Abstract
Salmonella enterica serovar Enteritidis strain E40 filaments were developed under conditions of a reduced water activity (aw) of 0.95 in tryptic soy broth (TSB) or tryptic soy agar (TSA) supplemented with 8% or 7% NaCl, respectively. Filament formation was accompanied by an increase of biomass without an increase in CFU and was affected by incubation temperature and the physical milieu. The greatest amount of filaments was recovered from TSA with 7% NaCl and incubation at 30°C. Within 2 h of transfer to fresh TSB, filaments started to septate into normal-sized cells, resulting in a rapid increase in CFU. S. Enteritidis E40 filaments were not more tolerant of low- or high-temperature stresses than nonfilamented control cells. However, there was greater survival of filaments in 10% bile salts after 24 to 48 h of incubation, during pH 2.0 acid challenge for 10 min, and under desiccation on stainless steel surfaces at 25°C and 75.5% relative humidity for 7 days. S. Enteritidis E40 filaments invaded and multiplied within Caco-2 human intestinal epithelial cells to a similar degree as control cells when a comparable CFU of filaments and control cells was used. S. Enteritidis E40 filaments established a successful infection in mice via intragastric inoculation. The filaments colonized the gastrointestinal tract and disseminated to the spleen and liver at levels comparable to those attained by control cells, even when animals were inoculated with 10- to 100-fold fewer CFU. To our knowledge this is the first demonstration of virulence of stress-induced Salmonella filaments in vitro and in vivo. Formation of filaments by Salmonella in food products and food processing environments is significant to food safety, because detection and quantitation of the pathogen may be compromised. The finding that these filaments are virulent further enhances their potential public health impact.
INTRODUCTION
Food-borne pathogens encounter myriad stresses in the pre-harvest and processing environments. In response to stress, bacteria trigger protection systems that induce or change metabolic pathways, modify the cell membrane, or produce proteins to protect the organism from the stress. In many cases, these stress responses result in cross-protection under different stress conditions. As a consequence, there is greater survival and prolonged persistence of the organism. Morphological change is commonly observed when bacteria are exposed to stress. For example, reduction in cell size is a typical starvation response and survival strategy for Vibrio species inhabiting the marine environment (14, 20). Size reduction was also observed in Escherichia coli O157:H7 grown in low-nutrient medium (6). In contrast, some bacteria elongate in response to unfavorable conditions. For example, E. coli and Salmonella enterica subsp. enterica serovars Enteritidis and Typhimurium developed filaments without septa when exposed to refrigeration temperatures (24, 27, 31). Upon transfer to 30°C or 37°C, the filaments fragmented into typical-sized cells. Clostridium acidiurici and E. coli elongated when grown at elevated temperatures (12, 32). Reduced water activity (aw) and desiccation can also lead to bacterial filament formation. S. Enteritidis and S. Typhimurium developed filaments when grown in broths with an aw of 0.93 to 0.98 due to addition of sucrose, glycerol, or NaCl, with some filaments reaching more than 200 μm in length (22). Similarly, S. Enteritidis elongated when grown on agar plates containing 6 or 8% NaCl (aw of 0.95 and 0.94, respectively), while air drying of S. Enteritidis on glass surfaces led to a low percentage of elongated cells (19). A strain of S. Choleraesuis formed filaments on agar containing various salts, but not in liquid media containing the same salts (36). It is unknown if the formation of filaments is a survival strategy or a consequence of stress. Regardless, the response appears to be conserved in several food-borne pathogens, which suggests that there is some benefit to filament formation.
In this paper, we describe the outgrowth, survival, and virulence of filaments of S. Enteritidis strain E40. Plate counts of Salmonella filaments underestimated the potential number of bacterial cells present in comparison to biomass and optical density measurements, which may impact estimates of infectious dose based on data from food-borne outbreak investigations. Additionally, the results from this study demonstrated that Salmonella filaments were invasive in Caco-2 cells and virulent in mice.
MATERIALS AND METHODS
Bacterial strain and filament development at reduced aw.
Salmonella enterica serovar Enteritidis strain E40 was originally isolated from a chicken ovary and maintained in 40% glycerol at −70°C. Working stock cultures were maintained on tryptic soy agar (TSA; Becton Dickinson, Sparks, MD) plates. Salmonella survival and filament formation at reduced aw were determined with tryptic soy broth (TSB; aw, 0.99) (Becton Dickinson) supplemented with 8% NaCl (TSB-8; aw, 0.95) and TSA (aw, 0.99) supplemented with 7% NaCl (TSA-7; aw, 0.95). The aw levels were measured by using an Aqualab model 4TE aw meter (Decagon Devices, Pullman, WA) according to the manufacturer's instructions.
Inoculum was prepared by transferring a colony from a TSA plate into 3 ml of TSB, followed by overnight incubation (20 to 24 h) at 37°C. Forty-microliter portions were added to wells of polystyrene 24-well plates (Falcon; Becton Dickinson, Franklin Lakes, NJ) containing 2 ml of TSB or TSB-8. The plates were incubated statically at 21, 30, or 37°C, and the optical density at 600 nm (OD600) and the number of CFU per milliliter were determined on plate count agar (PCA; Becton Dickinson) daily for 6 days. Cell morphology was assessed after cells were heat fixed on a glass slide and stained with 0.3% crystal violet (Becton Dickinson). Alternatively, cells were stained (LIVE/DEAD BacLight; Molecular Probes, Inc., Eugene, OR) and concentrated on a black 0.2-μm-pore-size membrane filter (Poretics Products). The membrane was placed on a glass slide and then covered with a drop of microscope oil and a glass coverslip. The stained cells were viewed using an Olympus BH2 microscope equipped with an Olympus model DP70 digital camera. Images were captured and processed using DP Controller version 1.2.1.108 software. The sizes and percentages of elongated cells were estimated from 10 fields of view. Salmonella Enteritidis strain E40 cells were normally 2 to 3 μm in length. Cells longer than 10 μm were considered filaments.
Filaments were produced on agar by spreading 100 μl of inoculum on TSA-7 plates. Control cells were grown on TSA without added NaCl. The plates were sealed with parafilm to prevent evaporation and incubated at 25, 30, or 37°C for 4 days. Salmonella cells were harvested by pipetting 2 ml of 0.01 M phosphate-buffered saline (PBS, pH 7.4) onto each plate and scraping cells off the agar surface with a sterile plastic rod. Viable counts were determined by plating on PCA. Strain E40 grown on TSA or TSA-7 at 30°C for 4 days was used for control (nonfilamented) and filamented cells, respectively, for use in survival, in vitro Caco-2 cell invasion, and in vivo mouse inoculation experiments.
Survival and growth of Salmonella filaments and control cells.
Strain E40 cells were harvested from TSA and TSA-7 plates and adjusted to a concentration of ∼7 log10 CFU/ml (equivalent to an OD600 of ∼0.01 for TSA-grown and ∼1.0 for TSA-7-grown cells).
(i) Temperature.
Forty-microliter aliquots of E40 control cells or filaments were added to wells containing 2 ml of TSB in 24-well plates. The plates were incubated statically at 4, 7, 25, or 37°C. Cultures were assayed for CFU/ml on PCA incubated at 37°C for 24 h and examined for cell morphology at predetermined times for each temperature. For higher temperatures (45 and 55°C), 10-ml tubes containing 900 μl of TSB were tempered to 45°C or 55°C, and 100 μl of E40 control cells or filaments was added to each tube. Tubes were removed from the water bath immediately after inoculation and periodically thereafter to determine the number of CFU/ml.
(ii) pH 2 and 4.
The acidity of TSB was adjusted with 6 N HCl to pH 2 or 4 and filter sterilized using a 0.22-μm filter unit (MillexGV). Tubes (15 ml) were filled with 9 ml of the appropriate medium and tempered to 37°C with shaking. One milliliter of E40 control cells or filaments was added to each tube. Samples were removed immediately after inoculation and periodically thereafter to determine CFU/ml and cell morphology.
(iii) Bile salts.
Bile salts (11 g; Sigma) containing 50% sodium cholate and 50% sodium deoxycholate were dissolved in 100 ml TSB with heat and filter sterilized. The TSB-bile salts mixture (9 ml) was inoculated with 1 ml of E40 control cells or filaments and incubated at 37°C. Samples were removed immediately after exposure to bile salts and periodically thereafter to determine CFU/ml and cell morphology.
(iv) Desiccation on stainless steel surfaces.
Stainless steel type 304 with a no. 4 finish (Temperature Systems, Madison, WI) was cut into 1-cm2 chips, washed with detergent (Micro International Products Corp., Trenton, NJ), and sterilized by autoclaving. Each chip was inoculated with 25 μl of E40 cells resuspended in 0.1× TSB. Inoculated chips were placed in 9-cm glass petri dishes, which were put in desiccators containing saturated MgCl2 · H2O or NaCl solutions to maintain 32.5 or 75.5% relative humidity (RH) (35), respectively, and incubated at 25°C. Chips were removed on days 1, 3, and 7 and placed in 1 ml PBS in a 15-ml tube with several glass beads. Salmonella cells were dislodged from the chips by vortexing for 15 s. The CFU/chip and cell morphology were determined.
Invasion and multiplication in Caco-2 cells.
Caco-2 cells were seeded at a density of approximately 20,000 cells per well in 24-well tissue culture plates (Becton Dickinson) containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 1% minimal essential medium with nonessential amino acids, 2 mM l-alanyl-l-glutamine, and 1 mM sodium pyruvate (Sigma) as previously described (8). The plates were incubated in a humidified atmosphere containing 5% CO2 at 37°C for 15 to 18 days to allow the Caco-2 cells to fully differentiate and form polarized monolayers. The tissue culture medium was aspirated from each well and replaced with 1 ml fresh tissue culture medium containing different concentrations of E40 control cells or filaments. Following incubation at 37°C for 1.5 h, the medium was removed. The monolayers were washed five times with warm (37°C) Hanks' balanced salt solution (HBSS) and then incubated an additional 2.5 h in tissue culture medium containing 50 μg/ml gentamicin (APP Pharmaceuticals) to kill any extracellular Salmonella. To determine invasion, half of the monolayers were washed five times with warm HBSS and lysed using 0.5% Triton X-100 (Calbiochem, EMD Chemicals, Gibbstown, NJ) in PBS. To assess intracellular growth, the remaining monolayers were washed with warm HBSS and incubated an additional 12 h in fresh tissue culture medium with 5 μg/ml gentamicin. The monolayers were then washed and lysed as described above. Serial dilutions of the cell lysates were plated in duplicate on sheep blood agar (SBA) plates (Becton Dickinson) and incubated at 37°C for 24 h. The resulting colonies were counted, and the CFU/ml of the cell lysates was determined.
Mouse inoculation.
Female inbred A/J mice (JAX Mice and Services, Bar Harbor, ME) were obtained at 5 to 6 weeks of age and housed under microisolator caps at the University of Wisconsin—Madison School of Veterinary Medicine animal care facility. Mice were acclimated for at least 1 week in this facility (until their average weight reached 15 g) before use in experiments. Mice received food and water ad libitum until 5 h prior to intragastric inoculation, at which time food was removed from the cage. This was done to minimize the possibility that food in the stomach might interfere with delivery of the inoculum and lead to aspiration into the lungs. Groups of mice (6 mice per treatment group) were anesthetized by intraperitoneal injection with sodium pentobarbital (0.75 mg per 25 g of body weight; APP Pharmaceuticals, Schaumburg, IL) as described previously (5). Once sedation occurred, the inoculum was introduced (in a total volume of 0.2 ml) via a 1.5-in. 24-gauge stainless steel feeding needle attached to a 1-ml syringe. At 1 to 5 days after inoculation, the mice were euthanized by asphyxiation with CO2 followed by exsanguination. Blood was collected into a syringe containing 0.1 ml sodium citrate (4%) as anticoagulant. The blood was then plated (undiluted and diluted 1:10 in PBS) in duplicate (0.1 ml) on SBA plates and incubated for 48 h at 37°C. The abdominal cavity was aseptically opened and portions of the spleen, liver, gallbladder, and cecum were removed, weighed in sterile weigh boats, and placed in sterile tissue grinders containing 1 ml cold sterile PBS. The tissues were homogenized, diluted in sterile PBS, and plated in duplicate onto SBA (spleen, liver, blood, and gallbladder), or XLD agar (Becton Dickinson) for cecum, followed by incubation at 37°C for 48 h. To detect fecal shedding of Salmonella, fresh fecal pellets collected from the mice in each cage were pooled and homogenized in PBS. After allowing the debris to settle, samples of the supernatants were removed, serially diluted in PBS, and plated on XLD agar plates, followed by incubation at 37°C for 48 h.
Data analysis.
For survival and growth studies, two independent trials were conducted with two replications per trial unless noted otherwise. The limit of detection was 0.70 log10 CFU/ml or chip, and this value was assigned to samples from which no survivors were detected. Analyses of growth and survival data were performed using Student's t test with Microsoft Excel. For Caco-2 cell studies, two independent trials were conducted with three replicate wells per trial. The limit of detection was 1.0 log10 CFU/ml of cell lysate. For mouse inoculation experiments, six mice were used per treatment. The limit of detection was 1.0 log10 CFU per ml of tissue homogenate or per g of feces. Samples from which no colonies were recovered were assigned a value of 0.95 log10 CFU per ml or per g for calculation of the mean ± standard error of the mean (SEM) for that treatment group. Caco-2 cell and mouse data were analyzed using a one-way analysis of variance and Tukey's comparison using Prism (GraphPad Software, Inc., San Diego, CA). Statistical significance for all comparisons was set at a P level of <0.05.
RESULTS
Filament formation at reduced aw and different temperatures.
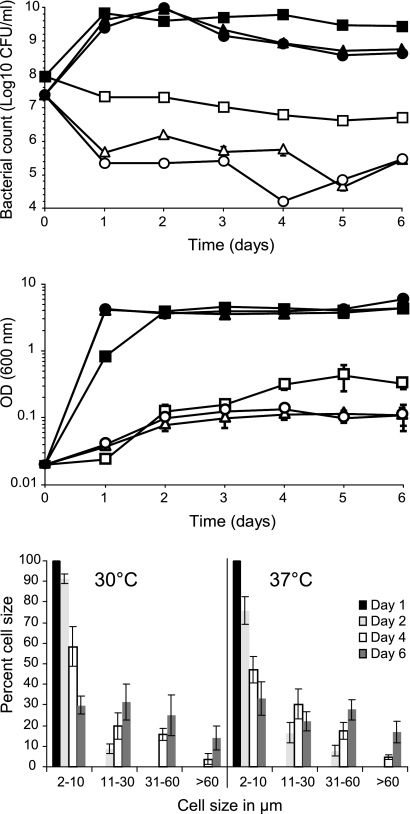
The viable numbers of strain E40 decreased over 6 days when incubated in TSB-8 (aw, 0.95) at 21°C, 30°C, or 37°C. A more rapid and greater decrease in CFU/ml (1.5 to 2.0 log10) was observed at 30°C and 37°C in comparison to 21°C. However, cultures at all three temperatures exhibited an increase in the OD600 that reflected an increase in biomass accompanied by the development of filaments at 30°C and 37°C (Fig. 1). The E40 control cells were generally 2 to 3 μm in length when grown in TSB. In TSB-8, they elongated and started to form filaments (defined in this study as >10 μm) (Fig. 2) after 2 days of incubation at 30°C or 37°C. The proportion of nonfilamentous cells (2 to 10 μm) decreased with time, while that of filaments increased. After 6 days, 70% and 67% of the cells were filamented at 30°C and 37°C, respectively (Fig. 1). The proportion of longer filaments (>30 μm) also increased with time. At 30°C, the longest filaments observed were ∼70 μm, while the longest filaments at 37°C were >100 μm. At 21°C less than 0.1% of the cells were over 10 μm (data not shown), and the longest filaments observed were about 15 μm.
Fig 1.
Growth of S. Enteritidis strain E40 in TSB (solid symbols) and TSB with 8% NaCl (open symbols) at 21°C (squares), 30°C (triangles), and 37°C (circles) as determined by CFU/ml (top) and OD at 600 nm (middle). The bottom graph shows percentages of cells of different lengths observed after 1, 2, 4, and 6 days at 30°C and 37°C on TSA with 7% NaCl.
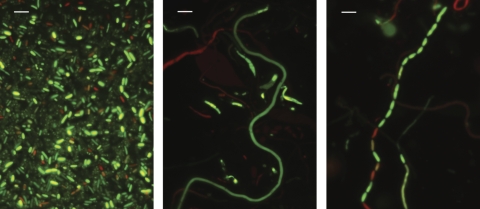
Fig 2.
Morphology of S. Enteritidis strain E40 control cells grown in TSB (left), filaments formed when grown in TSB with 8% NaCl (middle), and a septating filament following transfer to TSB (right). Bar, 5 μm.
The total number of E40 cells recovered from TSA-7 plates (aw, 0.95) after 4 days at 25°C, 30°C, and 37°C were approximately 7, 8, and 8 log10 CFU, respectively. In comparison, approximately 10 log10 CFU was recovered from TSA plates without NaCl at all three temperatures (data not shown). Approximately 70, 75, and 85% of the cells developed filaments on TSA-7 after incubation for 4 days at 25, 30, and 37°C, respectively. Filaments observed at 37°C were generally shorter than those at 25 and 30°C; about 7% of the cells at 37°C were >30 μm, with the longest measuring ∼60 μm. At 25 and 30°C, over 20% of the cells were >30 μm, and the longest filaments observed at these two temperatures were >100 μm. Because the greatest amount of filaments, in particular the longer ones, was recovered from TSA-7 plates incubated at 30°C, this method was used to develop E40 filaments for subsequent survival and virulence experiments.
Three other serovars, S. Agona, S. Typhimurium, and S. Tennessee, were examined, and they all developed filaments in TSB-8 and on TSA-7 at levels similar to that observed with S. Enteritidis E40 (data not shown).
Septation of filaments.
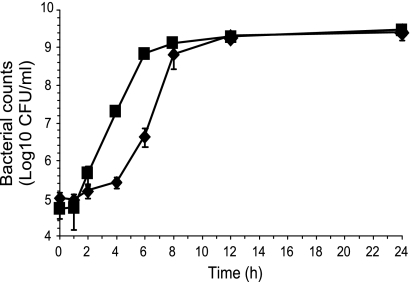
E40 filaments and nonfilamented (control) cells were inoculated into fresh TSB and incubated at 37°C. Filaments started to septate (Fig. 2) into normal-sized cells within 2 h. This resulted in a more rapid increase in CFU/ml than with control cells (Fig. 3). Septation continued to occur for the next 4 to 6 h; by 8 h less than 1% of the E40 cells remained filamented. Septation of filaments was also observed at 25°C, although it occurred at a lower rate than at 37°C (data not shown). When stained with the LIVE/DEAD BacLight, both green and red control cells and filaments were observed. However, it is interesting that occasionally during filamentation and septation (Fig. 2) different segments of a single filament fluoresced green or red. This suggests that the bacterial cell membrane remained intact (green) or was compromised (red) at different regions along the length of the filament.
Fig 3.
Growth of S. Enteritidis strain E40 control cells (diamonds) and filaments (squares) in TSB incubated at 37°C.
Survival at different temperatures.
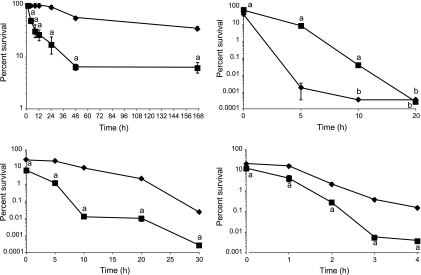
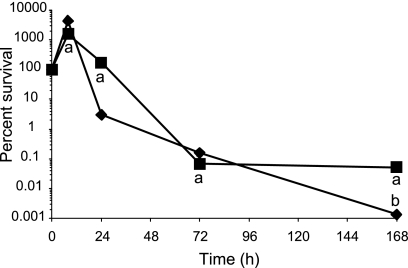
Survival of E40 filaments and control cells in TSB at 7°C is shown in Fig. 4. The filaments showed a reduction of 1.2 log10 CFU/ml after 48 h (6.4% survival), after which the viable counts remained stable through 168 h. Control cells showed higher survival than filaments at 7°C (P < 0.001), showing a reduction of 0.24 and 0.45 log10 CFU/ml after 48 h (55.6% survival) and 168 h (35.7% survival), respectively. Decreases in viability of both filaments and control cells were more gradual at 4°C than at 7°C (data not shown). After 8 days, survival of filaments and control cells was 11.8% and 56.9%, respectively. Filamentous cells remained as filaments at both temperatures. We extended the incubation at 4°C to 19 days to determine whether prolonged cold stress would induce filamentation by the E40 control cells, as was observed by Mattick et al. (24). No filamentation was observed.
Fig 4.
Survival of S. Enteritidis strain E40 control cells (diamonds) and filaments (squares) exposed to 7°C (top left), 55°C (bottom left), pH 2 (top right), and 10% bile salts (bottom right) in TSB. Letters above symbols: a, significantly different from control (P < 0.05); b, below detection limit of 0.70 log10 CFU/ml.
Rapid decreases in survival were observed for either filaments or control cells of E40 exposed to 55°C (Fig. 4). A decrease in CFU/ml was observed in samples retrieved immediately after inoculation (T0) into TSB at 55°C, resulting in survival of 6.2% and 25.3% for filaments and control cells, respectively. Survival of filaments (0.0003% after 30 min) was significantly less than that of control cells (0.02%; P < 0.005). When incubated at 45°C for 30 min, survival of filaments and control cells was 1.5% and 4.9%, respectively (P < 0.0001) (data not shown).
Survival at pH 2 and 4.
Decreases in CFU/ml were observed in E40 filament and control cell samples retrieved immediately after inoculation (T0) into TSB at pH 2, resulting in survival rates of 73.5% and 40.6%, respectively (Fig. 4). Survival of filaments after a 5- or 10-min exposure at pH 2 was significantly greater (P < 0.01) than that of control cells, although viable cells in both populations were not detected after 20 min. After 5 min, a reduction of 1.1 log10 CFU/ml (8.6% survival) in filaments was observed, compared to 4.8 log10 CFU/ml (0.002% survival) in control cells. After 10 min, the number of viable cells in filament populations was decreased by 3.4 log10 CFU/ml (0.04% survival), while viable control cells were not detected. At pH 4, both filaments and control cells grew to more than 8 log10 CFU/ml after 24 h (data not shown). During that time, most of the filaments had septated to form cells of regular length (2 to 3 μm), with fewer than 15% of the cells longer than 10 μm.
Survival in bile salts.
Survival of E40 filaments was significantly less (P < 0.01) than that of control cells during the first 4 h of exposure in TSB containing 10% bile salts (Fig. 4). Decreases in viability were observed in samples retrieved immediately after exposure to bile salts, resulting in 12.8% and 20.6% survival of filaments and control cells, respectively. After 4 h, survival of filaments and control cells was 0.004% and 0.15%, respectively. However, when incubation was extended to 24 h, growth was observed more frequently with filaments than control cells. Inoculation into 24-well plates containing TSB–10% bile salts with E40 filaments (2 trials; 12 wells per trial) resulted in growth in 16 of 24 wells after 24 h, and all 24 wells showed growth after 48 h of incubation. In contrast, only 5 of 24 wells inoculated with control cells showed growth after both 24 and 48 h. No filamented cells were observed in any of the control cultures or in the filament cultures that grew.
Desiccation on stainless steel at 32.5% and 75.5% RH.
The ability of E40 filaments and control cells to survive desiccation on stainless steel surfaces was examined. Viability of both filaments and control cells decreased with time at 32.5% RH, resulting in 3.8- and 5.8-log10 CFU per 1-cm2 chip reductions after 24 h and 168 h, respectively, for filaments and 4.4 and 5.3 log10 CFU per 1-cm2 chip reduction for control cells (data not shown). The viability of filaments and control cells was greater when the stainless steel chips were stored at 75.5% RH. After 8 h, the number of CFU increased by 1.1 and 1.6 log10 per chip for filament and control cells, respectively, resulting in survival of greater than 100% (Fig. 5). The increase in CFU was accompanied by septation of filaments, as the percentage of cells that were >10 μm in the filament population decreased from 70% to 35% during this time. Thereafter, survival of both filaments and control cells started to decline. After 168 h, survival of filaments was significantly greater (P < 0.01) than control cells, as viable Salmonella (2.74 log10 CFU/chip) could be recovered, while no survivors were detected from stainless steel surfaces inoculated with control cells. Control cells did not develop filaments under either desiccation condition.
Fig 5.
Survival of S. Enteritidis strain E40 control cells (diamonds) and filaments (squares) desiccated on stainless steel surfaces at 75.5% RH and 25°C. Letters below symbols: a, significantly different from control (P < 0.05); b, below detection limit of 0.70 log10 CFU/ml.
Invasion and multiplication in Caco-2 cells.
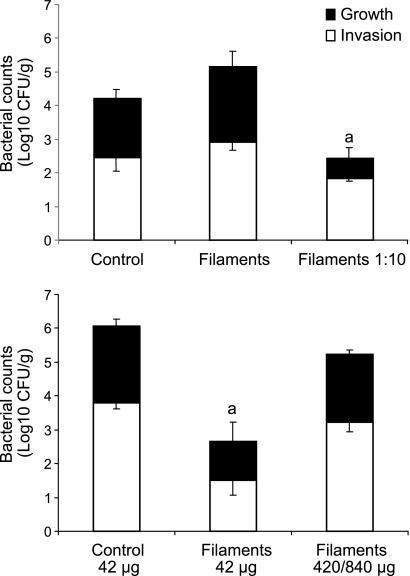
When fully differentiated Caco-2 cell monolayers were incubated with comparable CFU (∼7.4 log10 CFU) of E40 filaments or control cells, similar abilities of filaments and control cells to invade and multiply within the intestinal epithelial cells were observed (P > 0.05) (Fig. 6). When Caco-2 cells were incubated with 10-fold-fewer filaments (6.55 log10 CFU) than control cells, invasion was somewhat lower but not statistically different than control cells or in the incubation mixtures with higher number of filaments. In contrast, intracellular growth of Salmonella in Caco-2 cells (12-h incubation) was significantly less (P < 0.05) for the lower inoculum of filaments than for Caco-2 cells infected with control Salmonella cells or in the mixtures with the higher number of filaments.
Fig 6.
Invasion and intracellular growth of S. Enteritidis strain E40 control cells and filaments in Caco-2 cells. (Top) Differentiated Caco-2 cells were incubated with E40 control cells (7.49 log10 CFU) or filaments (7.43 log10 CFU for filaments and 6.55 log10 for filaments at 1:10) at 37°C for 1.5 h to allow invasion, followed by incubation with gentamicin (50 μg/ml) for 2.5 h to kill any extracellular Salmonella. Some of the wells were assayed for Caco-2 cell invasion; the others were incubated for 12 h in fresh tissue culture medium with gentamicin (5 μg/ml) and assayed for intracellular growth. Letter above right-most bar: a, intracellular growth was significantly different from control and filaments (P < 0.05). (Bottom) Differentiated Caco-2 cells were incubated with E40 control cells (42 μg [wet weight]) or filaments (42 μg or 420/840 μg [wet weight]) and assayed for invasion and intracellular growth as described above. For the greater mass of filaments, data were averaged from two separate trials in which 420 μg of E40 was used in one trial and 840 μg in the second. Letter above middle bar: a, invasion and intracellular growth was significantly different from control and filaments (420/840 μg [wet weight]) (P < 0.05).
Because suspensions of E40 filaments have a greater bacterial mass than a suspension containing comparable CFU of filaments, these experiments were repeated using comparable masses (wet weights) of filaments and control cells. When Caco-2 cells were incubated with 42 μg of filaments or control E40 cells, invasion and intracellular multiplication by the filaments were significantly reduced (P < 0.05) (Fig. 6). In contrast, when a greater biomass of filamentous cells (10- to 20-fold greater wet weight compared to control cells) was added, invasion and intracellular multiplication were not significantly different than control cells. Overall, these data demonstrate that E40 filaments can attach to and invade intestinal epithelial cells. The CFU recovered from the Caco-2 cells increased with time, which might reflect septation of the internalized filaments, intracellular multiplication of normal-sized cells, or both.
Infection of mice via the gastrointestinal tract.
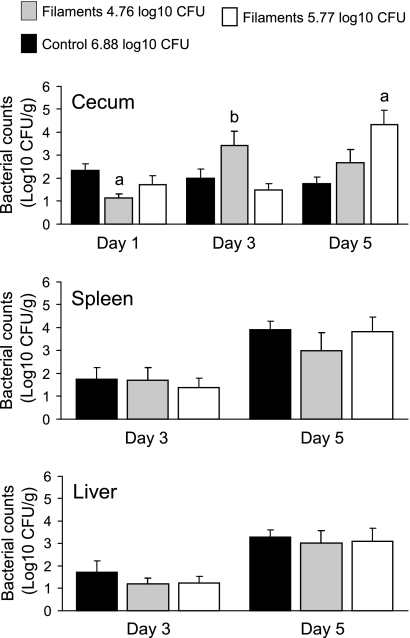
To assess virulence of filaments and control cells, mice were inoculated intragastrically with E40 filaments with 10- or 100-fold less CFU (5.77 or 4.76 log10 CFU) than mice that received control cells (6.88 log10 CFU). One day after inoculation, low-level colonization of the cecum was observed (Fig. 7), with no Salmonella recovered from the spleen or liver of any mouse regardless of whether it was inoculated with filaments or control cells. At 3 days after inoculation, the ceca of mice inoculated with filaments or control cells continued to be colonized. Paradoxically, mice inoculated with the lowest numbers of filaments displayed greater cecal colonization. On day 3 Salmonella was also recovered, albeit in low numbers, from the spleens and livers of a few mice inoculated with filaments or control cells. On day 5, similar CFU was recovered from the spleens and livers of mice inoculated with either filaments or control cells. At this point, colonization of the cecum was significantly greater (P < 0.05) for mice inoculated with the higher number (5.77 log10 CFU) of filaments.
Fig 7.
Recovery of Salmonella from the cecum, spleen, and liver of A/J mice inoculated intragastrically with S. Enteritidis strain E40 control cells (6.88 log10 CFU) or filaments (4.76 or 5.77 log10 CFU). Groups of six mice were euthanized at the indicated times, their tissues were removed, and the numbers of viable Salmonella were estimated by plate counts. Results are expressed as the mean ± SEM log10 CFU per g (wet weight) of tissue. Letters above bars: a, significantly different from control (P < 0.05); b, significantly different between the two filament groups (P < 0.05).
As an additional measure of intestinal colonization, fecal shedding of Salmonella was monitored daily throughout the experiment, using pooled fecal pellets from all mice in each cage as a composite sample (data not shown). On the day of inoculation, Salmonella was not recovered from any group of mice. On day 1, Salmonella was recovered in low numbers (1.6 to 2.0 log10 CFU/g) from fecal pellets of mice inoculated with control E40 cells or the lower concentration of filaments. On day 2, Salmonella was recovered in greater numbers (3.3 to 3.9 log10 CFU/g) from all groups of mice. On day 3 and thereafter, Salmonella was not recovered from the fecal pellets of mice inoculated with control cells. However, Salmonella was recovered through day 5 from mice inoculated with the higher concentration of filaments (5.77 log10 CFU) and through day 4 from mice inoculated with the lower concentration of filaments (4.76 log10 CFU).
DISCUSSION
Filamentation has been observed in diverse bacterial species exposed to unfavorable conditions. In many instances the biological relevance of filamentation is unclear, but filament formation has been implicated as a survival strategy. For example, filamentous bacteria are commonly observed in freshwater environments and are resistant to grazing by protist predators (3, 10, 26). Uropathogenic E. coli filaments formed during a urinary tract infection are protected from phagocytosis by polymorphonuclear leukocytes (18), while survival of Mycobacterium tuberculosis and S. enterica serovar Typhimurium in macrophages has been associated with filamentous phenotypes (2, 11, 29). Even with a diversity of examples, it is unknown if filament formation is a programmed response or a consequence of unfavorable environmental conditions.
In the pre-harvest and processing environment, food-borne pathogens encounter a multitude of stresses. In this study, we showed that filamentation by S. enterica serovar Enteritidis strain E40 when exposed to reduced-aw environments varied depending on temperature and the physical milieu. The ability of S. Enteritidis E40 filaments to survive unfavorable conditions compared to nonfilamented cells also varied depending on the specific stress condition. In particular, this study demonstrated that the filamentous E40 cells were virulent. They were able to invade and multiply intracellularly in Caco-2 cells and furthermore could colonize the gastrointestinal tract of mice and subsequently invade other organs.
Salmonella Enteritidis E40 developed filaments when incubated in either TSB or TSA supplemented with NaCl to achieve the same aw (0.95). Filamentation was affected by incubation temperature. Although the number of CFU/ml decreased in TSB-8 at both 30°C and 37°C, there was an increase in the OD600 that reflected an increase in biomass as filaments began to develop after 2 days of incubation. The proportion of filaments increased with incubation time. At 21°C, <0.1% of the cells was filamented. This is in contrast with the observation reported by Mattick et al. (22), who found that Salmonella filamented in Tryptone Soya broth supplemented with 8% NaCl at 21°C. This difference in results could be due to the different strains used. Our data confirmed the observations of Mattick et al. (22) and Kieboom et al. (19), indicating that filamentation results in an increase in biomass without an increase in viable counts. Plate counts of Salmonella filaments underestimated the potential number of bacterial cells, which may impact estimates of the level of contamination and infectious dose in food-borne outbreak investigations.
The matrix of the reduced-aw environment also had an effect. Both TSA-7 and TSB-8 had the same aw, 0.95, but much larger amounts of filaments could be recovered from TSA-7 plates. This could be partly due to how the Salmonella cell responds to surface growth on a reduced-aw medium compared to immersion in an aqueous solution. In TSB-8, the Salmonella cells were immersed in the hyperosmotic medium, while on TSA-7, the cells were under both osmotic stress due to the reduced-aw medium and matric stress due to exposure to the atmosphere (28). Different rates of drying under these two conditions could also affect survival and filamentation. It would be interesting to determine whether a food surface could be more conducive for filament formation by a bacterial contaminant than a liquid food environment.
It is unknown if the formation of filaments by S. Enteritidis E40 is a survival strategy or a consequence of osmotic stress. In addition to reduced-aw conditions, exposures to low or elevated temperatures and acid or alkaline pH levels lead to filament formation by Salmonella (19, 24, 25). Regardless, it appears to be a conserved response in several food-borne pathogens, including Listeria monocytogenes (1, 9, 16, 17), E. coli O157:H7 (25), and Bacillus cereus (21). The underlying mechanism(s) involved in filament formation by S. Enteritidis E40 is unclear. Proposed mechanisms have focused on the inhibition of bacterial septation and cell division. One of the best characterized is the SOS response, which is induced by DNA damage (33). The SOS system comprises more than 30 genes, one of which is sulA, which when induced transiently inhibits cell division (13) by preventing the polymerization of the FtsZ protein. The FtsZ ring that forms at the site of septation is a key step in cell division. In S. Typhimurium, expression levels of SOS response genes, including sulA, recA, and lexA, were upregulated during intracellular growth and filamentation in macrophages (7). We observed by Western blot analysis a 15-fold increase in RecA protein expression by S. Enteritidis E40 filaments compared to control cells (unpublished data). Whether this increased RecA expression is a consequence or cause of E40 filamentation or is associated with an SOS response is unknown. Henry et al. (11) found filament formation by S. Typhimurium within human melanoma cells to be sulA independent and occurred after FtsZ ring assembly. It resulted from a hisG mutation and required the activity of HisFH, a heterodimeric enzyme in the histidine biosynthetic pathway. Another mechanism was demonstrated with a cationic α-helical, cathelicidin-related antimicrobial peptide that impairs replication of S. Typhimurium in macrophages (30). Whether one of these processes or another yet-undefined mechanism leads to S. Enteritidis E40 filamentation remains to be determined.
Cross-protection is an important consequence of exposure to sublethal stress (34). Because the formation of filaments is associated with stress, it is reasonable to assume that stress protection processes are induced that frequently result in cross-protection; however, this has not been investigated in depth. For example, habituation of Salmonella to low aw increased its tolerance to heat (23) and sodium hypochlorite (19) compared to control cells. Of the various stress conditions tested in this study, growth of filament populations in the presence of 10% bile salts and the reduced rate of lethality at pH 2.0 were the most noticeable differences in comparison to control cells. These growth and survival properties may afford some benefit in the intestinal tract of a host.
A key characteristic of Salmonella-host interactions is the ability to invade and multiply intracellularly in host cells. We demonstrated that E40 filaments could invade and multiply within human intestinal epithelial Caco-2 cells. It is not known whether the filaments remained filamentous during invasion or septated into normal-size cells prior to invasion. Both filaments and septating E40 cells were observed adhering to Caco-2 cells (see Fig. S1 in supplemental material). The number of CFU recovered from filament-infected Caco-2 cells increased with time, which could reflect septation of internalized filaments, intracellular multiplication, or both. Our results are in contrast to those reported by Humphrey et al. (15), who concluded that S. Typhimurium filaments generated by overexpression of SulA were unable to invade Madin-Darby canine kidney (MDCK) cells after 15 min of incubation. Potential differences between stress-induced versus SulA-induced filaments, Salmonella serovar and strain, and cell type could account for the differences in results observed in the earlier study and in the present report. It is also possible that filaments require a longer time than control cells to invade, and the 1.5 h used in our study provided a sufficient time frame.
We also showed that E40 filaments can establish a successful infection in mice via intragastric inoculation. The degree to which they colonized the gastrointestinal tract and disseminated to the spleen and liver was comparable to that attained by control E40 cells. Filaments were able to multiply in the cecum, spleen, and liver, as the numbers of CFU recovered increased with infection time. In addition, they appeared to establish a more robust colonization in the intestinal tract than control cells, as the CFU recovered from the cecum after 5 days was significantly (P < 0.05) greater for mice inoculated with filaments than control cells, even though the inoculum level was 10-fold lower for filaments than control cells (Fig. 7). Presumably, septation of filaments in the gastrointestinal tract, combined with subsequent growth of the septated Salmonella cells, contributed to these results.
To our knowledge this is the first demonstration of virulence by stress-induced Salmonella filaments in vitro (i.e., Caco-2 cells) and in vivo (i.e., mice). Humphrey et al. (15) hypothesized that the presence of Salmonella filaments in food did not pose an infection risk until they started to septate. Our results suggest that ingestion of Salmonella filaments could lead to multiplication within intestinal cells and spread to other organs in the host. Salmonella Enteritidis E40 filaments are not unusually stress tolerant, but there are stress-specific differences. For example, filaments eventually grew after prolonged incubation in 10% bile salts, which could contribute to their survival and multiplication in the gastrointestinal tract. It is unknown if the formation of filaments by E40 is a survival strategy or a consequence of osmotic stress, and the underlying mechanism(s) of filamentation is unknown. If filament formation were a survival strategy, one would expect E40 filaments to be more tolerant to some of the other stresses tested in this study, due to cross-protection mechanisms. It is currently unknown whether Salmonella can develop filaments in food. Filaments in food or food processing environments could pose an increased food safety risk and impact epidemiological investigations, as plate counts would underestimate the potential number of Salmonella cells present. In addition, septation of filaments would lead to a rapid increase in cell numbers, thus increasing the total Salmonella load.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by USDA Agriculture and Food Research Initiative grant 2010-65201-20563 from the National Institute of Food and Agriculture and by the Food Research Institute and the Walter and Martha Renk Endowed Laboratory at the University of Wisconsin—Madison.
Footnotes
Published ahead of print 27 January 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Bereksi N, Gavini F, Bénézech T, Faille C. 2002. Growth, morphology and surface properties of Listeria monocytogenes Scott A and LO28 under saline and acid environments. J. Appl. Microbiol. 92: 556–565 [DOI] [PubMed] [Google Scholar]
- 2. Chauhan A, et al. 2006. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J. Bacteriol. 188: 1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corno G, Jürgens K. 2006. Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl. Environ. Microbiol. 72: 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reference deleted. [Google Scholar]
- 5. Czuprynski CJ, Faith NG, Steinberg H. 2003. A/J. mice are susceptible and C57BL/6 mice are resistant to Listeria monocytogenes infection by intragastric inoculation. Infect. Immun. 71: 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewanti R, Wong ACL. 1995. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int. J. Food Microbiol. 26: 147–164 [DOI] [PubMed] [Google Scholar]
- 7. Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47: 102–118 [DOI] [PubMed] [Google Scholar]
- 8. Faith NG, Kathariou S, Neudeck BL, Luchansky JB, Czuprynski CJ. 2007. A P60 mutant of Listeria monocytogenes is impaired in its ability to cause infection in intragastrically inoculated mice. Microb. Pathog. 42: 237–241 [DOI] [PubMed] [Google Scholar]
- 9. Giotis ES, Blair IS, McDowell DA. 2007. Morphological changes in Listeria monocytogenes subjected to sublethal alkaline stress. Int. J. Food Microbiol. 120: 250–258 [DOI] [PubMed] [Google Scholar]
- 10. Hahn MW, Moore ERB, Hogle MG. 1999. Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl. Environ. Microbiol. 65: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henry T, Garcia-del Portillo F, Gorvel JP. 2005. Identification of Salmonella functions critical for bacterial cell division within eukaryotic cells. Mol. Microbiol. 56: 252–267 [DOI] [PubMed] [Google Scholar]
- 12. Hoffman H, Frank ME. 1963. Temperature limits, genealogical origin, developmental course, and ultimate fate of heat-induced filaments in Escherichia coli microcultures. J. Bacteriol. 85: 1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huisman O, D'Ari R. 1981. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature 290: 797–799 [DOI] [PubMed] [Google Scholar]
- 14. Humphrey BA, Kjelleberg S, Marshall KC. 1983. Responses of marine bacteria under starvation conditions at solid-water interface. Appl. Environ. Microbiol. 45: 43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Humphrey S, et al. 2011. SulA-induced filamentation in Salmonella enterica serovar Typhimurium: effects on SPI-1 expression and epithelial infection. J. Appl. Microbiol. 111: 185–196 [DOI] [PubMed] [Google Scholar]
- 16. Isom LL, Khambatta ZS, Moluf JL, Akers DF, Martin SE. 1995. Filament formation in Listeria monocytogenes. J. Food Prot. 58: 1031–1033 [DOI] [PubMed] [Google Scholar]
- 17. Jørgensen F, Stephens PJ, Knøchel S. 1995. The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J. Appl. Bacteriol. 79: 274–281 [Google Scholar]
- 18. Justice SS, et al. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 101: 1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kieboom J, et al. 2006. Survival, elongation, and elevated tolerance of Salmonella enterica serovar Enteritidis at reduced water activity. J. Food Prot. 69: 2681–2686 [DOI] [PubMed] [Google Scholar]
- 20. Kjelleberg S, Humphrey BA, Marshall KC. 1983. Initial phases of starvation and activity of bacteria at surfaces. Appl. Environ. Microbiol. 45: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maier SK, Scherer S, Loessner MJ. 1999. Long-chain polyphosphate causes cell lysis and inhibits Bacillus cereus septum formation, which is dependent on divalent cations. Appl. Environ. Microbiol. 65: 3942–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattick KL, et al. 2000. Survival and filamentation of Salmonella enterica serovar Enteritidis PT4 and Salmonella enterica serovars Typhimurium DT104 at low water activity. Appl. Environ. Microbiol. 66: 1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mattick KL, Jørgensen F, Legan JD, Lappin-Scott HM, Humphrey TJ. 2000. Habituation of Salmonella spp. at reduced water activity and its effect on heat tolerance. Appl. Environ. Microbiol. 66: 4921–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mattick KL, Phillips LE, FJørgensen Lappin-Scott HM, Humphrey TJ. 2003. Filament formation by Salmonella spp. inoculated into liquid food matrices at refrigeration temperatures, and growth patterns when warmed. J. Food Prot. 66: 215–219 [DOI] [PubMed] [Google Scholar]
- 25. Mattick KL, Rowbury RJ, Humphrey TJ. 2003. Morphological changes to Escherichia coli O157:H7, commensal E. coli and Salmonella spp. in response to marginal growth conditions, with special reference to mildly stressing temperatures. Sci. Prog. 86: 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pernthaler J, et al. 1997. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl. Environ. Microbiol. 63: 596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phillips LE, Humphrey TJ, Lappin-Scott HM. 1998. Chilling invokes different morphologies in two Salmonella enteritidis PT4 strains. J. Appl. Microbiol. 84: 820–826 [DOI] [PubMed] [Google Scholar]
- 28. Potts M. 1994. Desiccation tolerance in prokaryotes. Microbiol. Rev. 58: 755–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberger CM, Finlay BB. 2002. Macrophages inhibit Salmonella Typhimurium replication through MEK/ERK kinase and phagocyte NADPH oxidase activities. J. Biol. Chem. 277: 18753–18762 [DOI] [PubMed] [Google Scholar]
- 30. Rosenberger CM, Gallo RL, Finlay BB. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. U. S. A. 101: 2422–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaw EK. 1968. Formation of filaments and synthesis of macromolecules at temperatures below the minimum for growth of Escherichia coli. J. Bacteriol. 95: 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terry DR, Gaffar A, Sagers RD. 1966. Filament formation in Clostridium acidiurici under conditions of elevated temperatures. J. Bacteriol. 91: 1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker GC, Smith BT, Sutton MD. 2000. The SOS response to DNA damage, p 131–144 In Storz G, Hengge-Aronis R. (ed), Bacterial stress responses. ASM Press, Washington, DC [Google Scholar]
- 34. Wesche AM, Gurtler JB, Marks BP, Ryser ET. 2009. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J. Food Prot. 72: 1121–1138 [DOI] [PubMed] [Google Scholar]
- 35. Winston PW, Bates DH. 1960. Saturated solutions for the control of humidity in biological research. Ecology 41: 232–237 [Google Scholar]
- 36. Yoshida S-I, Takezo U, Mizuguchi Y, Tanabe T. 1986. Salt-induced filamentous growth of a Salmonella strain isolated from blood. J. Clin. Microbiol. 23: 192–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.