Abstract
Theiler's murine encephalomyelitis virus (TMEV) results in a persistent central nervous system infection (CNS) and immune-mediated demyelination in mice. TMEV largely persists in macrophages (Mϕs) in the CNS, and infected Mϕs in vitro undergo apoptosis, whereas the infection of other rodent cells produces necrosis. We have found that necrosis is the dominant form of cell death in BeAn virus-infected BHK-21 cells but that ∼20% of cells undergo apoptosis. Mcl-1 was highly expressed in BHK-21 cells, and protein levels decreased upon infection, consistent with onset of apoptosis. In infected BHK-21 cells in which Mcl-1 expression was knocked down using silencing RNAs there was a 3-fold increase in apoptotic cell death compared to parental cells. The apoptotic program switched on by BeAn virus is similar to that in mouse Mϕs, with hallmarks of activation of the intrinsic apoptotic pathway in a tumor suppressor protein p53-dependent manner. Infection of stable Mcl-1-knockdown cells led to restricted virus titers and increased physical to infectious particle (PFU) ratios, with additional data suggesting that a late step in the viral life cycle after viral RNA replication, protein synthesis, and polyprotein processing is affected by apoptosis. Together, these results indicate that Mcl-1 acts as a critical prosurvival factor that protects against apoptosis and allows high yields of infectious virus in BHK-21 cells.
INTRODUCTION
Theiler's murine encephalomyelitis viruses (TMEV), members of the Cardiovirus genus in the family Picornaviridae, are enteric pathogens of mice. TMEV strains differ in neurovirulence following intracerebral inoculation of mice with the high-neurovirulence GDVII strain, causing extensive infection of cerebral cortical and hippocampus neurons and producing a rapidly fatal encephalitis, whereas the low-neurovirulence DA and BeAn strains result in a persistent central nervous system (CNS) infection primarily in microglia/macrophages (Mϕs) but also in oligodendrocytes, leading to immune-mediated demyelination. TMEV persistence in mice is recognized as a relevant experimental animal model for multiple sclerosis (4, 22, 29).
Depending on the specific cell type infected, TMEV induces at least two distinct cell death programs, necrosis and apoptosis, which can result in substantial differences in infectious viral yields. All rodent cell types except for Mϕs probably undergo necrosis because of the high viral yields produced (15); murine Mϕs undergo apoptosis with restricted viral yields, producing only a few PFU of virus per cell (14). Moreover, TMEV infection of mouse M1-D Mϕs induces apoptosis by activating the intrinsic apoptotic pathway (14, 32). In these cells, TMEV infection results in activation of p38 mitogen-activated protein kinase by 2 to 3 h postinfection (p.i.), followed by phosphorylation of tumor suppressor protein p53 Ser 15 at 3 to 6 h p.i. and stable p53 levels until 6 h p.i; activated p53 upregulates the transcription of the proapoptotic puma and noxa genes at 2 to 4 h p.i., and the levels of prosurvival Mcl-1 and A1 proteins become undetectable at 4 to 10 h p.i. (32, 33). Specific inhibition of phospho-p38 and inhibition of p53 by a genetic suppressor element led to a significant decrease in apoptosis (33). Degradation of prosurvival proteins was also shown to release Bax (32, 33), which forms homo-oligomers and translocates into and permeabilizes the mitochondrial outer membrane, releasing cytochrome c and initiating the caspase cascade.
Antiapoptotic Mcl-1, initially isolated from a human myeloblastic leukemia cell line (17), predominates in differentiated human Mϕs (20)., protecting against apoptosis during the initial steps of differentiation (7, 39). Overexpression of Mcl-1 in TMEV-infected M1-D cells reduced the cleavage of caspase-3 and caspase-9 to their active forms and inhibited apoptosis (33). Whether Mcl-1 endogenously regulates TMEV-induced cell death in other cells remains to be determined.
In the present study, BeAn virus infection in BHK-21 cells led to necrosis of the majority of cells but to apoptosis in ca. 20% of the cells through the intrinsic apoptotic pathway in a p53-dependent manner. Mcl-1, an antiapoptotic Bcl-2 family member, was highly expressed in BHK-21 cells, and infection resulted in gradual loss of Mcl-1, which is consistent with the onset of apoptosis. Mcl-1 knockdown by silencing RNAs (siRNAs) increased apoptotic cell death by ∼3-fold and concomitantly reduced infectious viral yields by at least 12-fold, indicating that Mcl-1 is an important regulator of cell death in infected BHK-21 cells. The availability of BHK-21 cells with stably knocked-down Mcl-1 expression provides a convenient tool to further define the step(s) in the viral life cycle impacted by apoptosis.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 7.5% tryptose phosphate, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml at 37°C in 5% CO2. Cells of the immature myelomonocytic cell line M1, derived from the SL mouse strain, were induced to differentiate into Mϕs with supernatants from mouse L929 fibroblasts and mouse P388D1 Mϕs as described previously (32). N20.1 mouse oligodendrocytes were grown in Dulbecco modified Eagle medium (DMEM)/F-12 medium containing 100 μg of streptomycin, 100 U of penicillin per ml, and 10% FBS at 37°C in 5% CO2 (37); the cells at this temperature were undifferentiated and consisted of immature oligodendrocytes. The origin and passage history of the BeAn virus stock has been described (27). Virus titers of clarified lysates of infected cells were determined by standard plaque assay in BHK-21 cells (27).
Reagents.
The following reagents and antibodies were purchased commercially: mouse anti-caspase 9, rabbit anti-caspase 3, rabbit anti-poly(ADP-ribose) polymerase (PARP), and rabbit anti-actin antibodies (Cell Signaling Technology, Beverly, MA); mouse anti-Bax, Mcl-1 siRNA, and Mcl-1 short hairpin RNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); goat anti-mouse IgG-horseradish peroxidase and goat anti-rabbit IgG-horseradish peroxidase (BD Pharmingen, San Diego, CA); and actinomycin D (Sigma, St. Louis, MO).
Virus infections.
After virus adsorption at a multiplicity of infection (MOI) of 10 for 45 min at 24°C, cell monolayers were washed twice with phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 0.5 mM MgCl2, followed by incubation in DMEM containing 1% FBS at 37°C for designated times. The end of the adsorption period was designated as time zero.
Fluorescence-activated cell sorting (FACS).
Subconfluent monolayers of BHK-21 cells in 35-mm six-well plates were infected with BeAn virus (MOI = 10) for 16 or 24 h; in some experiments, this was after transfection with Mcl-1 siRNAs for 24 h. Cell monolayers were washed with PBS and detached with trypsin, and 5 × 105 cells were stained with propidium iodide (PI) and annexin V-fluorescein isothiocyanate (Calbiochem, Darmstadt, Germany) according to the manufacturer's instructions. Incubation of cell monolayers with actinomycin D (3 μg/ml) provided positive controls for apoptosis. For each sample, 10,000 events were analyzed using a Beckman Coulter Epics Elite ESP and FlowJo 8.8.3 software.
Virus titers.
Cell monolayers (2 × 104 cells/well) in 96-well plates were infected with serial 10-fold dilutions of virus, and the viral cytopathic effect (CPE) was monitored under an inverted light microscope. On day 5 of incubation, the 50% tissue culture infective dose ml−1 was calculated as the reciprocal of the lowest 10-fold dilution resulting in CPE in more than 50% of the monolayers (24).
Cell viability assay.
WST-1 reagent (Roche Applied Science, Indianapolis, IN), a tetrazolium salt, was added to the medium of monolayer cultures in 96-well plates at indicated times and incubated for 1 to 2 h at 37°C in 5% CO2. Cell viability in samples was determined by absorbance at 420 nm (reference wavelength, 610 nm) for cleavage of the tetrazolium salt to formazan against a background control using a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, CA). Percent cell death values were calculated as the ratio of BeAn virus-infected to mock-infected cultures.
Apoptosis assay.
The number of apoptotic cells was determined using DAPI (4′,6′-diamidino-2-phenylindole) staining. Briefly, M1-D cells grown and infected on glass coverslips (Fisher Scientific Co., Pittsburgh, PA) were fixed in 4% paraformaldehyde for 15 min at room temperature, washed twice in PBS (pH 7.2), and incubated with DAPI for 5 min at a final concentration of 0.5 μg/ml. Coverslips were washed with 0.5% Tween 20 in PBS and distilled H2O and viewed with a Zeiss digital confocal microscope. At least three randomly chosen fields containing cells were photographed at ×200 magnification, and the percentage of cells with condensed chromatin and fragmented nuclei were determined by a blinded observer; each experiment was repeated twice.
Caspase activity assay.
Caspase-8 and -9 activities were assayed with Caspase-Glo reagent (Promega, Madison, WI) in BHK-21 cells seeded in six-well plates (3 × 105 cells per well), infected 24 h later with BeAn virus (MOI = 10), and harvested at 12 h p.i. An equal volume of Caspase-Glo reagent containing MG132 was added to infected cell pellets, which were transferred to white-walled 96-well plates, and incubated at room temperature for 1 h, and the luminescence was measured in a plate-reading luminometer (GloMax-multi detection system; Promega).
Assay for ROS.
Cellular reactive oxygen species (ROS) production was measured by flow cytometry using the ROS-sensitive dye 5- (and 6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Molecular Probes, Eugene, OR). Infected BHK-21 cells (12 h p.i.) were incubated with infection media containing 5 μmol of CM-H2DCFDA/liter for 30 min in the dark at 37°C before harvesting. Cells were treated with trypsin, washed with DMEM without pyruvate X2, suspended in 0.5 ml of PBS, and subjected to flow cytometry.
Northern hybridization.
Total RNA from cell cultures was ethanol precipitated, resuspended in RNA loading buffer (morpholinepropanesulfonic acid buffer), heated for 5 min at 95°C, and electrophoresed in a 1.2% agarose gel at 85 V for 2 h. The gel was soaked in 10× SSC for 15 min. RNA was passively transferred to a Hybond-N+ membrane (Amersham, Piscataway, NJ), treated with short-wavelength UV light for 2 min, and prehybridized with hybridization solution (Clontech, Mountain View, CA) for at least 1 h at 68°C. A random-primed DNA probe specific for BeAn virus was derived from a 1.5-kb BclI/NciI fragment of BeAn cDNA spanning nucleotides 4952 to 6492 by using the Prime-a-Gene labeling system (Promega). The α-32P-labeled probe was added to the prehybridization solution, and hybridization was carried out overnight at 42°C. Membranes were washed once with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.05% sodium dodecyl sulfate (SDS) for 30 min and three times with 0.1× SSC–0.1% SDS at 24°C for 20 min each time, dried, and exposed to a Molecular Dynamics PhosphorImager screen to detect BeAn virus with the [α-32P]deoxynucleoside triphosphate-labeled hybridized probe.
Pulse-labeling virus proteins.
Cell monolayers were infected with BeAn (MOI = 50) for 4 h with infection medium containing 2 μg of actinomycin D/ml. After rinsing, the cells were incubated with methionine-free DMEM and 2 μg of Actinomycin D/ml for 1 h, followed by incubation for 5 min in medium containing 30 μCi of [35S]methionine. Cells were rinsed and chased in the presence of 10 mM methionine for 0, 1, 5, 10, and 20 min. Next, the cells were harvested, lysed with 0.1 ml of lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 50 mM Tris [pH 7.5]), and centrifuged at 13,000 rpm for 20 min at 4°C. Aliquots were analyzed by SDS–12% polyacrylamide gel electrophoresis (PAGE), followed by autoradiography.
Estimation of the physical particle/PFU ratio.
To obtain an estimate of the physical particle/infectious particle ratio, the BeAn virus RNA concentration was determined from virus purified on sucrose gradients (13). The number of physical particles was calculated assuming that 1 mg of picornavirus RNA is equivalent to 7.2 × 1013 virus genomes (28) and to an equivalent number of virus particles. The number of physical particles was then related to the titer of purified virus, as determined by a standard plaque assay.
Electron microscopy.
M1-D cells were harvested, fixed with 3% glutaraldehyde in PBS and then in aqueous 2% osmium tetroxide, stained with 0.5% aqueous uranyl acetate, dehydrated with a graded ethanol series, and embedded in Epoxy Resin LX112 for processing and staining as described previously (32, 33).
Statistical analysis.
A paired Student t test was used to compare groups, and differences were considered significant at P < 0.05.
RESULTS
BeAn virus infection induces both necrotic and apoptotic cell death pathways in BHK-21 cells.
Since TMEV infection of BHK-21 cells is highly productive, with infectious virus yields on the order of 200 to 500 PFU/cell (27), these cells have been widely used to study the molecular virology and to purify the virus. Analysis of cell viability in BHK-21 cells infected with the BeAn strain of TMEV at (MOI = 10) revealed progressive virus-induced cell death, with 85% of the cells dying by 24 h p.i. (Fig. 1A). FACS analysis revealed that cell death was due to a combination of necrosis and apoptosis (Fig. 1B), with most cells dying by necrosis and ∼20% by apoptosis at 24 h p.i. (Fig. 1B). Moreover, the very high levels of ROS observed by 10 to 12 h p.i. (Fig. 1C) were consistent with necrosis since its production in cell death is thought to be higher in necrosis than apoptosis (10). Figure 1D shows representative electron micrographs of a more normal-appearing cell early in infection (left) and of necrotic (center) and apoptotic (right) cells at 16 h p.i. The necrotic cell is intact but markedly swollen, with little recognizable cytoplasmic and nuclear architecture remaining (in most such cells the cell membrane was ruptured); the apoptotic cell is slightly shrunken with condensed nuclear chromatin, blebbing of a part of its nucleus, and proliferation of vesicular membranes or viroplasm in the cytoplasm, the site of viral genome replication. Thus, infection of BHK-21 cells resulted primarily in necrosis, with only a minority of cells undergoing apoptosis.
Fig 1.
BeAn virus infection of BHK-21 cells (MOI = 10) led primarily to necrosis, with a minority of cells undergoing apoptosis. (A) Representative WST-1 cell survival assay revealed progressive cell death to 40 h p.i. Error bars indicate the standard deviations (SD). (B) FACS analysis of PI- and annexin V-stained cells showing a temporal profile of PI-positive cells, probably necrotic (majority of cells; upper quadrants) and apoptotic cells (minority of cells; lower right quadrant). (C) FACS analysis showing that infected cells develop high levels of reactive oxygen species (ROS) at 10 to 12 h p.i. (D) Electron microscopy of infected BHK-21 cells showing (i) a relatively normal-appearing cell prior to onset of apoptosis at 8 h p.i. (left), (ii) a necrotic cell at 16 h p.i., appearing swollen with little recognizable cytoplasmic or nuclear architecture (center), and (iii) an apoptotic cell at 16 h p.i., appearing mildly shrunken, with condensed nuclear chromatin and blebbing of part of its nucleus and perinuclear proliferating membranes representing viroplasm (“viral factory”) (right).
Mcl-1 expression in BHK-21 and mouse cell lines.
Based on the antiapoptotic role of Mcl-1 in BeAn virus-infected M1-D Mϕs (32, 33), we examined Mcl-1 expression in mouse cells, including five Mϕ and two fibroblast cell lines and in BHK-21 cells. Immunoblotting analysis revealed low levels of Mcl-1 in the murine cells compared to abundant expression in the hamster-derived BHK-21 cells (Fig. 2A). Because BHK-21 cell Mcl-1 consistently migrated above the mouse species in polyacrylamide gels, the nucleotide sequence was determined; Syrian hamster Mcl-1 has one less one amino acid (319 amino acids) and shares 93% sequence identity with its mouse counterpart (GenBank accession number U35623). Assessment of the expression of the five Bcl-2 antiapoptotic family members demonstrated abundant Bcl-xL expression in both BHK-21 and M1-D cell lines and Mcl-1 in BHK-21 cells but minimal expression of the other antiapoptotic proteins in BHK-21 cells (Fig. 2B). Because Mcl-1 expression was abundant and might not be regulated, BHK-21 cells were maintained for 36 h in medium containing 0.1% FBS before immunoblotting; Mcl-1 expression decreased at 24 to 36 h (Fig. 2C), indicating that Mcl-1 is regulated by other signaling pathways, such as ERK (reviewed in reference 16). Following BeAn virus infection (MOI = 10), Bcl-xL expression remained stable from 0 (end of adsorption period) to 12 h p.i., while that of Mcl-1 decreased by >50% at 10 to 12 h p.i. (Fig. 2D and E). Assuming steady-state Mcl-1 synthesis and degradation, this decrease in Mcl-1 expression at 10 to 12 h is consistent with the onset of apoptosis.
Fig 2.
Expression profile of Bcl-2 family antiapoptotic members determined by immunoblotting. (A) Mcl-1 expression in five mouse Mϕ cell lines, mouse L929 and murine embryonic fibroblasts (MEFs), and Syrian hamster BHK-21 cells. (B) Comparison of the expression of the five Bcl-2 family antiapoptotic proteins in BHK-21 and M1-D Mϕs, not the abundant Bcl-xL and Mcl-1 expression in BHK-21 cells. (C) Loss of Mcl-1 expression in BHK-21 cells grown in 0.1% FBS for 24 and 36 h. (D) Expression of Bcl-2 antiapoptotic proteins in BeAn virus-infected BHK-21 cells (MOI = 10) at the indicated times p.i.; the substantial reduction of Mcl-1 levels at 10 to 12 h p.i. is consistent with the induction of apoptosis at this time. (E) Densitometric analysis of Bcl-xL and Mcl-1 immunoblot data in infected BHK-21 cells over time p.i. normalized to β-actin.
Mcl-1 is antiapoptotic in BeAn virus-infected BHK-21 cells.
To further examine the role of Mcl-1 in apoptosis of virus-infected BHK-21 cells, we tested whether siRNA Mcl-1 knockdown would result in increased apoptosis. Mcl-1 expression was inhibited by ≥90% with Mcl-1-specific siRNAs, as determined by immunoblotting (Fig. 3A and B) in experiments where the transfection efficiency was ∼90% (data not shown). A representative FACS analysis of four experiments revealed a significant increase (P < 0.048) in apoptotic cell death in BeAn virus-infected cells transfected with Mcl-1-specific compared to scrambled siRNAs (Fig. 3C). Analysis of viral replication in BHK-21 cells with siRNA knocked-down expression of Mcl-1 revealed an ∼70-fold reduction in virus titers (Fig. 3D), indicating that apoptosis inhibited BeAn viral replication. siRNA knockdown of Bcl-xL expression also resulted in a substantial increase in apoptosis (Fig. 3E), indicating that Bcl-xL may also play an antiapoptotic role in infected BHK-21 cells.
Fig 3.
siRNA knockdown demonstrates an antiapoptotic role of Mcl-1 and Bcl-xL in BeAn virus-infected BHK-21 cells. (A) Timeline of cell death analysis in siRNA experiments. (B) Immunoblot analysis revealing 90% knock down of the amount of Mcl-1 with specific compared to scrambled siRNAs. (C) Representative FACS analysis of PI- and annexin V-stained cells revealed an ∼3-fold increase in apoptotic cells (right lower quadrant) after infection of Mcl-1 knockdown cells at 16 h p.i. (D) Infectious virus yields of Mcl-1 siRNA-transfected cells was significantly decreased at 16 h p.i. (P < 0.01) compared to viral yields of scrambled siRNA-transfected cells. Error bars indicate the SD. (E) FACS analysis of PI- and annexin V-stained cells demonstrated that both Mcl-1 and Bcl-xL function as antiapoptotic proteins in infected BHK-21 cells.
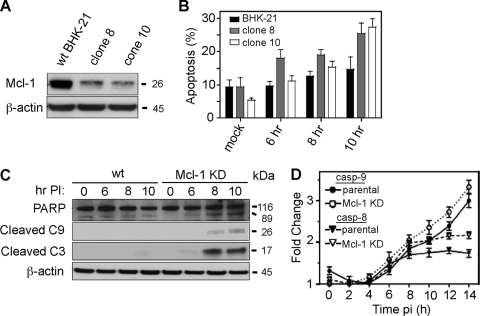
To further study the effect of Mcl-1 knockdown on BeAn virus replication, 22 stable, puromycin-resistant BHK-21 clones were generated, and two clones (clones 8 and 10) showing the greatest decrease in Mcl-1 expression (Fig. 4A) were infected with virus and assessed for apoptosis. Both clones showed increased levels of apoptosis at 6 and 8 h p.i. (P < 0.05) and levels were significantly higher at 10 h p.i. (P = 0.02) compared to infected parental cells (Fig. 4B) in an assay based on nuclear morphology of attached cells; because of CPE, the assay was not reliable ≥12 h p.i. BeAn infection of both clones resulted in PARP, caspase-9 and caspase-3 cleavage (shown for clone 10 in Fig. 4C) but not caspase-8 cleavage at 6 to 10 h p.i., suggesting that TMEV infection in BHK-21 cells activates the intrinsic signaling pathway similar to its action in murine Mϕs (14, 32). A caspase activity assay using a commercial luminescent reagent revealed an ∼3-fold activation of caspase-9 compared to ∼1.7-fold for caspase-8 (P = 0.01) (Fig. 4D); however, the increases in activity were probably minimal because of the still limited extent of apoptosis. In contrast to the immunoblotting results, knockdown of Mcl-1 did not lead to significantly increased initiator caspase activity (Fig. 4D).
Fig 4.
BeAn virus infection of BHK-21 cells with stable Mcl-1 knock-down induces apoptosis through the intrinsic pathway. (A) Two stable Mcl-1 knockdown clones (clones 8 and 10) showed a 90% reduction in Mcl-1 expression. (B) BeAn infection (MOI = 10) of clones 8 and 10 induced apoptosis beginning at 6 h p.i., as assessed based on nuclear morphology (DAPI staining) (error bars indicate the SD). (C) Immunoblot analysis revealed PARP and caspase-9 and caspase-3 cleavage in infected parental and Mcl-1 knockdown BHK-21 cells (shown for clone 10) at 8 and 10 h p.i. Note that the 17-kDa caspase-3 cleavage product is barely detectable at 10 h in parental cells but is clearly seen with a longer exposure time of the autoradiogram (data not shown). (D) Activation of caspase-9 but not caspase-8 over time in a Luc assay of parental and Mcl-1 knockdown BHK-21 cells (error bars indicate the SD).
Temporal analysis of virus titers in infected parental and Mcl-1 knockdown cells, indicated a shortened virus growth phase for infected clone 10 cells, peaking at 8 to 10 h p.i. and resulting in 90% reduction in infectious virus yields compared to parental cells (Fig. 5A). BeAn virus grown in knock-down cells yielded more defective virus particles per cell, i.e., a 16-fold increase in the ratio of physical particles to infectious particles (PFU) than in parental cells (5.2 × 104 versus 3.1 × 103) determined from virus purified at 24 h from infected cells (representative of three experiments). Northern blot analysis to determine whether the kinetics of single-stranded viral RNA (ss viral RNA) replication was also affected revealed no significant differences in parental and knockdown cells (Fig. 5B); Fig. 5C shows the densitometric scan of the Northern blot. Pulse-chase analysis at 5 h p.i. showed similar viral protein synthesis and polyprotein processing (Fig. 5D). These results indicate that Mcl-1 is an essential prosurvival factor that enables increased infectious virus yields by inhibiting apoptosis and by its impact on a step in the viral life cycle later than viral RNA replication, protein synthesis, or polyprotein processing.
Fig 5.
Stable Mcl-1 knockdown in BHK-21 cells does not affect viral RNA synthesis or translation but reduces infectious virus production of compared to parental cells. (A) Temporal kinetics of BeAn infection showing that peak virus titers in knock-down cells were significantly reduced at 8 to 12 h p.i. compared to parental cells (P = 0.04 at 12 h; error bars indicate the SD). (B) Northern blot analysis showing similar replication of ss viral RNA in both parental and knockdown cell lines. (C) Cells lines pulsed with [35S]methionine at 5.5 h p.i. for 5 min, followed by chases at the indicated times revealed similar protein synthesis, including polyprotein processing into the final structural and nonstructural viral proteins (labeled on the right).
Early activation of p53 in infected BHK-21 cells.
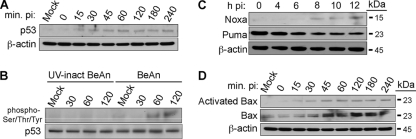
Mcl-1 expression is regulated by a variety of cytokines and growth factors through a number of signaling pathways (1, 14). Mcl-1 is also regulated by the p53 tumor suppressor protein (12), the activation of which is required for the induction of apoptosis in BeAn virus-infected M1-D Mϕs (33). Immunoblotting to determine whether p53 was also activated in infected BHK-21 cells showed increased p53 expression as early as 15 min p.i. (Fig. 6A). Ser-15 and Ser-20, two common p53 phosphorylation sites that are induced in BeAn-infected M1-D cells (33), did not differ in phosphorylation levels (not shown), suggesting that p53 may be activated by phosphorylation at other sites. Immunoblot analysis of immunoprecipitated p53 with monoclonal antibody to phospho-Ser, phospho-Tyr, and phospho-Thr residues showed that p53 was phosphorylated in infected BHK-21 cells but not in BHK-21 cells incubated with UV-inactivated virus (Fig. 6B). Similar analysis of the BH3-only proapoptotic proteins Noxa and Puma, which are transcriptionally upregulated by p53 (11, 12, 38) and activated in BeAn-infected M1-D Mϕs (33), revealed upregulation of only Noxa in infected BHK-21 cells (Fig. 6C). Immunoblotting analysis for Bax and Bak expression showed increased expression and activation of only Bax (Fig. 6D; Bak data not shown) at 30 min p.i. These data demonstrate the close similarity of the apoptotic cell death pathway in BHK-21 cells and M1-D Mϕs.
Fig 6.
BeAn virus-infected parental BHK-21 cells show p53 and Noxa activation upstream and Bax downstream of Mcl-1. (A) Immunoblot analysis showing after infection (MOI = 10) of parental BHK-21 cells detectable p53 from 15 to 240 min p.i. but not in mock-infected cells (later is not shown). (B) Immunoblot of p53 immunoprecipitated from infected cells early p.i. and incubated with mouse monoclonal antibody to phosphoserine/threonine/tyrosine demonstrated phosphorylation of p53 at 60 and 120 min p.i., whereas control, parental BHK-21 cells infected with UV-inactivated BeAn virus showed no p53phosphorylation. (C and D) Immunoblots showing that BeAn infection leads to activation of Noxa, but not Puma at 8 to 10 h and to activation of Bax at 30 min p.i. and increased amounts of total Bax (D).
BeAn infection induces necrosis in immature N20.1 oligodendrocytes.
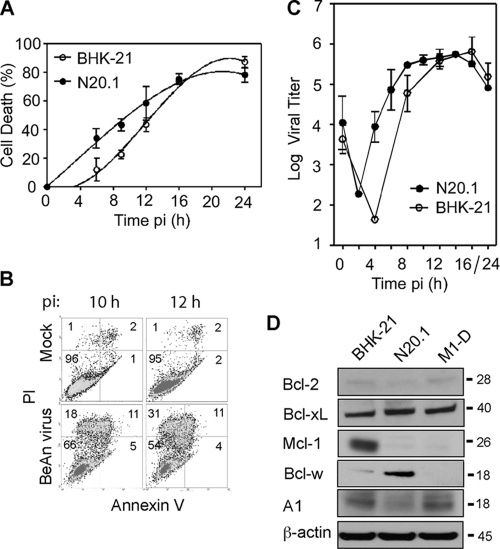
Unlike the restricted infection of Mϕs, infection of oligodendrocytes in mice appears to be highly productive (3). Analysis of cell death in the undifferentiated murine oligodendrocyte cell line N20.1 which is productively infected (35), showed a temporal profile similar to that in BHK-21 cells (Fig. 7A); however, FACS analysis of PI- and annexin V-stained cells indicated that essentially all of the cells underwent necrosis (Fig. 7B). As expected, virus infection of both cell lines resulted in one-step growth kinetics with similar high viral yields (Fig. 7C). Immunoblotting to examine the expression profile of the Bcl-2 antiapoptotic proteins in BHK-21, N20.1, and M1-D cells showed high levels of Mcl-1 only in BHK-21 cells, with just detectable levels in N20.1 and M1-D cells (Fig. 7D). Bcl-w was expressed at high levels in N20.1 cells, suggesting that Bcl-w may be a critical prosurvival protein in oligodendrocytes; however, the results of siRNA knockdown of Bcl-w were not conclusive (not shown).
Fig 7.
BeAn virus-induced necrosis in N20.1 oligodendrocyte cells. (A) Comparison of the temporal profile of virus induced-cell death of N20.1 and parental BHK-21 cells in a representative experiment using the Wst-1 assay. Error bars indicate the SD. (B) BeAn virus infection of N20.1 cells resulted primarily in necrotic cell death; 42% of the cells stained PI positive by 12 h p.i., whereas only 4% underwent apoptosis. (C) Comparison of one-step growth kinetics of BeAn virus in N20.1 and BHK-21 cells showed similar peaks of high virus titers by 12 to 14 h p.i. Error bars indicate the SD. (D) Immunoblot analysis of the five Bcl-2 family antiapoptotic proteins showed that Bcl-w was highly expressed in the N20.1 oligodendrocytes compared to BHK-21 and M1-D cells. The low expression level of Mcl-1 in M1-D cells was not detected in this immunoblot.
DISCUSSION
The mode of virus-induced cell death is critical in determining host range of a virus, i.e., the extent of virus production and viral spread in an animal host and dissemination to other animal hosts. In picornavirus infections where rapid viral replication kinetics usually predominate, necrosis generally has been associated with productive infections and apoptosis with restricted infections (6, 15, 18, 31, 34). TMEV infection has been shown to induce necrosis in rodent cells, such as BHK-21 and L929 cells, in association with high viral yields (15, 32), whereas murine Mϕs undergo apoptosis exclusively, with interruption of virus production and decreased yields of infectious virus progeny. An in-depth characterization of infection in BHK-21 cells has not been carried out, and the mechanism by which BeAn virus induces host-specific cell death to affect viral yields is not well understood. In the present study, we examined the mode of BeAn virus-induced cell death in BHK-21 cells, the role of Bcl-2 family prosurvival proteins, particularly Mcl-1, in regulating cell death, and the effect of apoptosis and necrosis on TMEV replication.
Our studies revealed the apoptosis of only a minority of BHK-21 virus-infected cells (∼20%), while a majority were PI positive (∼60%) and underwent necrosis (Fig. 1). The Bcl-2 multidomain, antiapoptotic protein Mcl-1 was highly expressed in BHK-21 cells, with a decrease in expression at 10 to 12 h p.i., which is consistent with the onset of apoptosis. siRNA knockdown of Mcl-1 resulted in an ∼3-fold increase in apoptosis (Fig. 2 and 3), indicating that Mcl-1 is important in regulating apoptosis in BHK-21 cells. Bcl-xL was also expressed at high levels (Fig. 2D) and, when knocked down by siRNAs, led to a similar increase in apoptosis (Fig. 3E). Knockdown of both Mcl-1 and Bcl-xL did not result in a greater percentage of apoptotic cells than either prosurvival molecule alone (not shown), possibly because either Mcl-1 or Bcl-xL is sufficient to counter most of the proapoptotic activity of Bax.
The apoptotic program that switched on at the time of exponential TMEV replication in BHK-21 cells was similar to that M1-D Mϕs (32, 33), with hallmarks of activation of the intrinsic apoptotic pathway in a tumor suppressor protein p53-dependent manner (Fig. 6A and B). Experiments in which Mcl-1 was stably knocked down to increase the otherwise low level of apoptosis in parental BHK-21 cells suggested that the subsequent activation of the Bcl-2 BH-only proapoptotic Noxa protein led to degradation of Mcl-1 (Fig. 6C and D), activation of Bax and, after the release of cytochrome c into the cytoplasm (not shown), the cleavage of caspase-9 and caspase-3 (Fig. 4C). The early activation of Bax, in contrast to its later activation in infected M1-D cells, was surprising but might be explained by the direct interaction of Bax with p53 inducing its homo-oligomerization and activation (9).
Analysis of one-step viral growth kinetics in Mcl-1 knockdown BHK-21 cells demonstrated a peak in viral growth at 8 to 10 h p.i. (∼2 h earlier than in parental cells), leading to 90% reduction in infectious viral yields at 24 h p.i. (Fig. 5A). While finding that viral RNA replication, protein synthesis, and polyprotein processing did not differ between stable Mcl-1 knockdown and parental cells (Fig. 5B and C) pointed to a defect in virion assembly possibly due to capsid protein cleavage from activated caspases, our preliminary experiments provide insufficient evidence of differences in the assembly of 5S protomers and 14S pentamers (assembly intermediates) or in 150S mature virions in cells with stable knockdown of Mcl-1 (unpublished data). Capsid proteins of feline calicivirus and Aleutian mink disease virus, a parvovirus, have been shown to be cleaved by caspases during infections in cell culture (2, 8). Recently, Bryant and Clem (5) reported that a p35 mutant of Autographa californica multiple nucleopolyhedrovirus, a baculovirus that infects lepidopteran insects, did not induce apoptosis despite caspase activity but did produce progeny virus with defects in stability and infectivity. This defect was rescued in the producer cells when virus was grown in the presence of zVAD-fmk (5). Caliciviruses, parvoviruses, baculoviruses, and TMEV are all nonenveloped viruses. Studies are in progress to identify a defect in TMEV virions or the capsid proteins grown in BHK-21 cells with stable knockdown of Mcl-1.
During persistent infection in the mouse CNS TMEV replication is observed primarily in Mϕs (19, 23, 30) and, to a lesser extent, in oligodendrocytes (3, 26). Blakemore et al. (3) found paracrystalline arrays of virions in oligodendrocytes by electron microscopy in chronically infected spinal cords, indicating that oligodendrocytes are productively infected and probably undergo necrosis. However, Tsunoda et al. (36) detected some TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling)-positive oligodendrocytes in mice chronically infected with another low-neurovirulence TMEV, DA virus. Our in vitro analysis using the N20.1 cell line, which consisted of primarily of immature oligodendrocytes (undifferentiated cells when grown at 37°C), showed that infection with BeAn virus led to necrosis with the production of high virus titers, similar to BHK-21 cells. N20.1 cells expressed high levels of another Bcl-2 antiapoptotic family protein, Bcl-w (Fig. 7D), which is highly expressed in brain and spinal cord tissues (21) and in neurons and glia (21, 25). Further studies of primary murine oligodendrocytes and other murine oligodendrocyte cells lines are needed.
ACKNOWLEDGMENTS
We thank Patricia Kallio for expert technical help, Robert P. Becker for the electron microscopic analysis, and Shannon Hertzler and Zhiguo Liang for helpful suggestions and nucleotide sequencing, respectively.
This study was supported by National Institutes of Health grant NS065945, the Modestus Bauer Foundation (H.L.L.), American Heart Postdoctoral Fellowship 10POST2710005 (K.-N.S.), and American Heart Predoctoral Fellowship 11PRE5150008 and Chicago Biomedical Consortium and Deiss Awards (S.Y.A.).
Footnotes
Published ahead of print 30 November 2011
REFERENCES
- 1. Akgul C. 2009. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell. Mol. Life Sci. 66:1326–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Molawi N, Beardmore VA, Carter MJ, Kass GEN, Roberts LO. 2003. Caspase-mediated cleavage of the feline calcivirus capsid protein. J. Gen. Virol. 84:1237–1244 [DOI] [PubMed] [Google Scholar]
- 3. Blakemore WF, Welsh CJ, Tonks P, Nash AA. 1988. Observations on demyelinating lesions induced by Theiler's virus in CBA mice. Acta Neuropathol. (Berl.) 76:581–589 [DOI] [PubMed] [Google Scholar]
- 4. Brahic M, Bureau JF, Michiels T. 2005. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu. Rev. Microbiol. 59:279–298 [DOI] [PubMed] [Google Scholar]
- 5. Bryant B, Clem RJ. 2009. Caspase inhibitor p35 is required for the production of robust baculovirus virions in Trichoplusia ni TN-368 cells. J. Gen. Virol. 90:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carthy CM, et al. 1998. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 72:7669–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chao JR, et al. 1998. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol. Cell. Biol. 18:4883–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng F, et al. 2010. The capsid proteins of Aleutian mink disease virus activate caspases and are specifically cleaved during infection. J. Virol. 84:2687–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chipuk JE, et al. 2004. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010–1014 [DOI] [PubMed] [Google Scholar]
- 10. Fiers W, Beyeart R, Declercq W, Vandenabeele P. 1999. More than one way to die: necrosis and reactive oxygen damage. Oncogene 18:7719–7730 [DOI] [PubMed] [Google Scholar]
- 11. Fischer SF, Belz GT, Strasser A. 2008. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haupt S, Berger M, Goldberg Z, Haupt Y. 2003. Apoptosis: the p53 network. J. Cell Sci. 116:4077–4085 [DOI] [PubMed] [Google Scholar]
- 13. Hertzler S, Luo M, Lipton HL. 2000. Mutation of predicted virion pit residues alters binding of Theiler's murine encephalomyelitis virus to BHK-21 cells. J. Virol. 74:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jelachich ML, Brumlage C, Lipton HL. 1999. Differentiation of M1 myeloid precursor cells into macrophages results in binding and infection by Theiler's murine encephalomyelitis virus (TMEV) and apoptosis. J. Virol. 73:3227–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jelachich ML, Lipton HL. 1996. Theiler's murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J. Virol. 70:6856–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi S, et al. 2007. Serine 64 phosphorylation enhances the antiapoptotic function of Mcl-1. J. Biol. Chem. 282:18407–18417 [DOI] [PubMed] [Google Scholar]
- 17. Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. 1993. MCL 1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. U. S. A. 90:3516–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuo R-L, Kung S-H, Hsu Y-Y, Liu W-T. 2002. Infection of enterovirus 71 or expression of its 2A protease induces apoptotic cell death. J. Gen. Virol. 83:1367–1376 [DOI] [PubMed] [Google Scholar]
- 19. Lipton HL, Twaddle G, Jelachich ML. 1995. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. J. Virol. 69:2525–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H, Perlman H, Pagliari LJ, Pope RM. 2001. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages: role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J. Exp. Med. 194:113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minami M, et al. 2000. Bcl-w expression is increased in brain regions affected by focal cerebral ischemia in the rat. Neurosci. Lett. 279:193–195 [DOI] [PubMed] [Google Scholar]
- 22. Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. 2004. Theiler's virus infection: a model for multiple sclerosis. Clin. Microbiol. Rev. 17:174–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pena-Rossi C, et al. 1997. Role of macrophages during Theiler's virus infection. J. Virol. 71:3336–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reed LJ, Muench HA. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 25. Reilly LA, et al. 2001. Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ. 8:486–494 [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez M, Leibowitz JL, Lampert PW. 1983. Persistent infection of oligodendrocytes in Theiler's virus-induced demyelination. Ann. Neurol. 13:426–433 [DOI] [PubMed] [Google Scholar]
- 27. Rozhon EJ, Kratochvil JD, Lipton HL. 1983. Analysis of genetic variation in Theiler's virus during persistent infection in the mouse central nervous system. Virology 128:16–32 [DOI] [PubMed] [Google Scholar]
- 28. Rueckert RR. 1976. On the structure and morphogenesis of picornaviruses, p 131–213 In Fraenkel-Conrat H, Wagner RR. (ed), Comprehensive virology: vol 6. Plenum Press, Inc., New York, NY [Google Scholar]
- 29. Sato F, Omura S, Martinez NE, Tsunoda I. 2011. Animal models of multiple sclerosis, p 55–79 In Minagar A. (ed), Neuroinflammation. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 30. Schlitt BP, Felrice M, Jelachich ML, Lipton HL. 2003. Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions. J. Virol. 77:4383–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shih S-R, Weng K-F, Li M-L. 2008. Viral protein synthesis is required for enterovirus 71 to induce apoptosis in human glioblastoma cells. J. Neurovirol. 14:53–61 [DOI] [PubMed] [Google Scholar]
- 32. Son K-N, Becker RP, Kallio P, Lipton HL. 2008. Theiler's virus-induced apoptosis in M1-D macrophages is Bax-mediated through the mitochondrial pathway, resulting in loss of infectious virus: a model for persistence in the mouse central nervous system. J. Virol. 82:4502–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Son K-N, Pugazhenthi S, Lipton HL. 2009. Activation of tumor suppressor protein p53 is required for Theiler's murine encephalomyelitis virus-induced apoptosis in M1-D macrophages. J. Virol. 83:10770–10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tolskaya EA, et al. 1995. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J. Virol. 69:1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trottier M, Kallio P, Wang W, Lipton HL. 2001. High numbers of viral RNA copies in the central nervous system of mice during persistent infection with Theiler's virus. J. Virol. 75:7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsunoda I, Kurtz CIB, Fujinami RS. 1997. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology 228:388–393 [DOI] [PubMed] [Google Scholar]
- 37. Verity AN, Bredesen D, Vonderscher C, Handley VW, Campagnonin AT. 1993. Expression of myelin protein genes and other myelin components in an oligodendrocyte cell line conditionally immortalized with a temperature-sensitive retrovirus. J. Neurochem. 60:577–587 [DOI] [PubMed] [Google Scholar]
- 38. Villunger A, et al. 2003. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302:1036–1038 [DOI] [PubMed] [Google Scholar]
- 39. Zhou P, Quian L, Kozopas KM, Craig RW. 1997. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 89:630–643 [PubMed] [Google Scholar]