In the first decade of this century, ever-increasing scientific and technological advances are revolutionizing our approaches to developing therapies that bring about the promise of personalized medicine and the possibility of regenerative interventions. Multidisciplinary research has led to a better understanding of four key areas of scientific and technological knowledge that are essential to the development of such innovative therapies for cardiovascular disease: (1) increased understanding of normal development and cell differentiation processes in vivo; (2) elucidating signaling pathways involved in these processes; (3) uncovering new technological approaches that can efficiently mimic these processes in vitro; and (4) most importantly, the identification and characterization of adequate sources of precursor cells that serve as the starting material for regenerative undertakings.
A normal vasculature is crucial for maintaining homeostasis and providing the necessary nutrients to cells of the human body. Therefore, impairment to the integrity of blood vessels will lead to various complications. Cardiac and peripheral vascular diseases have been the major causes of morbidity and mortality in the Western world.1 Currently available therapies rely on the implantation of stents or grafts for reconstruction of blood conduits. However, the availability of suitable venous and arterial grafts for implantation is a challenge, and furthermore these therapies may not be sufficient for complete recovery of function and integrity of the injured vasculature.2 Thus, alternative vascular drains that have the ability to mechanically and biologically fulfill the properties of native vessels are in high demand. Engineered cell-based vascular therapies, which include a combination of vascular cells, scaffolding, and signaling to form biologically active vessels, offer the possibility of permanent and effective treatments for many vascular diseases.
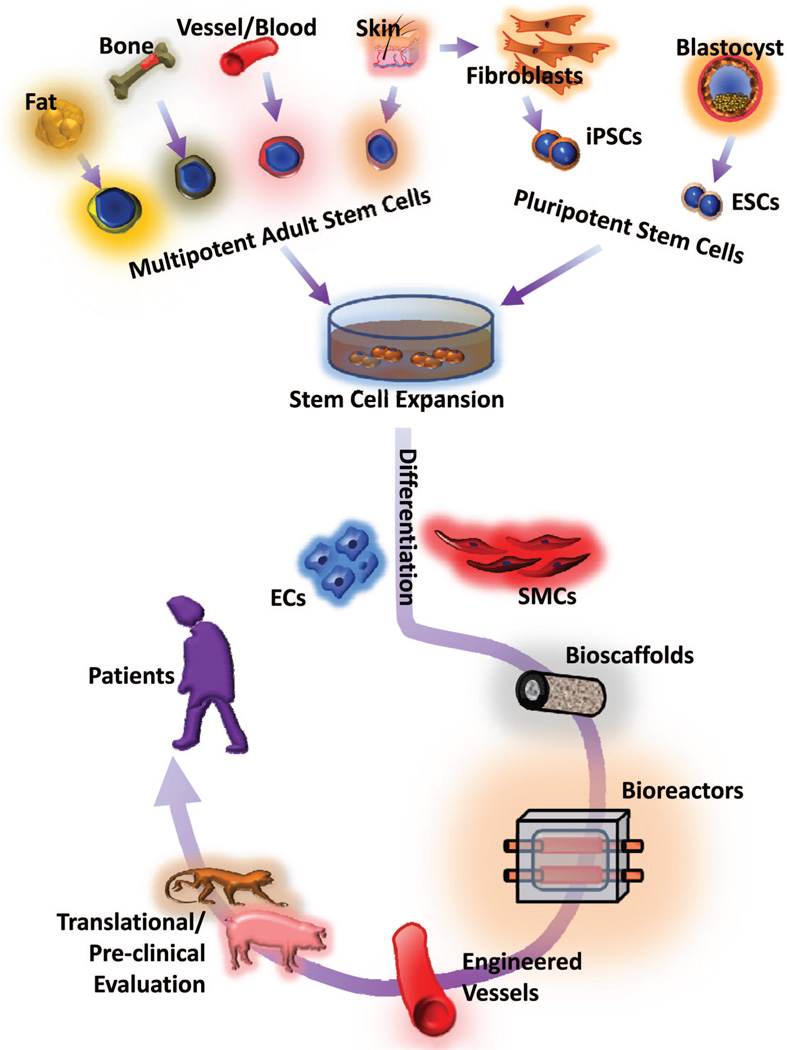
Vascular smooth muscle cells (SMCs), in addition to playing an important role in maintaining viability and activity of the vascular endothelium, also regulate blood pressure in response to various stimuli.3 Therefore, the tunica media, the tissue layer containing functional SMCs, is critical for the successful regeneration of damaged vascular tissue (Figure). Mature vascular SMCs isolated from donor tissue have been used for the construction of tissue-engineered blood vessels. However, primary SMCs from native vessels have a limited capacity for proliferation and expansion, making it necessary to explore alternative sources of SMCs or smooth muscle-like cells.
Figure.
Schematic of vascular engineering in vitro using stem cells for eventual application of engineered vessels to patients. ECs indicates endothelial cells; SMCs, smooth muscle cells; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells.
Through fate-mapping studies, at least 8 independent origins of vascular SMC progenitors have been identified in the developing embryo, indicating substantial variation in derived SMCs and features within the vascular system.4 In addition to the numerous independent origins of vascular SMCs, there are several adult stem cell sources of vascular SMCs for vascular engineering. Those stem cells include2 (1) mesenchymal stem cells (MSCs) from bone marrow, umbilical cord, adipose tissue, vessel wall, and placenta; and (2) endothelial progenitor cells from bone marrow, peripheral blood, umbilical cord blood, adipose tissue, and vessel wall (Figure).
Biopsied skin samples may serve as alternative vascular SMC sources for vascular engineering, as indicated by recent studies.5,6 Specifically, in this issue of ATVB, Steinbach et al provided detailed evidence that skin-derived precursor cells (SKPs) can be manipulated to differentiate into SMCs.6 In this study, adult SKPs derived from different species were differentiated in response to transforming growth factor (TGF) signaling, which is known to direct neural crest stem cells to a SMC lineage. Interestingly, under serum-free conditions, TGFβ-stimulated human SKPs can differentiate into SMCs with high efficiency. However, with respect to enhanced SMC differentiation, rat and human SKPs appear to have different responses to TGF signaling. Most studies have shown that TGF-β reduces MSC proliferation but does not enhance SMC differentiation from MSCs. For example, TGF-β3 was shown to differentiate rat bone marrow MSCs into SMCs.7 Furthermore, human MSCs cultured in the presence of TGF-β1 were also able to differentiate into SMCs.8 However, in terms of directed SMC differentiation, neural crests cell-derived SKPs,9 when compared with MSCs, may respond differently to TGF-β stimulation, in which c-Myb may partially contribute to this differential response.10 The study by Steinbach et al shows that TGF-β not only enhances SMC marker expression in human SKPs, but also promotes SMC proliferation, thus providing a promising source for efficient high-throughput SMC generation and the potential for future application in vascular engineering (Figure).
However, the detailed molecular mechanisms that initiate and control TGFβ-stimulated SKP-directed SMC differentiation are unclear. Thus, a detailed investigation of TGF-β function and expression profiles during the SKP to SMC differentiation process will lead to novel insights into the signaling pathways driving toward SMC differentiation. In addition, the effects of TGF-β on SKP telomerase activity and telomere length, which control the longstanding proliferative activity of cells,11 have not yet been tested. Thus, further understanding of the molecular mechanisms required to maintain and control SKPs is fundamentally important at the basic level and will enable advances in vascular engineering to be more effectively applied for treating disorders of the vessel wall.
Intriguingly, although the data in this study showed that SKP-derived SMCs displayed a high expression of SMC-specific markers, myocardin expression levels were low in SMCs differentiated from human SKPs, which is not consistent with the features of mature vascular SMCs. Thus, it remains to be determined whether in vitro culture conditions alter gene expression profiling in SMCs derived from SKPs. As discussed in the study by Steinbach et al, early expressed myocardin-related transcription factors may be compensating for the function of myocardin in these cells. Myocardin-related transcription factors may facilitate SKP-directed SMC differentiation and subsequent functional maturation even though SKP-derived SMCs maintain a low level of myocardin. Also, the possibility of genetic modification in SKPs has not yet been elucidated, and this could potentially limit the extent of SKP application in certain vascular diseases, particularly those in which a genetic mutation is evident.
More importantly, different vessels, or even different segments of the same vessel, are composed of SMC populations that arise from distinct sources of progenitors, each with its own unique developmental tract.4 Therefore, it is imperative to determine the in vivo short-term and long-term responses of reconstructed vessels containing SKP-derived SMCs and determine whether SKP-derived SMCs retain SKP epigenetic memory. This may impact the physiological processes necessary for proper vascular repair. Moreover, SKP-derived SMCs have yet to be studied for their biocompatibility within appropriate bioactive scaffolds, which will be the next logical step toward translating these findings as a viable therapy for patients afflicted with vascular diseases.
Additionally, other lines of ongoing technological approaches and alternative sources of SMC production for vascular engineering have become increasingly reliant on the utilization of pluripotent stem cells, which include embryonic stem cells and induced pluripotent stem (iPS) cells12 (Figure). iPS technology offers a transformative platform for generating an infinite potential of genetically modifiable and personalized pluripotent cells derived from autologous patient tissue.13 Recent proof-of-principle studies involving second-generation iPS technologies14– 16 and unique pluripotent features of iPS cells are paving the way for the realization of personalized vascular engineering and patient-specific transplantation treatment despite the undeniable remaining challenges regarding the application of personalized iPS cells.2
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusuma S, Gerecht S. Engineering blood vessels using stem cells: Innovative approaches to treat vascular disorders. Expert Rev Cardiovasc Ther. 2010;8:1433–1445. doi: 10.1586/erc.10.121. [DOI] [PubMed] [Google Scholar]
- 3.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 4.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinbach SK, El-Mounayri O, DaCosta RS, Frontini MJ, Nong Z, Maeda A, Pickering JG, Miller FD, Husain M. Directed differentiation of skin-derived precursors into functional vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2938–2948. doi: 10.1161/ATVBAHA.111.232975. [DOI] [PubMed] [Google Scholar]
- 7.Seruya M, Shah A, Pedrotty D, du Laney T, Melgiri R, McKee JA, Young HE, Niklason LE. Clonal population of adult stem cells: Life span and differentiation potential. Cell Transplant. 2004;13:93–101. doi: 10.3727/000000004773301762. [DOI] [PubMed] [Google Scholar]
- 8.Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- 9.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 10.Gadson PF, Jr, Dalton ML, Patterson E, Svoboda DD, Hutchinson L, Schram D, Rosenquist TH. Differential response of mesoderm- and neural crest-derived smooth muscle to tgf-beta1: Regulation of c-myb and alpha1 (i) procollagen genes. Exp Cell Res. 1997;230:169–180. doi: 10.1006/excr.1996.3398. [DOI] [PubMed] [Google Scholar]
- 11.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie C, Ritchie RP, Huang H, Zhang J, Chen YE. Smooth muscle cell differentiation in vitro: Models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol. 2011;31:1485–1494. doi: 10.1161/ATVBAHA.110.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. Piggybac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]