Abstract
HIV-1 neutralizing monoclonal antibodies (MAbs) define key targets for vaccine development and are being considered for passive prevention of infection. We analyzed the interaction of MAbs to two independent epitopes on the viral envelope glycoprotein. Potently neutralizing MAbs to the CD4 binding site and V1V2 region displayed no in vitro cross-competition and displayed additive, though not synergistic, neutralization activity. Predicted neutralization coverage of a combination of two MAbs reached 97% on a 208-isolate panel.
TEXT
Neutralizing antibodies are predicted to be a critical component of an effective HIV-1 vaccine (5, 18, 20) and have been shown to provide sterilizing protection in animal models (1, 11, 19). Neutralizing monoclonal antibodies (MAbs) have been isolated from B cells of HIV-infected donors with broad serum-neutralizing activity, and the characterization of these MAbs has helped define conserved regions of the HIV-1 envelope glycoprotein (Env) that can serve as templates for vaccine design. The Env targets defined by these MAbs include the CD4 binding site (CD4bs) of gp120 (4, 10, 27, 38, 39), a conserved peptidoglycan region of variable loops 1 and 2 (V1V2) (21, 35, 36), the membrane proximal region of gp41 (23, 32), and most recently, a peptidoglycan epitope in the V3 region of gp120 (24, 35). The potency and breadth of these new human MAbs have also suggested the possibility of their clinical use as therapeutic agents (30, 31) or as agents to prevent HIV-1 infection, including the prevention of mother-to-child transmission (22, 26). HIV-1 prevention could also be mediated by MAbs as microbicides (33) or by systemic levels of antibodies generated by gene-based vectors (2, 12).
The CD4bs MAbs VRC01 and VRC-PG04 and the V1V2 MAbs PG9 and PG16 are leading candidates for clinical use due to their broad neutralization, potency, and lack of self-reactivity (36, 38). The MAb VRC01 has been shown to neutralize 91% of 198 HIV-1 isolates tested (38) and to precisely target the CD4bs (40). VRC-PG04, isolated from a different donor, is structurally similar to VRC01, derives from the same variable heavy-chain gene precursor, and targets the CD4bs in a highly similar manner (39). PG9 and PG16, two somatic variant IgGs isolated from one donor, neutralized 79% and 73%, respectively, of 162 isolates tested (36). PG9 and PG16 target a glycan-dependent epitope mapping to the V1V2 region on the viral spike trimer (21, 36). Since the potential utility of MAbs to prevent HIV-1 infection would depend, in part, on their breadth of activity against circulating viral isolates, we tested the in vitro interaction and predicted the breadth of neutralization coverage of these MAbs, which target two distinct sites on the HIV-1 Env.
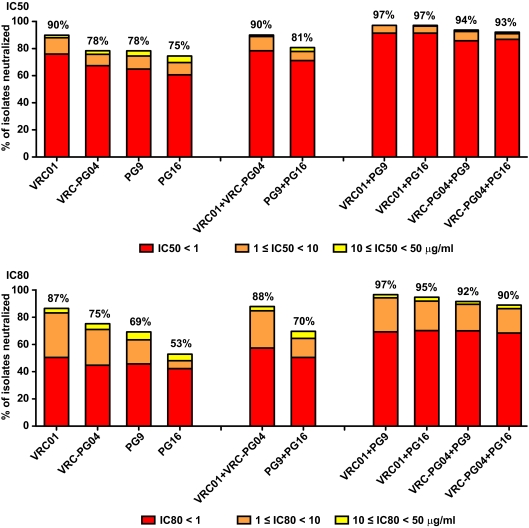
We determined the 50% inhibitory concentration (IC50) and IC80 neutralization titers of the VRC01, VRC-PG04, PG9, and PG16 MAbs against a panel of 208 HIV-1 Env pseudovirus isolates (190 for VRC-PG04). The panel covers the major genetic subtypes and circulating recombinant forms and consists almost entirely of primary isolate Envs (see Fig. S1 in the supplemental material) (3, 6, 13–16, 28, 38). Neutralization activity was measured using single round of infection Env pseudoviruses and TZM-bl target cells as previously described (29, 38). The percentage of HIV-1 isolates neutralized was determined for each MAb alone, and the predicted coverage of various MAb combinations was calculated (Fig. 1). The best combinations were those in which a CD4bs MAb and a V1V2 glycan-dependent MAb were paired. For example, VRC01 alone neutralized 90% of the panel at an IC50 of less than 50 μg/ml and 76% of the panel at an IC50 of less than 1 μg/ml, while VRC01 combined with PG16 neutralized 97% and 91%, respectively, at these cutoffs. Thus, the combination of these two MAbs has the potential to increase both breadth and potency of neutralization. In contrast, the combination of PG9 and PG16 (or the combination of VRC01 and VRC-PG04) was only marginally better than either MAb alone (Fig. 1). The same pattern held for IC80 values, with the broadest and most potent MAb pairs being those with distinct neutralization epitopes. Only 5 of 208 isolates were fully resistant to all four MAbs at an IC50 of less than 50 μg/ml; 4 of the 5 isolates were resistant to the MAb 2G12, and all 5 were resistant to the MAb 4E10 but moderately sensitive to CD4-Ig and to a polyclonal serum pool and/or HIV immune globulin and thus were not globally neutralization resistant. Even with a highly stringent definition of neutralization of an IC80 of <1 μg/ml, a combination of VRC01 and PG16 could still neutralize 70% of the isolates tested. These data are similar to recently published coverage calculations for these MAbs that used a different panel of 162 isolates (35) and compare favorably with coverage provided by the newly described PGT antibodies (35).
Fig 1.
Neutralization coverage of a panel of 208 global HIV-1 isolates (190 for VRC-PG04) by MAbs targeting independent epitopes on the Env glycoprotein. Within each stacked bar, red indicates the percentage of isolates neutralized at an IC of <1 μg/ml, orange indicates the percentage neutralized 1 ≤ IC <10 μg/ml, and yellow indicates the percentage neutralized at 10 ≤ IC <50 μg/ml. The value above each bar is the total percentage of isolates neutralized at an IC of <50 μg/ml. The top and bottom panels show data for IC50 and IC80 values, respectively.
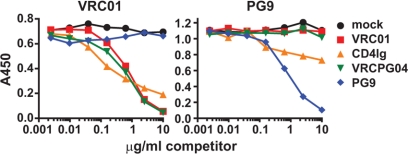
To determine whether the activity of the CD4bs MAbs was independent of the activity of the glycan-dependent V1V2 MAbs, we first performed competition enzyme-linked immunosorbent assays (ELISAs) (38) using a gp120 protein known to bind PG9 (since not all gp120s bind well to this MAb) as well as the other MAbs tested. We observed the expected cross-competition among the CD4bs MAbs VRC01 and VRC-PG04 and the CD4-Ig reagent (Fig. 2). PG9 had no impact on VRC01 binding, and similarly, PG9 binding to gp120 was unaffected by VRC01 and VRC-PG04. Consistent with previous reports on CD4-Ig or soluble CD4 (36, 38), CD4-Ig competed weakly with PG9 binding. Overall, these data indicate that PG9 and the CD4bs MAbs neither compete for nor enhance each other's binding to HIV-1 gp120.
Fig 2.
Independence of gp120 binding by MAbs to the CD4bs and V1V2 regions. Serial dilutions of MAbs or CD4-Ig were incubated with a single dilution of biotinylated VRC01 (left) or PG9 (right) on plates coated with gp120 from HIV-1 isolate ZM109. The results shown are from a representative competition ELISA experiment; two additional assays produced similar data.
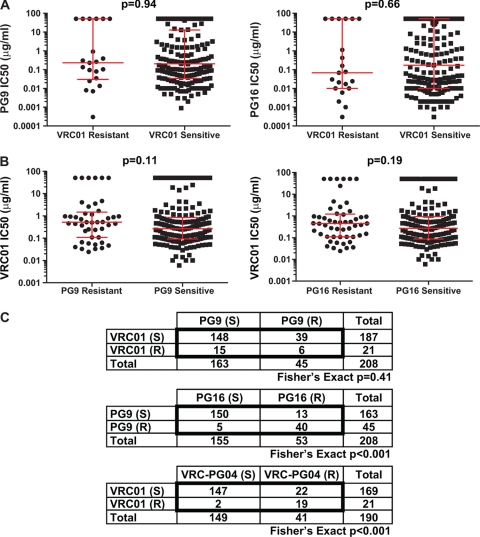
We next asked whether there was an association between viral resistance to CD4bs MAbs and viral resistance to V1V2 MAbs. We stratified the HIV-1 isolates into VRC01 neutralization-resistant and neutralization-sensitive categories and observed no significant differences in the IC50s of PG9 and PG16 against viruses in those categories (Fig. 3A). Similarly, VRC01 neutralization was not significantly different among viruses categorized as sensitive and those categorized as resistant to PG9 or PG16 (Fig. 3B). The same was true for VRC-PG04 when it was compared to PG9 and PG16 (Mann-Whitney-Wilcoxon U test; P > 0.05 for all comparisons). We then examined whether resistance to one MAb correlated with resistance to the others. Among 45 PG9-resistant isolates, we found the expected frequency of VRC01-sensitive viruses. Thirty-nine of 45 viruses (87%) were sensitive to VRC01, a frequency which is similar to the total frequency of VRC01-sensitive viruses in this data set (89%). Conversely, 15 of 21 (71%) VRC01-resistant viruses were sensitive to PG9. In a formal contingency analysis, we found no correlation between resistance to VRC01 and resistance to PG9 (Fig. 3C, top panel) at any of the neutralization cutoff values (P > 0.05 for all pairs; Fisher's exact test). Thus, resistance to the CD4bs MAbs is independent of resistance to PG9 and PG16. In contrast, resistance to PG9 was highly correlated with PG16 resistance, and the same held true for resistance to VRC01 and VRC-PG04 (Fig. 3C, bottom two panels).
Fig 3.
Resistance to CD4bs MAbs is independent from resistance to V1V2 MAbs. (A) Titers of PG9 and PG16 for isolates that are either sensitive or resistant (IC50 > 50 μg/ml) to VRC01. The P values were obtained using a Mann-Whitney-Wilcoxon U test. Red bars indicate the medians and interquartile ranges. (B) Titers of VRC01 for isolates that are either sensitive or resistant (IC50 > 50 μg/ml) to PG9 or PG16. (C) 2 × 2 contingency tables for isolates that are sensitive (S) or resistant (R) to the indicated MAb at an IC50 of >50 μg/ml.
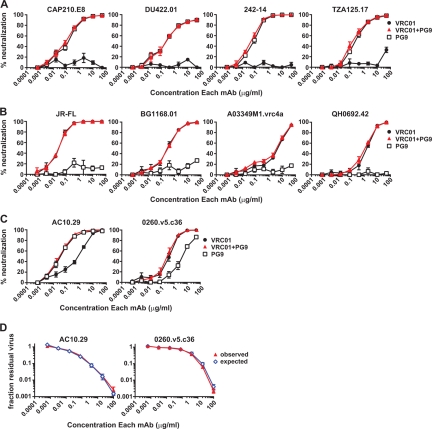
The coverage calculations in Fig. 1 are based on the assumption that a nonneutralizing antibody would not interfere with the action of a neutralizing antibody. We tested this assumption against a subset of viral isolates resistant to either VRC01 or PG9. These assays included the MAb VRC01 alone, the MAb PG9 alone, and a 1:1 mixture of the two. We chose four isolates that were VRC01 resistant and PG9 sensitive: CAP210.E8, DU422.01, 242-14, and TZA125.17. For these isolates, there was no significant difference between the neutralization curve of PG9 alone and that of PG9 in a 1:1 mixture with VRC01 (Fig. 4A). The IC50 and IC80 values were <2-fold different, which is well within the range of experimental variation for this assay. Similarly, four PG9-resistant and VRC01-sensitive strains (JR-FL, BG1168.01, A03349M1.vrc4a, and QH0692.42) were tested with VRC01 alone or VRC01 mixed 1:1 with PG9, and no difference in neutralization was observed (Fig. 4B). Thus, among HIV isolates resistant to one of the MAbs, the potency of the neutralizing MAb was unaffected by the addition of the MAb to which the isolate was resistant.
Fig 4.
In vitro combinations of VRC01 and PG9 show a lack of interference and show neutralization additivity. The percentage of neutralization was measured for VRC01, PG9, and a 1:1 mixture of the two against VRC01-resistant isolates (A) and PG9-resistant isolates (B). The means and standard errors of the means (SEM) are shown for three experiments. (C) Neutralization of dual-sensitive isolates by VRC01, PG9, and a 1:1 mixture of the two. The means and SEM are shown for six experiments using isolate AC10.29 and four experiments using isolate 0260.v5.c36. (D) Fractional product neutralization curves. Data from the experiment in panel C are plotted as the fraction of residual virus in wells containing the mixture (“observed”) and the product of the residual virus in the VRC01 and PG9 wells (“expected”) at each dilution.
Given their binding to distinct epitopes and the lack of cross-competition by ELISA, we tested if the combination of MAbs to the CD4bs and V1V2 region would mediate additive or synergistic neutralization. Antibody synergy may occur by one of several mechanisms, including the potential for one antibody to induce a conformational change that enhances the effect of a second antibody (17, 25, 34, 41). Neutralization by VRC01 and PG9 was tested on two viral isolates, AC10.29 and 0260.v5.c36, that each showed moderate sensitivity to both viruses and that lacked the plateau neutralization curves sometimes observed for PG9 and PG16 (21, 36). We compared neutralization by each MAb alone to that by a 1:1 mixture of VRC01 with PG9 (Fig. 4C). We then analyzed the data by the fractional product method (17, 37), in which the fraction of residual virus (Vn/Vo; e.g., the amount of virus in the well with antibody divided by the amount in the virus-only wells) for the 1:1 mixture is predicted by the equation (Vn/Vo)mix = (Vn/Vo)VRC01 × (Vn/Vo)PG9. This equation predicts the additive neutralization effect of two MAbs with independent modes of action (9, 37). The observed experimental data for the VRC01 and PG9 MAb mixture were very similar to the predicted data at all antibody dilutions, demonstrating an additive antibody interaction (Fig. 4D). We performed a further analysis using the method of Chou et al. (7–9) and found no consistent evidence of synergy or antagonism. Based on these analyses, we conclude that the combination of the MAbs VRC01 and PG9 mediates additive viral neutralization.
In summary, we assessed the interactions of potently neutralizing MAbs to the CD4bs and the V1V2 regions of HIV-1 Env. MAb binding and neutralization studies demonstrated that antibodies to these two Env targets do not cross-compete and that their combination can mediate additive viral neutralization. Independently, each of the MAbs displays potent neutralization against a majority of diverse HIV-1 strains, and the combination of MAbs VRC01 and PG9 provides a predicted coverage of 90% to 97% of viral strains, depending on the cutoff value used to define neutralization. Because they bind to independent epitopes, neutralization resistance to one MAb does not affect MAbs directed to the second epitope. These in vitro studies show the potential benefit of combining MAbs directed to distinct Env epitopes in providing broad coverage of globally diverse HIV-1 strains. In vitro studies using alternative formats, such as peripheral blood mononuclear cell (PBMC)-based neutralization, and in vivo passive-transfer studies in nonhuman primate models would further test this hypothesis. Ultimately, only clinical trials in human volunteers will definitively show the utility of this approach. Overall, these data support vaccine design efforts aimed at eliciting neutralizing antibodies to the CD4bs and V1V2 regions of Env and are encouraging for clinical use of the combinations of HIV-1 MAbs.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH.
We thank Ellen Turk and Chien-Li Lin for technical assistance, Shazad Majeed and Qiang Wang for spreadsheet scripting, and Xueling Wu for critical reading of the manuscript. We thank Francine McCutchan, George Shaw, Beatrice Hahn, David Montefiori, Feng Gao, Michael Thomson, Julie Overbaugh, Ronald Swanstrom, Lynn Morris, Linqi Zhang, Dennis Ellenberger, Carolyn Williamson, Kunxue Hong, S. Tovanabutra, E. Sanders-Buell, Jerome Kim, and the U.S. Military HIV Research Program for contributing the HIV-1 envelope plasmids used in our neutralization panel.
Footnotes
Published ahead of print 18 January 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Baba TW, et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 [DOI] [PubMed] [Google Scholar]
- 2. Balazs AB, et al. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blish CA, Nedellec R, Mandaliya K, Mosier DE, Overbaugh J. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 21:693–702 [DOI] [PubMed] [Google Scholar]
- 4. Burton DR, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 [DOI] [PubMed] [Google Scholar]
- 5. Burton DR, Stanfield RL, Wilson IA. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. U. S. A. 102:14943–14948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chong H, et al. 2008. Genetic and neutralization properties of HIV-1 env clones from subtype B/BC/AE infections in China. J. Acquir. Immune Defic. Syndr. 47:535–543 [DOI] [PubMed] [Google Scholar]
- 7. Chou T-C, Martin N. 2007. CompuSyn for drug combinations and for general dose-effect analysis, and user's guide. ComboSyn, Inc., Paramus, NJ [Google Scholar]
- 8. Chou TC. 2010. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 70:440–446 [DOI] [PubMed] [Google Scholar]
- 9. Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27–55 [DOI] [PubMed] [Google Scholar]
- 10. Corti D, et al. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5:e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hessell AJ, et al. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson PR, et al. 2009. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 15:901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keele BF, et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kulkarni SS, et al. 2009. Highly complex neutralization determinants on a monophyletic lineage of newly transmitted subtype C HIV-1 Env clones from India. Virology 385:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M, et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li M, et al. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 80:11776–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mascola JR, et al. 1997. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71:7198–7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mascola JR, Montefiori DC. 2010. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28:413–444 [DOI] [PubMed] [Google Scholar]
- 19. Mascola JR, et al. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 20. McElrath MJ, Haynes BF. 2010. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33:542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McLellan JS, et al. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mofenson LM. 2011. Prevention of mother-to-child HIV-1 transmission—why we still need a preventive HIV immunization strategy. J. Acquir. Immune Defic. Syndr. 58:359–362 [DOI] [PubMed] [Google Scholar]
- 23. Muster T, et al. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pejchal R, et al. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potts BJ, et al. 1993. Synergistic inhibition of HIV-1 by CD4 binding domain reagents and V3-directed monoclonal antibodies. Virology 197:415–419 [DOI] [PubMed] [Google Scholar]
- 26. Safrit JT, et al. 2004. Immunoprophylaxis to prevent mother-to-child transmission of HIV-1. J. Acquir. Immune Defic. Syndr. 35:169–177 [DOI] [PubMed] [Google Scholar]
- 27. Scheid JF, et al. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seaman MS, et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for neutralizing antibody assessment. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shu Y, et al. 2007. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine 25:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trkola A, et al. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615–622 [DOI] [PubMed] [Google Scholar]
- 31. Trkola A, et al. 2008. In vivo efficacy of human immunodeficiency virus neutralizing antibodies: estimates for protective titers. J. Virol. 82:1591–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trkola A, et al. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veazey RS, et al. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346 [DOI] [PubMed] [Google Scholar]
- 34. Verrier F, Nadas A, Gorny MK, Zolla-Pazner S. 2001. Additive effects characterize the interaction of antibodies involved in neutralization of the primary dualtropic human immunodeficiency virus type 1 isolate 89.6. J. Virol. 75:9177–9186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker LM, et al. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walker LM, et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Webb JL. 1963. Effects of more than one inhibitor, p 488–512 Enzyme and metabolic inhibitors, vol 1 Academic Press, New York, NY [Google Scholar]
- 38. Wu X, et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu X, et al. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou T, et al. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zwick MB, et al. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198–12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.