiles by lectin blot analysis revealed both glycoprotein induction and suppression in growth-arrested organisms. Our findings raise the possibility that blocking trophozoite growth with naturally occurring dietary lectins may influence the course of giardiasis. In addition, the study of cell cycle arrest by WGA may provide a model to study the regulation of cell division in lower eukaryotes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady P. G., Vannier A. M., Banwell J. G. Identification of the dietary lectin, wheat germ agglutinin, in human intestinal contents. Gastroenterology. 1978 Aug;75(2):236–239. [PubMed] [Google Scholar]
- Bretting H., Kabat E. A., Liao J., Pereira M. E. Purification and characterization of the agglutinins from the sponge Aaptos papillata and a study of their combining sites. Biochemistry. 1976 Nov 16;15(23):5029–5038. doi: 10.1021/bi00668a013. [DOI] [PubMed] [Google Scholar]
- Farthing M. J., Mata L., Urrutia J. J., Kronmal R. A. Natural history of Giardia infection of infants and children in rural Guatemala and its impact on physical growth. Am J Clin Nutr. 1986 Mar;43(3):395–405. doi: 10.1093/ajcn/43.3.395. [DOI] [PubMed] [Google Scholar]
- Galbraith W., Goldstein I. J. Phytohemagglutinins: A new class of metalloproteins. Isolation, purification, and some properties of the lectin from Phaseolus lunatus. FEBS Lett. 1970 Aug 17;9(4):197–201. doi: 10.1016/0014-5793(70)80354-4. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Clonal growth of Giardia lamblia trophozoites in a semisolid agarose medium. J Parasitol. 1980 Apr;66(2):350–352. [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S. Attachment of the flagellate Giardia lamblia: role of reducing agents, serum, temperature, and ionic composition. Mol Cell Biol. 1982 Apr;2(4):369–377. doi: 10.1128/mcb.2.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T. M., Ohtsubo M., Roberts J. M., Assoian R. K. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993 Dec 3;262(5139):1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- Hayles J., Nurse P. Cell cycle regulation in yeast. J Cell Sci Suppl. 1986;4:155–170. doi: 10.1242/jcs.1986.supplement_4.10. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Hewlett E. L., Pearson R. D. Lectin binding by Giardia lamblia. Infect Immun. 1981 Dec;34(3):733–738. doi: 10.1128/iai.34.3.733-738.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyne G. F., Boreham P. F., Parsons P. G., Ward C., Biggs B. The effect of drugs on the cell cycle of Giardia intestinalis. Parasitology. 1989 Dec;99(Pt 3):333–339. doi: 10.1017/s0031182000059047. [DOI] [PubMed] [Google Scholar]
- Islam A., Stoll B. J., Ljungström I., Biswas J., Nazrul H., Huldt G. Giardia lamblia infections in a cohort of Bangladeshi mothers and infants followed for one year. J Pediatr. 1983 Dec;103(6):996–1000. doi: 10.1016/s0022-3476(83)80739-2. [DOI] [PubMed] [Google Scholar]
- Jarroll E. L., Manning P., Lindmark D. G., Coggins J. R., Erlandsen S. L. Giardia cyst wall-specific carbohydrate: evidence for the presence of galactosamine. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):121–131. doi: 10.1016/0166-6851(89)90063-7. [DOI] [PubMed] [Google Scholar]
- Lorenzsonn V., Olsen W. A. In vivo responses of rat intestinal epithelium to intraluminal dietary lectins. Gastroenterology. 1982 May;82(5 Pt 1):838–848. [PubMed] [Google Scholar]
- Mannino R. J., Ballmer K., Burger M. M. Growth inhibition of transformed cells with succinylated concanavalin A. Science. 1978 Sep 1;201(4358):824–826. doi: 10.1126/science.210502. [DOI] [PubMed] [Google Scholar]
- Mannino R. J., Burger M. M. Growth inhibition of animal cells by succinylated concanavalin A. Nature. 1975 Jul 3;256(5512):19–22. doi: 10.1038/256019a0. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Yu G. S., Conteas C., Morrill P. R., McMorrow B. In vitro model of attachment of Giardia intestinalis trophozoites to IEC-6 cells, an intestinal cell line. Antimicrob Agents Chemother. 1991 Jan;35(1):29–35. doi: 10.1128/aac.35.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsigny M., Kieda C., Roche A. C., Delmotte F. Preparation and biological properties of a covalent antitumor drug--arm---carrier (DAC conjugate). FEBS Lett. 1980 Sep 22;119(1):181–186. doi: 10.1016/0014-5793(80)81026-x. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Sene C., Obrenovitch A., Roche A. C., Delmotte F., Boschetti E. Properties of succinylated wheat-germ agglutinin. Eur J Biochem. 1979 Jul;98(1):39–45. doi: 10.1111/j.1432-1033.1979.tb13157.x. [DOI] [PubMed] [Google Scholar]
- Moreno S., Hayles J., Nurse P. Regulation of the cell cycle timing of mitosis. J Cell Sci Suppl. 1989;12:1–8. doi: 10.1242/jcs.1989.supplement_12.1. [DOI] [PubMed] [Google Scholar]
- Nachbar M. S., Oppenheim J. D. Lectins in the United States diet: a survey of lectins in commonly consumed foods and a review of the literature. Am J Clin Nutr. 1980 Nov;33(11):2338–2345. doi: 10.1093/ajcn/33.11.2338. [DOI] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Olveda R. K., Andrews J. S., Jr, Hewlett E. L. Murine giardiasis: localization of trophozoites and small bowel histopathology during the course of infection. Am J Trop Med Hyg. 1982 Jan;31(1):60–66. doi: 10.4269/ajtmh.1982.31.60. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Pawlowski Z. S. Implications of parasite-nutrition interactions from a world perspective. Fed Proc. 1984 Feb;43(2):256–260. [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A. Immunochemical studies on lectins and their application to the fractionation of blood group substances and cells. CRC Crit Rev Immunol. 1979 Nov;1(1):33–78. [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989 Jan;8(1):73–82. doi: 10.1002/j.1460-2075.1989.tb03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. D., Alroy J., Lev B. I., Keusch G. T., Pereira M. E. Biology of Giardia lamblia. Detection of N-acetyl-D-glucosamine as the only surface saccharide moiety and identification of two distinct subsets of trophozoites by lectin binding. J Exp Med. 1988 Jan 1;167(1):73–88. doi: 10.1084/jem.167.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. D., Lev B. I., Kane A. V., Keusch G. T., Pereira M. E. Identification and characterization of taglin, a mannose 6-phosphate binding, trypsin-activated lectin from Giardia lamblia. Biochemistry. 1987 Dec 29;26(26):8669–8675. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Okada S. Stationary phase of cultured mammalian cells (L5178Y). J Cell Biol. 1967 Nov;35(2):285–294. doi: 10.1083/jcb.35.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Taniguchi T., Nagai K., Saitoh H., Yamamura H. The lectin wheat germ agglutinin stimulates a protein-tyrosine kinase activity of p72syk in porcine splenocytes. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1325–1329. doi: 10.1016/s0006-291x(05)81340-3. [DOI] [PubMed] [Google Scholar]




