Abstract
Thermal ablation using radiofrequency is a new, minimally invasive modality employed as an alternative to surgery in patients with benign thyroid nodules and recurrent thyroid cancers. The Task Force Committee of the Korean Society of Thyroid Radiology has developed recommendations for the optimal use of radiofrequency ablation for thyroid nodules. These recommendations are based on a comprehensive analysis of the current literature, the results of multicenter studies, and expert consensus.
Keywords: Thyroid, radiofrequency; Thyroid, ethanol; Thyroid, US; Thyroid, nodules; Thyroid, recurrent cancers; Thyroid, intervention
INTRODUCTION
Thermal ablation using radiofrequency (RF) is a new, minimally invasive modality that may be an alternative to surgery in patients with benign thyroid nodules. RF ablation has shown efficacy and safety in the treatment of thyroid nodules (1-8). In 2009, the Korean Society of Thyroid Radiology (KSThR), an organization of thyroid radiologists in Korea primarily involved in the diagnosis and treatment of thyroid nodules, proposed the first set of recommendations for RF ablation of thyroid nodules (9). These recommendations were formulated by an organized task force committee that included specialists of thyroid RF ablation in Korea. These recommendations included only patient selection and the efficacy of RF ablation. Because new information has become available since 2009 from clinical studies of RF ablation in patients with benign thyroid nodules and recurrent thyroid cancers, the task force committee members suggested the need to revise earlier recommendations. The KSThR therefore organized a committee to revise these earlier recommendations, and this committee prepared recommendations for RF ablation of thyroid nodules and recurrent thyroid cancers on June 3, 2011. The revised recommendations include sections dealing with indications for RF ablation, pre-procedural evaluations, RF ablation procedures, post-procedural monitoring, efficacy, and safety. A PubMed Medline search was performed with the keywords "radiofrequency" and "thyroid" up to October 2011. Because there are only a few controlled clinical studies (6, 10) of RF ablation, the recommendations regarding some issues are based on expert opinions. This limitation needs to be overcome in the future by further investigations.
The goal of these recommendations is to provide the best scientific evidence available and a consensus expert opinion regarding the use of RF ablation of the thyroid in clinical practice.
INDICATIONS
Radiofrequency ablation can be used to treat both benign thyroid nodules and inoperable, recurrent thyroid cancers in the operation bed and lymph nodes (1-8, 10-17). The KSThR does not recommend thyroid RF ablation for follicular neoplasms or primary thyroid cancers because there is no evidence of treatment benefit by RF ablation in follicular neoplasms or primary thyroid cancers (2, 18). Caution should be taken in regard to the use of thyroid RF ablation in pregnant women, patients with serious heart problems, and those with contralateral vocal cord palsy (19-23).
1. Benign thyroid nodules
Indications for RF ablation of benign thyroid nodules include patients with nodule-related clinical problems (2-5, 12, 14):
1) Symptom (neck pain, dysphasia, foreign body sensation, discomfort, and cough) score can be self-measured by patients using a 10 cm visual analogue scale (grade 0-10) (6, 7, 24)
2) Cosmetic score (1, 4, 6, 7, 24) can be measured by a physician (6-8). Cosmetic score (1, no palpable mass; 2, no cosmetic problem but palpable mass; 3, a cosmetic problem on swallowing only; and 4, a readily detected cosmetic problem)
3) Patients with autonomously functioning thyroid nodules (AFTN) causing problems related to thyrotoxicosis (1, 4, 5, 12, 25-29)
Patients with nodules with a maximum diameter > 2 cm that continue to grow, may be considered for thyroid RF ablation based on symptoms and clinical concerns. We recommend that patients with cystic thyroid nodules that re-grow after simple aspiration should be treated first with ethanol ablation (EA) rather than RF ablation (7, 8, 24, 30, 31). The efficacy and complications were similar in both EA and RF ablation groups, but the number of treatment sessions was fewer in the EA group. In addition, EA is a simple and less expensive procedure (8).
2. Recurrent thyroid cancers (operation bed and lymph nodes)
Surgery is a standard treatment for recurrent thyroid cancers, followed by radioactive iodine and thyroid hormone therapy. RF ablation, however, can be used in patients at high surgical risk and in patients who refuse to undergo repeated surgery (11, 13, 16, 17).
PRE-PROCEDURAL EVALUATIONS
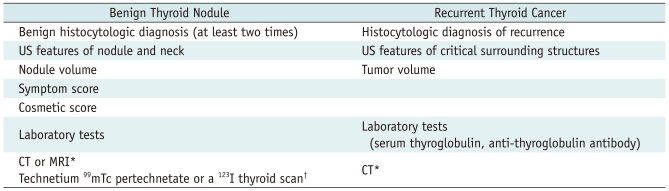
Prior to treatment of benign thyroid nodules (Table 1), thyroid nodules should be confirmed as benign on at least two separate ultrasound (US)-guided fine needle aspiration and/or core needle biopsy (32, 33). Caution should be taken in performing the RF ablation of thyroid nodules with malignant US features, even though there are benign results on fine needle aspiration or core needle biopsy (2, 34-40). Symptom and cosmetic scores should be determined before RF ablation for comparison with follow-up data (3, 6-8, 12, 24, 41).
Table 1.
Preprocedural Check List for RF Ablation
Note.-*Selectively indicated, †Indicated for AFTN. RF = radiofrequency, US = ultrasound
Ultrasound examination is important to characterize the nodule and evaluate the critical surrounding anatomic structures (2). The size, characteristics, proportion of solid component, and internal vascularity of each nodule should be evaluated. Three orthogonal nodule diameters, including the largest diameter, should be measured by US, and nodule volume could be calculated using the equation: V = πabc/6, where V is the volume, a is the maximum diameter, and b and c are the other two perpendicular diameters. (2, 6, 8)
Laboratory tests usually include a complete blood count, a blood coagulation battery, as well as measurements of thyrotrophin, thyroid hormones, thyroid auto-antibodies, and calcitonin concentrations (6, 42). If any serum concentrations are abnormal, RF ablation should be performed only after performing procedures to correct these abnormal test results. When platelets and blood coagulation test results are abnormal, we should document whether a patient has taken medications such as anti-platelets and anticoagulants or has any disease causing coagulopathy. When serum concentration of thyrotropin is elevated, further tests should be performed to document whether a patient has hypothyroidism. When serum concentration of thyrotrophin is reduced, we should document whether a patient is being treated with thyroid hormones or has hyperthyroidism. If a thyroid hormone was prescribed to control the growth of thyroid nodules, we recommend that this drug be stopped before RF ablation.
If hyperthyroidism or AFTN is suggested, a technetium 99mTc pertechnetate or a 123I thyroid scan may be helpful for diagnosis (5, 12, 43). When serum concentration of calcitonin is elevated, an additional thorough assessment of thyroid nodules is necessary before RF ablation therapy because high serum calcitonin may suggest a medullary carcinoma (25, 29, 44). If the concentrations of thyroid antibodies are elevated before ablation, patients should be told about the possibility of hypothyroidism after the procedure. It remains unclear, however, whether hypothyroidism is caused by RF ablation or thyroid antibodies (12, 42, 45). CT or MRI examinations may help evaluate intrathoracic thyroid nodules.
Prior to treatment of recurrent thyroid cancers (Table 1), tumor recurrence should be confirmed by US-guided fine needle aspiration (16). US-guided fine needle aspiration cytology and measurements of washout thyroglobulin (Tg) concentration are methods diagnostic for recurrent thyroid cancers (16, 46-50). US examination is important to evaluate the recurred tumor and the critical surrounding structures. The size, volume, and vascularity of the recurred tumor should be assessed by US (11, 13, 16, 17). Laboratory tests usually include a complete blood count, a blood coagulation battery, and measurements of thyrotrophin, thyroid hormones, thyroglobulin, and anti-thyroglobulin antibody (6, 11, 13, 16, 17, 42). A neck CT may be selectively used for the evaluation of a recurrent tumor prior to RF ablation.
Informed consent forms should include the following content (2):
1) Ablated thyroid nodules decrease slowly in size for several months to years
2) Number of expected treatment sessions
3) Possibility of regrowth of the treated nodule and the need for additional treatment
4) Patients may experience various degrees of pain during the ablation
5) Complications of RF ablation
6) Patients should inform the physician about their history of thyroid surgery, the side effects of any drugs they are taking, and whether they are taking drugs such as antiplatelets, anticoagulants and thyroid hormones.
7) Further observation or admission may be required after RF ablation, depending on the patients' condition after ablation.
Patients taking drugs associated with a bleeding tendency should be told to discontinue those drugs before RF ablation: 7-10 days for aspirin or clopidogrel, 3-5 days for warfarin and 4-6 hours for heparin. Patients can take heparin 2-6 hours after RF ablation, warfarin on the night after the procedure and aspirin (or clopidogrel) the next day (51). However, physicians should compare the benefits of RF ablation with its potential complications related to the interruption of these drugs. If required, patients should consider changing warfarin to heparin, which has a shorter half-life (1-2 hours) (51). Moreover, patients should fast for at least 6 hours before each procedure. We recommend that a venous line be installed before the procedure for drug delivery.
PROCEDURE, MONITORING AND FOLLOW-UP
We recommend that RF ablation be performed using a 'trans-isthmic approach' and a 'moving shot technique' under local anesthesia because these techniques are useful for the safe and effective RF ablation procedure (2, 6, 12, 41, 52). A modified, straight, internally cooled electrode is suitable for use in the moving shot technique (12, 41).
Physicians should monitor blood pressure, pulse rate and the voice of each patient by regular conversations during the procedure. If a voice change is suspected, the physician should stop the procedure immediately (41, 53). Although pain was found to be the most common complaints during RF ablation, it was relieved rapidly when the generator output was reduced or turned off (2, 6, 12, 41, 53). Painkillers and sedatives can relieve pain during the ablation (1), but we do not recommend the painkillers or sedatives during the ablation because the early detection of complications is impossible in patients under deep sedation, which disturbs communication.
Ultrasound monitoring is important for detecting hemorrhage (2, 41). If a hematoma is too large or pain is too severe due to hemorrhage, manual compression may be helpful, allowing the procedure to continue. Hematomas usually disappear completely within 2 weeks. When a large hematoma develops, the RF procedure should be delayed for 1-2 weeks (41).
Patient condition should be monitored after the procedure (2, 41). Patients with severe pain, edema, skin burn, vomiting, dyspnea, or voice change should be continuously monitored. The decision to admit a patient should be at the discretion of each physician (42).
During the follow-up periods, painkillers and steroids can be used to relieve symptoms (42). Color-Doppler US is a primary imaging modality during follow-up. In addition, patients can be monitored by CT, MRI, thyroid scans, and thyroid function tests. Color-Doppler US is useful for detecting any undertreated or regrowing portion of the ablated nodules (2, 7, 12, 41). We recommend that patients be followed-up at 1-2, 6 and 12 months after RF ablation, as well as every 6-12 months thereafter, depending on the status of the treated nodules (2, 6, 12, 41). Additional treatments may be indicated in patients with incompletely resolved clinical concerns or if a viable growing portion of the nodule is detected on US (2, 41, 52).
EFFICACY
Efficacy of RF ablation for benign thyroid nodules can be evaluated by symptom score, cosmetic score, % volume reduction ([initial volume - final volume] × 100/initial volume) (2, 6, 12), and therapeutic success rate (volume reduction > 50%) (8). Color-Doppler US is helpful for the evaluation of presence of vascularity within the ablated nodule, which suggests a possible undertreated area of a nodule (41, 52). For AFTN, we additionally recommend evaluation with a thyroid scan, serum thyroid hormone, and thyrotrophin concentration (1, 4, 12). The efficacy of RF ablation for recurrent thyroid cancers can be evaluated by % volume reduction and serum thyroglobulin concentrations (11, 13, 16, 17).
Radiofrequency ablation cannot remove thyroid nodules immediately; rather, it induces the necrosis and involution of thyroid nodules, resulting in volume reduction and improvements in clinical symptoms, with the efficacy of RF ablation similar to that of thyroid surgery (2-4). Hyperthyroidism caused by AFTN can be cured or improved by RF ablation (1, 4, 12). Although most patients have shown improvements after a single session of RF ablation, others may require several sessions to achieve complete ablation (2-4, 6, 12, 14). The reduction in nodule volume after RF ablation has been found to range from 33-58% at one month and from 51% to 92% at six months (1-4, 6, 7, 12, 54). Laser ablation has been used for the treatment of benign thyroid nodules (18, 26, 42, 55-63). When we compared the efficacy of two treatment tools, the efficacy of RF ablation appears to be slightly superior to that of laser ablation (10, 41, 54). RF ablation can be used for locoregional control of cancer or improvement of cancer related symptoms in patients with recurrent thyroid cancer who have a high surgical risk and refuse repeated surgery (11, 13, 17). RF ablation of recurrent thyroid cancers in the neck resulted in a mean volume reduction of 56% to 93% (13, 16), with 42% to 58% of nodules completely disappearing (11, 16, 17), 64% of patients experiencing symptom improvement (13), and serum thyroglobulin concentration decreasing (11, 13, 16, 17). However, long-term follow-up data has not been published yet.
SAFETY
Physicians who perform thyroid RF ablation should understand the broad spectrum of complications and the steps they can take to prevent or minimize complications and sequelae (52, 53). Reported complications of thyroid RF ablation include pain, hemorrhage, voice change, skin burn, hypothyroidism, hyperthyroidism, infection and nodule rupture (1-4, 6, 9, 12). RF ablation does not require general anesthesia, does not induce scarring of the neck, and when performed by well-trained physicians, is associated with a low complication rate (1-6).
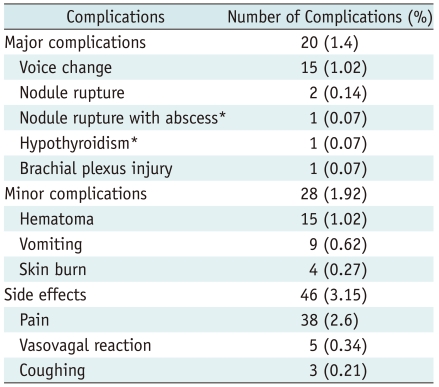
In November 2008, the KSThR organized a task force to evaluate the complications and side effects of thyroid RF ablation of benign nonfunctioning thyroid nodules (53). This task force collected data on complications from 13 hospitals in Korea and evaluated them according to the guidelines of the Society of Interventional Radiology (65, 66). These complications and side effects are listed in Table 2 (53). Complications were reported in 48 patients (3.3%) and major complications in 20 (1.4%). Among the reported sequelae are hypothyroidism in one patient and abscess formation and tumor rupture in one (53). During the ablation, most patients complain of various degrees of pain in the neck and/or radiating to the head, ear, shoulders, chest, back, or teeth. However, pain decreases rapidly when the generator output is reduced or turned off (2, 6, 41, 53). We recommend that patients may be prescribed painkillers for 2-3 days to reduce post-procedural pain (41).
Table 2.
Complications and Side Effects of Thyroid RF Ablation from Multicenter Study of Korean Society of Thyroid Radiology (53)
Note.-Number in parentheses is percentage of complications of all patients. *Complications with remaining sequela. RF = radiofrequency
Voice change is a major complication of RF ablation, caused by damage to the recurrent laryngeal or vagus nerve (67, 68). In most cases, voice change is detected during or immediately after ablation. Voice changes are usually transient, with most patients recovering within 3 months (41, 53). To prevent voice change, we recommend that RF ablation should be performed using the 'trans-isthmic approach' and the 'moving shot technique'. Using these techniques, unit-by-unit ablation of conceptual ablation units by moving the electrode and undertreatment of the danger triangle (i.e. the area of the thyroid nodule adjacent to the recurrent laryngeal nerve), may minimize voice changes (41, 52, 53). The cervical portion of the vagus nerve is located within the carotid sheath, usually between the common carotid artery and internal jugular vein, however a bulging large thyroid nodule may alter the location of the vagus nerve, making it closer to the thyroid nodule (67-70). We recommend checking the location of the vagus nerve before RF ablation. There is a potential risk of a sympathetic ganglion damage. If extrathyroidal penetration of a electrode is not detected during the ablation procedure, it may induce ablation injury of sympathetic ganglion because it is located posterior to common carotid artery near the thyroid gland. To avoid this complication, continuous and cautious US-guided tracing of the electrode tip is mandatory during the RF ablation.
Hematomas, resulting from electrode-induced mechanical injury, can occur in the perithyroidal, supcapsular, and intranodular locations (53). Hematomas can usually be controlled by mild compression of the neck for several minutes, with most hematomas disappearing within 1 or 2 weeks (41). Since serious hematomas may compress the airways, close observation is recommended during and after procedure. Serious hemorrhage may be prevented by careful monitoring of the electrode tip (41).
Transient thyrotoxicosis may occur after RF ablation, but it is usually normalized within one month (2). Hypothyroidism has been reported in patients with nonfunctioning thyroid nodules and those with AFTN (12, 53). Consequently, thyroid function tests are recommended during follow-up for these patients (2, 3, 12, 42, 53). To prevent an infection or abscess, the puncture site should be sterilized before RF ablation and prophylactic antibiotics can be used.
Thyroid nodule rupture after RF ablation may be suspected in patients complaining of sudden neck bulging and pain at the treatment site (41, 53, 71). Prior to nodule rupture, the ablated thyroid nodule gradually decreases in size. US examination usually shows a breakdown of the anterior thyroid capsule and the formation of a new nodule in the anterior neck. Delayed sudden perinodular hemorrhage might be a cause of nodule rupture. Spontaneous improvement without treatment is possible, but surgery is required when an abscess forms (41, 53, 71).
During thyroid RF ablation, skin burns have been reported only at the electrode puncture site (3, 13, 53). Application of an ice bag during the ablation may prevent skin burns at the electrode puncture site. The risk of burns at the pad attachment site is relatively low, because RF energy is lower in the thyroid than in the liver (53). Nausea and vasovagal reflection may be caused by severe tension, pain, or hypersensitivity to lidocaine (53).
Life-threatening complications, including injury to the trachea (72) and heart attack (19, 20, 22, 23, 73), have been reported in patients undergoing RF ablation of tumors other than those of the thyroid gland. However, these complications, as well as esophageal rupture, have not been reported yet. Coughing can be induced by thermal propagation to the trachea and is managed by stopping the ablation (41, 53). To prevent thermal injury to the esophagus, patients should be asked to swallow cold water during the ablation of a conceptual unit adjacent to the esophagus (19, 21, 41, 53).
To prevent life-threatening complications, the operators should strictly trace the electrode tip during the procedure, and should have knowledge of the neck's anatomy and experience of an image-guided intervention.
CONCLUSION
Thyroid RF ablation is an effective and safe treatment modality in patients with benign thyroid nodules. RF ablation may be as effective as surgery if it is performed by experienced physicians in optimally selected patients. RF ablation may also have an effective complementary role in the management of recurrent thyroid cancers.
Acknowledgments
The authors thank Eun Ju Ha for her assistance in the writing of this manuscript.
References
- 1.Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34:784–791. doi: 10.1016/j.ultrasmedbio.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250. doi: 10.1007/s00330-008-0880-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16:361–367. doi: 10.1089/thy.2006.16.361. [DOI] [PubMed] [Google Scholar]
- 4.Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19:219–225. doi: 10.1089/thy.2008.0202. [DOI] [PubMed] [Google Scholar]
- 5.Baek JH, Jeong HJ, Kim YS, Kwak MS, Lee D. Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid. 2008;18:675–676. doi: 10.1089/thy.2007.0274. [DOI] [PubMed] [Google Scholar]
- 6.Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194:1137–1142. doi: 10.2214/AJR.09.3372. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA) World J Surg. 2010;34:1488–1493. doi: 10.1007/s00268-010-0565-6. [DOI] [PubMed] [Google Scholar]
- 8.Sung JY, Kim YS, Choi H, Lee JH, Baek JH. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol. 2011;196:W210–W214. doi: 10.2214/AJR.10.5172. [DOI] [PubMed] [Google Scholar]
- 9.Baek JH, Na DG, Lee JH, Jung SL, Sung JY, Sim J, et al. Korean society of thyroid radiology recommendations for radiofrequency ablation of thyroid nodules. 2009. Available at: http://thyroidimaging.kr/
- 10.Huh JY, Baek JH, Choi H, Kim JK, Lee JH. Efficacy of additional treatment session of radiofrequency ablation for symptomatic benign thyroid nodules: A prospective randomized study. Radiology. 2012 doi: 10.1148/radiol.12111300. In press. [DOI] [PubMed] [Google Scholar]
- 11.Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244:296–304. doi: 10.1097/01.sla.0000217685.85467.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33:1971–1977. doi: 10.1007/s00268-009-0130-3. [DOI] [PubMed] [Google Scholar]
- 13.Park KW, Shin JH, Han BK, Ko EY, Chung JH. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol. 2011;18:2564–2568. doi: 10.1245/s10434-011-1619-1. [DOI] [PubMed] [Google Scholar]
- 14.Spiezia S, Garberoglio R, Di Somma C, Deandrea M, Basso E, Limone PP, et al. Efficacy and safety of radiofrequency thermal ablation in the treatment of thyroid nodules with pressure symptoms in elderly patients. J Am Geriatr Soc. 2007;55:1478–1479. doi: 10.1111/j.1532-5415.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- 15.Sung JY, Baek JH, Kim YS, Jeong HJ, Kwak MS, Lee D, et al. One-step ethanol ablation of viscous cystic thyroid nodules. AJR Am J Roentgenol. 2008;191:1730–1733. doi: 10.2214/AJR.08.1113. [DOI] [PubMed] [Google Scholar]
- 16.Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W331–W336. doi: 10.2214/AJR.10.5345. [DOI] [PubMed] [Google Scholar]
- 17.Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001;130:971–977. doi: 10.1067/msy.2001.118708. [DOI] [PubMed] [Google Scholar]
- 18.Papini E, Guglielmi R, Gharib H, Misischi I, Graziano F, Chianelli M, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21:917–920. doi: 10.1089/thy.2010.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 20.Rhim H, Dodd GD, 3rd, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24:41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 21.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123–134. doi: 10.1148/rg.231025054. discussion 134-136. [DOI] [PubMed] [Google Scholar]
- 22.Tong NY, Ru HJ, Ling HY, Cheung YC, Meng LW, Chung PC. Extracardiac radiofrequency ablation interferes with pacemaker function but does not damage the device. Anesthesiology. 2004;100:1041. doi: 10.1097/00000542-200404000-00053. [DOI] [PubMed] [Google Scholar]
- 23.Nemcek AA. Complications of radiofrequency ablation of neoplasms. Semin Intervent Radiol. 2006;23:177–187. doi: 10.1055/s-2006-941448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang SW, Baek JH, Kim JK, Sung JY, Choi H, Lim HK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: Role of radiofrequency ablation. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.02.039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16(Suppl 1):1–43. doi: 10.4158/10024.GL. [DOI] [PubMed] [Google Scholar]
- 26.Døssing H, Bennedbaek FN, Hegedüs L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid. 2003;13:885–888. doi: 10.1089/105072503322401104. [DOI] [PubMed] [Google Scholar]
- 27.Fassi J, Lambertini R, Farias P, Blejman O, Rosa Diez G, Algranati S, et al. Treatment of uremic hyperparathyroidism with percutaneous ethanol injection. Nephron Clin Pract. 2005;101:c53–c57. doi: 10.1159/000086222. [DOI] [PubMed] [Google Scholar]
- 28.Guglielmi R, Pacella CM, Bianchini A, Bizzarri G, Rinaldi R, Graziano FM, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid. 2004;14:125–131. doi: 10.1089/105072504322880364. [DOI] [PubMed] [Google Scholar]
- 29.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 30.Bennedbaek FN, Hegedüs L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–5777. doi: 10.1210/jc.2003-031000. [DOI] [PubMed] [Google Scholar]
- 31.Kim DW, Rho MH, Kim HJ, Kwon JS, Sung YS, Lee SW. Percutaneous ethanol injection for benign cystic thyroid nodules: is aspiration of ethanol-mixed fluid advantageous? AJNR Am J Neuroradiol. 2005;26:2122–2127. [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, Son EJ, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010;254:292–300. doi: 10.1148/radiol.2541090460. [DOI] [PubMed] [Google Scholar]
- 33.Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. Value of repeated fine needle aspirations of the thyroid: an analysis of over ten thousand FNAs. Thyroid. 2007;17:1061–1066. doi: 10.1089/thy.2007.0159. [DOI] [PubMed] [Google Scholar]
- 34.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 36.Jung SL, Jung CK, Kim SH, Kang BJ, Ahn KJ, Kim BS, et al. Histopathologic findings related to the indeterminate or inadequate results of fine-needle aspiration biopsy and correlation with ultrasonographic findings in papillary thyroid carcinomas. Korean J Radiol. 2010;11:141–148. doi: 10.3348/kjr.2010.11.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 38.Lee YH, Kim DW, In HS, Park JS, Kim SH, Eom JW, et al. Differentiation between benign and malignant solid thyroid nodules using an US classification system. Korean J Radiol. 2011;12:559–567. doi: 10.3348/kjr.2011.12.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Algin O, Algin E, Gokalp G, Ocakogğlu G, Erdogğan C, Saraydaroglu O, et al. Role of duplex power Doppler ultrasound in differentiation between malignant and benign thyroid nodules. Korean J Radiol. 2010;11:594–602. doi: 10.3348/kjr.2010.11.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SH, Park CS, Jung SL, Kang BJ, Kim JY, Choi JJ, et al. Observer variability and the performance between faculties and residents: US criteria for benign and malignant thyroid nodules. Korean J Radiol. 2010;11:149–155. doi: 10.3348/kjr.2010.11.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baek JH, Lee JH, Valcavi R, Pacella CM, Rhim H, Na DG. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540. doi: 10.3348/kjr.2011.12.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20:1253–1261. doi: 10.1089/thy.2010.0189. [DOI] [PubMed] [Google Scholar]
- 43.Burch HB, Shakir F, Fitzsimmons TR, Jaques DP, Shriver CD. Diagnosis and management of the autonomously functioning thyroid nodule: the Walter Reed Army Medical Center experience, 1975-1996. Thyroid. 1998;8:871–880. doi: 10.1089/thy.1998.8.871. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Kim BS, Jung SL, Lee JW, Yang PS, Kang BJ, et al. Ultrasonographic findings of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Korean J Radiol. 2009;10:101–105. doi: 10.3348/kjr.2009.10.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monzani F, Caraccio N, Goletti O, Lippolis PV, Casolaro A, Del Guerra P, et al. Five-year follow-up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: a study of 117 patients. Clin Endocrinol (Oxf) 1997;46:9–15. doi: 10.1046/j.1365-2265.1997.d01-1752.x. [DOI] [PubMed] [Google Scholar]
- 46.Jeon SJ, Kim E, Park JS, Son KR, Baek JH, Kim YS, et al. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: correlations with US features. Korean J Radiol. 2009;10:106–111. doi: 10.3348/kjr.2009.10.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heilo A, Sigstad E, Fagerlid KH, Håskjold OI, Grøholt KK, Berner A, et al. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2750–2755. doi: 10.1210/jc.2010-2952. [DOI] [PubMed] [Google Scholar]
- 48.Kim MJ, Kim EK, Kim BM, Kwak JY, Lee EJ, Park CS, et al. Thyroglobulin measurement in fine-needle aspirate washouts: the criteria for neck node dissection for patients with thyroid cancer. Clin Endocrinol (Oxf) 2009;70:145–151. doi: 10.1111/j.1365-2265.2008.03297.x. [DOI] [PubMed] [Google Scholar]
- 49.Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006;91:1364–1369. doi: 10.1210/jc.2005-1705. [DOI] [PubMed] [Google Scholar]
- 50.Baloch ZW, Barroeta JE, Walsh J, Gupta PK, Livolsi VA, Langer JE, et al. Utility of Thyroglobulin measurement in fine-needle aspiration biopsy specimens of lymph nodes in the diagnosis of recurrent thyroid carcinoma. Cytojournal. 2008;5:1. doi: 10.1186/1742-6413-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwok A, Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009;104:3085–3097. doi: 10.1038/ajg.2009.469. quiz 3098. [DOI] [PubMed] [Google Scholar]
- 52.Ha EJ, Baek JH, Lee JH. The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2011;18:310–314. doi: 10.1097/MED.0b013e32834a9168. [DOI] [PubMed] [Google Scholar]
- 53.Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342. doi: 10.1148/radiol.11110416. [DOI] [PubMed] [Google Scholar]
- 54.Wallace LB, Berber E. Percutaneous and video-assisted ablation of endocrine tumors: liver, adrenal, and thyroid. Surg Laparosc Endosc Percutan Tech. 2011;21:255–259. doi: 10.1097/SLE.0b013e3182266f52. [DOI] [PubMed] [Google Scholar]
- 55.Døssing H, Bennedbaek FN, Bonnema SJ, Grupe P, Hegedüs L. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol. 2007;157:95–100. doi: 10.1530/EJE-07-0094. [DOI] [PubMed] [Google Scholar]
- 56.Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules - a randomised study. Eur J Endocrinol. 2005;152:341–345. doi: 10.1530/eje.1.01865. [DOI] [PubMed] [Google Scholar]
- 57.Døssing H, Bennedbaek FN, Hegedüs L. Beneficial effect of combined aspiration and interstitial laser therapy in patients with benign cystic thyroid nodules: a pilot study. Br J Radiol. 2006;79:943–947. doi: 10.1259/bjr/40698061. [DOI] [PubMed] [Google Scholar]
- 58.Døssing H, Bennedbæk FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165:123–128. doi: 10.1530/EJE-11-0220. [DOI] [PubMed] [Google Scholar]
- 59.Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules: one versus three treatments. Thyroid. 2006;16:763–768. doi: 10.1089/thy.2006.16.763. [DOI] [PubMed] [Google Scholar]
- 60.Døssing H, Bennedbaek FN, Karstrup S, Hegedüs L. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation--initial experience. Radiology. 2002;225:53–57. doi: 10.1148/radiol.2251011042. [DOI] [PubMed] [Google Scholar]
- 61.Pacella CM, Bizzarri G, Guglielmi R, Anelli V, Bianchini A, Crescenzi A, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217:673–677. doi: 10.1148/radiology.217.3.r00dc09673. [DOI] [PubMed] [Google Scholar]
- 62.Papini E, Guglielmi R, Bizzarri G, Pacella CM. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract. 2004;10:276–283. doi: 10.4158/EP.10.3.276. [DOI] [PubMed] [Google Scholar]
- 63.Spiezia S, Vitale G, Di Somma C, Pio Assanti A, Ciccarelli A, Lombardi G, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid. 2003;13:941–947. doi: 10.1089/105072503322511346. [DOI] [PubMed] [Google Scholar]
- 64.Hegedüs L. Therapy: a new nonsurgical therapy option for benign thyroid nodules? Nat Rev Endocrinol. 2009;5:476–478. doi: 10.1038/nrendo.2009.152. [DOI] [PubMed] [Google Scholar]
- 65.Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243–S246. [PubMed] [Google Scholar]
- 66.Lewis CA, Allen TE, Burke DR, Cardella JF, Citron SJ, Cole PE, et al. Quality improvement guidelines for central venous access. The Standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol. 1997;8:475–479. doi: 10.1016/s1051-0443(97)70592-x. [DOI] [PubMed] [Google Scholar]
- 67.Park JK, Jeong SY, Lee JH, Lim GC, Chang JW. Variations in the course of the cervical vagus nerve on thyroid ultrasonography. AJNR Am J Neuroradiol. 2011;32:1178–1181. doi: 10.3174/ajnr.A2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ha EJ, Baek JH, Lee JH, Kim JK, Shong YK. Clinical significance of vagus nerve variation in radiofrequency ablation of thyroid nodules. Eur Radiol. 2011;21:2151–2157. doi: 10.1007/s00330-011-2167-6. [DOI] [PubMed] [Google Scholar]
- 69.Gibson A. Bilateral Abnormal Relationship of the Vagus Nerve in its Cervical Portion. J Anat Physiol. 1915;49:389–392. [PMC free article] [PubMed] [Google Scholar]
- 70.Giovagnorio F, Martinoli C. Sonography of the cervical vagus nerve: normal appearance and abnormal findings. AJR Am J Roentgenol. 2001;176:745–749. doi: 10.2214/ajr.176.3.1760745. [DOI] [PubMed] [Google Scholar]
- 71.Shin JH, Jung SL, Baek JH, Kim JH. Rupture of benign thyroid tumors after radio-frequency ablation. AJNR Am J Neuroradiol. 2011;32:2165–2169. doi: 10.3174/ajnr.A2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi JW, Kwak SH, Yoo SM, Song IS, Lee HY, Lee JB, et al. Ultrasound-guided radiofrequency ablation of thyroid gland: a preliminary study in dogs. J Korean Radiol Soc. 2005;52:333–341. [Google Scholar]
- 73.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]