Abstract
The nematode Caenorhabditis elegans has been a powerful experimental organism for almost half a century. Over the past 10 years, researchers have begun to exploit the power of C. elegans to investigate the biology of a number of human pathogens. This work has uncovered mechanisms of host immunity and pathogen virulence that are analogous to those involved during pathogenesis in humans or other animal hosts, as well as novel immunity mechanisms which appear to be unique to the worm. More recently, these investigations have uncovered details of the natural pathogens of C. elegans, including the description of a novel intracellular microsporidian parasite as well as new nodaviruses, the first identification of viral infections of this nematode. In this review, we consider the application of C. elegans to human infectious disease research, as well as consider the nematode response to these natural pathogens.
INTRODUCTION
Host-pathogen interactions can be studied on many levels, given that not all interactions lead to disease and those that do have a complex progression that leads to this state. As such, there are a number of ways of investigating these interactions; molecular approaches are complemented by animal studies, which examine the infection at the whole-organism level. Amid ever-growing concerns for the welfare of animals in scientific research, there is a heightened need to find organisms in which to study such interactions ethically and on a large scale. Therefore, the discovery that a number of simple and genetically tractable model organisms, such as Arabidopsis thaliana (16), Drosophila melanogaster (19), Caenorhabditis elegans (48), and zebrafish (Danio rerio) (75), are susceptible to a number of human pathogens has been a remarkable advance in this field. This work continues to reveal common mechanisms of immunity across animals and plants, including the identification of universal defense genes and the pathways that control their expression in response to infection (64). Here we consider how one such model, the nematode C. elegans, has provided insights into the components from both the host and the microbe that underlie the host-pathogen interface.
CAENORHABDITIS ELEGANS AND IMMUNITY
C. elegans is a free-living nematode that is found in soil and in compost heaps. The population is dominated by self-fertilizing hermaphrodites (XX) with a rare occurrence of males (X0), who have a distinct morphology. The animals were first adopted as a laboratory model by Sydney Brenner over 40 years ago (10) for studies of development and behavior, work which resulted in the Nobel Prize in Physiology or Medicine being awarded to Brenner and his colleagues in 2002 (20). In the intervening period, C. elegans has been used as a model in which to study a wide range of biological phenomena, and consequently there are vast amounts of genotypic and phenotypic data available to investigators.
C. elegans offers a number of benefits as a model host for studying innate immunity. Providing that the pathogen of choice is a suitable nutritional source for the animals, it can simply be substituted in place of the normal feeding bacterium Escherichia coli OP50; thus, the primary site of the infection is the intestine, although there are some exceptions (Fig. 1). Phenotypes such as animal survival, motility, pathogen burden, and so forth can subsequently be easily and noninvasively examined. Although C. elegans has no cell-mediated immunity, work by a number of groups has revealed a complex innate immune approach for disease resistance comprising avoidance behaviors (58, 60) and physical barriers (25). For systemic immunity, the animal is believed to depend purely upon the secretion and action of antimicrobial molecules, including lectins (43, 54, 66, 81), lysozymes (6, 17, 27, 43, 44, 50, 54, 67, 77, 81), and antibacterial factors (34, 62). Both lines of defense have been shown to be regulated by a number of signaling pathways, of which the p38 and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinases (MAPKs), insulin signaling/DAF-2, and transforming growth factor β (TGF-β)/DBL-1 pathways are the most significant (see reference 30 for a recent review).
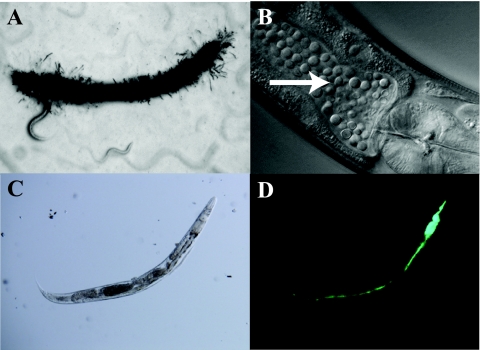
Fig 1.
Infections of C. elegans. Microscopic images of various infections of C. elegans. are shown. (A) D. coniospora infection in a wild-type worm at day 2. Note the characteristic hyphal penetration throughout the animal. (B) C. neoformans infection in a wild-type worm at day 5, where the intestine is packed with proliferating yeast cells (arrow). (C and D) Bright-field (C) and fluorescence (D) images of a representative wild-type animal at day 3 of an S. Typhimurium infection (with green fluorescent protein used for detection). Here, the pharyngeal structure has been destroyed by the infection and the intestine is distended and full of bacteria. (Panels C and D were reprinted from reference 44.)
C. ELEGANS AS A NATURAL HOST
Currently, the response to infection by four natural pathogens of this host have been described in detail: the Gram-negative bacterium Microbacterium nematophilum, the fungus Drechmeria coniospora, the microsporidian parasite Nematocida parisii, and most recently a nodavirus-like Orsay virus.
MICROBACTERIUM NEMATOPHILUM
M. nematophilum was discovered through chance contaminations of C. elegans laboratory cultures, as infected animals displayed an unusual and visible tail swelling, or deformed anal region (Dar), previously believed to be a spontaneous, and seemingly heritable, morphological mutation that arose during a routine genetic cross. However, later analysis of these animals and others demonstrated that the Dar phenotype was the result of a novel pathogen of C. elegans (26). These bacteria establish a specific rectal infection owing to their strong extracellular adherence to the cuticle that, in turn, causes a localized swelling response in the host (26, 52). Additional work showed that this Dar phenotype was a consequence of the limited activation of the ERK MAPK cascade in the region, perhaps as a defense mechanism raised against the infection (52), although this remains unclear (24, 26). Although not lethal, the animals develop slowly when feeding on pure M. nematophilum lawns and show signs of constipation (26). The isolation of a series of mutant animals resistant to the infection, exhibiting a bacterially unswollen (Bus) phenotype, demonstrated that a number of C. elegans genes are responsible for the Dar response. These mutations have implications for both the host (capability to elicit a swelling response) and the bacterium (ability to adhere and colonize) (24). Some of the Bus mutants were not colonized by the pathogen, indicating that mutations in the genes responsible for the formation of the cuticle may have prevented the establishment of an infection (24, 26, 55). In addition, microarray-based studies have identified a set of 68 genes induced upon M. nematophilum infection that are arranged in clusters on the C. elegans genome (54), including a number of proteins with C-type lectin domains, lysozymes, and other putative pathogen receptor molecules.
DRECHMERIA CONIOSPORA
Drechmeria coniospora is a nematode parasite that adheres to the mouth and vulva of animals and penetrates throughout the worm by means of proteinaceous hyphae (31). This colonization of C. elegans triggers an immune response in the host, predominantly through the induction of neuropeptide-like proteins (NLPs) (13). Some of the 32 NLP genes, which were identified via their homology to other invertebrate neuropeptides, are thought to act as nonclassical neurotransmitters, while others have gained alternative functions. Upon D. coniospora infection, a cluster of these genes, the nlp-29 cluster, is induced by the activity of the p38 MAPK cascade (13, 59). The proteins localize to the epidermis of the animal (61), where they can respond to fungus-induced or mechanically induced epidermal wounding.
Another group of antimicrobial molecules activated in response to infection with D. coniospora are the caenacin (for Caenorhabditis bacteriocin, or CNC) proteins (13, 83). Despite being structurally related to the NLP immune gene products, the CNC proteins are a discrete group of antimicrobials whose genes located in a different gene cluster on the same arm of chromosome V. The artificial overexpression of these peptides renders the animal resistant to fungal infection, and their induction in response to D. coniospora infection is dose dependent upon TGF-β signaling, by means of the C. elegans homologue dbl-1 (83).
NEMATOCIDA PARISII
Nematocida parisii is a recently identified microsporidian parasite of C. elegans. It was discovered when a newly isolated wild C. elegans strain from a compost heap in Franconville, France, was found to be infected with an unknown pathogen that could be transferred horizontally through an animal culture, but not vertically. N. parisii proceeds through its entire infection cycle, from meront to spore, within the nematode. While spores are the infectious stage, meronts appear to do the most damage to the host. For further information on the microsporidian life cycle, see reference 76.
The host response to this infection appears to be unique; the infection did not induce fundamental response genes known to be crucial to other pathogenic infections, nor did the abolition of vital components of the immune-signaling pathways (p38 MAPK and insulin signaling/DAF-2 pathways) have any effect on animal survival. Further, other wild isolates of C. elegans from France, Portugal, and India were found to harbor different strains of the parasite, indicating that it may be a relatively common natural pathogen of Caenorhabditis nematodes (78).
ORSAY VIRUS
The recent identification of a natural viral infection in C. elegans which can replicate and transmit through many generations has begun to address the lack of description and previous conundrum concerning the total lack of known C. elegans viruses (18). In a similar manner to the identification of N. parisii, natural populations of C. elegans and Caenorhabditis briggsae animals were isolated that exhibited unusual intestinal morphologies. The infection could be cleared by bleaching treatment and could be transmitted horizontally by applying dead infected animals or “infectious extracts,” including those passed through a 0.2-μm filter. Analysis of the infected intestines by electron microscopy identified virus-like particles of 20 nm in diameter, and subsequent molecular analysis described two phylogenetically related, small, positive-sense RNA viruses belonging to the Nodaviridae family: Orsay virus, which could only infect C. elegans strains (to some extent the laboratory strain Bristol N2 as well as its original natural isolate strain), and Santeuil virus, which was C. briggsae specific (although this virus appeared to only infect its corresponding C. briggsae isolate and, interestingly, not the AF16 wild-type laboratory strain). Surprisingly, the cellular effects of the viruses on their respective caenorhabditid natural isolates did not result in a change in life span or brood size, although the authors noted that progeny production was slowed (18).
A role for RNA interference (RNAi) in the defense against the infection was identified by deep sequencing of the infected animals versus uninfected controls. This analysis pulled out small sense and antisense RNA molecules that mapped to viral RNA and may therefore represent viral cleavage products and host response effectors, respectively. Furthermore, N2 animals, in which the Argonaute protein is mutated (rendering the animals incapable of initiating RNAi), exhibit higher viral loads and intestinal disruption than their wild-type counterparts (18).
This description of a novel virus, which naturally infects, replicates, and persists in C. elegans cultures, is very promising for the continuing success of C. elegans as an organism in which to examine host-pathogen interactions, not only from the perspective of studying viral infections in C. elegans, but also due to the entirely native RNAi response that has been identified.
C. ELEGANS AS A MODEL HOST
Despite several decades of intensive study, it is only recently that natural pathogens of C. elegans have been described. Instead, animals have been infected with a range of human pathogens, and these infection models have been used to dissect factors on both sides of the equation required for successful pathogenesis. Characteristic features are summarized in Table 1. It should be noted that many other pathogens have been shown to infect C. elegans, aside from those discussed in detail in this review. Of course, natural infections of C. elegans have coevolved and adapted with the worm in its environment, whereas many of the “artificial” pathogens that have been modeled in C. elegans either grow optimally at 37°C or induce specific virulence traits at this temperature. Exposure to this temperature for extended periods of time is lethal to C. elegans; thus, assays are always carried out at lower temperatures. This contrast will potentially limit the range of mammalian virulence factors that can be studied in the worm.
Table 1.
Key features of C. elegans infections
| Source (type of infection) | Pathogen | Key feature(s) | Molecular and experimental aspects | Reference(s) |
|---|---|---|---|---|
| Natural | M. nematophilum | Swelling of tail region (Dar); nonlethal | Induces ERK MAPK response; infection limited to rectal area | 26, 52, 54 |
| D. coniospora | Fungal infection: hyphae penetrate entire worm | Induces NLP and CNC response genes; difficult to grow or control infectious dose | 13, 31, 59, 61, 83 | |
| N. parisii | Intracellular parasite | Horizontal transfer of infection; unique immune response; cannot be cultured in vitro | 78 | |
| Nodavirus | Intestinal structure disrupted; life span unchanged | Induces natural RNAi response; horizontally transmitted | 18 | |
| Human (bacterial) | P. aeruginosa | Medium-dependent fast and slow killing, which are toxin and infection based, respectively | Induces p38 MAPK response; killing mechanism is strain dependent | 15, 36, 42, 71 |
| S. enterica | Persistent bacterial infection | Primarily an extracellular infection in C. elegans, unlike in mammals | 3, 38 | |
| S. marcescens | Grossly distended intestine; 6-day survival | Triggers inducible immune response; may be a natural host-pathogen interaction | 37, 43, 65 | |
| E. coli | Nonpathogenic food source; pathogenic strains exhibit fast and slow killing | Results in a behavioral conditioning response; type III secretion system not required (unlike mammalian host) | 7, 8, 12, 47 | |
| S. aureus | Intact bacteria overwhelm animal; not persistent until infection threshold reached | bar-1 and egl-5 response is key; conservation of virulence factors between C. elegans and mammals | 22, 28, 29, 68 | |
| Human (fungal) | C. albicans | Persistent lethal infection | Coinfection model particularly informative | 9, 55, 57, 70 |
| C. neoformans | Rapid infection, accumulation of yeast; not persistent | Mechanism of pathogenesis unclear; does not disseminate in C. elegans (unlike mammalian host) | 51, 72, 79 |
PSEUDOMONAS AERUGINOSA
The Gram-negative bacterium P. aeruginosa is ubiquitous in the environment and a common opportunistic pathogen of both animals and plants. In the worm, its mechanism of pathogenesis was demonstrated to be medium dependent; when grown on a minimal medium, strain PA14 caused an infection-like process in the intestine of the animal, killing it over the course of several days, termed “slow” killing. However, PA14 was also found to kill C. elegans in a matter of hours, termed “fast” killing, when grown on a rich medium (42, 71).
Slow killing is completely dependent upon the accumulation and active replication of bacteria in the animal's gut, yet animals can recover from the infection if removed from the pathogen source following a brief exposure and providing that the threshold has not been exceeded, which appears to reflect the capacity of this pathogen for inducing intestinal pathology (29). Once the pathogenic exposure has reached this threshold, the infection becomes persistent and the animals are unable to recover (71). This is in stark contrast to the lethal toxin-based fast killing exhibited on rich media, which is believed to be mediated by phenazines, pigment compounds secreted by pseudomonads (42).
Animals were infected with a second P. aeruginosa clinical strain, PAO1, which is known to be less pathogenic than PA14, and a third mechanism of killing was described, that of a rapid and lethal paralysis mediated by an unidentified diffusible toxin (15). It has since been described that PAO1 fast killing is mediated by cyanide poisoning (21). A further means of C. elegans killing by P. aeruginosa is that of “red death,” the presence of a red material in the C. elegans pharynx and intestine in nematodes exposed to physiological stress (such as starvation or heat shock) and subsequently infected with PAO1 grown on low-phosphate medium (82). Transcriptomic analysis of PAO1 grown on high- or low-phosphate media allowed identification of 323 genes that were upregulated in response to low phosphate and which may cause the red death phenotype. Three regulatory systems were found to be essential in the induction of red death in both worms and mice: the bacteria activate their phosphate uptake system through PhoB, induce a number of quorum-sensing-associated genes, including phenazines, and activate pyoverdin biosynthesis in order to acquire iron and initiate phosphate signaling (82).
Interestingly, P. aeruginosa appears to deliberately target the C. elegans immune response by activation of signaling through the DAF-2 receptor, resulting in reduced expression of a range of immunity factors (17, 35). Given this adaptation, there is some potential that P. aeruginosa may be a natural pathogen of C. elegans.
From the host perspective, an RNAi screen of chromosome I complemented by a candidate library and other candidates found through the construction of a C. elegans interactome identified 59 genes required for strong induction of clec-85 (5), which is highly expressed by C. elegans during the pathogenesis of P. aeruginosa (6). Some of these genes may represent novel targets for pharmaceutical development, as these immuno-modulated “control” genes identified in C. elegans have also been found to have a role in regulating cytokine production during the mammalian defense response against E. coli in murine macrophages (5).
SALMONELLA ENTERICA
The genus Salmonella encompasses a number of species of Gram-negative bacteria capable of causing enteric disease in animals and humans. One of these, S. enterica serovar Typhimurium, has been extensively investigated in the C. elegans model, in which it produces a persistent and eventually fatal colonization of the gut lumen (3, 38), although there has been one report of potential invasion into gut epithelial cells (32).
During Salmonella infection, animals show a strong endomitotic oocyte phenotype, where the parent animal dies containing fertilized eggs that never develop (3), indicating that germ line development may somehow be involved with S. Typhimurium infection. The nature of this involvement remains unclear; there was one report of germ line apoptosis being required for resistance to S. Typhimurium-mediated killing (1), although this result was not replicated by others (32).
As with C. elegans susceptibility to P. aeruginosa (36), animals with mutations in the p38 MAPK cascade are hypersensitive to S. Typhimurium infection (2) due to misregulation of Salmonella-elicited programmed cell death (2). Interestingly, several classical S. Typhimurium virulence factors (PhoP/PhoQ, SPI-1, and SPI-2) are induced by the bacterium upon colonization of C. elegans (4) and have been shown to be essential for virulence in the worm model (74). Interestingly, the type III secretion system (T3SS) and “wild-type” lipopolysaccharide (LPS) are both required for full virulence in both C. elegans and murine models of infection (2), suggesting that several aspects of this disease are conserved between mammalian and nematode hosts.
ENTEROCOCCUS FAECALIS
The Gram-positive coccus E. faecalis is a commensal bacterium of the human gastrointestinal tract. However, it is an opportunistic pathogen that can cause a number of diseases, most notably endocarditis. There is also widespread multidrug resistance in this organism, which is proving to be a health concern (56).
In the worm, E. faecalis kills adults with a 50% lethal time of around 4 days, after establishing a persistent infection in the animals' intestines (22). This infection arose from the proliferation of intact bacteria in the gut from just a minimal inoculum, resulting in a grossly distended intestine (22). The authors suggested that the thick Gram-positive nature of the cell wall conferred resistance of the bacterium to the action of the C. elegans grinder (22).
A number of virulence factors were described in this work; in particular, the cytolysin Cyl, which is a key determinant of pathogenesis in other animal models, is also important for killing in the worm model (22). Furthermore, the quorum-sensing response regulatory locus fsr/gelE-sprE is also crucial for C. elegans pathogenesis, as fsr mutants are severely impaired in their ability to kill (22, 41, 69) despite seemingly normal colonization of the host (69).
Interestingly, E. faecalis infection has also revealed a role for reactive oxygen species (ROS) in C. elegans immunity in a manner that is analogous to the reactive oxygen burst in mammalian phagocytes (11, 80). Thus, ROS as an effector of pathogen resistance is conserved between worms and mammals, even if the upstream regulators (DAF-16/p38 MAPK and inflammatory cascades, respectively) are different (11, 80).
SERRATIA MARCESCENS
It was a study with the Gram-negative bacterium S. marcescens that first suggested that C. elegans has an inducible immune response (43). S. marcescens is an environmental bacterium that causes disease in a number of organisms: plants, invertebrates, and vertebrate hosts. In humans it is an opportunistic pathogen associated with hospital-acquired infections and, as many strains are intrinsically antibiotic resistant, the pathogen represents an ongoing public health challenge (37).
In C. elegans, S. marcescens establishes a persistent intestinal infection, arising from an avoidance of the pharyngeal grinder, leading to intestinal distension and death within 6 days (37, 43). Notably, a small number of bacteria have been observed in the uterus, although this is a very rare event (37). The C. elegans response to the bacterium involves the upregulation of a number of putative pathogen response proteins, including lysozymes and lectins, a subset of which appeared to be largely under the control of the DBL-1/TGF-β pathway (49). Interestingly, this gene set is distinct from that induced upon infection with the natural nematode pathogen M. nematophilum, although the gene families induced were similar, suggesting that C. elegans can mount responses tailored to the specific infection and must therefore have a pathogen recognition system of some sort (43, 54).
ESCHERICHIA COLI
In the laboratory, nematodes are cultured using E. coli OP50 as the sole food source. This is a nonpathogenic food source, and animals live for 2 to 3 weeks under these conditions (10). However, there is evidence that even OP50 is pathogenic to both aging animals under standard growth conditions and to younger animals when grown on rich media (12, 23). This is analogous to the situation in humans and other mammals, in which E. coli is found in the intestine as part of the commensal gastrointestinal flora. There, it is typically harmless and can even offer protection to the host against other virulent pathogens, but it can cause severe disease in immunocompromised individuals (14).
As a model, C. elegans has been applied as a pathogenesis model for understanding the molecular basis of enteropathogenic E. coli (EPEC) infection. Killing of C. elegans by EPEC correlated with the accumulation of bacteria in the animal gut over a few days, rather than with a toxin-mediated mechanism (47). The C. elegans-EPEC model has its limitations, however, as other virulence factors required in mammalian systems, such as the T3SS, are not essential for the C. elegans model; hence, the modes of EPEC infection of invertebrate and vertebrate hosts appear to be far removed (47).
STAPHYLOCOCCUS AUREUS
S. aureus is a common Gram-positive bacterium that causes a range of minor infections, which occasionally become serious, in many animals (40). In C. elegans, intact bacteria accumulate in the gut of the animal, and it is this colonization that eventually overwhelms the host, disrupting the gut epithelium, then destroying internal organs, and ultimately leading to death (22, 29, 68). Interestingly, this systematic destruction was not dependent upon the S. aureus cytolysins, as bacterial strains lacking these virulence factors still caused the same cytopathology as the wild type (29). However, nematodes can be rescued from the lethality of the infection by transfer to a nonpathogenic food source, provided this occurs early enough during the infection, enabling the clearing of the bacteria from the animals' intestines (68). Like M. nematophilum, S. aureus induces a Dar (deformed anal region) phenotype that is dependent upon both β-catenin and ERK MAPK signaling (29).
Isolates of S. aureus with mutations for crucial mammalian virulence factors, such as the global virulence regulators agr and sarA, are attenuated in C. elegans killing, indicating that these regulators and their downstream targets, V8 protease and alpha-hemolysin, are also required for full pathogenesis in the worm model (68). From the host perspective, nematodes that are unable to signal through either the p38 MAPK cascade or the β-catenin (bar-1) pathway are more susceptible to S. aureus than wild-type animals (28, 68). The importance of this pathway in S. aureus pathogenesis is mirrored in higher vertebrates, where β-catenin activates NF-κB-mediated immune gene expression (28). Although the worm does not have NF-κB, several of the downstream regulatory targets are conserved, and thus it is possible that these signal pathways may act via alternative transcription factors to regulate immunity in an analogous fashion.
CANDIDA ALBICANS
The commensal fungus C. albicans is the causative agent of candidiasis, an opportunistic infection that figures highly in hospital-acquired infections, predominantly through the formation of biofilms on hospital devices (33). C. albicans was first described as a persistent and lethal infection of C. elegans as part of a study that was seeking a pathogenicity assay that could be used to identify antifungal compounds (9). The C. elegans killing model was a particularly effective assay that has since been adapted to facilitate high-throughput screening of these compounds (53). It has been shown that both the yeast and hyphal forms of the fungus are pathogenic to C. elegans (62, 63), with a quite-distinct immune response raised by the host to the infection, notably involving two caenacin genes, cnc-4 and cnc-7, and the antibacterial factor abf-2 (62). The response was further found to be predominantly under the control of the p38 MAPK pathway (62).
The C. albicans-C. elegans interaction has been exploited further by use of a coinfection model to examine how this eukaryotic pathogen interacts with a number of prokaryotic infections. Following an initial 4-h infection with C. albicans, animals were subsequently infected with Acinetobacter baumannii, P. aeruginosa (57), or S. Typhimurium (70). In all cases, the secondary Gram-negative bacterial infection was found to inhibit the formation of fungal filaments, a key virulence determinant in C. albicans pathogenesis (57, 70). Further, this inhibition was mediated by a secretory bacterial molecule, since bacterial supernatants also limited the ability of C. albicans to form filaments. Interestingly, the inhibitory activity of these bacterium against C. albicans could be recapitulated in vitro, both in culture and in biofilm formation (57, 70); thus, the C. elegans model may be particularly informative for greater understanding of pathogen-pathogen interactions.
CRYPTOCOCCUS NEOFORMANS
C. neoformans is an encapuslated yeast that is ubiquitous in the environment. As a pathogen, it causes disease in a number of animals. In humans it is primarily a pathogen of the immunocompromised, notably coinfecting AIDS patients.
Killing of C. elegans by C. neoformans is rapid (2 to 7 days) (51, 79), although the yeast cells remain within the intestine, and animals can be rescued by early transfer to normal culture conditions. The mechanism of pathogenesis is not clear, but a number of genes and features required for mammalian pathogenesis are also essential for the worm model (51, 72). A surprising exception was the finding that acapsular yeast, which are avirulent in mammals, retain virulence in the worm model (51). This, coupled with the discovery that heat-killed yeast also kill C. elegans, suggested that the pathogenesis for the worm may be mediated by a toxic interaction between the host and pathogen.
In the mammalian host, macrophages have an essential role in eliminating Cryptococcus infection. Two host scavenger receptors, SCARF1 and CD36, are required to recognize β-glucans on the invading yeast and elicit a host defense by activating macrophages. In C. elegans, the orthologues of these receptors, CED-1 and C03F11.3, are crucial to activating a defense response following recognition of the pathogen (45). This highly specific conservation between two seemingly divergent host groups underscores the significance of innate immunity in response to fungal pathogens.
Interestingly, recent work from our laboratory identified a complex genetic trade-off in the worm immune system, such that changes that increased susceptibility to killing by C. neoformans through the loss of the immune genes lys-7 and abl-1 simultaneously enhanced tolerance to S. Typhimurium, suggesting that some aspects of C. elegans immunity may provide specialized and opposing antimicrobial activities (44).
CONCLUSION
C. elegans is susceptible to a wide range of bacterial and fungal pathogens which vary in the mechanisms and rate at which they kill host animals. The ease of culture and genetic malleability of C. elegans makes it an attractive model for high-throughput screening, both in order to identify attenuated and hypervirulent strains and as a first stage for testing novel pharmaceutical compounds. However, the application of the C. elegans model toward human disease has significant limitations that must be recognized. First, although there is some conservation of the mechanisms of pathogenesis between C. elegans and higher vertebrates (5), there are huge differences. A critical mechanism of virulence in mammalian hosts is for these pathogens to become internalized and then spread throughout the host, whereas in C. elegans most infections studied thus far do not result in intracellular colonization or dissemination, features which are critical to most serious human infections.
Next, there are considerable differences between the immunity profiles of C. elegans and of higher vertebrates. The lack of cell-mediated immunity in C. elegans makes it dependent upon the secretion of antimicrobial peptides to counter a pathogen attack. Higher vertebrates, on the other hand, have both a more complex innate system and an additional, highly specialized, adaptive system, which together permit great versatility in the immune response. However, there is some conservation in the pathways that control the immune response in both animals; because the vertebrate response has likely evolved from a common predecessor to the primitive C. elegans response, there are still large inconsistencies. One example is the Toll-like receptor pathway, which represents a significant arm of innate immunity that was first identified in Drosophila (46). In the vertebrate immune response it has a fundamental role, and yet its function in C. elegans is not yet fully understood (2, 39, 60, 73).
Despite these drawbacks, however, the wealth of genetic resources available for study of C. elegans and the opportunity to study early infection processes noninvasively offer significant advantages to the study of host-pathogen interactions. The recent identification of natural pathogens of C. elegans will potentially be of enormous benefit in validating this model and furthering our understanding of the mechanisms of infection. In addition, this work opens up an exciting new field in which C. elegans can be utilized in order to examine immune responses in relation to the evolution of the immune system throughout the animal and plant kingdoms.
ACKNOWLEDGMENTS
This work was supported by a Medical Research Council (MRC) studentship award to E.K.M. and by Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/F000138/1.
Footnotes
Published ahead of print 27 January 2012
REFERENCES
- 1. Aballay A, Ausubel FM. 2001. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc. Natl. Acad. Sci. U. S. A. 98: 2735–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aballay A, Drenkard E, Hilbun LR, Ausubel FM. 2003. Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr. Biol. 13: 47–52 [DOI] [PubMed] [Google Scholar]
- 3. Aballay A, Yorgey P, Ausubel FM. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10: 1539–1542 [DOI] [PubMed] [Google Scholar]
- 4. Alegado RA, Tan MW. 2008. Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell. Microbiol. 10: 1259–1273 [DOI] [PubMed] [Google Scholar]
- 5. Alper S, et al. 2008. Identification of innate immunity genes and pathways using a comparative genomics approach. Proc. Natl. Acad. Sci. U. S. A. 105: 7016–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA. 2007. Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol. Cell. Biol. 27: 5544–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anyanful A, et al. 2005. Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol. Microbiol. 57: 988–1007 [DOI] [PubMed] [Google Scholar]
- 8. Anyanful A, Easley KA, Benian GM, Kalman D. 2009. Conditioning protects C. elegans from lethal effects of enteropathogenic E.coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5: 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breger J, et al. 2007. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 3: 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenner S. 1974. Genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chavez V, Mohri-Shiomi A, Maadani A, Vega LA, Garsin DA. 2007. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 176: 1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Couillault C, Ewbank JJ. 2002. Diverse bacteria are pathogens of Caenorhabditis elegans. Infect. Immun. 70: 4705–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Couillault C, et al. 2004. TLR-independent control of innate immunity in Caenorhabdtis elegans by the TIR domain adapter protein TIR-1, an ortholog of human SARM. Nat. Immunol. 5: 488–494 [DOI] [PubMed] [Google Scholar]
- 14. Crossman LC, et al. 2010. A commensal gone bad: complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407. J. Bacteriol. 192: 5822–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Darby C, Cosma CL, Thomas JH, Manoil C. 1999. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 96: 15202–15207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11: 539–548 [DOI] [PubMed] [Google Scholar]
- 17. Evans EA, Kawli T, Tan MW. 2008. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 4: e1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Felix MA, et al. 2011. Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biol. 9: 31000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrandon D, Imler JL, Hetru C, Hoffmann JA. 2007. The Drosphila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7: 862–874 [DOI] [PubMed] [Google Scholar]
- 20. Frängsmyr T. (ed). 2003. Les Prix Nobel. The Nobel Prizes 2002. The Nobel Foundation, Stockholm, Sweden [Google Scholar]
- 21. Gallagher LA, Manoil C. 2001. Pseudomonas aeruginosa kills Caenorhabditis elegans by cyanide poisoning. J. Bacteriol. 183: 6207–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garsin DA, et al. 2001. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. U. S. A. 98: 10892–10897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garsin DA, et al. 2003. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300: 1921. [DOI] [PubMed] [Google Scholar]
- 24. Gravato-Nobre MJ, et al. 2005. Multiple genes affect sensitivity of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics 171: 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gravato-Nobre MJ, Stroud D, O'Rourke D, Darby C, Hodgkin J. 2011. Glycosylation genes expressed in seam cells determine complex surface properties and bacterial adhesion to the cuticle of Caenorhabditis elegans. Genetics 187: 141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodgkin J, Kuwabara PE, Corneliussen B. 2000. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode Caenorhabditis elegans. Curr. Biol. 10: 1615–1618 [DOI] [PubMed] [Google Scholar]
- 27. Huffman DL, et al. 2004. Mitogen-activated protein kinase pathways defends against bacterial pore-forming toxins. Proc. Natl. Acad. Sci. U. S. A. 101: 10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irazoqui JE, Ng A, Xavier RJ, Ausubel FM. 2008. Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc. Natl. Acad. Sci. U. S. A. 105: 17469–17474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irazoqui JE, et al. 2010. Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 6: e1000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Irazoqui JE, Urbach JM, Ausubel FM. 2010. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 10: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jansson H-B. 1994. Adhesion of conidia of Drechmeria coniospora to Caenorhabidits elegans wild type and mutants. J. Nematol. 26: 430–435 [PMC free article] [PubMed] [Google Scholar]
- 32. Jia K, et al. 2009. Autophagy genes protect against Salmonella typhimurium infection and mediate signaling-regulated pathogen resistance. Proc. Natl. Acad. Sci. U. S. A. 106: 14564–14569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kabir MA, Hussain MA. 2009. Human fungal pathogen Candida albicans in the postgenomic era: an overview. Expert Rev. Anti Infect. Ther. 7: 121–134 [DOI] [PubMed] [Google Scholar]
- 34. Kato Y, et al. 2002. abf-1 and abf-2, ASABF-type antimicrobial peptide genes in Caenorhabditis elegans. Biochem. J. 361: 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawli T, Tan MW. 2008. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat. Immunol. 9: 1415–1424 [DOI] [PubMed] [Google Scholar]
- 36. Kim DH, et al. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297: 623–626 [DOI] [PubMed] [Google Scholar]
- 37. Kurz CL, et al. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22: 1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Labrousse A, Chauvet S, Couillault C, Kurz CL, Ewbank JJ. 2000. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr. Biol. 10: 1543–1545 [DOI] [PubMed] [Google Scholar]
- 39. Liberati NT, et al. 2004. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc. Natl. Acad. Sci. U. S. A. 101: 6593–6598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lindsay JA. 2010. Genomic variation and evolution of Staphylococcus aureus. Int. J. Med. Microbiol. 300: 98–103 [DOI] [PubMed] [Google Scholar]
- 41. Maadani A, Fox KA, Mylonakis E, Garsin DA. 2007. Enterococcus faecalis mutations affecting virulence in the Caenorhabditis elegans model host. Infect. Immun. 75: 2634–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96: 47–56 [DOI] [PubMed] [Google Scholar]
- 43. Mallo GV, et al. 2002. Inducible antibacterial defense system in C. elegans. Curr. Biol. 12: 1209–1214 [DOI] [PubMed] [Google Scholar]
- 44. Marsh EK, van den Berg MCW, May RC. 2011. A two-gene balance regulates Salmonella typhimurium tolerance in the nematode Caenorhabditis elegans. PLoS One 6: e16839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Means TK, et al. 2009. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 206: 637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1: 135–145 [DOI] [PubMed] [Google Scholar]
- 47. Mellies JL, Barron AMS, Haack KR, Korson AS, Oldridge DA. 2006. The global regulator Ler is necessary for enteropathogenic Escherichia coli colonization of Caenorhabditis elegans. Infect. Immun. 74: 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Millet ACM, Ewbank JJ. 2004. Immunity in Caenorhabditis elegans. Curr. Opin. Immunol. 16: 4–9 [DOI] [PubMed] [Google Scholar]
- 49. Mochii M, Yoshida S, Morita K, Kohara Y, Ueno N. 1999. Identification of transforming growth factor-beta-regulated genes in Caenorhabditis elegans by differential hybridization of arrayed cDNAs. Proc. Natl. Acad. Sci. U. S. A. 96: 15020–15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murphy CT, et al. 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–284 [DOI] [PubMed] [Google Scholar]
- 51. Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99: 15675–15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nicholas HR, Hodgkin J. 2004. The ERK MAPK cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr. Biol. 14: 1256–1261 [DOI] [PubMed] [Google Scholar]
- 53. Okoli I, et al. 2009. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS One 4: e7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Rourke D, Baban D, Demidova M, Mott R, Hodgkin J. 2006. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 16: 1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palaima E, et al. 2010. The Caenorhanditis elegans bus-2 mutant reveals a new class of O-glycans affecting bacterial resistance. J. Biol. Chem. 285: 17662–17672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palmer KL, Kos VN, Gilmore MS. 2010. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 13: 632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peleg AY, et al. 2008. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 105: 14585–14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pradel E, et al. 2007. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 104: 2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pujol N, et al. 2008. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 18: 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pujol N, et al. 2001. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr. Biol. 11: 809–821 [DOI] [PubMed] [Google Scholar]
- 61. Pujol N, et al. 2008. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 4: e1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pukkila-Worley R, Ausubel FM, Mylonakis E. 2011. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. 7: e1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. 2009. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell 8: 1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rahme LG, et al. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268: 1899–1902 [DOI] [PubMed] [Google Scholar]
- 65. Schulenburg H, Ewbank JJ. 2004. Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schulenburg H, Hoeppner MP, Weiner J, Bornberg-Bauer E. 2008. Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans. Immunobiology 213: 237–250 [DOI] [PubMed] [Google Scholar]
- 67. Shapira M, et al. 2006. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 103: 14086–14091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sifri CD, Begun J, Ausubel FM, Calderwood SB. 2003. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71: 2208–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sifri CD, et al. 2002. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect. Immun. 70: 5467–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tampakakis E, Peleg AY, Mylonakis E. 2009. Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica serovar Typhimurium. Eukaryot. Cell 8: 732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tan MW, Mahajan-Miklos S, Ausubel FM. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 96: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang RJ, et al. 2005. Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a Caenorhabditis elegans progeny-based approach. Infect. Immun. 73: 8219–8225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tenor JL, Aballay A. 2008. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep. 9: 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tenor JL, McCormick BA, Ausubel FM, Aballay A. 2004. Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr. Biol. 14: 1018–1024 [DOI] [PubMed] [Google Scholar]
- 75. Trede NS, Langenau DM, Traver D, Look AT, Zon LI. 2004. The use of zebrafish to study immunity. Immunity 20: 367–379 [DOI] [PubMed] [Google Scholar]
- 76. Troemel ER. 2011. New models of microsporidiosis: infections in zebrafish, C. elegans and honey bee. PLoS Pathog. 7: e1001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Troemel ER, et al. 2006. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2: 1725–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Troemel ER, Felix M-A, Whiteman NK, Barriere A, Ausubel FM. 2008. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 6: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van den Berg MCW, Woerlee JZ, Ma HS, May RC. 2006. Sex-dependent resistance to the pathogenic fungus Cryptococcus neoformans. Genetics 173: 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van der Hoeven R, McCallum KC, Cruz MR, Garsin DA. 2011. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 7: e1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank JJ. 2007. Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 8: R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zaborin A, et al. 2009. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc. Natl. Acad. Sci. U. S. A. 106: 6327–6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zugasti O, Ewbank JJ. 2009. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat. Immunol. 10: 249–256 [DOI] [PubMed] [Google Scholar]