Abstract
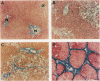
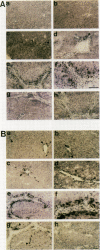
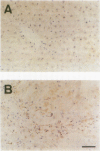
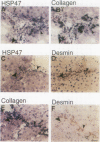
HSP47 is a collagen-binding stress protein and is assumed to act as a collagen-specific molecular chaperone during the biosynthesis and secretion of procollagen in the living cell. The synthesis of HSP47 has been reported to correlate with that of collagen in several cell lines. We examined the expression of HSP47 mRNA during the progression of carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Northern blot analysis revealed that the expression of HSP47 mRNA was markedly induced during the progression of fibrosis in parallel with alpha 1(I) and alpha 1(III) collagen mRNAs. By in situ hybridization, the distribution of HSP47 transcripts was similar to that of alpha 1(I) collagen and was observed only in cells lining collagen fibrils. These collagen-positive cells were confirmed to be Ito cells by immunohistochemistry for desmin. The absence of high levels of HSP47 mRNA in the liver of rats treated with only a single administration of CCl4 indicated that the induction of HSP47 mRNA was not due to the direct effect of CCl4 as a stressor, but was due to the progression of liver fibrosis. The function of HSP47 in liver fibrosis will also be discussed.
Full text
PDF

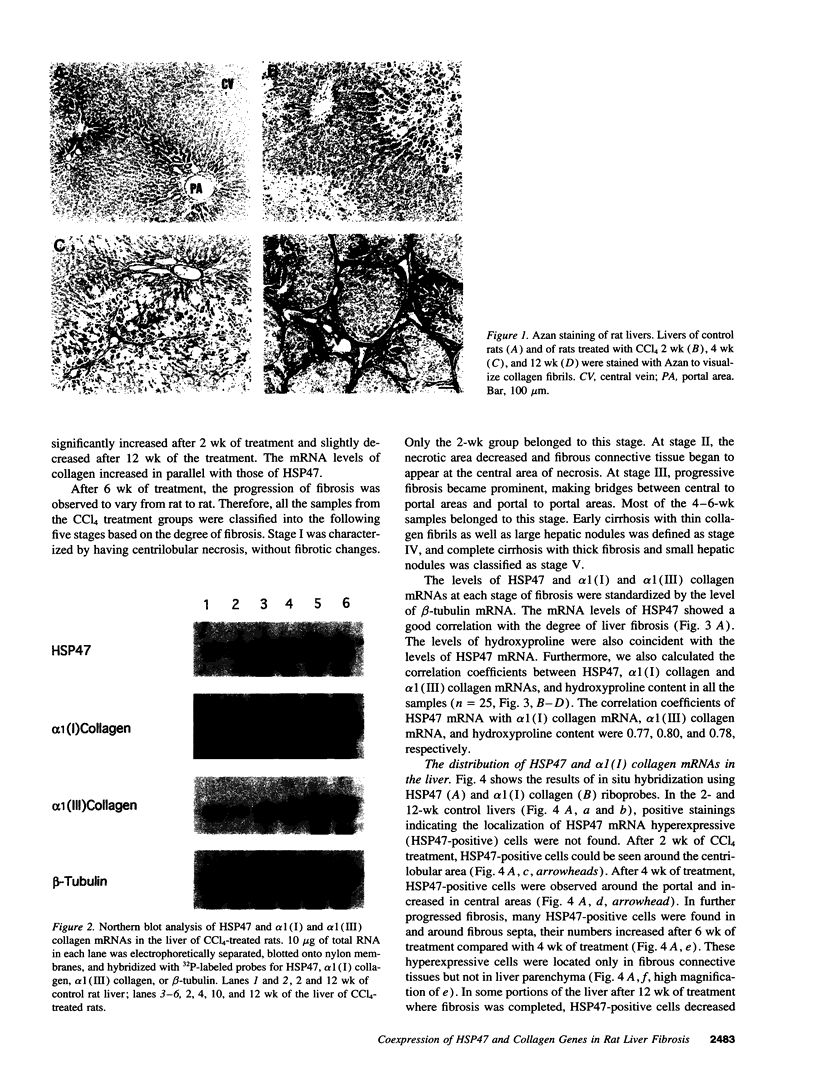
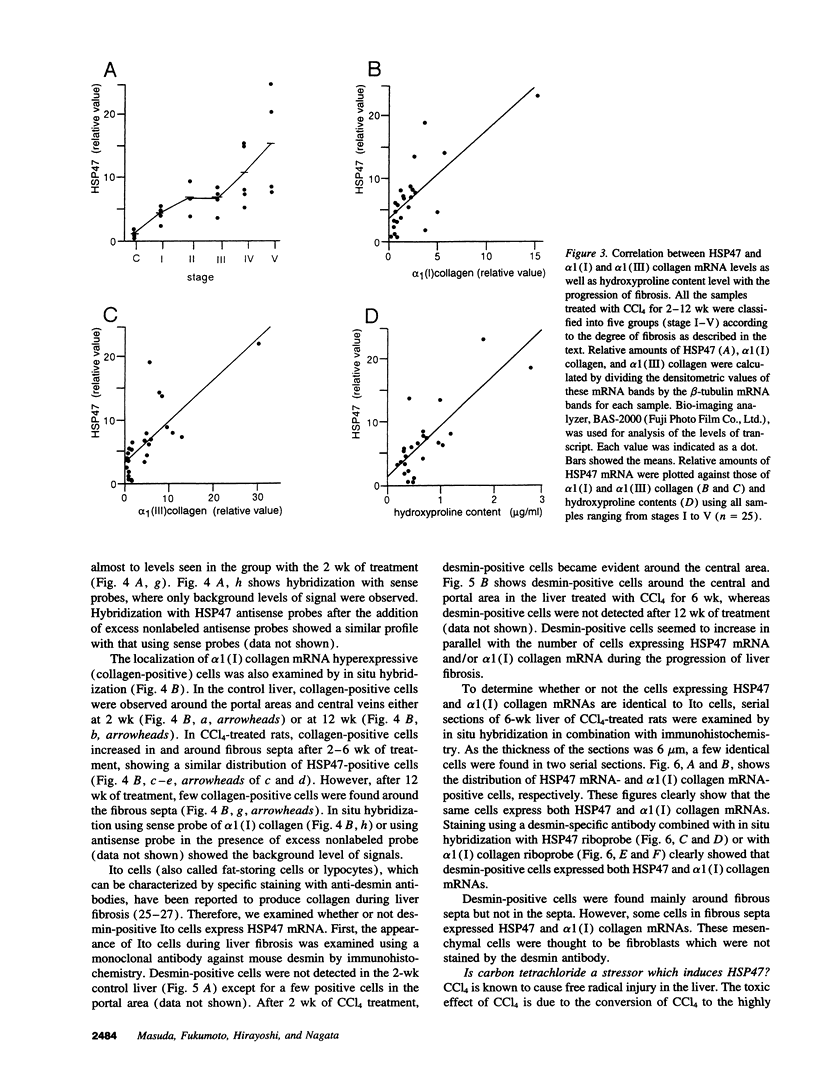
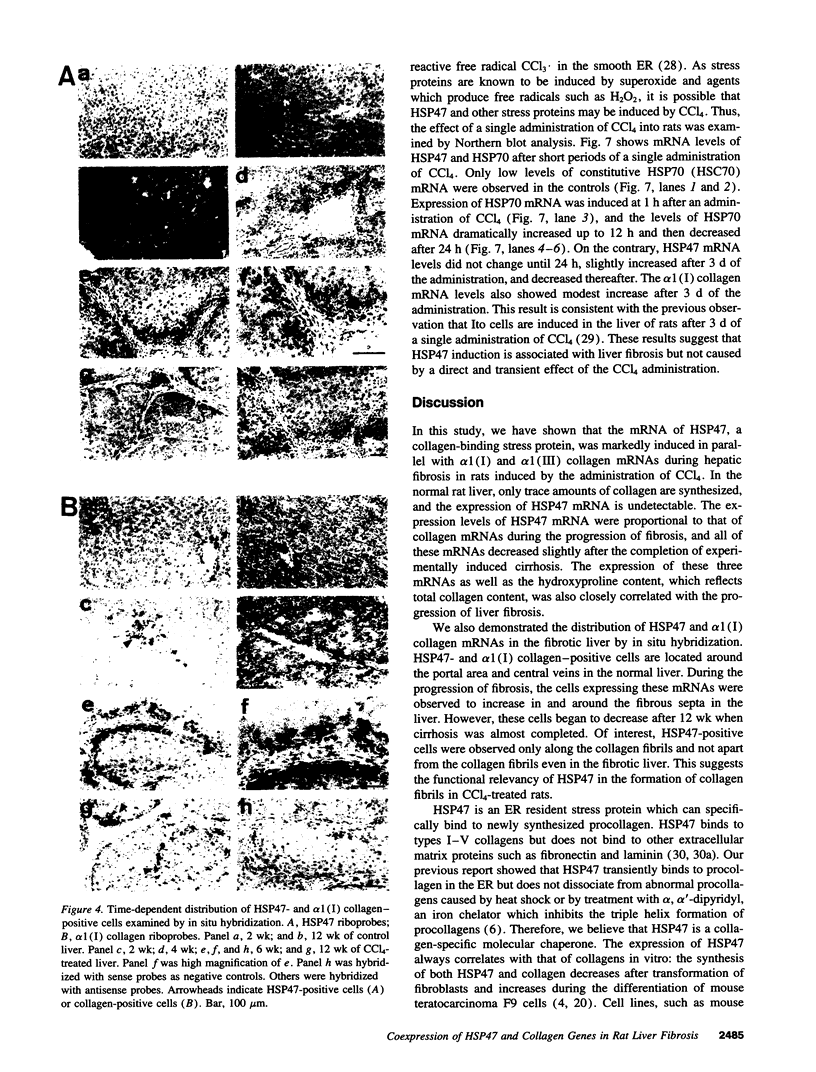

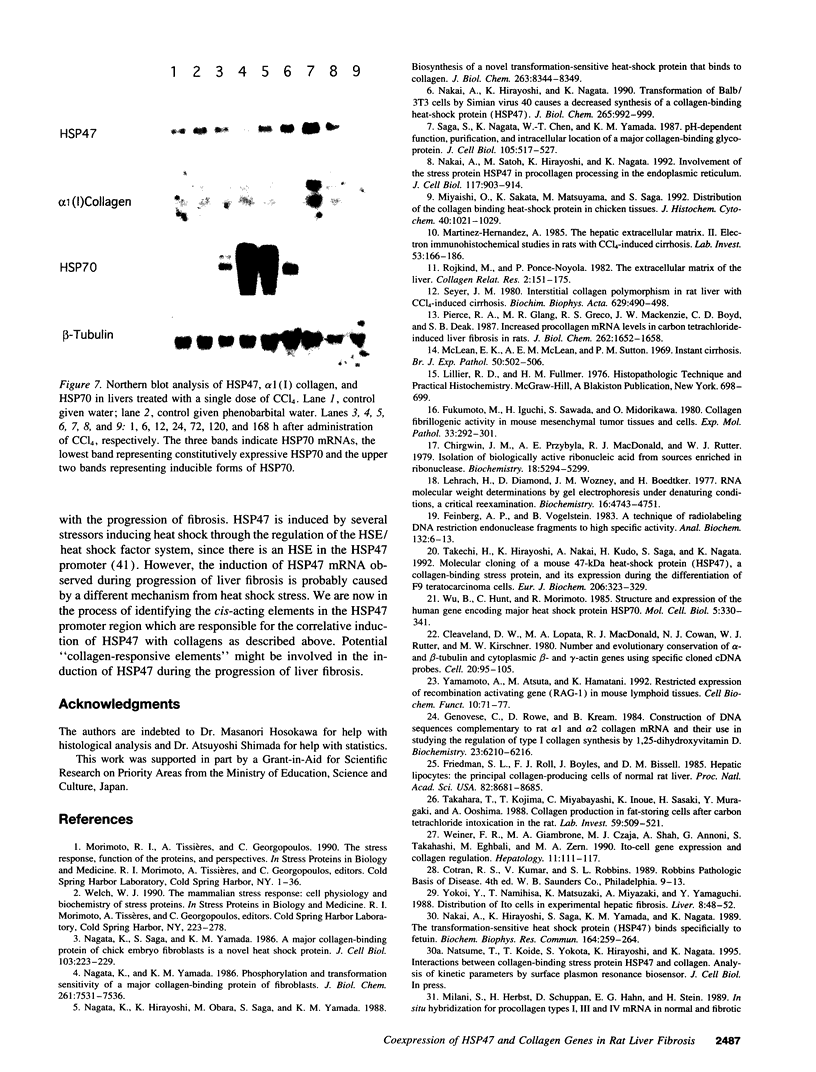

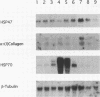
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armendariz-Borunda J., Katayama K., Seyer J. M. Transcriptional mechanisms of type I collagen gene expression are differentially regulated by interleukin-1 beta, tumor necrosis factor alpha, and transforming growth factor beta in Ito cells. J Biol Chem. 1992 Jul 15;267(20):14316–14321. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chojkier M. Hepatocyte collagen production in vivo in normal rats. J Clin Invest. 1986 Aug;78(2):333–339. doi: 10.1172/JCI112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojkier M., Lyche K. D., Filip M. Increased production of collagen in vivo by hepatocytes and nonparenchymal cells in rats with carbon tetrachloride-induced hepatic fibrosis. Hepatology. 1988 Jul-Aug;8(4):808–814. doi: 10.1002/hep.1840080419. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto M., Iguchi H., Sawada S., Midorikawa O. Collagen fibrillogenic activity in mouse mesenchymal tumor tissues and cells. Exp Mol Pathol. 1980 Dec;33(3):292–301. doi: 10.1016/0014-4800(80)90027-1. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Takechi H., Yokota S., Hirayoshi K., Nagata K. Structure of the gene encoding the mouse 47-kDa heat-shock protein (HSP47). Gene. 1993 Apr 30;126(2):187–193. doi: 10.1016/0378-1119(93)90366-b. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- McLean E. K., McLean A. E., Sutton P. M. Instant cirrhosis. An improved method for producing cirrhosis of the liver in rats by simultaneous administration of carbon tetrachloride and phenobarbitone. Br J Exp Pathol. 1969 Oct;50(5):502–506. [PMC free article] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Miyaishi O., Sakata K., Matsuyama M., Saga S. Distribution of the collagen binding heat-shock protein in chicken tissues. J Histochem Cytochem. 1992 Jul;40(7):1021–1029. doi: 10.1177/40.7.1607635. [DOI] [PubMed] [Google Scholar]
- Nagata K., Hirayoshi K., Obara M., Saga S., Yamada K. M. Biosynthesis of a novel transformation-sensitive heat-shock protein that binds to collagen. Regulation by mRNA levels and in vitro synthesis of a functional precursor. J Biol Chem. 1988 Jun 15;263(17):8344–8349. [PubMed] [Google Scholar]
- Nagata K., Saga S., Yamada K. M. A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J Cell Biol. 1986 Jul;103(1):223–229. doi: 10.1083/jcb.103.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Yamada K. M. Phosphorylation and transformation sensitivity of a major collagen-binding protein of fibroblasts. J Biol Chem. 1986 Jun 5;261(16):7531–7536. [PubMed] [Google Scholar]
- Nakai A., Hirayoshi K., Nagata K. Transformation of BALB/3T3 cells by simian virus 40 causes a decreased synthesis of a collagen-binding heat-shock protein (hsp47). J Biol Chem. 1990 Jan 15;265(2):992–999. [PubMed] [Google Scholar]
- Nakai A., Hirayoshi K., Saga S., Yamada K. M., Nagata K. The transformation-sensitive heat shock protein (hsp47) binds specifically to Fetuin. Biochem Biophys Res Commun. 1989 Oct 16;164(1):259–264. doi: 10.1016/0006-291x(89)91711-7. [DOI] [PubMed] [Google Scholar]
- Nakai A., Satoh M., Hirayoshi K., Nagata K. Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J Cell Biol. 1992 May;117(4):903–914. doi: 10.1083/jcb.117.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa H., Evarts R. P., Hsia C. C., Thorgeirsson S. S. Transforming growth factor-beta 1 and type I procollagen transcripts during regeneration and early fibrosis of rat liver. Lab Invest. 1990 Aug;63(2):171–180. [PubMed] [Google Scholar]
- Nakatsukasa H., Nagy P., Evarts R. P., Hsia C. C., Marsden E., Thorgeirsson S. S. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990 Jun;85(6):1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R. A., Glaug M. R., Greco R. S., Mackenzie J. W., Boyd C. D., Deak S. B. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987 Feb 5;262(4):1652–1658. [PubMed] [Google Scholar]
- Rojkind M., Ponce-Noyola P. The extracellular matrix of the liver. Coll Relat Res. 1982 Mar;2(2):151–175. doi: 10.1016/s0174-173x(82)80031-9. [DOI] [PubMed] [Google Scholar]
- Saga S., Nagata K., Chen W. T., Yamada K. M. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J Cell Biol. 1987 Jul;105(1):517–527. doi: 10.1083/jcb.105.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibapa K., Saito M., Umeda M., Enaka K., Tsukada Y. Native collagen formation by liver parenchymal cells in culture. Nature. 1976 Jul 22;262(5566):316–318. doi: 10.1038/262316a0. [DOI] [PubMed] [Google Scholar]
- Seyer J. M. Interstitial collagen polymorphism in rat liver with CCl4-induced cirrhosis. Biochim Biophys Acta. 1980 May 22;629(3):490–498. doi: 10.1016/0304-4165(80)90154-3. [DOI] [PubMed] [Google Scholar]
- Takahara T., Kojima T., Miyabayashi C., Inoue K., Sasaki H., Muragaki Y., Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988 Oct;59(4):509–521. [PubMed] [Google Scholar]
- Takechi H., Hirayoshi K., Nakai A., Kudo H., Saga S., Nagata K. Molecular cloning of a mouse 47-kDa heat-shock protein (HSP47), a collagen-binding stress protein, and its expression during the differentiation of F9 teratocarcinoma cells. Eur J Biochem. 1992 Jun 1;206(2):323–329. doi: 10.1111/j.1432-1033.1992.tb16930.x. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M., Takada A., Takase S. Characterization of desmin-positive rat liver sinusoidal cells. Hepatology. 1987 Mar-Apr;7(2):277–284. doi: 10.1002/hep.1840070212. [DOI] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Atsuta M., Hamatani K. Restricted expression of recombination activating gene (RAG-1) in mouse lymphoid tissues. Cell Biochem Funct. 1992 Jun;10(2):71–77. doi: 10.1002/cbf.290100202. [DOI] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Kuroda H., Komatsu I., Miyazaki A., Watanabe S., Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984 Jul-Aug;4(4):709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Matsuzaki K., Miyazaki A., Yamaguchi Y. Distribution of Ito cells in experimental hepatic fibrosis. Liver. 1988 Feb;8(1):48–52. doi: 10.1111/j.1600-0676.1988.tb00966.x. [DOI] [PubMed] [Google Scholar]