Abstract
High-mobility group box 1 (HMGB1) is a nuclear protein that has been implicated in the myocardial inflammation and injury induced by ischemia-reperfusion (I/R). The purpose of the present study was to assess the role of HMGB1 in myocardial apoptosis induced by I/R. In vivo, myocardial I/R induced an increase in myocardial HMGB1 expression and apoptosis. Inhibition of HMGB1 (A-box) ameliorated the I/R-induced myocardial apoptosis. In vitro, isolated cardiac myocytes were challenged with anoxia-reoxygenation (A/R; in vitro correlate to I/R). A/R-challenged myocytes also generated HMGB1 and underwent apoptosis. Inhibition of HMGB1 attenuated the A/R-induced myocyte apoptosis. Exogenous HMGB1 had no effect on myocyte apoptosis. However, inhibition of HMGB1 attenuated myocyte TNF-α production after the A/R was challenged; surprisingly, HMGB1 itself did not induce myocyte TNF-α production. Exogenous TNF-α induced a moderate proapoptotic effect on the myocytes, an effect substantially potentiated by coadministration of HMGB1. It is generally accepted that apoptosis induced by TNF-α is regulated by the balance of activation of c-Jun NH2-terminal kinase (JNK) and NF-κB. Indeed, in the present study, TNF-α increased the phosphorylation status of JNK and p65, a subunit of NF-κB; HMGB1 greatly potentiated TNF-α-induced JNK phosphorylation. Furthermore, inhibition of JNK (SP-600125) prevented the myocyte apoptosis induced by a TNF-α/HMGB1 cocktail. Finally, A/R increased HMGB1 production in both wild-type and toll-like receptor 4-deficient myocytes; however, deficiency in toll-like receptor 4 diminished A/R-induced myocyte apoptosis, TNF-α, and JNK activation. Our results indicate that myocyte-derived HMGB1 and TNF-α work in concert to promote I/R-induced myocardial apoptosis through JNK activation.
Keywords: cardiomyocyte, cell death, high-mobility group box 1/toll-like receptor 4 axis, cytokine
the most efficient means of limiting ischemia-induced cardiomyocyte death (necrosis and apoptosis) is to reperfuse the myocardium. However, reperfusion itself causes injury to the myocardium, including induction of myocardial apoptosis (3, 9). Therefore, understanding the mechanism(s) involved in reperfusion injury is critical to reduce or eliminate reperfusion-induced injury.
High-mobility group box 1 (HMGB1) protein is an alarmin that is released from activated immune cells, as well as stressed and/or necrotic cells, including cardiac myocytes, in response to tissue injury (1, 19, 32, 34). It has been shown to play an important role in ischemia-reperfusion (I/R)-induced injury to the brain, liver, and heart (1, 17, 30). In all of these organs, inhibition of HMGB1 offers protection against I/R-induced infarction and cell necrosis, whereas exogenous administration of HMGB1 worsens I/R-induced injury (1, 17, 30). In myocardial I/R, HMGB1 has been proposed to be the link between initial myocyte necrosis and/or activated immune cells (e.g., macrophages) and the myocardial infarction induced by I/R (1). However, in addition to myocyte necrosis, I/R also induces myocyte apoptosis (2, 7, 18). To our knowledge, the role of HMGB1 in I/R-induced myocyte apoptosis has not been evaluated. Thus the major objective of the present study was to assess the role of HMGB1 in I/R-induced myocyte apoptosis in vivo and to address the mechanisms involved using an in vitro model, specifically anoxia-reoxygenation (A/R) of isolated cardiac myocytes. We show that I/R-induced myocardial apoptosis and A/R-induced cardiomyocyte apoptosis can be substantially blunted by inhibition of HMGB1. To our knowledge, this is the first demonstration that HMGB1 can modulate I/R- or A/R-induced myocyte apoptosis and prompted us to assess some of the potential mechanisms involved using the in vitro model of A/R-challenged myocytes.
I/R induces free radical generation and initiates a cytokine cascade within the affected tissue and further leads to downstream events, including apoptosis(8, 14). One of the cytokines being studied extensively in I/R-induced injury is TNF-α. However, the role of the TNF-α in I/R-induced injury is still ambivalent. The general consensus held is that excessive TNF-α after I/R activates TNF-α receptor type 1 (TNFR1), which causes injury, while interaction of basal levels of TNF-α with its type 2 receptor (TNFR2) offers protection to myocardium from I/R injury (14, 15, 20). With respect to apoptosis, it is generally believed that TNF-α interaction with TNFR1 induces apoptosis by activation of c-Jun NH2-terminal kinase (JNK) (6, 28). However, it has been reported that treatment of cells with TNF-α can activate NF-κB (31), which could lead to antiapoptotic gene (e.g., SOD) expression (26). Thus TNF-α-induced apoptosis is strongly regulated by the balance of activation of JNK and NF-κB (16, 31). In the present study, we assessed cross talk between the HMGB1 and TNF-α/JNK/NF-κB pathway in the myocardial apoptosis induced by I/R. Our in vitro studies indicate that HMGB1 does not directly induce apoptosis and TNF-α production in A/R-challenged myocytes. Furthermore, our findings indicate that 1) TNF-α production in myocytes with A/R was amplified by HMGB1, and 2) HMGB1 potentiates TNF-α-induced myocyte apoptosis by greatly potentiating the TNF-α-induced JNK activation and only slight potentiating effect on NF-κB activation.
MATERIALS AND METHODS
Mice
C57BL/6 mice were obtained from Charles River Canada (St. Constant, PQ, Canada). Mice deficient in toll-like receptor 4 (TLR4−/− on C57BL/10 background, stock no. 03752) purchased from Jackson laboratories (Bar Harbor) were further backcrossed to C57BL/6 background. The mice were housed in Victoria Research Laboratories Vivarium Service with a 12:12-h light-dark cycle and free access to rodent chow and tap water. The mice were used for in vivo experiments, as well as a source for cardiac myocytes for in vitro experiments. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The experimental protocols were reviewed and approved by the University of Western Ontario Animal Care and Use Committee (protocol no. 2008-053).
Neonatal Cardiac Myocytes
Neonatal cardiac myocytes were isolated and cultured as previously described (24, 26, 34). Briefly, hearts were harvested, minced, and digested with collagenase II (160 U/ml, Worthington). After a washing step, the cells were suspended in M199 with 10% fetal calf serum. The myocytes were enriched by a preplating approach (to remove contaminating cells, e.g., endothelial cells, myocardial fibroblasts) before being seeded into cell culture plates. After 72 h in culture, the cells had formed a confluent monolayer, consisting of 95% cardiomyocytes beating in synchrony, and were used in experiments at this time.
I/R model.
The mouse model of myocardial I/R involved ligation of the left anterior descending artery, as described previously (25). Briefly, mice were anesthetized with ketamine (150 mg/kg) and xylazine (5 mg/kg) subcutaneously, given atropine (0.05 mg sc) to reduce airway secretions, and artificially ventilated. Postthoracotomy, the LAD was occluded using a suture with a piece of tubing interposed between the artery and suture, and the thorax was closed. Thirty minutes later, the thorax was reopened, the tubing removed, and the suture cut. The thorax was closed again, and the heart allowed to reperfusion for 24–48 h. Myocardial HMGB1 expression and myocardial apoptosis were assessed. As a control, sham-operated mice underwent the same surgical procedure, except for arterial occlusion. In some experiments, A-box (600 μg/mouse) was given intraperitoneally 2 and 24 h after the I/R protocol. This dose of A-box has previously been used in animal models of sepsis (34, 36).
A/R model.
As an in vitro correlate to I/R, isolated cardiac myocytes were exposed to A/R, as described previously (24, 25, 34). Briefly, confluent beating mouse cardiac myocyte monolayers were exposed to anoxia for 30 min and then reoxygenated for up to 48 h. As a control, cardiac myocytes were exposed to normoxia instead of anoxia (normoxia-reoxygenation). In some experiments, A-box (10 μg/ml) (11) was given to myocytes 2 and 24 h after reoxygenation.
Assays
HMGB1 expression.
HMGB1 expression in myocardial tissue was evaluated by immunohistochemistry (34). In brief, the myocardial tissue was processed for paraffin embedding. After deparaffinization, rehydration, and antigen retrieval with sodium citrate (pH 6.0), tissue sections were probed with a rabbit anti-mouse HMGB1 primary antibody. The HMGB1 protein was identified using an ABC system (Vectastain universal ABC kit) in accordance with the manufacturer's instructions, followed by an assessment with a microscope (Zeiss Axiovert 200M) for myocardial HMGB1 expression. Myocardial hematoxylin and eosin (H&E) staining was performed using a standard protocol.
The expression of HMGB1 in cardiac myocytes was assessed by Western blot of cardiomyocyte lysates, as previously described (34). Using a rabbit anti-mouse HMGB1 polyclonal antibody, specific bands were visualized with an ECL plus detection kit. The releasing of HMGB1 by cardiac myocytes was measured by collecting the supernatants and using a two-step sandwich ELISA kit, according to the manufacturer's instructions (34).
TNF-α.
Cardiomyocyte releasing of TNF-α was determined by collecting supernatant and was measured with a mouse TNF-α ELISA set (34).
Myocardium/cardiomyocyte apoptosis.
Myocardium apoptosis was assessed with an in situ cell death detection kit (Roche), and cardiomyocyte apoptosis was assessed with both caspase-3 activity and cell death ELISA assay kits (Roche), according to the manufacturer's instructions (5, 12).
JNK and p65 phosphorylation.
Lysates of myocytes were resolved on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blocked (5% nonfat milk) membranes were used for detection of phosphorylated and total JNK or p65 with specific antibodies, respectively. Myocyte JNK or p65 phosphorylation status was expressed as a ratio of phosphorylated to total JNK or p65 protein (37).
Reagents
Collagenase type II was from Worthington Biochemical, Lakewood, NJ; recombinant full length HMGB1 and anti-mouse HMGB1 polyclonal antibody were from ABCAM, Cambridge, MA (catalog no. ab18256); polyvinylidene difluoride membrane was from Bio-Rad Laboratories, Mississauga, ON; ECL plus Western blotting detection kit was from GE Healthcare, Mississauga, ON; HMGB1 ELISA kit was from Shino-Test; mouse TNF-α ELISA set was from BD Biosciences; antibodies against phosphorylated and total JNK (catalog nos. 4671S and 9258S) and phosphorylated and total p65 (catalog nos. 3031S and 3034) were from Cell Signaling Technology, Danvers, MA; and SP-600125 was from EMD Chemicals, Gibbstown, NJ (catalog no. 420119).
The HMGB1 inhibitor, A-box, was generated as previously described (11, 34). Briefly, plasmid (pGEX-5X-2) containing coding for A-box (a gift of Dr. C.-Y. Wang, Medical College of Georgia) or control plasmid was transformed into E. coli BL21 (DE3) and incubated in 2YT medium containing ampicillin (100 mg/ml) for 3–4 h at 37°C. Fusion protein A-box or glutathione S-transferase was induced by 0.5 mM isopropyl d-thiogalctopyranoside and purified by using Glutathione Sepharose affinity column. The purified A-box or glutathione S-transferase was passed over polymyxin B columns to remove any contaminating LPS.
Statistical Analysis
All data are expressed as means ± SE. Statistical analysis was performed using ANOVA and Student's t-test (with a Bonferroni correction for multiple comparisons).
RESULTS
HMGB1 Contributes to I/R- and A/R-induced Cardiomyocyte Apoptosis
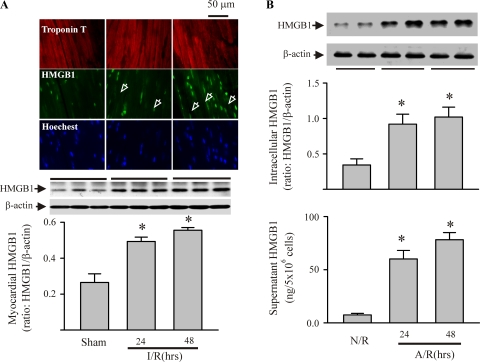
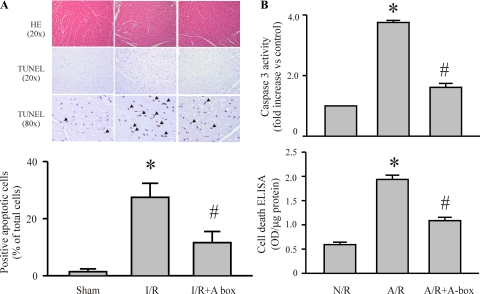
To determine the role of HMGB1 in I/R-induced myocardial injury, we first assessed the HMGB1 expression in myocardium after I/R challenge and in isolated cardiac myocytes after an A/R challenge (an in vitro correlate of I/R). As shown in Fig. 1A, myocardial HMGB1 expression was increased at 24 and 48 h after an I/R challenge. These findings are in accordance with previous observations in a similar mouse model of myocardial I/R (1). As shown in Fig. 1B, cardiomyocytes had increased intracellular levels of HMGB1 and had released HMGB1 into the surrounding media 24 and 48 h after an A/R challenge. Furthermore, I/R induced an increase in myocyte apoptosis in the ischemic myocardium (area at risk), and inhibition of HMGB1 (A-box) attenuated the I/R-induced apoptosis (Fig. 2A). In addition, A/R challenge of cardiac myocytes resulted in increased caspase-3 activity and apoptotic cell death, which was largely prevented by the HMGB1 inhibitor (Fig. 2B). To our knowledge, this is the first demonstration that myocardial HMGB1 production may play a role in I/R-induced myocardial apoptosis.
Fig. 1.
Ischemia-reperfusion (I/R)-induced myocardial expression of high-mobility group box 1 (HMGB1) in vivo and anoxia-reoxygenation (A/R)-induced myocyte production and release of HMGB1 in vitro. A: mice were challenged with I/R or sham procedures. Mouse hearts were harvested 24 and 48 h after the reperfusion for detection of HMGB1 protein by immunohistochemistry and Western blot. Top: immunohistochemistry using fluorescence microscopy. Troponin T-antibody targeted myocytes, Hoechst targeted nuclei, and HMGB1-antibody targeted HMGB1. Images are representatives of three separate experiments (original magnification ×63). Arrowheads indicate cytoplasmic HMGB1. Middle and bottom: Western blot assessment for myocardial HMGB1: exact blots (middle), and densitometric analyses (bottom). Values are means ± SE; n = 3 mice/group. *P < 0.05 compared with sham. B: isolated cardiac myocytes were challenged with A/R or normoxia-reoxygenation (N/R). Myocytes and their supernatants were harvested 24 and 48 h after reoxygenation for evaluation of HMGB1 expression with Western blot (top) and release of HMGB1 extracellularly by ELISA (bottom). For the Western blot assay, representative blots are shown at top, and densitometry analyses are shown at bottom. Values are means ± SE; n = 4. *P < 0.05 compared with N/R.
Fig. 2.
Inhibition of HMGB1 attenuates I/R-induced myocardial apoptosis in vivo and myocyte apoptosis in vitro. A: mice were challenged with I/R and A-box (600 μg/mouse), or saline was given (intraperitoneally) 2 and 24 h after reperfusion. The hearts were harvested 48 h after reperfusion, and sections were prepared for myocardial hematoxylin and eosin (HE) staining and corresponding immunohistochemical staining for myocardial apoptosis (in situ cell death analyses), as described in the text. Top: sections at are representatives of HE and in situ cell death analyses in the area at risk of left ventricle. Arrowheads indicate apoptotic cells. Bottom: quantitative analysis of myocardial apoptosis is shown in the histogram. For each section, 10 high-power fields were counted for apoptotic cells. Values are means ± SE; n = 3 hearts/group. *P < 0.05 compared with sham. #P < 0.05 compared with I/R. TUNEL, terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling. B: cardiac myocytes were challenged with N/R or A/R, and A-box (10 μg/ml) was added to the myocytes 2 h after reoxygenation. Cardiomyocyte apoptosis was assessed 48 h later by measuring caspase-3 activity and using a cell death ELISA kit. OD, optical density. Values are means ± SE; n = 3. *P < 0.05 compared with N/R; #P < 0.05 compared with A/R.
A/R-challenged Myocytes Secrete TNF-α and HMGB1 Potentiates TNF-α-induced Cardiomyocyte Apoptosis
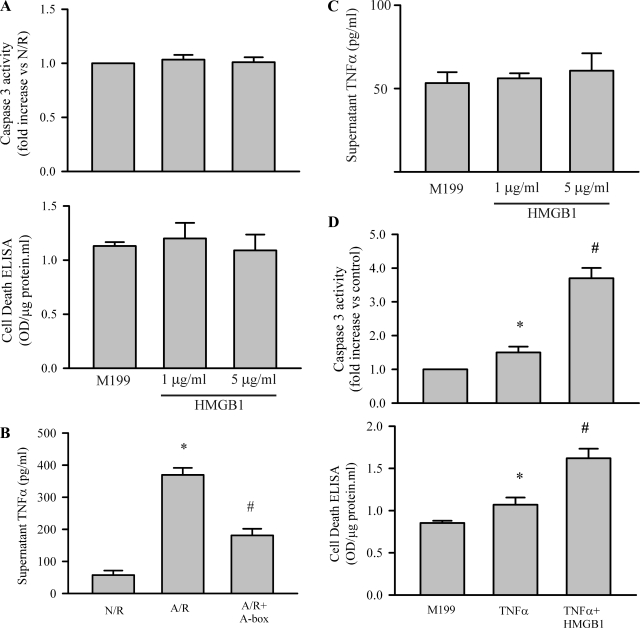
The results presented in Figs. 1 and 2 indicate that A/R challenge of cardiomyocytes can be a useful model to study the cellular mechanisms in myocyte apoptosis, and the results obtained should be relevant to the I/R-induced myocyte apoptosis noted in vivo. A previous study indicated that HMGB1 could induce apoptosis in PC12 cells in a dose-dependent manner (1–5 μg/ml) (13). However, exogenous administration of HMGB1 (1 and 5 μg/ml) to cardiac myocytes did not affect caspase-3 activity or apoptotic cell death (Fig. 3A), indicating that HMGB1 was not directly inducing myocyte apoptosis. This finding suggests that other factors generated by myocytes after an A/R challenge must be involved. Previous studies indicate that myocardial I/R increases TNF-α, and this cytokine is the prototype ligand for the receptor-mediated apoptotic pathway (2, 16, 23). Thus we assessed whether myocytes challenged with A/R could generate TNF-α. As shown in Fig. 3B, myocytes increased TNF-α production after A/R, which was attenuated by A-box. Similar to a previous report (1), administration of HMGB1 (1 and 5 μg/ml) to cardiac myocytes did not increase myocyte TNF-α production (Fig. 3C). As shown in Fig. 3D, challenge of cardiac myocytes with TNF-α slightly increased myocyte caspase-3 activity and apoptotic cell death (25–50%). Furthermore, the proapoptotic effects of TNF-α were significantly potentiated by HMGB1 (100–300%).
Fig. 3.
Interaction of HMGB1 and TNF-α in the A/R-induced myocyte apoptosis. A: HMGB1 was incubated with isolated cardiomyocytes at the doses indicated for 24 h, and apoptosis was assessed with caspase-3 activity and a cell death ELISA kit. n = 3. B: cardiomyocytes were challenged with N/R or A/R, with or without A-box (10 μg/ml), which was added to the myocytes 2 h after reoxygenation. Releasing of TNF-α into the supernatant was measured 48 h after reoxygenation using a mouse TNF-α ELISA assay set. n = 3. *P < 0.05 compared with N/R. #P < 0.05 compared with A/R. C: cardiomyocytes were treated with HMGB1 as indicated in A for 24 h, and myocyte TNF-α production was assessed. D: cardiomyocytes were exposed to M199 or M199 containing either TNF-α (40 ng/ml) or TNF-α with HMGB1 (1 μg/ml) for 24 h. Subsequently, cardiomyocyte apoptosis was determined. n = 4. *P < 0.05 compared with M199. #P < 0.05 vs TNF-α. Values are means ± SE.
Modulation of TNF-α-induced Myocyte Apoptosis and the JNK and NF-κB Pathways by HMGB1
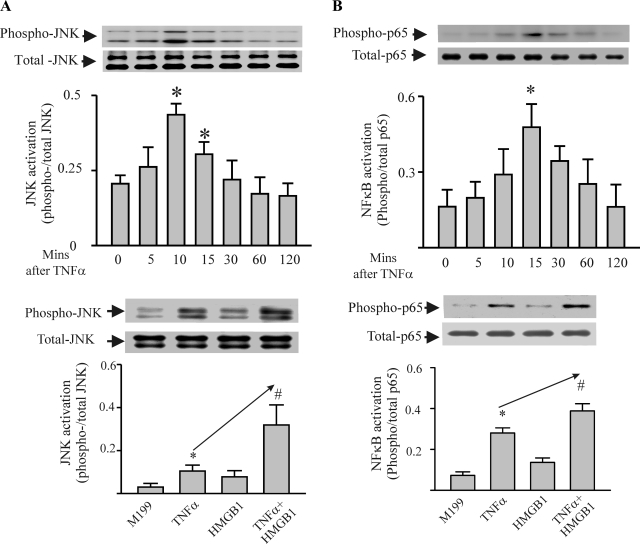
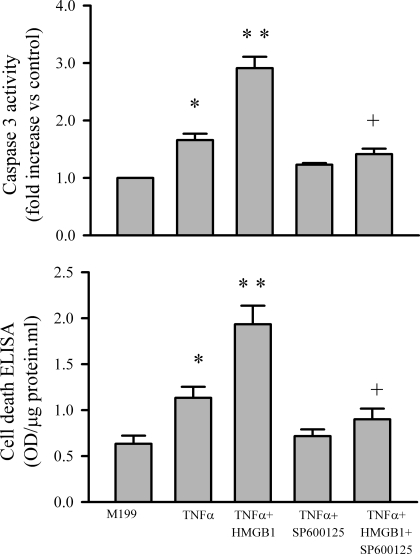
It is generally agreed that excessive TNF-α induces apoptosis by activation of JNK (6, 28). It is also believed that TNF-α is a potent activator of NF-κB, which is antiapoptotic (31). In this study, we assessed whether the HMGB1 could modulate the effect of TNF-α through the JNK and NF-κB. As shown in Fig. 4A, TNF-α increased phosphorylation of JNK (Fig. 4A, top) in cardiomyocytes, which was potentiated by HMGB1, although exogenous HMGB1 itself did not alter phosphorylation status of JNK (Fig. 4A, bottom). The HMGB1/TNF-α cocktail increased myocyte JNK phosphorylation by 300% compared with TNF-α only. As shown in Fig. 4B, TNF-α also activated NF-κB of myocytes, as indicated by increased p65 (a subunit of NF-κB) (top). However, HMGB1/TNF-α cocktail only increased NF-κB activation by <50% compared with TNF-α treatment (bottom). To further demonstrate that the potentiation effect of HMGB1 was mediated by JNK, myocytes were pretreated by JNK inhibitor (SP-600125, 10 μM). As shown in Fig. 5, inhibition of JNK prevented myocyte apoptosis induced by TNF-α or TNF-α/HMGB1 cocktail.
Fig. 4.
HMGB1 significantly potentiates TNF-α-induced activation of JNK, but not NF-κB in cardiac myocytes. A, top: time course of TNF-α-induced activation of JNK (JNK phosphorylation). TNF-α (40 ng/ml) was added to cardiomyocytes, and, at the times indicated, the myocytes were harvested for assessment of the phosphorylation status of JNK by Western blot. n = 3. *P < 0.05 compared with time 0. Bottom: cardiac myocytes were treated with M199 or one of TNF-α (40 ng/ml), HMGB1 (1 μg/ml), or TNF-α/HMGB1 cocktail for 10 min. The cells were harvested for assessment of the phosphorylation status of JNK by Western blot. n = 3. *P < 0.05 compared with M199. #P < 0.05 compared with TNF-α. B, top: time course of TNF-α-induced activation of NF-κB (p65 phosphorylation). Cardiac myocytes were treated with TNF-α as above and harvested for assessment of the phosphorylation status of p65 by Western blot at the times indicated. n = 3. *P < 0.05 compared with time 0. Bottom: cardiac myocytes were treated with M199 or one of TNF-α (40 ng/ml), HMGB1 (1 μg/ml), or TNF-α/HMGB1 cocktail for 15 min. The cells were harvested for assessment of the phosphorylation status of p65 by Western blot. n = 3. *P < 0.05 compared with M199. #P < 0.05 compared with TNF-α. Values are means ± SE.
Fig. 5.
Effect of JNK inhibition on TNF-α or TNF-α/HMGB1-induced myocyte apoptosis. Cardiomyocytes were pretreated with the JNK inhibitor (SP-600125; 10 μM) for 30 min and subsequently exposed to M199 or M199 containing either TNF-α (40 ng/ml) or TNF-α/HMGB1 (1 μg/ml) cocktail. After a 24-h incubation period, cardiomyocyte apoptosis was assessed with caspase-3 activity (top) and a cell death ELISA kit (bottom). Values are means ± SE; n = 3. *P < 0.05 compared with M199. **P < 0.05 compared with TNF-α. +P < 0.05 compared with TNF-α + HMGB1.
Role of HMGB1/TLR4 Axis in A/R-induced Induction of Myocyte Apoptosis
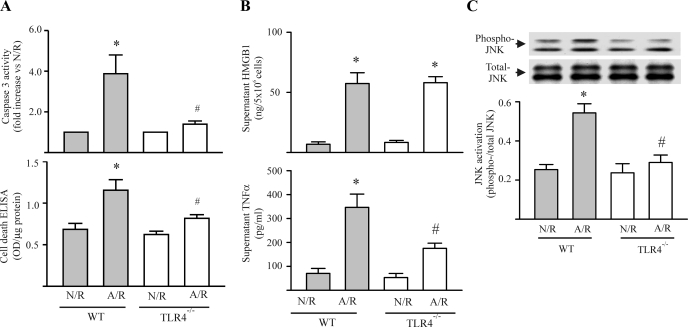
It has been proposed that TLR4 is an important mediator of I/R injury in a variety of organs (10, 29, 33). Deletion of TLR4 attenuates the I/R-induced myocardial injury (10, 38). Since TLR4 is one of the receptors with which HMGB1 can interact (19, 21), we addressed whether HMGB1 was inducing myocyte apoptosis via the TLR4 receptor. As shown in Fig. 6A, the A/R-induced increase in caspase-3 activity and apoptotic cell death was prevented when myocytes derived from TLR4-deficient (TLR4−/−) mice were used in the assays. We further measured HMGB1 and TNF-α production by myocytes after A/R challenge. As shown in Fig. 6B, A/R increased HMGB1 production in wild-type and TLR4−/− cardiomyocytes. However, compared with myocytes derived from wild-type mice, TNF-α production was attenuated in TLR4−/− myocytes. Furthermore, as shown in Fig. 6C, A/R-induced JNK activation was diminished when the cardiomyocytes were derived from TLR4−/− mice. These findings indicate that the HMGB1/TLR4 axis is critical to the A/R-induced induction of myocyte apoptosis.
Fig. 6.
The A/R-induced myocyte apoptosis is dependent on myocyte toll-like receptor 4 (TLR4). Wild-type (WT) and TLR4-deficient (TLR4−/−) cardiomyocytes were challenged with N/R or A/R, and myocyte apoptosis (A), HMGB1 and TNF-α production (B), and JNK phosphorylation (C) were assessed. A: cardiomyocyte apoptosis was determined 48 h after the reoxygenation. TLR4 deficiency attenuated A/R-induced myocyte apoptosis. n = 3. *P < 0.05 compared with WT-N/R. #P < 0.05 compared with WT-A/R. B: myocyte HMGB1 and TNF-α production were determined 24 h after reoxygenation. Top: A/R challenge to myocytes increase HMGB1 production in both WT and TLR4-deficient myocytes. Bottom: A/R-induced TNF-α production by cardiomyocytes was attenuated by deficiency in TLR4. n = 3. *P < 0.05 compared with N/R within each group. #P < 0.05 compared with WT-A/R. C: JNK phosphorylation was determined in A/R-challenged cardiomyocytes 10 min after the reoxygenation. TLR4 deficiency attenuated A/R-induced myocyte JNK phosphorylation. n = 3. *P < 0.05 compared with WT-N/R. #P < 0.05 compared with WT-A/R. Values are means ± SE.
DISCUSSION
HMGB1 has been implicated in I/R-induced injury to the brain, liver, and heart (1, 17, 30). In myocardial I/R, HMGB1 has been proposed to be the link between initial myocyte necrosis and/or activated macrophages and the development of myocardial infarction (1). However, another important feature of I/R-induced myocardial injury is myocyte apoptosis (4, 7). To our knowledge, the modulatory role of HMGB1 on I/R-induced apoptosis has not been assessed in the heart, or for that matter any other major organ system. Herein, we show that inhibition of HMGB1 substantially blunts I/R-induced myocyte apoptosis in a mouse model of I/R (Fig. 2A) and isolated cardiomyocytes challenged with A/R (Fig. 2B). Furthermore, we provide the following lines of evidence to indicate that HMGB1 may serve to potentiate TNF-α-induced apoptosis in A/R-challenged myocytes. First, HMGB1 does not directly induce apoptosis (Fig. 3A). Second, HMGB1 plays a role in TNF-α production by myocytes challenged with A/R (Fig. 3B). Third, TNF-α-induced myocyte apoptosis is potentiated by HMGB1 via greater impact on activation of JNK than on NF-κB (Figs. 4 and 5). Finally, our results indicate that HMGB1 was involved in the myocyte apoptosis-induced by A/R through interaction with TLR4 (Fig. 6).
In the heart, the I/R-induced HMGB1 production by myocytes has been linked to an inflammatory pathway (e.g., macrophage mediated) that exacerbates myocardial injury (1). We have previously shown that an A/R challenge can convert myocytes to a proinflammatory phenotype, i.e., they generate inflammatory mediators, which promote polymorphonuclear neutrophil transendothelial migration (24). Herein, we noted that A/R-challenged myocytes could also generate HMGB1 and TNF-α (Figs. 1 and 3B). Indeed, inhibition of HMGB1 significantly decreased the TNF-α production by cardiomyocytes after A/R (Fig. 3B). This finding suggests that HMGB1 derived from ischemic myocytes amplifies myocyte TNF-α production. While this is one of the possible mechanisms in myocardial apoptosis by HMGB1, it is also possible that HMGB1 could have impacts on infiltrated inflammatory cells (e.g., macrophages) after I/R. The increased TNF-α production by macrophages in the presence of HMGB1 could exaggerate I/R-induced myocardial injury (1).
It is generally believed that excessive TNF-α could induce myocyte apoptosis by activation of JNK signaling (16, 31). On the other hand, TNF-α could turn on antiapoptotic genes (e.g., SOD) through activation of NF-κB (26, 31). Thus the effect of TNF-α on myocyte apoptosis is mainly dependent on the balance of the JNK and NF-κB activation. Our data indicate myocyte apoptosis induced by TNF-α alone was relatively minor, but the degree of myocyte apoptosis was much more dramatic in the presence of both HMGB1 and TNF-α (Fig. 3D). Although TNF-α could activate both JNK and NF-κB (31), we found HMGB1 greatly potentiated the TNF-α-induced JNK activation, while only showing a mild impact on NF-κB activation. Thus the net effect of HMGB1 is to promote myocardial apoptosis. Since both HMGB1 and TNF-α are produced in the I/R-challenged myocardium (1, 8, 22), our findings suggest that they could work in concert to promote myocyte apoptosis in vivo. Previous studies indicate that the proinflammatory effects of HMGB1 are a result of binding with the cytokine, IL-1β (27). However, the binding of HMGB1 to TNF-α appears to be minimal (27), and thus an extracellular interaction between HMGB1 and TNF-α is not a likely explanation for our findings. A cross talk between intracellular pathways by receptors for HMGB1 and TNF-α is most likely based on myocytes deficient in TLR4, challenged with an A/R, which resulted in much less apoptosis (Fig. 6).
HMGB1 can interact with toll-like receptors (e.g., TLR4) and the receptor for advanced glycation end products (RAGE). A previous study indicated that the myocardial inflammation and injury sustained after an I/R challenge could be attributed to HMGB1 interacting with RAGE (1). Since wild-type, but not RAGE−/−, macrophages could generate inflammatory cytokines in response to HMGB1, it was proposed that HMGB1/RAGE axis played an important role in the I/R-induced myocardial inflammation and subsequent injury. In the present study, we also noted that the A/R-induced myocyte apoptosis is most likely dependent on the HMGB1/TLR4 axis (Figs. 2 and 6). One possible explanation is that HMGB1 interaction with macrophages requires RAGE, whereas HMGB1 interaction with myocytes requires TLR4. This seems unlikely in view of studies indicating that HMGB1 can interact with TLR4 on macrophages, and the HMGB1/TLR4 axis is involved in macrophage cytokine production (35). Until further studies unravel the precise role of the different HMGB1 receptors in cellular responses, both RAGE and TLR4 should be considered in the overall myocyte injury (both necrosis and apoptosis), incurred after an I/R challenge in vivo.
Previous studies have demonstrated that TLR4 is pivotal in I/R-induced myocardial injury (10, 38). Deletion of TLR4 blunts I/R-induced p38 and JNK signaling, while augmenting ERK and AMPK signaling, resulting in protection to myocardium from I/R injury (38). In addition, it has been identified that TLR4 deficiency results in activation of PI3K/Akt pathway, thus reducing myocardial injury after I/R (10). In the present study, we addressed the role of HMGB1 in myocardial injury induced by I/R. We noted deficiency in TLR4 in cardiomyocytes prevented the A/R-induced JNK activation (Fig. 6C). This finding agrees with the previous report that deletion of TLR4 alters A/R-induced activation of cell-signaling components (10). However, our results do not support that HMGB1 is involved in A/R-induced activation of cell signaling component in the early phase of A/R. We noted A/R-induced activation of JNK occurred 10 min after A/R, whereas increase in HMGB1 expression by myocytes did not happen until 24 h after the A/R.
Collectively, our findings indicate that HMGB1 plays an important role in myocyte apoptosis induced by I/R in vivo and A/R in vitro. Further, the HMGB1 released from A/R-challenged myocytes is involved in myocyte TNF-α generation. Subsequently, HMGB1 interacts with TLR4, and HMGB1 and TNF-α work in concert to induce myocyte apoptosis by greatly activating JNK.
GRANTS
The study was supported by grants from Heart and Stroke Foundation of Ontario (NA-6316) and the Canadian Institutes of Health Research (MOP-81303) to T. Rui.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117: 3216–3226, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol 38: 47–62, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, Song F, Qu W, Gomez T, Zou YS, Yan SF, Schmidt AM, Ramasamy R. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation 113: 1226–1234, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol 14: 170–175, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res 72: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J 26: 1257–1267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res 61: 414–426, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Gross GJ, Auchampach JA. Reperfusion injury: does it exist? J Mol Cell Cardiol 42: 12–18, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol 178: 7317–7324, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, Wang S, Zheng X, Wang CY, Gong F. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am J Transplant 7: 799–808, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Kewalramani G, Puthanveetil P, Wang F, Kim MS, Deppe S, Abrahani A, Luciani DS, Johnson JD, Rodrigues B. AMP-activated protein kinase confers protection against TNF-α-induced cardiac cell death. Cardiovasc Res 84: 42–53, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Kikuchi K, Kawahara K, Biswas KK, Ito T, Tancharoen S, Morimoto Y, Matsuda F, Oyama Y, Takenouchi K, Miura N, Arimura N, Nawa Y, Meng X, Shrestha B, Arimura S, Iwata M, Mera K, Sameshima H, Ohno Y, Maenosono R, Yoshida Y, Tajima Y, Uchikado H, Kuramoto T, Nakayama K, Shigemori M, Hashiguchi T, Maruyama I. Minocycline attenuates both OGD-induced HMGB1 release and HMGB1-induced cell death in ischemic neuronal injury in PC12 cells. Biochem Biophys Res Commun 385: 132–136, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Kleinbongard P, Schulz R, Heusch G. TNFalpha in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev 16: 49–69, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Kurrelmeyer KM, Michael LH, Baumgarten G, Taffet GE, Peschon JJ, Sivasubramanian N, Entman ML, Mann DL. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci U S A 97: 5456–5461, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15: 36–42, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J 21: 3904–3916, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol 38: 21–33, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331–342, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Mann DL. Tumor necrosis factor-induced signal transduction and left ventricular remodeling. J Card Fail 8: S379–S386, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16: 413–419, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol 38: 3–13, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Rui T, Cepinskas G, Feng Q, Ho YS, Kvietys PR. Cardiac myocytes exposed to anoxia-reoxygenation promote neutrophil transendothelial migration. Am J Physiol Heart Circ Physiol 281: H440–H447, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Dale AE, Xenocostas A, Kvietys PR. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res 65: 719–727, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Rui T, Kvietys PR. NFkappaB and AP-1 differentially contribute to the induction of Mn-SOD and eNOS during the development of oxidant tolerance. FASEB J 19: 1908–1910, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180: 2531–2537, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J 20: 1589–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation 114: I270–I274, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201: 1135–1143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell 116: 491–497, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 255: 320–331, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T. The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol 184: 1492–1498, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107: 11942–11947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A 101: 296–301, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang M, Wu J, Martin CM, Kvietys PR, Rui T. Important role of p38 MAP kinase/NF-κB signaling pathway in the sepsis-induced conversion of cardiac myocytes to a proinflammatory phenotype. Am J Physiol Heart Circ Physiol 294: H994–H1001, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Zhao P, Wang J, He L, Ma H, Zhang X, Zhu X, Dolence EK, Ren J, Li J. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J Cell Mol Med 13: 1513–1525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]