Abstract
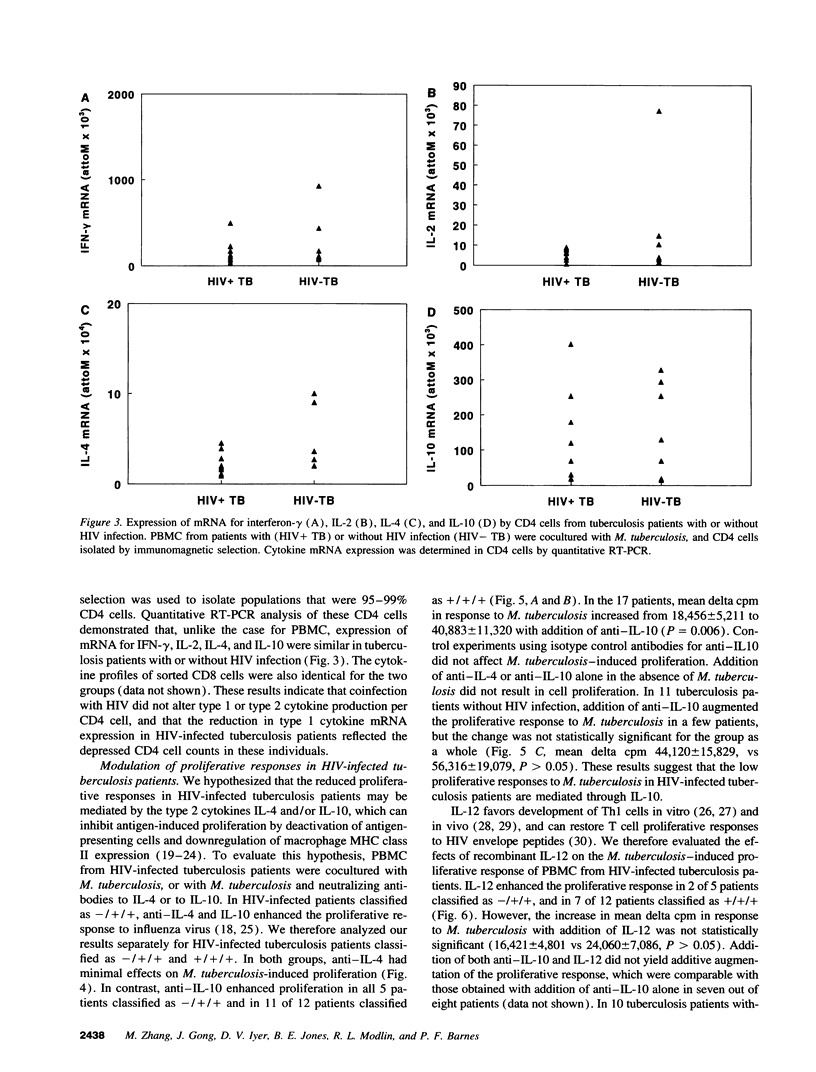
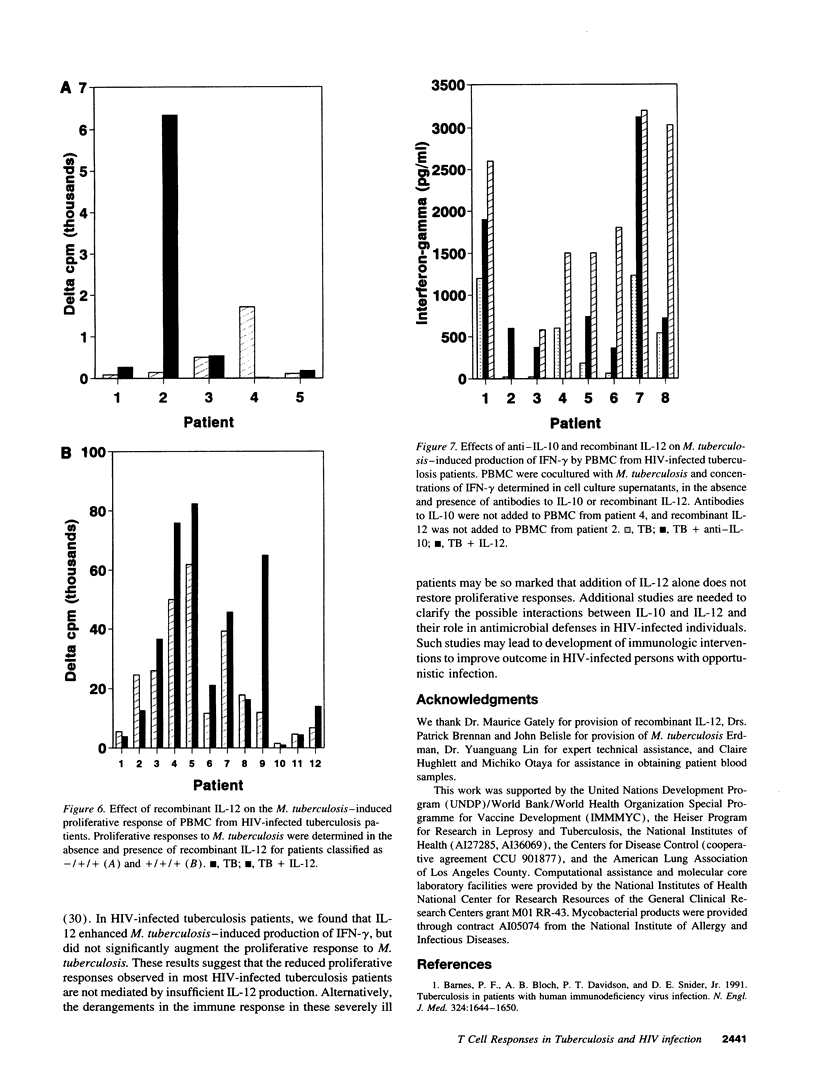
Tuberculosis causes more extensive and life-threatening disease in patients with HIV infection than in immunocompetent persons. To investigate the hypothesis that these severe manifestations of tuberculosis may be due to alterations in cytokine production, we evaluated cytokine patterns in HIV-infected tuberculosis patients. Upon stimulation with Mycobacterium tuberculosis in vitro, PBMC from HIV-infected tuberculosis patients had reduced proliferative and type 1 responses, compared with HIV-seronegative tuberculosis patients. The reduction in proliferative responses was independent of the CD4 cell count, but the reduced type 1 response was a direct result of CD4 cell depletion. There was no enhancement of type 2 cytokine production in HIV-infected patients, although production of IL-10 was prominent in all tuberculosis patients. In HIV-infected tuberculosis patients, M. tuberculosis-induced proliferative responses were significantly enhanced by neutralizing antibodies to IL-10 but not by antibodies to IL-4 or by recombinant IL-12. The M. tuberculosis-induced type 1 response was augmented both by antibodies to IL-10 and by recombinant IL-12. Tuberculosis in the context of HIV infection is characterized by diminished type 1 responses, probably induced by immunosuppressive cytokines produced by macrophages/monocytes, rather than by type 2 cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniskis D., Amin K., Barnes P. F. Pleuritis as a manifestation of reactivation tuberculosis. Am J Med. 1990 Oct;89(4):447–450. doi: 10.1016/0002-9343(90)90374-m. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Barnes P. F., Lu S., Abrams J. S., Wang E., Yamamura M., Modlin R. L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993 Aug;61(8):3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Bermudez L. E., Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993 Jul;61(7):3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagnolli M. M., Lin B. Y., Young D., Herrmann S. H. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992 Dec 15;149(12):3778–3783. [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Hakim F. T., Venzon D. J., Blatt S., Hendrix C. W., Wynn T. A., Shearer G. M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993 Mar;91(3):759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Berzofsky J. A., Pinto L. A., Wynn T. A., Blatt S. P., Dolan M. J., Hendrix C. W., Wolf S. F., Shearer G. M. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993 Dec 10;262(5140):1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989 Dec;84(6):1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Wynn T. A., Berzofsky J. A., Blatt S. P., Hendrix C. W., Sher A., Coffman R. L., Shearer G. M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994 Feb;93(2):768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley C. L., Small P. M., Schecter G. F., Schoolnik G. K., McAdam R. A., Jacobs W. R., Jr, Hopewell P. C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992 Jan 23;326(4):231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- Denis M., Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993 Nov 15;151(10):5425–5430. [PubMed] [Google Scholar]
- Di Perri G., Cruciani M., Danzi M. C., Luzzati R., De Checchi G., Malena M., Pizzighella S., Mazzi R., Solbiati M., Concia E. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet. 1989 Dec 23;2(8678-8679):1502–1504. [PubMed] [Google Scholar]
- Edlin B. R., Tokars J. I., Grieco M. H., Crawford J. T., Williams J., Sordillo E. M., Ong K. R., Kilburn J. O., Dooley S. W., Castro K. G. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992 Jun 4;326(23):1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Fischl M. A., Daikos G. L., Uttamchandani R. B., Poblete R. B., Moreno J. N., Reyes R. R., Boota A. M., Thompson L. M., Cleary T. J., Oldham S. A. Clinical presentation and outcome of patients with HIV infection and tuberculosis caused by multiple-drug-resistant bacilli. Ann Intern Med. 1992 Aug 1;117(3):184–190. doi: 10.7326/0003-4819-117-3-184. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Wolitzky A. G., Quinn P. M., Chizzonite R. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell Immunol. 1992 Aug;143(1):127–142. doi: 10.1016/0008-8749(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993 May 1;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. L., He S. H., Rios M. J., Wick E. A. Interleukin-4 inhibits human macrophage activation by tumor necrosis factor, granulocyte-monocyte colony-stimulating factor, and interleukin-3 for antileishmanial activity and oxidative burst capacity. J Infect Dis. 1992 Feb;165(2):344–351. doi: 10.1093/infdis/165.2.344. [DOI] [PubMed] [Google Scholar]
- Hopewell P. C. Impact of human immunodeficiency virus infection on the epidemiology, clinical features, management, and control of tuberculosis. Clin Infect Dis. 1992 Sep;15(3):540–547. doi: 10.1093/clind/15.3.540. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Kazura J. W., Hazlett F. E., Jr, Boom W. H. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993 Nov 1;151(9):4857–4864. [PubMed] [Google Scholar]
- Pirmez C., Yamamura M., Uyemura K., Paes-Oliveira M., Conceiço-Silva F., Modlin R. L. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993 Apr;91(4):1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPER W. H., WARING J. J. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc. 1955 May;71(5):616–634. doi: 10.1164/artpd.1955.71.5.616. [DOI] [PubMed] [Google Scholar]
- Robertson M. J., Soiffer R. J., Wolf S. F., Manley T. J., Donahue C., Young D., Herrmann S. H., Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992 Mar 1;175(3):779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993 Apr 23;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- Selwyn P. A., Hartel D., Lewis V. A., Schoenbaum E. E., Vermund S. H., Klein R. S., Walker A. T., Friedland G. H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989 Mar 2;320(9):545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- Sieling P. A., Abrams J. S., Yamamura M., Salgame P., Bloom B. R., Rea T. H., Modlin R. L. Immunosuppressive roles for IL-10 and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. J Immunol. 1993 Jun 15;150(12):5501–5510. [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]


