Abstract
BST-2/tetherin is an interferon (IFN)-inducible host restriction factor that inhibits the release of many enveloped viruses and functions as a negative-feedback regulator of IFN production by plasmacytoid dendritic cells. Currently, mechanisms underlying BST2 transcriptional regulation by type I IFN remain largely unknown. Here, we demonstrate that the BST2 promoter is a secondary target of the IFN cascade and show that a single IRF binding site is sufficient to render this promoter responsive to IFN-α. Interestingly, expression of IRF-1 or virus-activated forms of IRF-3 and IRF-7 stimulated the BST2 promoter even under conditions where type I IFN signaling was inhibited. Indeed, vesicular stomatitis virus could directly upregulate BST-2 during infection of mouse embryonic fibroblasts through a process that required IRF-7 but was independent from the type I IFN cascade; however, in order to achieve optimal BST-2 induction, the type I IFN cascade needed to be engaged through activation of IRF-3. Furthermore, using human peripheral blood mononuclear cells, we show that BST-2 upregulation is part of an early intrinsic immune response since TLR8 and TLR3 agonists, known to trigger pathways that mediate activation of IRF proteins, could upregulate BST-2 prior to engagement of the type I IFN pathway. Collectively, our findings reveal that BST2 is activated by the same signals that trigger type I IFN production, outlining a regulatory mechanism ensuring that production of type I IFN and expression of a host restriction factor involved in the IFN negative-feedback loop are closely coordinated.
INTRODUCTION
The immune system has evolved many mechanisms aimed at controlling viral infections. Among these, production of interferons (IFN) triggers an antiviral state that is established through induction of host intrinsic antiviral proteins, such as PKR, RNase L, and ISG15, which inhibit virus infection at specific steps of their life cycle (59). Recently, a novel IFN-inducible host factor, bone marrow stromal cell antigen-2 (BST-2) (also referred as tetherin/CD317/HM1.24/ILT7L), with a potent antiviral activity against human immunodeficiency virus (HIV-1) was identified (53, 69). The membrane-associated BST-2 protein was found to inhibit the release of newly formed HIV-1 particles by directly cross-linking virions to the infected cell surface (56). BST-2 was also shown to exert its antiviral activity on a broad range of enveloped viruses, including retroviruses (all classes), filoviruses (Ebola and Marburg viruses), arenaviruses (Lassa and Machupo virus), paramyxoviruses (Nipah virus), gammaherpesviruses (Kaposi's sarcoma-associated herpesvirus), and rhabdoviruses (vesicular stomatitis virus [VSV]) (17).
While BST-2 is expressed at high levels at the surface of plasmacytoid dendritic cells (pDCs) and some cancer cells, it is expressed at relatively lower levels in bone marrow stromal cells, terminally differentiated B cells, macrophages, and T cells. Importantly, it can be induced by type I IFN in a number of transformed cell lines as well as in primary cell cultures of human and murine origins (5, 6, 22, 48, 53). Indeed, analysis of the human BST2 promoter region revealed multiple cis-regulatory elements for transcription factors such as canonical type I and II IFN response elements (IFN-stimulated response element [ISRE] and γ-activated site [GAS], respectively), and binding sites for the signal transducer and activator of transcription (STAT), IFN regulatory factors (IRF), NF-κB, and NF-AT (47, 55), consistent with the notion that BST-2 is an effector of the IFN antiviral response.
Induction of an antiviral state by IFN is a highly regulated process involving interaction with specific receptors expressed at the surface of nearly all cells. The major pathway for interferon signaling involves activation of tyrosine kinases of the Janus family (Jak kinases) and tyrosine phosphorylation/activation of STAT proteins. For instance, type I IFNs (IFN-α and IFN-β) induce tyrosine phosphorylation of STAT1 and STAT2 and formation of the interferon-stimulated gene factor 3 complex (ISGF3), comprised of phosphorylated STAT1 and STAT2 in association with IRF-9. ISGF3, in turn, translocates to the nucleus, where it binds ISRE on IFN-regulated gene promoters to initiate transcription (39, 57). Type I IFN-inducible genes (ISG) can be divided into two groups: immediate-early response genes, which are induced very rapidly after IFN signaling and respond directly to ISGF3 activation, and delayed genes, also known as secondary targets of the IFN activation cascade, which are induced more slowly since they require de novo protein synthesis (46). Among the immediate-early IFN-response genes are STAT1, IFNB, and IFN regulatory factors, such as IRF1 and IRF7. While these IRFs are critical for the subsequent production of IFN, they are also involved in the activation of many IFN-response-delayed genes and, as such, allow a positive-feedback loop of IFN responses to come into effect (40, 46). IRF molecules are also involved in the intrinsic cellular immune responses that are triggered during pathogen recognition. For instance, viral recognition is believed to occur through concerted signals induced by engagement of several types of pattern recognition receptors. These include a variety of membrane-bound Toll like-receptors (TLR), such as TLR3, TLR7, TLR8, and TLR9, as well as soluble cytosolic sensors like RIG-I, MDA5, and ZBP1 (2, 34, 52). These cellular sensors recognize single- or double-stranded RNA and DNA species and signal through their constituent adaptor, ultimately leading to activation of IRF proteins, namely, IRF-3 or IRF-7, and induction of IFN production (27, 66). Noticeably, it was recently reported that BST-2 may act as a ligand for the ILT7 receptor expressed specifically on human pDCs (6). Binding of BST-2 to ILT7 was shown to trigger signaling via the ILT7-FcεRIγ complex, which negatively regulates TLR7- and TLR9-induced type I IFN secretion by pDCs, thus raising the possibility that BST-2 could modulate the pDC IFN responses through ILT7 in a negative-feedback fashion (6).
Despite recent reports highlighting the role of BST-2 as a broad-spectrum antiviral factor and a negative regulator of IFN responses, the molecular mechanisms controlling its transcriptional induction by type I IFN remain largely unknown. In this study, we performed a functional analysis of the human BST2 promoter and found that the presence of a single IRF binding site was sufficient to elicit the full type I IFN induction of this promoter. Although BST-2 induction by type I IFN was found to be dependent on STAT1 phosphorylation, we show that its expression can be upregulated by IRF-1 as well as IRF-3 and IRF-7 mutants that mimic activated forms of these proteins typically found in virus-infected cells, in the absence of functional IFN signaling. Indeed, using VSV infection of mouse embryonic fibroblasts (MEF), we provide evidence that infection itself is sufficient to upregulate BST-2 in a process that involves IRF-7; however, in order to achieve optimal BST-2 induction, the type I IFN cascade needs to be engaged through activation of IRF-3. Finally, we demonstrate that BST2 induction in human peripheral blood mononuclear cells (PBMCs) could be achieved by a variety of TLR agonists and in some cases prior to detectable type I IFN signaling. Taken together, our findings establish that intrinsic cellular innate immunity, through activation of IRF proteins, can directly trigger BST-2 expression.
MATERIALS AND METHODS
Antibodies and reagents.
Mouse monoclonal antibodies (Abs) against IRF-1 and STAT1 were purchased from Santa Cruz Biotechnologies and BD Biosciences, respectively. Rabbit polyclonal anti-actin and anti-Flag Abs were obtained from Sigma. Rabbit polyclonal Abs against phosphorylated STAT1_Tyr701 were purchased from Cell Signaling, while those directed against BST-2 were previously described (3). Anti-mouse BST-2 allophycocyanin (APC)-labeled Abs and its APC-labeled isotype control were purchased from eBiosciences. The mouse monoclonal Abs for multiparametric cell surface staining of human cells, anti-CD3 Pacific Blue, anti-CD4 PerCP/Cy5.5, and anti-CD8 APC/Cy7 Abs, were purchased from Biolegend, while anti-CD14 PE/Texas Red and anti-CD303 (BDCA2)-APC were purchased from Caltag and Miltenyi, respectively. Viability was assessed by Aqua live-dead cell staining (Invitrogen) according to the manufacturer's recommendations. Human recombinant tumor necrosis factor alpha (rTNF-α), rIFN-β1, rIFN-γ, phorbol myristate acetate (PMA), and ionomycin were obtained from Sigma. Human rIFN-α2a and mouse rIFN-α11 were obtained from PBL. Final concentrations used for the cytokines and drugs as well as the range of tested doses were the following: human rIFN-α2a, 1,000 U/ml (range, 100 to 5,000 U/ml); human rIFN-β1, 500 U/ml (range, 100 to 5,000 U/ml); human rIFN-γ, 1,000 U/ml (range, 100 to 5,000 U/ml), human rTNF-α, 40 ng/nl (range, 10 to 100 ng/ml); mouse rIFN-α11, 1,000 U/ml (range, 100 to 2,500 U/ml); PMA, 10 ng/ml (range, 1 to 50 ng/ml); and ionomycin, 1 μM (range, 0.1 to 5 μM). Human monocyte colony-stimulating factor was purchased from R&D Systems, while B18R was purchased from eBiosciences.
TLR agonists and their respective controls, naked poly(C) low molecular weight (pIC L) and high molecular weight (pIC H), imiquimod (IMIQ), single-stranded RNA40 (ss40) and its negative control (ss41), as well as type A CpG oligonucleotide (CpGA) (ODN2216) and its negative control (CpGA ctrl) (ODN2216 ctrl), were obtained from Invivogen. Final concentrations used for the TLR agonists were as follows: pICs, 5 μg/ml; IMIQ, 2.5 μg/ml; ss40/ss41, 5 μg/ml; and ODNs, 5 μM.
Plasmids.
pGL4.70-Actin_renLuc was constructed by subcloning the 1,200-bp region of the chicken β-actin/rabbit β-globin hybrid promoter from the pCAGGS-GFP plasmid (F. Charron, IRCM, Montreal, Canada) into pGL4.70 (Invitrogen). Construction of reporter plasmids containing the wild-type (WT) or mutated forms of the BST2 promoter was done as follows; a 1.45-kb DNA fragment containing the predicted BST2 promoter region was amplified by PCR using primer set −1409F and +50R (see Table S1 in the supplemental material) and HeLa cell genomic DNA as a template. The DNA sequence of the amplified fragment was confirmed by automated DNA sequencing prior to cloning into the KpnI and HindIII sites of the pGL4.17 vector (Invitrogen). This plasmid (pGL4.17-BST2_ffLucFL, designated FL) was used as the parental plasmid to generate a series of mutation and deletion constructs by PCR (see Fig. 5A). Primer sets used to generate the mutation and deletion constructs can be found in Table S1. All constructs were confirmed by automated DNA sequencing. Human IRF-1 cDNA expression plasmid was obtained from Origene. Expression plasmids pcDNA3.1_HA-Stat1, pcDNA3.1_HA-Stat1-Y701F, pFLAG-CMV2-IRF3, pFLAG-CMV2-IRF3-5D, pFLAG-CMV2-IRF7 and pFLAG-CMV2-IRF7_Δ247-467 were a generous gift from J. Hiscott (McGill University, Montreal, Canada). The lentiviral vector construct, pdl.PIV5, encoding the V protein of parainfluenza virus type 5 (PIV5) was a generous gift from Roger Everett (18).
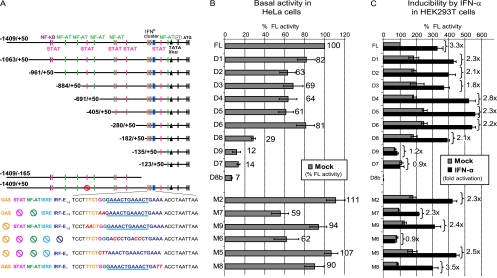
Fig 5.
Delineation and identification of cis-acting elements responsible for BST2 promoter activation by type I IFN. (A) Schematic representation of pGL4.17-BST2_ffLuc(FL), deletion mutants D1 to D9, and point mutations in the full-length promoter (M2, M5 to M9). Close-up of the IFNR cluster highlights different predicted TFBS, with nucleotides colored in red denoting mutations. Crossed circles point at TFBS that were inactivated by mutation. (B and C) Above-mentioned promoter reporter constructs together with pGL4.70-Actin_renLuc (200 ng each) were cotransfected in either (B) HeLa cells or (C) HEK293T cells (105 cells in both cases). Samples were collected 48 h posttransfection under the indicated conditions and analyzed for dual-luciferase activity. Results were plotted as firefly luciferase activity (standardized to control Renilla luciferase activity) and expressed as percentages of pGL4.17-BST2_ffLuc (FL) basal activity (100%). For HeLa cells, percent luciferase activity is depicted for each construct. For HEK293T cells, fold increase after IFN-α treatment relative to basal condition is indicated for each construct. Error bars represent the standard deviation (SD) calculated from results of four independent experiments.
Cell culture.
HeLa, HEK293T, BHK-21, 2fTGH, U3A, U3AR, U3A-701 cells, and primary MEF as well as IFN-α/β reporter cell lines were cultivated in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). HEK293T and HeLa cells were obtained from ATCC. 2fTGH, U3A, U3AR, and U3A-701 were a generous gift from G. R. Stark (Lerner Research Institute, Cleveland, OH) (37, 50). HEK-blue human IFN reporter and B16-blue mouse IFN reporter cell lines (Invivogen) were provided by M. J. Tremblay (Université Laval, Quebec, Canada) and N. Sonenberg (McGill University, Montreal, Canada), respectively. All primary MEF were a generous gift from K. Mossman (McMaster University, Hamilton, Ontario, Canada) (27, 61). HEK293T cells stably expressing the V protein of PIV5 and lacking STAT1 were established by lentiviral vector transduction. Briefly, recombinant pdl.PIV5 lentiviral particles were generated by transfecting HEK293T cells with the pdl.PIV5 construct together with psPAX2, a plasmid encoding HIV-1 Gag/Pol, Tat, and Rev, and pVSVg, a vector expressing the G glycoprotein of VSV, as described previously (4). Two days posttransfection, the culture medium containing the lentiviral particles was used to transduce HEK293T cells and PIV5 V protein-expressing cells (HEK293T-V5V) were selected using puromycin. Peripheral blood samples were obtained from healthy adult donors who gave written informed consent in accordance with the Declaration of Helsinki under research protocols approved by the research ethics review board of the Institut de Recherches Cliniques de Montréal (IRCM). Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque centrifugation as recommended by the manufacturer (GE Healthcare) and cultured in RPMI 1640 medium supplemented with 10% FBS. Human primary monocytes were purified from freshly isolated PBMCs by negative selection using immunomagnetic beads according to the manufacturer's instructions (Monocyte isolation kit II; Miltenyi Biotec). Monocytes were allowed to adhere to six-well plates for 90 min at 37°C in RPMI 1640 medium supplemented with antibiotics and glutamine but without FBS. Nonadherent cells were removed by washing with phosphate-buffered saline (PBS). DMEM supplemented with 10% FBS and human monocyte colony-stimulating factor was used during macrophage culture.
Transient-transfection and luciferase assay.
For both ectopic protein expression and promoter studies, cells were transfected using Lipofectamine-2000 (Invitrogen). Unless otherwise stated, medium was changed 24 h posttransfection. For promoter studies, pGL4.17-BST2_ffLuc promoter reporter-derived plasmids were cotransfected with pGL4.70-Actin_renLuc. The final concentration of transfected DNA was kept constant in all cotransfection assays. Luciferase activities were measured in duplicate using the Dual-Glo luciferase assay system (Promega) following the manufacturer's recommendations.
Surface antigen staining and flow cytometry analysis.
BST-2 cell surface staining and flow cytometry analysis were performed as previously described (3). Briefly, cells were washed in PBS and stained with the specific anti-BST2 rabbit serum for 60 min at 4°C. Rabbit preimmune (PI) serum served as a negative staining control. After incubation, cells were washed and stained using the appropriate fluorochrome-coupled secondary Abs for 45 min at 4°C. In mouse cells, APC-coupled anti-mouse BST-2 Abs and its isotype control were used. Fluorescence intensities were acquired using either a FACSCalibur flow cytometer (BD Biosciences) or a Cyan ADP flow cytometer (Beckman). Data were analyzed using FlowJo software v.8.7 (Treestar).
Surface phenotyping by flow cytometry.
Surface phenotyping was performed using multiparametric surface staining. Briefly, freshly isolated PBMCs or monocyte-derived macrophages (MDMs) (2 × 106 cells/ml and 0.5 × 106 cells/ml, respectively) were stimulated or mock-stimulated for the stated period of time at 37°C. Cells were then washed with cold PBS/EDTA/FBS, blocked with human IgG, and stained with the appropriate fluorochrome-conjugated surface cellular marker Abs for 60 min at 4°C. The CD4+ and CD8+ subpopulations of T cells were analyzed from a T cell population defined as CD3+/CD14−. The monocytes and macrophages were defined as CD3−/CD14+, while the pDC population was defined as CD303+/CD3−. Standard cell surface BST-2 labeling was combined with this staining. Cells were washed, resuspended in PBS, and analyzed using the Cyan flow cytometer with FlowJo software.
Steady-state detection of proteins by Western blotting.
Cells were lysed in RIPA-DOC buffer (140 mM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4, 1% Nonidet-P40, 0.5% sodium dodecyl sulfate, 1.2 mM deoxycholate [DOC], pH 7.2) supplemented with a cocktail of protease inhibitors (Complete; Roche Diagnostics). Proteins from lysates were resolved on 10 to 12.5% SDS-PAGE Tricine gels and analyzed by Western blotting as described previously (19).
Quantification of type I IFN.
Reporter cell lines HEK-Blue IFN-α/β cells and B16-Blue IFN-α/β (InvivoGen) were used for the detection of bioactive human and mouse type I IFNs, respectively. These cell lines harbor a reporter gene expressing the secreted embryonic alkaline phosphatase under the control of the IFN-inducible ISG54 promoter. Detection of released type I IFN was performed according to the manufacturer's recommendations. Data were expressed as optical density (OD) value or protein concentration. Type I IFN concentration (U/ml) was extrapolated from the linear range of a standard curve generated using known amounts of type I IFN.
Vesicular stomatitis virus infection.
Red fluorescence protein-tagged VSV-MΔ51 (Indiana serotype) was a gift from J. Bell (University of Ottawa, Ottawa, Canada) (11). VSV was propagated in BHK-21 cells, and titers were determined by plaque assay in the same cells (62). MEF cells were infected with VSV at a multiplicity of infection of 1. After 1 h of adsorption at 37°C, fresh complete medium was added. Samples were collected at the indicated time.
Statistical analysis.
The two-tailed paired Student t test was used for statistical comparison of data, and P < 0.05 was considered significant.
RESULTS
The BST2 promoter region contains multiple overlapping regulatory sequences.
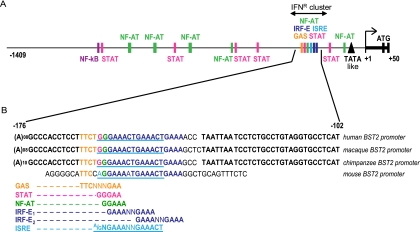
The primary transcription start site (TSS) of BST2 was previously mapped 34 bp upstream of the translation initiation site using human normal lung fibroblasts (47). To investigate the transcriptional regulation of the human BST2 gene, a 1.45-kb DNA fragment containing noncoding sequences upstream from the TSS as well as the 5′ untranslated region was selected for promoter studies. Bioinformatics analyses using TFSEARCH and MatInspector software (7, 24) predicted a putative nontraditional TATA box (TAATAAAG or TATA-like, from position −41 to −33, where +1 corresponds to the TSS), and several potential transcription factor-binding sites (TFBS) such as NF-AT, NF-κB, and general STAT binding sites (Fig. 1A). Analysis of the sequence also revealed the potential presence of multiple overlapping consensus sequence motifs related to IFN responses, including two IRF binding elements (IRF-E1 and IRF-E2), one ISRE, and one GAS element. Often, IRF-E, GAAANNGAAA (42, 67), can be found nested within ISRE sites, (A/G)NGAAANNGAAACT (10). Indeed, BST2's predicted IRF-E1 is nested within its predicted ISRE. However, it is important to point out that the IRF-E2 region displays a much less conserved similarity to a canonical ISRE site than IRF-E1. This region, spanning nucleotide positions −157 to −135, was designated the IFN response cluster (IFNR cluster) (Fig. 1A). Although noncoding in either DNA strand, this region is highly conserved, as no single nucleotide polymorphisms (SNPs) mapping to the IFNR cluster were found in the National Center for Biotechnology Information (NCBI) SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). Alignment of the IFNR region (Fig. 1B) revealed that this 22-nucleotide (nt) region is also conserved in all primate genomes analyzed and has 86% homology with the mouse genome, a further indication that it most likely plays an important role in BST2 promoter regulation.
Fig 1.
Predicted regulatory elements in the BST2 promoter region. (A) Schematic representation of the BST2 promoter region. (B) Nucleotide sequence alignment of BST2 IFNR clusters from primates and mouse. Nucleotides marked in bold are conserved among species. As indicated, colored and underlined nucleotides denote putative regulatory elements. Abbreviations used: ISRE, IFN-stimulated response element; IRF, IFN regulatory factor; IRF-E, IRF element; GAS, γ-activated site; STAT, signal transducer and activator of transcription.
Interferons are key activators of BST2 in human blood-derived primary cells.
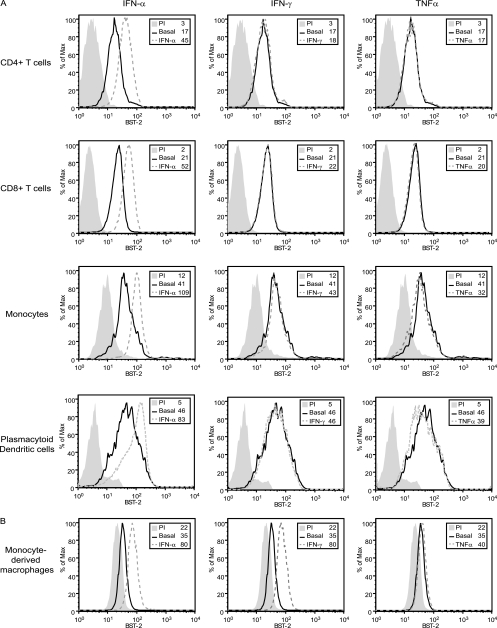
In order to gain a broad view of BST-2 expression in response to various stimuli, a panel of different human blood-derived primary cells was analyzed by flow cytometry. We first examined the surface expression levels of BST-2 in CD4+ and CD8+ T cells, monocytes, and pDCs from freshly isolated PBMCs as well as in MDMs. All tested cell types expressed BST-2 to different extents (Fig. 2). These data are consistent with recent reports that showed that monocytes displayed approximately 10 times more BST-2 molecules on a per-cell basis than T lymphocytes in healthy subjects (25, 33), but they contrast with results of a recent study that did not detect basal BST-2 expression in leukocyte subsets (16). No significant upregulation was observed after treatment with inducers of NF-κB (TNF-α and PMA) or NF-AT (ionomycin) pathways, even after different induction times (3 to 48 h) and increasing doses (Fig. 2 and data not shown). Only type I IFNs (IFN-α2a and IFN-β) were capable of upregulating BST-2 expression in all cell types (Fig. 2A and B and data not shown for IFN-β). Interestingly, stimulation of BST-2 expression by type II IFN (IFN-γ) was observed only in MDM cultures and was in fact similar in magnitude to that observed with type I IFN (Fig. 2B). Overall, these analyses performed on human primary lymphoid and myeloid cells confirm that type I IFNs are universal regulators of BST2.
Fig 2.
Effect of IFNs and TNF-α on BST-2 expression in human blood-derived primary cells. Human PBMCs or MDMs were exposed to IFN-α, IFN-γ, or TNF-α for 24 h, and BST-2 cell surface expression was analyzed by flow cytometry. (A) Multiparametric flow cytometry analysis after ex vivo staining of freshly isolated PBMCs. BST-2 cell surface expression on CD4+ T cells, CD8+ T cells, monocytes, and pDCs is shown. (B) Flow cytometry analysis of BST-2 expression at the surface of MDMs. A representative histogram of at least 3 independent experiments using cells from different donors is shown. Histograms represent at least 104 gated live cells under all conditions. Gray-filled histograms represent staining with rabbit PI Abs; nonfilled histograms represent staining with anti-BST-2 Abs. Solid lines represent basal condition, while dashed lines depict the indicated treatment conditions. Corresponding mean fluorescence intensity (MFI) values are shown for each condition.
Upregulation of cell surface BST-2 levels by type I IFN is dependent on STAT1 phosphorylation.
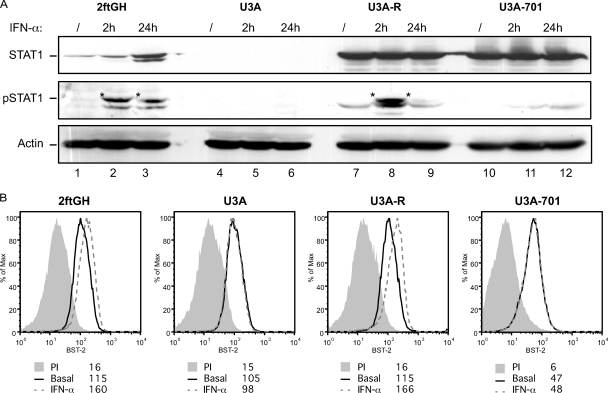
In order to assess the role of STAT1 phosphorylation in the IFN-mediated activation of BST-2 expression, we analyzed the induction of BST-2 protein by IFN-α in the STAT1-deficient human fibrosarcoma cell line U3A (10) (Fig. 3A). As controls, we used STAT1-expressing parental 2fTGH cells, U3A-R, a rescue cell line in which expression of STAT1 was stably reestablished through introduction of a construct encoding STAT1 under the control of a human cytomegalovirus immediate-early gene promoter, as well as U3A-701 cells, which express a phosphorylation-defective mutant of STAT1 (37, 50) (Fig. 3A). While IFN-α treatment upregulated BST-2 in parental 2fTGH and U3A-R cells, this induction was completely abrogated in U3A and U3A-701 cells lacking functional STAT1 (Fig. 3B), suggesting that stimulation of BST-2 expression by IFN-α requires phosphorylation of STAT1. As such, BST2 behaves as a classical ISG.
Fig 3.
Type I IFN-mediated upregulation of BST-2 in cells lacking functional STAT1. (A) Western blot of whole-cell extracts from control or IFN-α-treated (2 or 24 h) 2ftGH, U3A, U3A-R, and U3A-701 cells, revealed as indicated with α-STAT1-P (Tyr701), α-STAT1, and α-actin specific Abs. *, pSTAT1 specific band. (B) The above-described cells were treated with IFN-α for 24 h, and BST-2 cell surface expression was analyzed by flow cytometry. A representative histogram of at least three independent experiments is shown. Data were analyzed and plotted as described in Fig. 2.
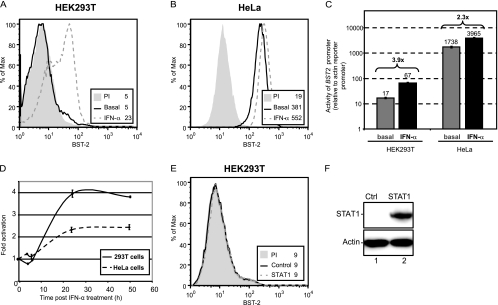
The BST2 promoter is a secondary target of the IFN activation cascade.
In order to investigate human BST2 regulation by type I IFN in model cell lines, the entire 1.45-kb BST2 promoter region (Fig. 1A) driving a reporter firefly luciferase gene was cloned into the pGL-4.17 plasmid to generate pGL4.17-BST2_ffLuc (FL). A control plasmid with a synthetic chicken β-actin promoter driving Renilla luciferase, pGL4.70-Actin_renLuc, was used for transfection efficiency control, internal normalization, and standardization. For these promoter studies, we took advantage of the differential BST-2 expression reported in HEK293T cells (no basal expression, inducible by type I IFN) (Fig. 4A) and HeLa cells (high basal expression, upregulated by type I IFN, Fig. 4B) (53). As shown in Fig. 4C, the BST2 full-length promoter region was sufficient to drive expression of the firefly luciferase gene when transiently transfected into HEK293T and HeLa cells. Interestingly, transfection of pGL4.17-BST2_ffLucFL (FL) in HeLa cells resulted in considerable levels of luciferase activity, over 1,700 times higher than that of the control actin reporter (Fig. 4C). In contrast, and correlating with the lack of detectable basal expression of endogenous BST-2, BST2 promoter activity in HEK293T cells was substantially lower (approximately 100 times less active than in HeLa cells). In both cell lines, the BST2 promoter was inducible by type I IFN and the degree of stimulation was comparable to that observed when measuring surface BST-2 (Fig. 4A to C). Consistent with previous data on the expression of BST-2 mRNA levels following type I IFN induction (8), a time course analysis of promoter activation by IFN-α revealed a delayed kinetics (Fig. 4D). Promoter activity peaked 24 h post-IFN-α treatment and remained constant for at least an additional 26 h in both HeLa and HEK293T cells. Given that direct gene activation by the ISGF3 complex is a very rapid process, requiring only phosphorylation and nuclear translocation events, the delayed kinetics of BST2 promoter activation by type I IFN suggests that a factor other than the ISGF3 complex is involved in type I IFN-mediated BST2 promoter activation. However, this factor should be encoded by an IFN-inducible gene and its expression should be dependent on STAT1 phosphorylation (Fig. 3B). Results from a previous report (8) prompted us to first examine whether IFN-inducible STAT1 was responsible for BST2 gene expression in our system. Ectopic expression of WT STAT1 did not result in increased levels of BST-2 (Fig. 4E and F) or BST2 promoter activation (data not shown) in HEK293T cells. Taken together, these results are consistent with the notion that the BST2 promoter is a secondary target of the type I IFN activation cascade and as such requires prior expression of a STAT1 phosphorylation-dependent factor in order to be activated.
Fig 4.
The BST2 promoter is activated by type I IFN with a delayed kinetics. HEK293T (A) and HeLa (B) cells were treated with IFN-α for 24 h, and BST-2 cell surface expression was analyzed by flow cytometry. (C and D) Activity of the BST2 promoter relative to the β-actin promoter. pGL4.17-BST2_ffLuc(FL) and pGL4.70-Actin_renLuc (200 ng each) were cotransfected in duplicate in either HEK293T or HeLa cells (105 cells). (C) Samples were collected under basal conditions or after 24 h of IFN-α treatment. The luciferase signal obtained with pGL4.70-Actin_renLuc was set as 1.0 (numbers above the bars represent fold increase of promoter activation by IFN-α). (D) Samples were collected at different time intervals post-IFN-α treatment and analyzed for dual-luciferase activity. Firefly luciferase activity driven by the BST2 promoter was expressed relative to control Renilla luciferase activity. Fold increase was calculated relative to the value obtained at time zero, which was set at 1. (E) HEK293T cells transfected with a STAT1-encoding plasmid (1 μg of plasmid per 2 × 105 cells) were analyzed for BST-2 cell surface expression by flow cytometry, 48 posttransfection. (F) Western blot of whole-cell extracts from the indicated transfected cells revealed with α-STAT1 and α-actin Abs. The data shown are representative of results obtained in at least three independent experiments.
BST2 promoter activation by type I IFN is dependent on IRF elements.
To define the boundaries of the minimal BST2 promoter and to identify cis-acting elements that govern the transcriptional activity of BST2, we generated a series of truncated promoter constructs (constructs D1 to D9, Fig. 5A) and tested their ability to direct luciferase expression in both HeLa (for basal expression) and HEK293T cells (for IFN-inducible expression). Sequential 5′ deletions from nucleotides −1063 to −280 (D1 to D6) did not substantially alter constitutive or inducible luciferase expression compared to the full-length promoter (Fig. 5). The D8 deletion construct displayed a lower basal luciferase activity in HeLa cells but, nevertheless, preserved its ability to respond to IFN in HEK293T cells (Fig. 5). In contrast, constructs D7, D8b, and D9, which lack the IFNR cluster, revealed very low or undetectable basal activity in HeLa cells and were not inducible by IFN-α in HEK293T cells, suggesting that the minimal promoter region responsive to IFN induction lies between positions −182 and −135, a region containing the IFNR cluster. To further confirm our findings, a panel of specific point mutations was generated within the full-length promoter construct to assess the functional role of specific predicted cis-acting elements. All the selected mutant promoter constructs had basal activities in HeLa cells that were comparable to those of the full-length promoter or the D1-D6 deletion constructs (Fig. 5A and B). Mutations outside the IFNR cluster (M2) as well as mutations within the IFNR cluster affecting the GAS element (M9) or NFAT/STAT binding sites (M7) did not affect type I IFN induction (Fig. 5C). In contrast, mutations affecting all remaining key regulatory sites in the IFNR cluster, including the two IRF-Es and ISRE (M6), essentially abolished the response to IFN-α. Importantly, IFN responsiveness was rescued when IRF-E2 was the only cis-acting element remaining in the IFNR cluster (M5). Interestingly, the two IRF binding sites appeared to be synonymous, since a promoter construct missing only IRF-E2 (M8) was fully inducible (Fig. 5C). Taken together, these results indicate that the presence of a single IRF binding site in the BST2 promoter is sufficient to elicit activation by type I IFN.
IRF proteins can induce BST-2 upregulation in the absence of type I IFN signaling.
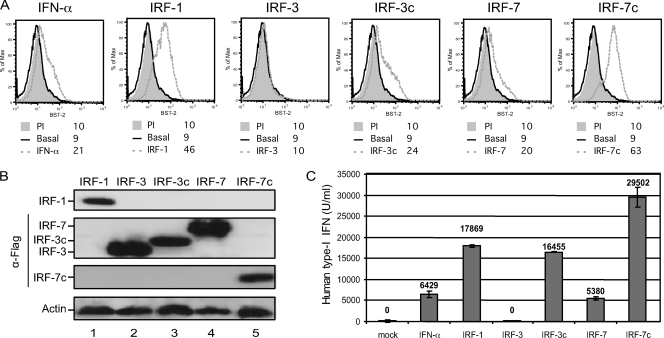
Having shown the importance of IRF-E binding sites in the BST2 promoter response to IFN-α, we next assessed the effect of different IRF proteins on BST2 promoter-directed gene expression. IRF-1 and IRF-7 are both encoded by genes upregulated by the ISGF3 complex upon treatment with IFN-α. Expression of IRF-1 or virus-induced activation of IRF-7 by phosphorylation was shown to stimulate expression of type I IFN genes, as well as several ISGs (49, 58, 66). A constitutive active form of IRF-7 (IRF-7Δ247-467, referred to throughout this paper as IRF-7c) can activate IFNA gene transcription by more than a 1,000-fold compared to WT IRF-7 (44). IRF-3 is another member of the IRF family, which is associated with the IFN antiviral response. IRF-3 can be found in an inactive form in the cytoplasm of most cells; however, as for IRF-7, virus-induced phosphorylation of IRF-3 activates the transcription of IFN-β, as well as other ISGs. An IRF-3-5D mutant (referred to in this paper as IRF-3c), in which serine or threonine residues at positions 396, 398, 402, 404, and 405 were replaced by the phosphomimetic aspartate amino acid, was shown to behave as a constitutively active form of the protein, thus inducing strong activation of the IFNB promoter in the absence of virus induction (43, 45). Transfection of HEK293T cells with IRF-1-, IRF-3c-, and IRF-7c-encoding constructs induced expression of endogenous BST-2 (Fig. 6A and B), although, as expected, it also caused a considerable release of type I IFN as measured by a sensitive reporter cell-based assay (Fig. 6C). In contrast, ectopic expression of WT IRF-3 had no effect on BST-2 expression and did not cause release of type I IFN (Fig. 6C), whereas treatment with IFN-α or ectopic expression of WT IRF-7 induced BST-2 expression to comparable levels (Fig. 6A and B). Noteworthy, these similar effects of IFN-α and IRF-7 on BST-2 expression correlated very well with the levels of type I IFN detected in cell supernatants under these conditions (Fig. 6C).
Fig 6.
Effect of IRF proteins on BST-2 surface expression. HEK293T cells were treated for 24 h with IFN-α or transfected for 48 h with IRF-1 or Flag-tagged IRF-3-, IRF-3c-, IRF-7- and IRF-7c-expressing plasmids (500 ng of plasmid per 2 × 105 cells). (A) BST-2 cell surface expression as measured by flow cytometry. (B) Western blot of whole HEK293T cells extracts revealed with α-IRF-1 specific Abs and reprobed with α-Flag and α-actin Abs. (C) Cell supernatant for each condition mentioned above was collected and tested for the presence of type I IFN (U/ml) using HEK-Blue human IFN-α/β reporter cells. Error bars represent the SD calculated from results of two independent experiments.
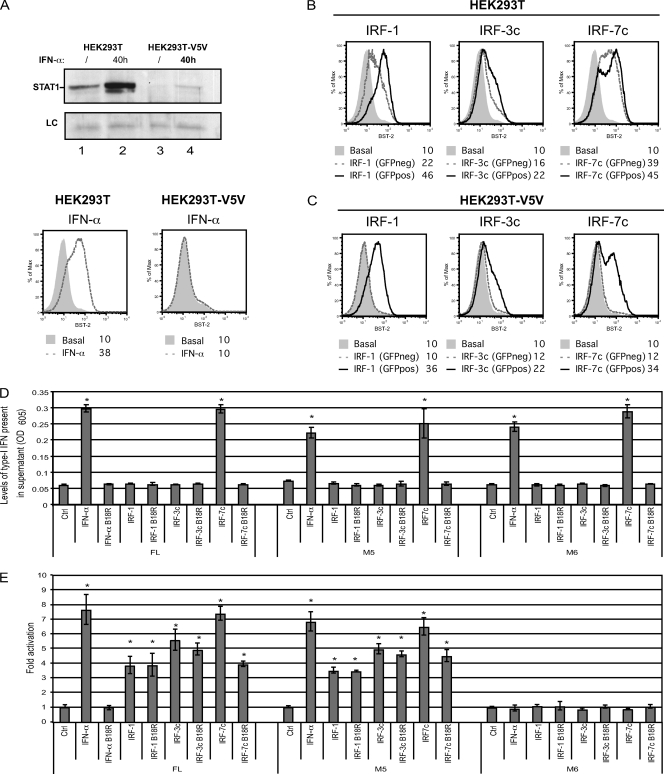
In order to determine whether IRF proteins are acting directly on the BST2 promoter or indirectly through the release of type I IFN, the effect of IRF proteins on surface BST-2 was assessed in STAT1-depleted HEK293T cells. To achieve efficient STAT-1 depletion, we took advantage of the function of the V protein of parainfluenza virus type 5 (PIV5), which targets STAT1 for efficient proteasome-mediated degradation (12). As shown in Fig. 7A (upper panel), transduction of HEK293T cells with a lentiviral vector encoding PIV5 V5 protein (HEK293T-V5V) led to an almost complete depletion of STAT-1 (compare lanes 3 and 1) and to a strong reduction of the steady-state levels of the protein upon treatment with type I IFN (compare lanes 4 and 2). Importantly, the PIV5 V protein-mediated STAT1 depletion made HEK293T-V5V cells unresponsive to IFN-α-induced upregulation of surface BST-2 (Fig. 7A, bottom panel). IRF1, IRF3c, or IRF7c was transiently coexpressed with green fluorescent protein (GFP) in HEK293T or HEK293T-V5V cells and cell surface BST-2 levels were evaluated on GFP-positive and GFP-negative cells to discriminate direct effects of IRFs from bystander effects mediated by the release of interferons. Consistent with the data of Fig. 6, expression of IRF1, IRF3c, or IRF7c in HEK293T control cells upregulated BST-2 in both GFP-positive and -negative cells (Fig. 7B). In contrast, when similar experiments were performed in type I IFN-unresponsive HEK293T-V5V cells (Fig. 7A), BST-2 upregulation was detected only in the IRF-expressing GFP-positive cells (Fig. 7C), suggesting that neither STAT1 nor type I IFN production is required for the IRF-dependent induction of the BST2 gene. Indeed, we further confirmed the direct activating effect of these IRFs using BST2 promoter-directed luciferase activity under conditions where type I IFN released in the extracellular medium was neutralized by the B18R protein, a vaccinia virus-encoded secreted protein with a potent type I IFN neutralizing activity (65). Constructs expressing IRF-1, IRF-3c, or IRF-7c were cotransfected in HEK293T cells with pGL4.70-Actin_renLuc and either pGL4.17-BST2_ffLucFL (FL), pGL4.17-BST2_ffLucM5 (M5), or pGL4.17-BST2_ffLucM6 (M6) constructs (Fig. 5A). Due to the large quantity of type I IFN released after IRF expression (Fig. 6C), we optimized the amounts of promoter reporter and IRF construct transfected and collected cells earlier after transfection (24 h) in order to achieve maximum activation of the BST2 promoter yet minimal release of IFN. Indeed, under these conditions, IRF-1 and IRF-3c ectopic expression resulted in undetectable levels of functional type I IFN in the extracellular medium whereas type I IFN released from cells expressing IRF-7c or treated with IFN-α was efficiently neutralized by B18R (Fig. 7D). Consistent with the upregulating effect of IRF proteins on BST-2 expression at the cell surface (Fig. 6A), ectopic expression of IRF proteins resulted in activation of the full-length BST2 promoter (Fig. 7E). Interestingly, while a BST2 promoter that contains a single IRF binding sites (M5) was still responsive to IRF expression, a non-IFN-inducible promoter construct that contained mutations affecting all putative IFN regulatory elements (M6) remained unresponsive (Fig. 7E), thus demonstrating the specificity of the experimental system. Importantly, activation of the BST2 promoter was observed when type I IFN was not detected in the supernatant or, when detected, was successfully neutralized by B18R (Fig. 7D and E). As an additional control we tested whether B18R-treated supernatant from IRF-transfected cells contained soluble factors that could upregulate surface BST-2 expression on fresh HEK293T cells and indeed ruled out this possibility (data not shown). Overall, these results indicate that several members of the IRF family, including IRF-1 and activated forms of IRF-3 and IRF-7, can directly induce BST-2 expression. Moreover, since the difference between pGL4.17-BST2_ffLucM5 and pGL4.17-BST2_ffLucM6 promoter constructs resides in a single IRF element, these results further confirm that induction of BST2 by type I IFN is likely achieved through engagement of at least one IRF-E binding site.
Fig 7.
Effect of IRF proteins on BST-2 expression in the absence of type I IFN signaling. (A to C) Effect of IRF proteins on BST-2 expression at the cell surface in STAT-1-depleted HEK293T cells. (A) Whole-cell extracts from untreated (/) or IFN-α treated (40 h) HEK293T and HEK293T-V5V cells were subjected to Western blotting, as indicated, using α-STAT1 specific Abs (upper panel). An unspecific band is also shown as a loading control (LC). BST-2 cell surface expression was measured by flow cytometry in both cell lines following IFN-α treatment (lower panels). HEK293T (B) or HEK293-V5V (C) cells were cotransfected with GFP- and either IRF-1-, IRF-3c-, or IRF-7c-expressing plasmids (500 ng of plasmid per 2 × 105 cells). BST-2 cell surface expression was analyzed in nontransfected GFP-negative (GFPneg) and transfected GFP-positive (GFPpos) cells by flow cytometry, 48 posttransfection. The data shown are representative of results obtained in three independent experiments. (D and E) Effect of IRF proteins on BST2 promoter-directed expression in the presence of the type I IFN neutralizing agent, B18R. HEK293T cells (2 × 105) were cotransfected with pGL4.70-Actin_renLuc (200 ng) and low doses of either IRF-1- (300 ng), IRF-3c- (300 ng), and IRF-7c- (150 ng) expressing plasmids or empty plasmid (Ctrl, 300 ng) as well as with BST2 promoter reporter constructs, pGL4.17-BST2_ffLucFL, M5, or M6 (200 ng each) in duplicate. Following a medium change 3 h posttransfection, half the IRF-expressing samples received medium containing B18R (0.1 μg/ml). Mock-transfected samples were treated either with IFN-α or with IFN-α preincubated 5 min with B18R (0.1 μg/ml). All samples were collected 24 h posttransfection. (D) Supernatant for each condition mentioned above was collected and tested for the presence of type I IFN using HEK-Blue human IFN-α/β reporter cells. Levels of type I IFN are plotted as OD605. (E) Samples were then analyzed for their dual-luciferase activity. Results were plotted as firefly luciferase activity (standardized to control Renilla luciferase activity) and expressed relative to the basal activity detected in cells transfected with empty plasmid, which was set at 1. Error bars represent the SD calculated from results of four independent experiments. Statistical significance compared to control samples: *, P < 0.01.
VSV infection upregulates BST-2 levels at the surface of MEF through IRF-7 prior to IFN production.
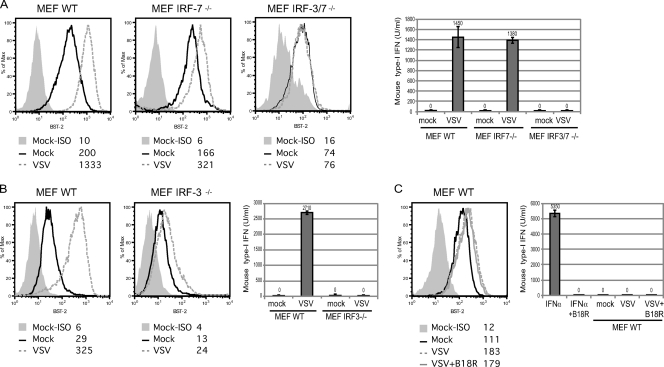
Since IRF-3 and IRF-7 transcription factors are directly activated following virus infection, we sought to investigate whether BST2 induction is one of the early cellular responses to viral infection, together with type I IFN production. Primary cultures of MEF from WT mice (MEF WT), IRF-3 null mice (MEF IRF-3−/−), IRF-7 null mice (MEF IRF-7−/−), or IRF-3/IRF-7 double knockout (KO) mice (MEF IRF-3/7−/−) were used to evaluate BST-2 expression prior to and following infection with VSV, a well-known inducer of IFN responses (64). Initial characterization of BST-2 expression at the surface of primary MEF revealed a heterogeneity that was passage dependent. At very early passages, both WT MEF and KO MEF displayed positive and negative cell populations with regard to BST-2 expression (see Fig. S1 in the supplemental material), which stabilized into a single homogenous population of BST-2-expressing cells with cell passage. The actual passage number required to obtain this homogenous cell population varied with each particular MEF culture but was achieved for all prior to senescence and immortalization (Fig. 8A, early cell passage; Fig. 8B, intermediate cell passage). It is important to note that as observed with the other cell types used in this study, all the primary MEF lines upregulated BST-2 upon exposure to type I IFN (see Fig. S1), indicating that the signaling pathways triggered by exogenous IFN are functional in these cells.
Fig 8.
Effect of VSV infection of mouse embryonic fibroblasts on BST-2 expression. BST-2 cell surface expression in primary cultures of the indicated MEF infected with VSV-MΔ51 or mock infected as measured by flow cytometry. Detection of type I IFN (U/ml) in supernatants for each tested condition using B16-Blue mouse IFN-α/β reporter cells. Collection at 24 h postinfection (pi) of lower-passage MEF WT, MEF IRF-7−/−, and MEF IRF-3/7−/− (A), 24 hpi of intermediate-passage MEF WT, and MEF IRF-3−/− (B), and 8 hpi of lower-passage MEF WT in the presence or absence of B18R (0.1 μg/ml) (C). As controls, fixed amounts of mouse IFN-α11 alone or preincubated 5 min with B18R (0.1 μg/ml) were added to the mouse IFN-reporter cell line (B16-Blue IFN-α/β). The data shown are representative of results obtained in at least three independent experiments. Gray-filled histograms represent staining with APC-labeled isotype (ISO) control; nonfilled histograms represent staining with APC-labeled anti-mouse BST-2 Abs with solid black, dashed gray and solid gray lines representing mock-infected cultures, VSV-infected cultures, and VSV-infected cultures in the presence of B18R, respectively. Corresponding MFI values are shown for each condition.
In order to evaluate the VSV-induced cellular antiviral response, we took advantage of a red fluorescence protein-tagged variant of VSV with a deletion of a methionine residue at position 51 in the matrix protein (VSV-MΔ51), which inactivates the matrix's ability to block transport of IFN and other ISG mRNAs from the nucleus during the course of viral infection. Using fluorescence microscopy, we determined that similar levels of infection were achieved in all MEF lines (data not shown). In fact, as shown in Fig. 8A, infection of MEF WT with VSV-MΔ51 led to production of type I IFN in the extracellular medium 24 h postinfection and resulted, as expected, in a strong upregulation of BST-2. Although VSV-infected MEF IRF-7−/− cells produced comparable amounts of type I IFN, BST-2 upregulation was less pronounced in these cells (Fig. 8A), suggesting a potential direct contribution of IRF-7 in BST-2 upregulation upon VSV sensing by infected cells and an indirect involvement of IRF-3 through activation of IFN production. Consistent with this possibility, neutralizing mouse IFN released from infected MEF IRF-7−/− with B18R completely abolished BST-2 upregulation (data not shown). MEF IRF-3−/− cultures were not homogeneous for surface BST-2 at early passages but only at intermediate passages and were therefore compared to WT MEF collected at a similar passage (Fig. 8B). Interestingly, even though, as previously reported (23), infection of MEF IRF-3−/− did not result in IFN production, it still induced a significant upregulation of BST-2 (Fig. 8B). Importantly, both IFN production and BST-2 upregulation were completely abolished in MEF IRF-3/7−/− infected with VSV (Fig. 8A), providing further evidence for a direct role of IRF-7 in BST-2 expression during host responses to viral infection.
In order to further demonstrate a direct effect of VSV on BST-2 expression during infection of MEF WT, samples were collected earlier after infection (8 hours postinfection [hpi]) when the amounts of type I IFN released were below detection (Fig. 8C). Nevertheless, as an additional control, experiments were also performed in the presence of B18R. A significant upregulation of BST-2 was noted under these conditions (Fig. 8C), although as expected, its magnitude was reduced compared to that observed when the infection was allowed to progress further and type I IFN was readily detected in the culture supernatant (less than 2-fold in the absence of IFN signaling in Fig. 8C versus over 6-fold in the presence of IFN signaling in Fig. 8A). Taken together, these results suggest that VSV can directly upregulate BST-2 expression early after infection through a process that requires IRF-7. These data also indicate that BST-2 expression can be further enhanced during VSV infection of MEF by engagement of the type I IFN positive-feedback loop through activation of IRF-3.
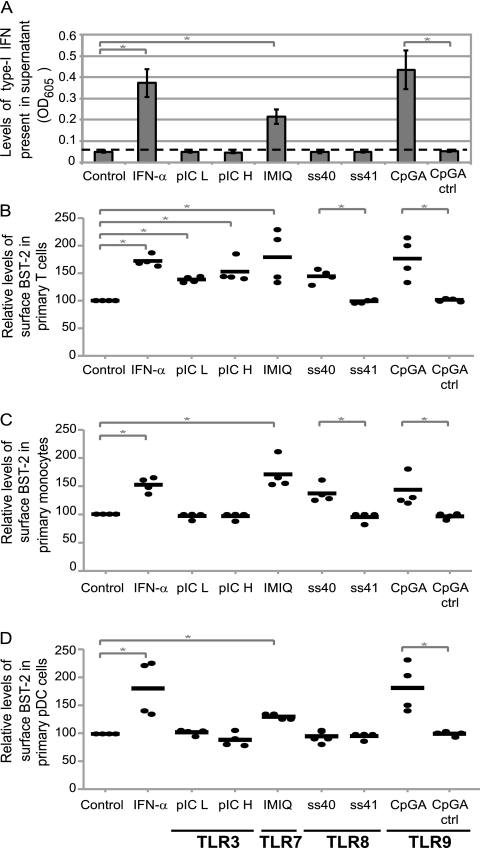
Agonists of TLRs upregulate BST-2 expression in human PBMCs.
Since viral sensing by the intrinsic cellular immune response occurs through concerted signals by several pattern recognition receptors, including TLRs, we next sought to test whether agonists of several TLR pathways could upregulate BST-2 expression in human PBMC cultures. To that end, the following TLR pathway agonists were tested: naked polyinosine-poly(C) (pIC) low molecular weight (pIC L) and high molecular weight (pIC H) for TLR3, imiquimod (IMIQ) for TLR7, single-stranded RNA40 (ss40) and its negative control (ss41) for TLR8, as well as type A CpG oligonucleotide (CpGA) and its negative control (CpGA ctrl) for TLR9 (34). PBMC cultures were treated with the different TLR agonists for a very short interval of time (5 h) to ensure detection of early cellular responses. BST-2 expression at the cell surface was measured in specific cell subtypes by multiparametric flow cytometry analysis, while release of type I IFN was monitored in the extracellular medium as described above. Agonists of TLR7 (IMIQ) and TLR9 (CpGA) induced a rapid and potent production of type I IFN (Fig. 9A). As expected, under these conditions, all tested cell types exhibited higher levels of surface BST-2 relative to controls (Fig. 9B to D). Interestingly, some agonists caused upregulation of BST-2 expression before detection of type I IFNs in a cell type-specific manner (Fig. 9). Hence, the TLR8 agonist, ss40, caused IFN-independent BST-2 upregulation in TLR8-positive T cells (both CD4+ and CD8+ T cells) and monocytes but not in TLR8-negative pDCs (compare Fig. 9B and C with D). On the other hand, TLR3 agonists induced an IFN-independent upregulation of BST-2 expression only in TLR3-positive T cells and not in TLR3-negative monocytes or pDCs (compare Fig. 9B with C and D). As expected, the negative controls for the ss40 and CpGA agonists, namely, ss41 and CpGA ctrl, caused neither a release of type I IFN nor BST-2 upregulation, thus underlining the specificity of our experimental system (Fig. 9). Overall, these results indicate that BST-2 upregulation is part of an early intrinsic immune response since TLR8 and TLR3 agonists, known to trigger pathways that mediate activation of different IRF proteins (15, 26, 35) could upregulate BST-2 in the absence of type I IFN signaling.
Fig 9.
Effect of TLR agonists on BST-2 expression in human PBMCs. PBMCs from healthy donors were treated as indicated for 5 h. Specific TLR signaling pathways induced by agonists are specified at the bottom of the figure. (A) Supernatant for each of the indicated conditions was tested for the presence of type I IFN using HEK-Blue human IFN-α/β reporter cells. Relative levels of type I IFN are plotted as OD605. Error bars represent the SD calculated from results for four independent donors. (B to D) Multiparametric flow cytometry analysis after ex vivo staining of freshly isolated PBMCs. Expression of surface BST-2 in T cells (B), monocytes (C), and pDC subsets (D). MFI values for surface BST-2 (minus isotypic control MFI) were calculated for each condition and expressed as percentages relative to results for control untreated cells for that particular donor and for each cell subset. Values obtained for each donor are marked with a dot, and bars represent mean of all readings. Statistical significance compared to control samples: *, P < 0.05.
DISCUSSION
Many studies have suggested that BST-2 expression could be induced by type I IFN (5, 6, 14, 25, 36, 48) and type II IFN (6, 14, 68, 71) in different cell types of human and mouse origin. Consistent with these findings, analysis of the putative promoter region of human, nonhuman primate, and mouse BST2 identified several highly conserved TFBS that would support this type of regulation (Fig. 1). Indeed, analysis of BST2 induction by type I and II IFNs in different subsets of human PBMCs as well as in MDMs showed that while type II IFN selectively induced BST-2 expression in MDMs, type I IFNs upregulated BST-2 in CD4+ and CD8+ T cells as well as in monocytes, pDCs, and MDMs (Fig. 2). These findings indicate that while the different TFBS contained in the BST2 promoter region might be activated in a cell type-specific manner, type I IFNs represent universal inducers of BST-2 expression in blood-derived human immune cells.
The first step of the type I IFN cascade involves the phosphorylation of STAT1, a posttranslational modification required to form the ISGF3 complex that recognizes ISRE sites on target genes (39). The data presented here indeed show that BST2 induction by type I IFN is dependent on STAT1 phosphorylation since cell lines defective in STAT1 (U3A) or unable to phosphorylate STAT1 (U3A-701) lost their ability to upregulate BST-2 in the presence of type I IFN (Fig. 3). This dependence on STAT1 phosphorylation together with the delayed IFN-α induction of both BST2 promoter (Fig. 4D) and BST-2 mRNA expression (8) imply that a factor directly regulated by ISGF3 or by at least phosphorylated STAT1 needs to be expressed prior to BST2 induction. Furthermore, our mutational analysis of the BST2 promoter in HEK293T cells identified a 100-bp sequence responsible for BST2 induction by type I IFN and demonstrated that STAT and ISRE sites were not required for induction (Fig. 5). Importantly, the presence of at least one single IRF-E was found to be sufficient for BST2 promoter activation by type I IFN (Fig. 5 and 7E). The lack of requirement for ISRE and STAT sites (Fig. 5) is indeed consistent with our previous conclusion that the ISGF3 complex may not be acting directly on the BST2 promoter and that an IFN-inducible gene is required for its regulation. Even though it was previously shown that IFN-inducible nonphosphorylated STAT1 was sufficient to induce BST-2 upregulation in certain cancer cells (8), we did not find that this was indeed the case in our model HEK293T cell line (Fig. 4E and F).
The key role played by IRF-E in IFN-α-mediated BST-2 induction suggests that IFN regulatory factors (IRFs) might be involved in BST2 transcriptional induction by type I IFN. IRFs constitute a family of nine transcription factors that bind to IRF-E and include IRF-1, IRF-2, IRF-3, IRF-4/Pip/ICSAT, IRF-5, IRF-6, IRF-7, ICSBP/IRF-8, and ISGF3γ/p48/IRF-9. Among the IRF family members, IRF-1, IRF-3, and IRF-7 have been established as essential factors required for the production of type I IFN and IFN-inducible genes in response to viral or cytokine stimulation (27, 49, 66). Furthermore, IRF-1, IRF-2, IRF-5, and IRF-7 are type I IFN-inducible genes themselves (66). We provided evidence that ectopic expression of IRF-1 induced BST2 promoter activation as well as BST-2 expression in HEK293T cells (Fig. 6). This is in line with a previous report, which showed that IRF-1 can induce BST-2 expression in cancer cell line C32 (68), although this study could not discriminate whether the effect of IRF-1 was direct or indirect through the release of IFN. In that regard, using HEK293T cells depleted for STAT1 we showed that expression of IRF-1 protein, in the absence of the IFN-α/ISGF3 signaling cascade, was sufficient to upregulate BST-2 expression (Fig. 7C). These results were further confirmed using vaccinia virus-encoded type I IFN soluble receptor B18R as a mean to neutralize type I IFN (Fig. 7E). The facts that a single IRF-E is necessary for BST2 promoter responsiveness to type I IFN induction and that IRF-1 could activate BST-2 expression strongly suggest that IRFs are likely the IFN-induced gene product required for activating the BST2 promoter following IFN signaling. Even though our results suggest that IRF-7 is not likely to be a candidate in this process, since MEF IRF-7−/− cells are still upregulating BST-2 upon treatment with type I IFN (see Fig S1 in the supplemental material), we cannot exclude the possibility of binding redundancy as previously reported for the IFNB and BLIMP1 promoters (38, 61).
One interesting finding from our deletion analysis of the human BST2 promoter is that sequences upstream from the IFNR cluster appear to determine a high basal level of expression in HeLa cells (Fig. 5A and B). Interestingly, HeLa cells were reported to produce significant levels of HEB (HeLa E box-binding protein) (30), a protein that belongs to the E-protein class of basic helix-loop-helix transcription factor. The proposed binding site for transcription factors of this family, the E-box [CA(G/C)CTG], is found five times within the sequences upstream of the BST2 promoter IFNR cluster region. Notably, the first E-box (position −1335, CACCTG) is eliminated in deletion D1 while the last (position −212, CAGCTG) is lost in deletion D8, and in both instances a significant drop in the basal activity of the BST2 promoter reporter constructs was observed in HeLa cells (Fig. 5B). Whether the E-box sequences play a role in the basal activity of the BST2 promoter will be interesting to follow up on. In that regard, E2-2, another E protein preferentially expressed in the murine and human pDC, a cell type which expresses high basal levels of BST-2, has been identified as a specific transcriptional regulator of the pDC lineage that can directly activate multiple pDC-enriched genes, including IRF-7 and BST2 (9). In fact, in E2-2 KO mice, dendritic cells accumulate as an immature population which does not express BST-2 (9). Whether the high levels of BST-2 detected in some cell types at steady state are the result of higher expression of IRF-7 or of more specific lineage factors like E2-2 remains to be determined.
IRF-3 and IRF-7 are responsible for the production of type I IFN after virus sensing through a well-described biphasic approach (28). While activated IRF-3 is thought to regulate the early phase of the innate virus sensing, characterized by IFN-β production, IRF-7 appears responsible for the IFN cascade amplification (60). Ultimately, during the late phase of the response, IRF-3 and IRF-7 together activate IFNA and IFNB promoters, resulting in subsequent ISG activation. However, early reports also indicated that a number of ISGs could be directly activated by virus infection, in the absence of IFN production (1, 70). Consistent with this possibility, we showed that ectopic expression of constitutive active forms of IRF-3 and IRF-7 that mimic those found in infected cells can activate the BST2 promoter and upregulate BST-2 in the absence of STAT1 and IFN signaling in HEK293T cells (Fig. 7). Importantly, we provided evidence that infection of MEF with VSV is capable of directly upregulating BST-2 expression by a process dependent on the presence of IRF-7 (Fig. 8). As expected, our results also revealed that BST-2 expression could be further enhanced by the engagement of the IRF-3-dependent type I IFN-positive feedback loop (Fig. 8). BST2 is therefore part of a number of ISG genes, such as the 561 gene, IRF-1, ISG15 (previously known as IFI-15K), IFIT2 (ISG54 or IFI-54K), and IFIT1 (ISG56 or IFI-56K) (1, 70), whose expression can be directly activated during viral infection to increase host survival, as reported for Newcastle disease virus. IRF-7 potential to activate ISGs independently of IFN signaling was previously reported for other genes, including IRF7 itself, IFNB, MXA, CXCL10, and TRAIL (13, 51, 54). Nevertheless, since IRF-1 is among the genes proposed to be induced following viral infection in the absence of IFN signaling, we cannot completely exclude that IRF-1 could also be an effector during this VSV-induced cellular response. Overall, as previously shown for type I IFNs (28), the transcriptional regulation of BST2 is also controlled by IRF proteins soon after viral infection. This notion highlights that a close coordination of BST-2 and type I IFN expression prevails during the early cellular response to virus infection.
The pathways that mediate IRF-3 and IRF-7 activation and type I IFN production during pathogen infection are initiated by the intracellular recognition of pathogen-associated molecular patterns by cellular sensors such as membrane-bound TLRs. Viral molecular patterns are recognized by endosomal TLR3, TLR7, TLR8, and TLR9 and lead to a robust type I IFN production (34). Indeed, it was previously shown that human PBMCs produce high levels of type I IFN in response to a 24-h treatment with TLR7 and TLR9 agonists. Interestingly, while treatment with TLR8 agonists resulted in a less potent response, treatment with TLR3 agonists did not result in production of type I IFN (20). To determine whether human BST-2 upregulation was part of a very early cellular response occurring prior to IFN production and signaling, PBMCs were collected 5 h posttreatment with TLR agonists. Under these conditions, TLR7 and TLR9 agonists released detectable levels of type I IFN but TLR3 or TLR8 agonists did not (Fig. 9). Interestingly, BST-2 upregulation was observed after TLR8 agonist treatment in primary T cells and monocytes, cell types known to express this receptor, but not in TLR8-negative cells, such as pDCs (Fig. 9) (29, 31, 32). Furthermore, TLR3 agonists caused BST-2 upregulation in TLR3-positive T cells but not in TLR3-negative monocytes or pDCs (29, 31, 32) (Fig. 9), underlining the specificity of these responses and ruling out the possibility of a bystander BST2 activation resulting from an IFN signaling that would be below the detection limits of our sensitive reporter system. It was previously established that TLR8 is able to activate IRF-7 (26, 35) while TLR3 mainly acts through IRF-3 (15), although it is also capable to activate IRF-7 as well (63). Hence, consistent with the results obtained with VSV-infected MEF (Fig. 8), our data in human primary cells reveal that BST-2 upregulation is part of a very early intrinsic innate immune response since some TLR agonists could upregulate BST-2 prior to detectable type I IFN signaling (Fig. 9).
Type I IFN production by pDCs is part of the antiviral innate immune response that prevents local viral dissemination at the portal of entry. Following an initial viral infection, IFN responses are normally transient and self-limiting, since prolonged and uncontrolled IFN exposure can interfere with normal hematopoiesis (41) and as a result increase the risk of autoimmune diseases (21). Consequently, mechanisms that ensure a specific and restricted IFN response to viruses are important to minimize undesirable side effects. BST-2 interaction with its cognate receptor, ILT7, is thought to provide a strong negative signal that controls IFN released by pDCs (6). Given the efficient and robust induction of BST2 by type I IFN in a broad variety of cell types, the interaction between BST-2 and ILT7 could be considered an important negative feedback mechanism aimed at preventing prolonged IFN production after viral infection. On the other hand, expression of BST-2 in virus-infected cells in response to virus-induced IRF-7 and potentially IRF-3 activation would ensure that host cells actively maintain BST-2-mediated virus restriction mechanisms as long as needed even after the initial IFN response is resolved.
In conclusion, this study provides insight into the signaling mechanisms regulating BST2 induction by innate immunity responses. The important role of type I IFNs, IRF-1, IRF-3 and IRF-7 in the regulation of BST2 adds to our understanding of the IFN-mediated antiviral response and the delicate regulatory loops that control its strength and duration.
Supplementary Material
ACKNOWLEDGMENTS
We thank E. Massicotte, J. Lord, and R. Bacon-Savard for expert technical assistance, the IRCM clinic staff and all donors for providing blood samples, and K. Mossman, J. Hiscott, G. Stark, M. J. Tremblay, N. Sonenberg, R. D. Everett, and J. Bell for providing valuable reagents. Finally, we thank members of the CIHR Team in HIV Pathogenesis for helpful discussions.
E.A.C. is the Canada Research Chair in Human Retrovirology.
This work was supported by grants from CIHR (HET 85519 and MOP111226) and the FRSQ AIDS network to E.A.C.
Footnotes
Published ahead of print 1 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Bandyopadhyay SK, Leonard GT, Jr, Bandyopadhyay T, Stark GR, Sen GC. 1995. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J. Biol. Chem. 270:19624–19629 [DOI] [PubMed] [Google Scholar]
- 2. Barber GN. 2011. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr. Opin. Immunol. 23:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bego MG, Dube M, Mercier J, Cohen EA. 2009. Effect of calcium-modulating cyclophilin ligand on human immunodeficiency virus type 1 particle release and cell surface expression of tetherin. J. Virol. 83:13032–13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belzile JP, et al. 2007. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 3:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blasius AL, et al. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177:3260–3265 [DOI] [PubMed] [Google Scholar]
- 6. Cao W, et al. 2009. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 206:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartharius K, et al. 2005. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942 [DOI] [PubMed] [Google Scholar]
- 8. Cheon H, Stark GR. 2009. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. U. S. A. 106:9373–9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cisse B, et al. 2008. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darnell JE, Jr, Kerr IM, Stark GR. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421 [DOI] [PubMed] [Google Scholar]
- 11. Diallo JS, et al. 2010. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol. Ther. 18:1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Didcock L, Young DF, Goodbourn S, Randall RE. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928–9933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Domizio J, et al. 2009. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood 114:1794–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douglas JL, et al. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J. Virol. 83:7931–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doyle S, et al. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251–263 [DOI] [PubMed] [Google Scholar]
- 16. Erikson E, et al. 2011. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc. Natl. Acad. Sci. U. S. A. 108:13688–13693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. 2010. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 18:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everett RD, Young DF, Randall RE, Orr A. 2008. STAT-1- and IRF-3-dependent pathways are not essential for repression of ICP0-null mutant herpes simplex virus type 1 in human fibroblasts. J. Virol. 82:8871–8881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finzi A, Brunet A, Xiao Y, Thibodeau J, Cohen EA. 2006. Major histocompatibility complex class II molecules promote human immunodeficiency virus type 1 assembly and budding to late endosomal/multivesicular body compartments. J. Virol. 80:9789–9797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh TK, et al. 2006. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I Comparison with T cell receptor-induced responses. Cell. Immunol. 243:48–57 [DOI] [PubMed] [Google Scholar]
- 21. Gota C, Calabrese L. 2003. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity 36:511–518 [DOI] [PubMed] [Google Scholar]
- 22. Goto T, et al. 1994. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood 84:1922–1930 [PubMed] [Google Scholar]
- 23. Hata N, et al. 2001. Constitutive IFN-alpha/beta signal for efficient IFN-alpha/beta gene induction by virus. Biochem. Biophys. Res. Commun. 285:518–525 [DOI] [PubMed] [Google Scholar]
- 24. Heinemeyer T, et al. 1998. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 26:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Homann S, Smith D, Little S, Richman D, Guatelli J. 2011. Upregulation of BST-2/tetherin by HIV infection in vivo. J. Virol. 85:10659–10668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honda K, et al. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 101:15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honda K, et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777 [DOI] [PubMed] [Google Scholar]
- 28. Honda K, Yanai H, Takaoka A, Taniguchi T. 2005. Regulation of the type I IFN induction: a current view. Int. Immunol. 17:1367–1378 [DOI] [PubMed] [Google Scholar]
- 29. Hornung V, et al. 2002. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 30. Hu JS, Olson EN, Kingston RE. 1992. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol. Cell. Biol. 12:1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabelitz D. 2007. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 19:39–45 [DOI] [PubMed] [Google Scholar]
- 32. Kadowaki N, et al. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawai S, et al. 2006. Construction of a conventional non-radioisotope method to quantify HM1.24 antigens: correlation of HM1.24 levels and ADCC activity of the humanized antibody against HM1.24. Leuk. Res. 30:949–956 [DOI] [PubMed] [Google Scholar]
- 34. Kawai T, Akira S. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawai T, et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068 [DOI] [PubMed] [Google Scholar]
- 36. Kuhl BD, Sloan RD, Donahue DA, Liang C, Wainberg MA. 2011. Vpu-mediated tetherin antagonism of ongoing HIV-1 infection in CD4(+) T-cells is not directly related to the extent of tetherin cell surface downmodulation. Virology 417:353–361 [DOI] [PubMed] [Google Scholar]
- 37. Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. 1997. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278:1630–1632 [DOI] [PubMed] [Google Scholar]
- 38. Kuo TC, Calame KL. 2004. B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J. Immunol. 173:5556–5563 [DOI] [PubMed] [Google Scholar]
- 39. Levy DE, Kessler DS, Pine R, Darnell JE., Jr 1989. Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 3:1362–1371 [DOI] [PubMed] [Google Scholar]
- 40. Levy DE, Marie I, Smith E, Prakash A. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22:87–93 [DOI] [PubMed] [Google Scholar]
- 41. Lin Q, Dong C, Cooper MD. 1998. Impairment of T and B cell development by treatment with a type I interferon. J. Exp. Med. 187:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin R, Genin P, Mamane Y, Hiscott J. 2000. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 20:6342–6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin R, Heylbroeck C, Pitha PM, Hiscott J. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin R, Mamane Y, Hiscott J. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 275:34320–34327 [DOI] [PubMed] [Google Scholar]
- 45. Lin R, Mamane Y, Hiscott J. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19:2465–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marie I, Durbin JE, Levy DE. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsuda A, et al. 2003. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene 22:3307–3318 [DOI] [PubMed] [Google Scholar]
- 48. Miyagi E, Andrew AJ, Kao S, Strebel K. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyamoto M, et al. 1988. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell 54:903–913 [DOI] [PubMed] [Google Scholar]
- 50. Muller M, et al. 1993. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 12:4221–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakaya T, et al. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 283:1150–1156 [DOI] [PubMed] [Google Scholar]
- 52. Nakhaei P, Genin P, Civas A, Hiscott J. 2009. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin. Immunol. 21:215–222 [DOI] [PubMed] [Google Scholar]
- 53. Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430 [DOI] [PubMed] [Google Scholar]
- 54. Ning S, Huye LE, Pagano JS. 2005. Regulation of the transcriptional activity of the IRF7 promoter by a pathway independent of interferon signaling. J. Biol. Chem. 280:12262–12270 [DOI] [PubMed] [Google Scholar]
- 55. Ohtomo T, et al. 1999. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 258:583–591 [DOI] [PubMed] [Google Scholar]
- 56. Perez-Caballero D, et al. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Platanias LC. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375–386 [DOI] [PubMed] [Google Scholar]
- 58. Reis LF, Harada H, Wolchok JD, Taniguchi T, Vilcek J. 1992. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 11:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sato M, et al. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106–110 [DOI] [PubMed] [Google Scholar]
- 61. Sato M, et al. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539–548 [DOI] [PubMed] [Google Scholar]
- 62. Sen GC, Herz RE. 1983. Differential antiviral effects of interferon in three murine cell lines. J. Virol. 45:1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siednienko J, Halle A, Nagpal K, Golenbock DT, Miggin SM. 2010. TLR3-mediated IFN-beta gene induction is negatively regulated by the TLR adaptor MyD88 adaptor-like. Eur. J. Immunol. 40:3150–3160 [DOI] [PubMed] [Google Scholar]
- 64. Stojdl DF, et al. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275 [DOI] [PubMed] [Google Scholar]
- 65. Symons JA, Alcami A, Smith GL. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81:551–560 [DOI] [PubMed] [Google Scholar]
- 66. Tamura T, Yanai H, Savitsky D, Taniguchi T. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26:535–584 [DOI] [PubMed] [Google Scholar]
- 67. Tanaka N, Kawakami T, Taniguchi T. 1993. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol. Cell. Biol. 13:4531–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsukamoto N, et al. 2009. Impairment of plasmacytoid dendritic cells for IFN production by the ligand for immunoglobulin-like transcript 7 expressed on human cancer cells. Clin. Cancer Res. 15:5733–5743 [DOI] [PubMed] [Google Scholar]
- 69. Van Damme N, et al. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wathelet MG, Berr PM, Huez GA. 1992. Regulation of gene expression by cytokines and virus in human cells lacking the type-I interferon locus. Eur. J. Biochem. 206:901–910 [DOI] [PubMed] [Google Scholar]
- 71. Yoo H, Park SH, Ye SK, Kim M. 2011. IFN-gamma-induced BST2 mediates monocyte adhesion to human endothelial cells. Cell. Immunol. 267:23–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.