Abstract
Purpose
Osteogenesis imperfecta (OI) has been treated with bisphosphonates for many years, with some clear clinical benefits. In adults, there are reports of a new pattern of atraumatic subtrochanteric fractures with bisphosphonate treatment. This study assesses if bisphosphonate treatment leads to an altered pattern of femoral fractures.
Methods
Retrospective review of imaging for a cohort of 176 bisphosphonate-treated OI patients to identify the locations of femoral fractures over a two-year period, as compared to a historical control group managed pre-bisphosphonates.
Results
Sixteen femoral fractures were identified in this time period in the bisphosphonate-treated group. All but two were within the subtrochanteric region. In comparison, the historical group—composed of 26 femoral fractures—had a more widespread fracture pattern, with the most frequent location being the mid-diaphysis. Many of the subtrochanteric fractures in the treatment group occurred with minimal trauma.
Conclusions
It appears that concerns over the treatment of the adult osteoporotic population with bisphosphonates are amplified and mirrored in OI. It is possible that the high bending moments in the proximal femur together with altered mechanical properties of cortical bone secondary to the use of this group of drugs increase the risk of this type of injury, which warrants further modification of surgical management of the femur.
Keywords: Osteogenesis imperfecta, Bisphosphonates
Introduction
Osteogenesis imperfecta (OI) is a condition characterised by bone fragility and multiple fractures, which causes considerable morbidity in affected individuals [1]. The many genetic defects in type I collagen that are generally responsible lead to a phenotypically broad spectrum of disease [2, 3]. The fracture risk arises from decreased bone density, abnormal mineralisation of extracellular matrix, disuse osteoporosis, and altered bone geometry, leading in most cases to transverse or short oblique patterns [4]. Lower limb fractures and in particular femoral fractures are most common due to their load bearing during normal gait patterns [5, 6].
The use of bisphosphonates has revolutionised the medical management of OI [7]. Their mechanism of action comes from a structure closely resembling pyrophosphate, which via its effects on surface lipoproteins ultimately results in the inhibition of osteoclast resorption of bone, altering bone turnover [8, 9]. There are other effects, including those on osteoblasts, osteocytes, and chondroblasts [10–15]. Indirect measures of bone quality (bone mineral density) increase with bisphosphonate use, as do cortical thickness and vertebral body size [16–20]. Randomized and controlled trials have also shown reductions in fracture rates and bone pain in some cases [21–24]. However, these findings are not universal [25], and not necessarily linked to a better quality of life [26], although BMD has been considered a guide to functional outcome [27]. Further studies are needed to confirm the reduction in long bone fracture incidence [28]—a difficult undertaking considering the rarity of this condition. There have been concerns raised regarding potential long-term complications, due to their effects on bone [29]. These include delayed remodelling during fracture healing [30, 31], reduced longitudinal growth [32], teratogenicity, and osteopetrosis [33].
Concern regarding the potential long-term effects of bisphosphonates has received increasing attention in the treatment of osteoporotic adults, with a number of cases where atypical subtrochanteric fractures have occurred in treated patients [34–37]. Many cases are bilateral and occur in those treated with bisphosphonates over a long period of time; there is an associated picture of thickening of the subtrochanteric bone which occurs in an elliptoid pattern associated with these fractures. Some studies suggest that the cortical thickening seen in these subtrochanteric fractures is due to repeated microfractures accumulating in this area, where bisphosphonate-mediated bone turnover effects lead to progressive failure under mechanical stress, often preceded by prodromal pain [38–40]. Recent population-based studies suggest an increased risk of subtrochanteric fracture in adults treated with bisphosphonates, which is, however, dwarfed by the overall reduced fracture rate in treated patients [41, 42]. This has not been corroborated in other population-based studies [43] and a review of three randomized controlled trials [44]. Some authors have suggested a treatment holiday to minimise potential complications [45].
Children treated with bisphosphonates may therefore be at increased risk of such fracture types. The aim of this study was to review a cohort of OI patients treated with bisphosphonates in order to assess the femoral fracture patterns seen.
Patients and methods
Approval from the Hospital Research and Audit Department was obtained. An existing database of patients currently under treatment with bisphosphonates was utilised to identify patients treated for OI. Using the digital radiograph archive, all imaging available for these patients over a two-year period from 2007 to 2009 was reviewed. Radiographs of femora as part of a skeletal survey or as isolated imaging were reviewed to assess the prevalence of acute femoral fractures in this time period. Other long bone or axial fractures were not assessed. Where fractures were identified, case notes were obtained for demographics on the patients and details of the fracture. The presentation, mode of injury, and length and type of bisphosphonate treatment were all recorded, as was the classification of the type of OI according to Sillence. Whether affected femora were rodded or not was also recorded, together with whether the mechanism of injury was high or low energy. Total body and spinal bone mineral density Z scores were also obtained for the time of injury.
A historical control group of OI patients, treated initially in childhood by surgical techniques, were used as a comparison. Case notes for this group were obtained and radiological reports for femoral fractures in those not treated with bisphosphonates were reviewed. Radiographs were also examined. Results for the locations of the fractures were plotted graphically for a direct visual demonstration of the trends.
Results
One hundred and seventy-six patients with moderate or severe OI in total were currently receiving treatment with oral risedronate or cyclical intravenous pamidronate during the two-year study period. These patients represent the entire bisphosphonate caseload at our unit treated during the two-year period described. The dose of pamidronate varied from 3 to 12 mg/kg/year given over 2–3 consecutive days with 1–4 treatments per year. Patients treated with risedronate received 0.2–2 mg/kg per week. Treatment holidays are not routinely used, except where surgery for long bone realignment is planned. Of these patients, 133 patients had imaging available for review, either as radiographic follow-up for surgical procedures, acute presentations, DEXA scans or skeletal surveys. A total of 43 had no imaging in this timescale.
Eleven patients had 16 femoral fractures on the radiographs available (Table 1). Of the 11, three were Sillence type III and the remainder type IV. Five had sustained bilateral fractures over the study period. All but two had been treated with bisphosphonates for more than six years. All fractures were either short oblique (25%) or transverse (75%). Figure 1 represents the location of each fracture in each of the patients in both the bisphosphonate-treated and control patients. In terms of the mechanism of injury, those classified as high energy made up 31% of all fractures. Those included in this group were injuries where a definite fall had occurred that could cause a fracture in an unaffected individual. Examples include a fall getting out of a swimming pool and a fall whilst dancing. Low-energy injuries included all fractures where the mechanism would not be associated with a fracture in an unaffected individual. In two cases there was no history of injury prior to the onset of pain, after which the fracture was diagnosed.
Table 1.
Demographics of femoral fractures in osteogenesis imperfecta patients treated with bisphosphonates
| Age at fracture (years) | Sex | Rodded bone | Z score spine/total body | OI type (Sillence) | Duration of bisphosphonate therapy | Dose of pamidronate/risedronate | Fracture pattern | Fracture level | Level of energy |
|---|---|---|---|---|---|---|---|---|---|
| 9.5 | F | Yes | −0.2/0.8 | IV | 9.0 years (P) | 1.5 mg/kg/3 days ×3 | Oblique | Subtrochanteric | Low |
| 14.4 | M | Yes | 0.9/−0.8 | III | 9.4 years (P) | 1.25 mg/kg/3 days ×4 | Oblique | Subtrochanteric | Low |
| 19.5 | M | Yes | 0.4/−2.7 | IV | 7.5 years (P) | 1.0 mg/kg/3 days ×4 | Transverse | Subtrochanteric | Low |
| 18.3 | M | Yes | IV | 6.3 years | 1.0 mg/kg/3 days ×4 | Transverse | Subtrochanteric | Low | |
| 16.2 | M | No | −2.0/−1.4 | IV | 6.2 years (P) | Transverse | Subtrochanteric | Low | |
| 17.2 | F | Yes | 0.4/−0.8 | III | 6.1 years (P) | 1.0 mg/kg/3 days | Transverse | Mid-shaft | High |
| 18.6 | F | Yes | −0.5/−0.4 | 7.5 years | Transverse | Lesser troc | Low | ||
| 8.6 | M | No | 1.1/0.4 | IV | 6.4 years (P) | 1.0 mg/kg/3 days ×4 | Oblique | Subtrochanteric | Low |
| 2.7 | F | No | 0.4/0.67 | 2.6 years (P) | 1.0 mg/kg/3 days ×4 | Transverse | Subtrochanteric | Low | |
| 3.2 | F | No | 0.45/0.74 | IV | 3.1 years (P) | Transverse | Subtrochanteric | Low | |
| 16.4 | M | Yes | −3.3/−4.3 | III | 8.4 years (R) | 1.0–2.0 mg/kg/week | Transverse | Subtrochanteric | Low |
| 16.8 | M | Yes | −5.8/−3.5 | IV | 8.5 years (R) | 1.0–2.0 mg/kg/week | Transverse | Subtrochanteric | High |
| 17.3 | M | Yes | −5.9/−3.7 | IV | 9.0 years | Transverse | Subtrochanteric | Low | |
| 9.0 | F | No | −4.0/1.5 | IV | 5.1 years (R) | 1.0–2.0 mg/kg/week | Oblique | Subtrochanteric | High |
| 9.9 | F | Yes | −2.8/−2.1 | IV | 6.0 years | Transverse | Subtrochanteric | High | |
| 13.8 | F | No | −0.8/−1.5 | IV | 3.4 years (R) | 1.0–2.0 mg/kg/week | Transverse | Subtrochanteric | High |
R risedronate, P pamidronate shaded rows constitute fractures in the same patient
Fig. 1.
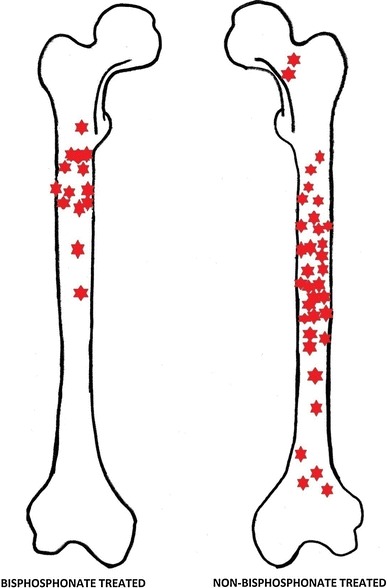
Graphical representation of fracture locations in bisphosphonate and non-bisphosphonate-treated patients
In three cases, the fracture occurred through a previous osteotomy with radiological evidence of union prior to the current fracture (union was defined as bridging callus visible on four cortices on an AP and lateral radiograph of the femur). One fracture had occurred through a mid-diaphyseal healed fracture. 38% of the fractures occurred in rodded femora. Fractures tended to be associated with pan-cortical thickening of sub-trochanteric bone, although—due to the small differences in the amount of thickening and despite standardised views—the absence of a calibration sphere meant that this could not be accurately measured (Figs. 2, 3). Ten of the 16 fractured femora had a telescopic rod in situ, and none had a pathological sagittal or coronal bow, although coxa vara was (as would be expected) a common finding.
Fig. 2a–c.
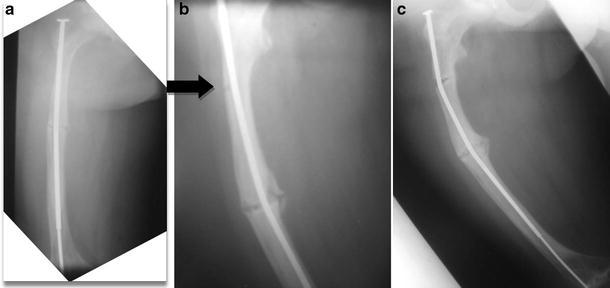
A type IV adolescent OI male patient. a Initial radiograph post corrective osteotomy and telescopic rod insertion, b unicortical break identified (black arrow), c break develops into a complete fracture with associated rod bowing
Fig. 3.
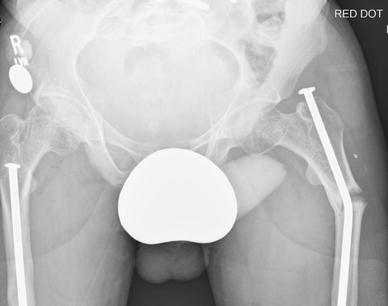
A subtrochanteric fracture in an adolescent Sillence type IV OI patient sustained whilst dancing. The elliptoid thickening in the region of the fracture corresponds to similar findings in adult bisphosphonate-treated patients
The comparison group consisted of a historical group of OI patients treated prior to the advent of bisphosphonates. A total of 45 were identified with complete data, all with moderate or severe OI. All had reached skeletal maturity at the time of this study. Fractures were recorded from the original treatment until discharge from the children’s hospital to the adult sector at 18 years of age. This cohort consisted of patients referred for consideration of surgical treatment of OI, with either acute fractures, recurrent fractures, or deformity. The radiographic review therefore consisted of unrodded femora without significant deformity, or rodded femora for the indications mentioned above. Over the course of treatment within our institution, a total of 34 femoral fractures occurred in the 26 patients. Apart from one segmental midshaft fracture, all injuries were short oblique or transverse fractures. Two femoral neck and four distal fractures were included in the group, although the majority were in the mid-diaphyseal region. 73% of the fractures occurred in patients already treated with intramedullary rodding of the femur; these were telescopic rods in nearly all cases (Table 2). Due to the retrospective data available and the small number of historical cases with complete data to allow a comparison, this cohort consists of similar cases of moderate and severe OI that in the present era would qualify for treatment with bisphosphonates, but which differ from the treatment group in that all fractures sustained were recorded during the entire treatment, and are therefore similar only in the underlying severity of bone disease.
Table 2.
Non-bisphosphonate-treated OI patient classification and fracture details
| OI type | Number of patients | Low-energy injuries (%) | Fractures in rodded bones (%) |
|---|---|---|---|
| I | 7 | 86 | 71 |
| III | 8 | 75 | 75 |
| IV | 11 | 82 | 73 |
| Total | 26 | 81 | 73 |
Statistical analysis was not possible based on existing classification systems, primarily because the Pediatric Comprehensive Classification of Long Bone Fractures only differentiates on fracture location by describing the proximal and distal metaphysis and the diaphysis; this would not allow any differences in the location within the diaphysis to be differentiated. For this reason, and due to the small numbers within the study, only trends are inferred from the resultant fracture patterns (Fig. 1).
Discussion
The benefits of bisphosphonates are not in doubt, either in adult populations or in the various clinical situations where children require treatment. Experience in our institution certainly suggests that treatment has reduced OI fracture rates and bony pain in treated patients [46]. OI is a different clinical entity compared to osteoporosis, the main indication for the use of bisphosphonates in adult populations. For this reason, identifying a change in the pattern of femoral fractures within a relatively small cohort is a finding of concern, and warrants further investigation. This adds to an already controversial topic; current issues include the choice, dose, and method of administration of bisphosphonate, as well as the perioperative medical treatment regime that is least likely to interfere with osteotomies and fracture healing.
The length and method of treatment is in itself a subject of debate in the care of osteoporotic adults. At present there is insufficient research to guide on whether “drug holidays” may be of benefit in reducing the rate of atypical fractures. These treatment holidays may themselves put patients at risk of further fractures.
With increasing bisphosphonate use, there appears to have been a change in the pattern of fractures seen in our cohort of patients, with more proximal fractures seen in the femur, and some developing insidiously without any history of trauma. In the adult osteoporotic population, a review of over 14,000 patients in three randomized trials treated with bisphosphonates identified no significant increase in atypical fractures, although firm conclusions could not be drawn because it was felt to be underpowered [44]. A further population-based case–control study has identified that there is an increase in subtrochanteric fractures in those treated with bisphosphonates for more than five years [42]. The trend demonstrated in our cohort highlights a trend that far exceeds that seen in the adult population, and the underlying OI abnormalities are the likely cause (Fig. 4).
Fig. 4.
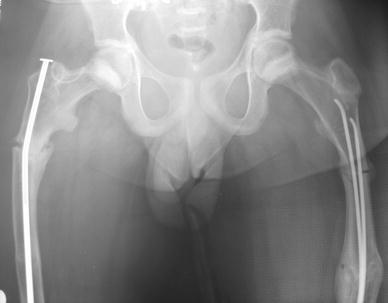
A type IV adolescent male patient who sustained bilateral sequential atraumatic subtrochanteric fractures. One side was treated with elastic nailing. Zebra lines from bisphosphonate use are visible around the proximal femora and pelvis
A possible hypothesis for this altered pattern can be inferred from animal studies, and the basic science of developing bone growth. OI is a condition associated with high bone turnover. Although the main effect of bisphosphonates is modifying the osteoclastic resorption of bone, they also affect osteoblasts [10–15] and chondroblasts [47], and in vivo studies suggest better longitudinal bone growth [48, 49]. How exactly the risk of fracture may be reduced with bisphosphonate treatment is not fully understood. The bone of patients with untreated OI is hypermineralised, but despite this, treatment with bisphosphonates appears to increase bone mass by cortical and trabecular thickening; it may alter the cross-sectional geometry, but it does not alter the material properties of bone [50]. Intramembranous ossification, such as that involved in appositional growth of cylindrical bones such as the femur, are mainly osteoblast mediated, with some evidence to suggest that bisphosphonates affect the quality of the organic matrix, leading to bone with weaker mechanical properties [51, 52]. Studies of OI patient iliac bone samples have suggested that intrinsic properties are unaffected [53]. There is also evidence to suggest bisphosphonates bind selectively to areas of increased bone turnover, which may result in patchy areas of uptake. This may also play a role, and may be exacerbated, if there is an element of stress shielding in rodded bones.
A recent study looking at the effect of bisphosphonates on the BRTL mouse OI model corroborates that the brittleness of bone is not altered by treatment; histological analysis of mid-shaft and proximal bisphosphonate-treated bone identified areas of weakness secondary to unmineralised cartilage. Altered osteoblast function was also identified, and an overall reduction in bone strength with continued treatment was noted; the stiffness, toughness, Young’s modulus, and moment of inertia remains the same with treatment [54, 55].
It is likely that weakness to bending moments within the proximal femur due to the mechanisms explained above result in this altered fracture pattern.
We believe that the findings of this study identify concerns regarding the long-term use of bisphosphonates and a changing pattern of fractures, amplifying as well as mirroring the findings of other groups among adults. OI is a completely different disease entity when compared to the main clinical indications for bisphosphonate use in adults, and the response of bone to the use of this group of drugs would be expected to demonstrate similar trends, but not a large increase in the incidence of pathological fractures. The findings suggest that there is a much increased risk of fracture pattern alteration in this skeletal dysplasia, which warrants further investigation including clinical trials and histological analysis of cortical bone from treated OI patients.
It also warrants an altered attitude towards surgical management of the femur. As this particular region appears at increased risk of fracture, efforts should focus on maintaining a mechanical axis that is as normal as possible. In particular, excessive coxa vara, which is commonly seen in more severely affected OI patients, increases the moment arm at the subtrochanteric level, which may in turn allow lateral microfractures to develop. In addition, corrective osteotomies at this level, if possible, should be avoided when planning corrective surgery, as healing in this group of patients treated with bisphosphonates is known to be altered in a deleterious manner. Any patient reporting pain in the upper thigh should have the proximal femur imaged and radiological changes described previously should be sought. Screening radiographs may be indicated to assess and predict fracture occurrence in this area.
There are limitations to this observational study. It does not compare fracture incidence in bisphosphonate-treated patients to untreated patients. A review of radiographs is not guaranteed to identify all femoral fractures, as some patients may have been imaged in other hospitals because the centre is a tertiary referral unit. There is matching of the underlying OI type with the historical comparison made, although the use of a method based on available historical data and the small number of femoral fractures sustained in the treatment group only allow a trend to be demonstrated. However, we do believe that, as a comparison, it does highlight a different pattern of fractures (Fig. 1) which must be considered together with the other controversies regarding the medical management of OI.
References
- 1.Shapiro F. Consequences of an osteogenesis imperfecta diagnosis for survival and ambulation. J Pediatr Orthop. 1985;5:456–462. doi: 10.1097/01241398-198507000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Roughley PJ, Rauch F, Glorieux FH (2003) Osteogenesis imperfecta—clinical and molecular diversity. Eur Cell Mater 5:41–47; discussion 47 [DOI] [PubMed]
- 3.King JD, Bobechko WP. Osteogenesis imperfecta—an orthopaedic description and surgical review. J Bone Joint Surg Br. 1971;53-B:72–89. [Google Scholar]
- 4.Dent JA, Paterson CR. Fractures in early childhood: osteogenesis imperfecta or child abuse? J Pediatr Orthop. 1991;11(2):184–186. doi: 10.1097/01241398-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Fritz JM, Guan Y, Wang M, Smith PA, Harris GF. A fracture risk assessment model of the femur in children with osteogenesis imperfecta (OI) during gait. Med Eng Phys. 2009;31(9):1043–1048. doi: 10.1016/j.medengphy.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Garrison JG, Slaboch CL, Niebur GL. Density and architecture have greater effects on the toughness of trabecular bone than damage. Bone. 2009;44(5):924–929. doi: 10.1016/j.bone.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glorieux FH. Experience with bisphosphonates in osteogenesis imperfecta. Paediatrics. 2007;119(Suppl 2):S163–S165. doi: 10.1542/peds.2006-2023I. [DOI] [PubMed] [Google Scholar]
- 8.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo H, Samson-Fang L. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2008;51:17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 10.Roelofs AJ, Coxon FP, Ebetino FH, Lundy MW, Henneman ZJ, Nancollas GH, Sun S, Blazewska KM, Bala JL, Kashemirov BA, Khalid AB, McKenna CE, Rogers MJ. Fluorescent risendronate analogues reveal bisphosphonate uptake by bone marrow monocytes and localization around osteocytes in vivo. J Bone Miner Res. 2010;25(3):606–616. doi: 10.1359/jbmr.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2002;60:6001–6007. [PubMed] [Google Scholar]
- 12.Fromigue O, Body JJ. Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002;25:539–546. doi: 10.1007/BF03345497. [DOI] [PubMed] [Google Scholar]
- 13.Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004;25:4105–4115. doi: 10.1016/j.biomaterials.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 14.von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–6949. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 15.Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G. Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone. 1998;22:455–461. doi: 10.1016/S8756-3282(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 16.Poyrazoglu S, Gunoz H, Darendeliler F, Bas F, Tutunculer F, Eryilmaz SK, Bundak R, Saka N. Successful results of pamidronate treatment in children with osteogenesis imperfecta with emphasis on the interpretation of bone mineral density for local standards. J Pediatr Orthop. 2008;28(4):483–487. doi: 10.1097/BPO.0b013e318173a923. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin H, Rauch F, Bishop NJ, Montpetit K, Ruck-Gibis J, Travers R, Glorieux FH. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85(5):1846–1850. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 18.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 19.Bajpai A, Kabra M, Gupta N, Sharda S, Ghosh M. Intravenous pamidronate therapy in osteogenesis imperfecta: response to treatment and factors influencing outcome. J Pediatr Orthop. 2007;27(2):225–227. doi: 10.1097/bpo.0b013e3180316d06. [DOI] [PubMed] [Google Scholar]
- 20.Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, Hill SC, Gerber LH, Marini JC. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005;20(6):977–986. doi: 10.1359/JBMR.050109. [DOI] [PubMed] [Google Scholar]
- 21.Sakkers R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2 year randomised placebo-controlled study. Lancet. 2004;363:1427–1431. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 22.Gatti D, Antoniazzi F, Prizzi R, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res. 2005;20:758–763. doi: 10.1359/JBMR.041232. [DOI] [PubMed] [Google Scholar]
- 23.Seikaly MG, Kopanati S, Salhab N, et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. J Pediatr Orthop. 2005;25:786–791. doi: 10.1097/01.bpo.0000176162.78980.ed. [DOI] [PubMed] [Google Scholar]
- 24.Antoniazzi F, Zamboni G, Lauriola S, Donaldi L, Adami S, Tato L. Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. J Pediatr. 2006;149:174–179. doi: 10.1016/j.jpeds.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Letocha AD, Cintas HL, Troendle JF, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005;20:977–986. doi: 10.1359/JBMR.050109. [DOI] [PubMed] [Google Scholar]
- 26.Kok DH, Sakkers RJ, Janse AJ, Pruijs HE, Verbout AJ, Castelein RM, Engelbert RH. Quality of life in children with osteogenesis imperfecta treated with oral bisphosphonates (olpadronate): a 2-year randomized placebo-controlled trial. Eur J Pediatr. 2007;166(11):1155–1161. doi: 10.1007/s00431-006-0399-2. [DOI] [PubMed] [Google Scholar]
- 27.Huang RP, Ambrose CG, Sullivan E, Haynes RJ. Functional significance of bone density measurements in children with osteogenesis imperfecta. J Bone Joint Surg Am. 2006;88(6):1324–1330. doi: 10.2106/JBJS.E.00333. [DOI] [PubMed] [Google Scholar]
- 28.Phillipi CA, Remmington T, Steiner RD (2008) Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 4:CD005088 [DOI] [PubMed]
- 29.Marini JC. Do bisphosphonates make children’s bones better or brittle? N Engl J Med. 2003;349(5):423–426. doi: 10.1056/NEJMp038103. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Mori S, Li J, Kaji Y, Akiyama T, Kawanishi J, Norimatsu H. Long-term effect of incadronate disodium (YM-175) on fracture healing of femoral shaft in growing rats. J Bone Miner Res. 2001;16(3):429–436. doi: 10.1359/jbmr.2001.16.3.429. [DOI] [PubMed] [Google Scholar]
- 31.Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in paediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004;19(11):1779–1786. doi: 10.1359/JBMR.040814. [DOI] [PubMed] [Google Scholar]
- 32.Smith EJ, Little DG, Briody JN, McEvoy A, Smith NC, Eisman JA, Gardiner EM. Transient disturbance in physeal morphology is associated with long-term effects of nitrogen-containing bisphosphonates in growing rabbits. J Bone Miner Res. 2005;20(10):1731–1741. doi: 10.1359/JBMR.050604. [DOI] [PubMed] [Google Scholar]
- 33.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349(5):457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 34.Yoon RS, Hwang JS, Beebe KS. Long-term bisphosphonate usage and subtrochanteric insufficiency fractures: a cause for concern? J Bone Joint Surg Br. 2011;93(10):1289–1295. doi: 10.1302/0301-620X.93B10.26924. [DOI] [PubMed] [Google Scholar]
- 35.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89(3):349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 36.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39(2):224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 37.De Das S, Setiobudi T, Shen L, De Das S. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010;92(5):679–686. doi: 10.1302/0301-620X.92B5.22941. [DOI] [PubMed] [Google Scholar]
- 38.Edwards MH, McCrae FC, Young-Min SA. Alendronate-related femoral diaphysis fracture—what should be done to predict and prevent subsequent fracture of the contra lateral side? Osteoporos Int. 2010;21(4):701–703. doi: 10.1007/s00198-009-0986-y. [DOI] [PubMed] [Google Scholar]
- 39.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22(5):346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 40.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358(12):1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 41.Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop. 2009;80(4):413–415. doi: 10.3109/17453670903139914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305(8):783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 43.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24(6):1095–1102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 44.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362(19):1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 45.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 46.Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, Knowles E, Hill C, Hall C, Chapman S, Sprigg A, Rigby A. A randomized, controlled dose-ranging study of risendronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res. 2010;25(1):32–40. doi: 10.1359/jbmr.090712. [DOI] [PubMed] [Google Scholar]
- 47.Evans KD, Lau ST, Oberbauer AM, Martin RB (2003) Alendronate affects long bone length and growth plate morphology in the oim mouse model for osteogenesis imperfecta. Bone 32(3):268–274 [DOI] [PubMed]
- 48.Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Paediatrics. 2003;111(5 Pt 1):1030–1036. doi: 10.1542/peds.111.5.1030. [DOI] [PubMed] [Google Scholar]
- 49.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110(9):1293–1299. doi: 10.1172/JCI0215952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao SH, Evans KD, Oberbauer AM, Martin RB. Bisphosphonate treatment in the oim mouse model alters bone modelling during growth. J Biomech. 2008;41(16):3371–3376. doi: 10.1016/j.jbiomech.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kashii M, Hashimoto J, Nakano T, Umakoshi Y, Yoshikawa H. Alendronate treatment promotes bone formation with a less anisotropic microstructure during intramembranous ossification in rats. J Bone Miner Metab. 2008;26(1):24–33. doi: 10.1007/s00774-007-0782-8. [DOI] [PubMed] [Google Scholar]
- 52.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 53.Weber M, Roschger P, Fratzl-Zelman N, Schöberl T, Rauch F, Glorieux FH, Fratzl P, Klaushofer K. Pamidronate does not adversely affect bone intrinsic material properties in children with osteogenesis imperfecta. Bone. 2006;39(3):616–622. doi: 10.1016/j.bone.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 54.Uveges TE, Kozloff KM, Ty JM, Ledgard F, Raggio CL, Gronowicz G, Goldstein SA, Marini JC. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res. 2009;24(5):849–859. doi: 10.1359/jbmr.081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shahnazari M, Yao W, Dai W, Bob Wang B, Ionova-Martin SS, Ritchie RO, Heeren D, Burghardt AJ, Nicolella DP, Kimiecik MG, Lane NE. Higher doses of bisphosphonates further improve bone mass, architecture, and strength but not the tissue material properties in aged rats. Bone. 2010;46(5):1267–1274. doi: 10.1016/j.bone.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


