Abstract
Neutrophil adherence to endothelial cells (ECs) under conditions of flow occurs in successive steps, including selectin-dependent primary adhesion and CD18-dependent secondary adhesion. We used a parallel-plate flow chamber to assess the steps in T cell adherence in vitro. On monolayers of L cells transfected with the EC adhesion molecules E-selectin, vascular cell adhesion molecule-1 (VCAM-1), or intercellular adhesion molecule-1 (ICAM-1), E-selectin was capable of mediating only primary adhesion, ICAM-1 was capable of mediating only secondary adhesion, and VCAM-1 was capable of mediating both primary and secondary adhesion. Studies using human umbilical vein EC monolayers stimulated for 24 h with IL-1 also revealed distinct primary and secondary steps in T cell adhesion under flow, and the secondary adhesion was inhibited > 90% by blocking both VCAM-1/alpha 4 beta 1 integrin and ICAM-1/CD18 integrin pathways. However, the primary adhesion under conditions of flow could not be attributed to any of the mechanisms known to support adhesion of leukocytes to ECs. Alone, this pathway was shown to mediate T cell rolling and was a necessary prerequisite for engagement of the two integrin pathways in this system. Thus, T cell adherence to 24-h IL-1-stimulated human umbilical vein ECs at venular wall shear stresses involves at least two successive steps, with clear molecular distinctions from the mechanisms accounting for neutrophil/EC adhesion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
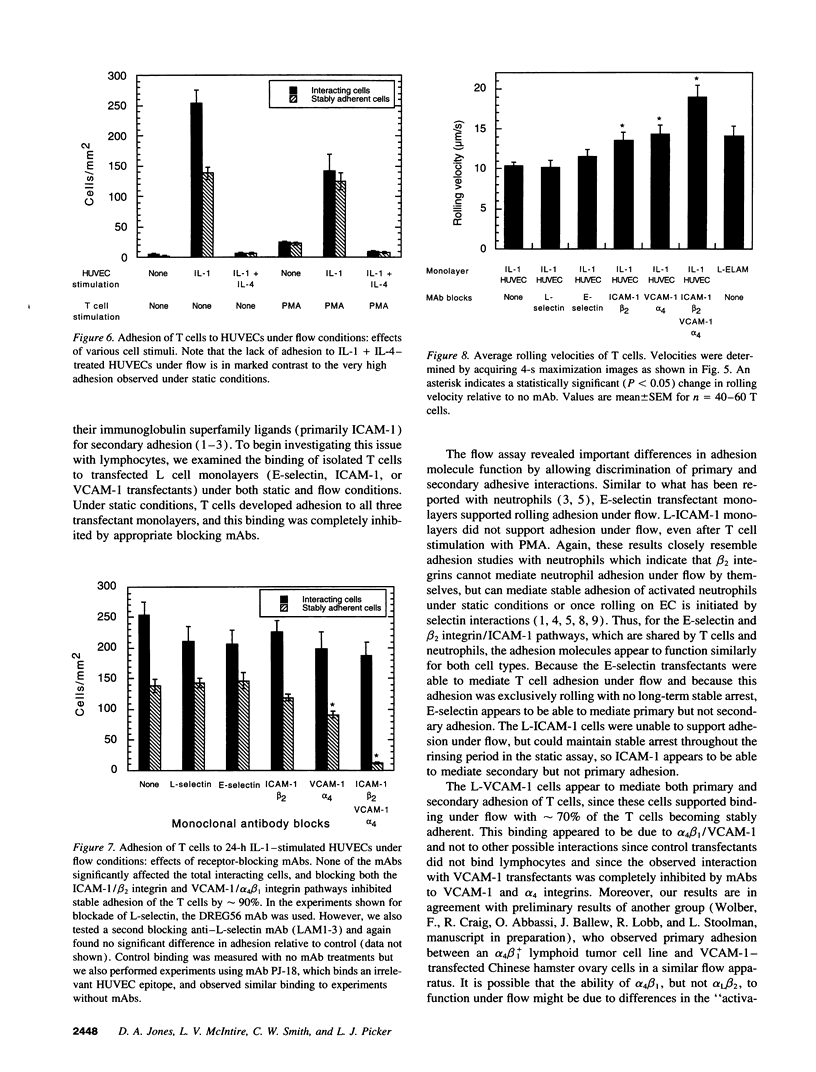
- Abbassi O., Kishimoto T. K., McIntire L. V., Anderson D. C., Smith C. W. E-selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest. 1993 Dec;92(6):2719–2730. doi: 10.1172/JCI116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbassi O., Lane C. L., Krater S., Kishimoto T. K., Anderson D. C., McIntire L. V., Smith C. W. Canine neutrophil margination mediated by lectin adhesion molecule-1 in vitro. J Immunol. 1991 Oct 1;147(7):2107–2115. [PubMed] [Google Scholar]
- Airas L., Salmi M., Jalkanen S. Lymphocyte-vascular adhesion protein-2 is a novel 70-kDa molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol. 1993 Oct 15;151(8):4228–4238. [PubMed] [Google Scholar]
- Atherton A., Born G. V. Relationship between the velocity of rolling granulocytes and that of the blood flow in venules. J Physiol. 1973 Aug;233(1):157–165. doi: 10.1113/jphysiol.1973.sp010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargatze R. F., Butcher E. C. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J Exp Med. 1993 Jul 1;178(1):367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C., Berg E. L., Briskin M. J., Andrew D. P., Kilshaw P. J., Holzmann B., Weissman I. L., Hamann A., Butcher E. C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993 Jul 16;74(1):185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H., Ottaway C. A. Dynamics of lymphocyte-endothelial interactions in vivo. Science. 1986 Jan 24;231(4736):402–405. doi: 10.1126/science.3941903. [DOI] [PubMed] [Google Scholar]
- Briscoe D. M., Cotran R. S., Pober J. S. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992 Nov 1;149(9):2954–2960. [PubMed] [Google Scholar]
- Hammer D. A., Apte S. M. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992 Jul;63(1):35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Abbassi O., McIntire L. V., McEver R. P., Smith C. W. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993 Oct;65(4):1560–1569. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell Immunol. 1991 Jan;132(1):201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., McIntire L. V., Eskin S. G. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987 Nov;70(5):1284–1290. [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Neutrophils roll on E-selectin. J Immunol. 1993 Dec 1;151(11):6338–6346. [PubMed] [Google Scholar]
- Ley K., Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991 Oct;69(4):1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- Masinovsky B., Urdal D., Gallatin W. M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990 Nov 1;145(9):2886–2895. [PubMed] [Google Scholar]
- Michie S. A., Streeter P. R., Bolt P. A., Butcher E. C., Picker L. J. The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol. 1993 Dec;143(6):1688–1698. [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Dame M. K., Lane C. L., Smith C. W., Anderson D. C., Ward P. A. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Perry M. A., Granger D. N. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Invest. 1991 May;87(5):1798–1804. doi: 10.1172/JCI115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Bergstresser P. R., Terstappen L. W. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993 Feb 1;150(3):1122–1136. [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992 Sep 4;257(5075):1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- Schmidt E. E., MacDonald I. C., Groom A. C. Interactions of leukocytes with vessel walls and with other blood cells, studied by high-resolution intravital videomicroscopy of spleen. Microvasc Res. 1990 Jul;40(1):99–117. doi: 10.1016/0026-2862(90)90011-f. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Luscinskas F. W., Kansas G. S., Munro J. M., Griffin J. D., Gimbrone M. A., Jr, Tedder T. F. Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991 Oct 15;147(8):2565–2573. [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Tözeren A., Ley K. How do selectins mediate leukocyte rolling in venules? Biophys J. 1992 Sep;63(3):700–709. doi: 10.1016/S0006-3495(92)81660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R. L-selectin-IgG chimera--in vitro and in vivo. Agents Actions Suppl. 1993;41:103–109. [PubMed] [Google Scholar]
- Weston S. A., Parish C. R. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990 Oct 4;133(1):87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., Berg E. L., Michie S. A., Brown D. A., Karolak D., Ramezani L., Berger E. M., Arfors K. E., Butcher E. C. L-selectin mediates neutrophil rolling in inflamed venules through sialyl LewisX-dependent and -independent recognition pathways. Blood. 1993 Jul 1;82(1):182–191. [PubMed] [Google Scholar]



