Abstract
Gut microbiota have recently been implicated in the pathogenesis of the obesity and its related metabolic diseases. Avariety of factors including diet, genetic background, environment and host innate and adaptive immune responses define an individual’s gut microbiota. In this review we outline potential mechanisms by which gut microbiota can contribute to the development of obesity focusing on specific processes such as microbial energy extraction, microbiota induced-inflammation and regulation of appetite. We review the current understanding of each of these processes on regulating metabolism and examine potential therapeutic strategies for the treatment or prevention of the metabolic syndrome. We explore the hypothesis that alteration in gut microbiota may be an initial event leading to altered feeding behavior and/or systemic inflammation, ultimately leading to weight gain and the metabolic syndrome.
Obesity is a growing epidemic in many developed and developing countries including the United States (1, 2). The prevalence of obesity in adults has increased more than 75% since 1980 with currently more than half of the US population is overweight (2). At its simplest level, obesity stems from an alteration in energy balance that can result from increased energy intake and/or reduced expenditure thus favoring energy storage. Factors that can promote this imbalance are widely appreciated to include genetics, high-fat food, high-fructose diet, and physical inactivity (3, 4). Less appreciated, and the focus of this brief review, is the possibility that the gut microbiota might play a role in the pathophysiology of obesity (5–7).
The human gastrointestinal (GI) tract harbors a large diverse community of bacteria collectively referred to as the gut microbiota, which is increasingly appreciated to play an intricate role in health and well-being, particularly in terms of digestive health and immune function (8). The study of the entire microbial communities using metagenomics approaches estimates that gastrointestinal tract in an adult human contains approximately 1012 micro-organisms per milliliter of luminal content and harbors approximately 15, 000 species of bacteria (9). Amongst these species, Bacteriodetes and Firmicutes, account for more than 90% of all the phylotypes of colonic bacteria. Methanobrevibacter Smithi is a hydrogen consuming methanogen that dominates the Archae domain of gut microbiota. The gut microbiome is established during the first year of life and is influenced by multiple host and external factors including diet and antibiotics. Recent studies show that obese and lean people are different in their gut microbiota composition, specifically in the proportion of Bacteriodetes and Firmicutes. In obese people the Firmicutes are dominant and when they lose weight, the proportion of Firmicutes become more like lean people (10). Some studies have demonstrated that gut microbiota have a substantial correlation with obesity through regulation of metabolism (5, 7, 10, 11). This review explores the recent advances in understanding the role of gut microbiota in pathogenesis of obesity and regulation of satiety. Specifically, we review some of the major processes by which gut microbiota can influence weight gain, including modulation of the inflammatory response to microbiota, nutrient extraction of calories by microbiota and also microbial influence of satiety through the brain-gut axis.
Role of Gut Microbiota in Energy Harvesting
Gut microbiota are involved in energy harvest through breaking down complex dietary macromolecules, synthesizing micronutrients, fermenting indigestible food substances, assisting in absorption of electrolytes, and growth and differentiation of the intestinal and colonic epithelium through regulating the diverse aspects of cellular differentiation and gene expression (8, 12). Such bioactivities result in the microbiota regulating nutrient acquisition and energy extraction. The gut microbiota provides enzymes involved in the utilization of non-digestible carbohydrates, cholesterol reduction and biosynthesis of vitamins (K and B group), isoprenoids and amino acids (e.g. lysine and threonine) (8, 12). In particular, the ability of the commensal microbiota to utilize complex dietary polysaccharides which would otherwise be inaccessible to human subjects, to generate short-chain fatty acids (SCFA) seems to contribute to the ability of the host to harvest energy from the diet (5). In addition, the commensal microbiota and its metabolites regulate the expression of genes involved in the processing and absorption of dietary carbohydrates and complex lipids in the host adipose tissue, favoring fat storage. Gut microbiota can also modulate serum lipids by taking part in bile acid metabolism (5, 13, 14).
Gut microbiota can facilitate extraction of calories from ingested dietary substances that can then be stored as adipose tissue or provide nutrients for microbial growth. The greater the energy extraction efficiency of the microbiota the more predisposition an individual may have towards the development of obesity (15). Studies conducted in germ-free animals, indicate gut microbiota have a profound influence on the onset and progression of human disease such as obesity and particularly, energy regulation and fat storage (5). This is thought to be achieved by diverse mechanisms including improvement of diet macronutrient utilization, generation of metabolites involved in energy balance and regulation of host gene expression. Backhed et al. demonstrated that conventionally reared mice have a 40% higher body fat content than germ-free mice even though they consume less food than their germ-free counterparts (5). Colonization of germ-free mice with microbiota from normal mice resulted in a 60 % increase in total body fat (5, 6). The microbial colonization increased the host’s ability to both harvest energy from the diet and store this energy in adipocytes. Specifically, the microbiota promoted absorption of monosacharrides from the gut through activation of the signaling protein ChREBP. There was also an increase in hepatic lipogenesis due to an increase in the expression of LPL and SREBP-1, genes influencing fatty acid synthesis. Microbiota from genetically obese leptin deficient mice, the ob/ob mice, contained genes encoding enzymes that break down indigestible dietary polysaccharides. Transferring the bacteria from the ob/ob mice to germ free mice led to greater fat gain compared to mice receiving microbiota from the lean mice (11).
Thus, by fermentation of indigestible dietary polysaccharides, increased intestinal absorption of monosaccharides and short chain fatty acids and increased hepatic lipogenesis, microbiota can influence the development of obesity. In a study examining energy harvesting in lean and obese individuals fed varied caloric diets, it was noted that an increase energy harvest correlated with the predominance of a Firmicutes and a reduction in Bacteriodetes, demonstrating the cross talk between the nutrients and flora ultimately modulating nutrient assimilation (16).
Methanogenic Archaea can also contribute to increased energy extraction. In a study by Samuel and Gordon they found that colonization with Methanobrevibacter Smithii increased the efficiency of energy extraction from dietary polysaccharides and consequent adiposity (17). Thus M. Smithii could be a potential therapeutic target for reducing energy harvesting in obese humans.
Role of gut microbiota in inflammatory signaling influencing obesity
Acomplex mucosal immune system defends the intestine from potentially pathogenic bacteria that can be members of the gut microbiota. Indeed, one of the powerful triggers for inflammation is the presence of microorganisms in sites where they do not belong. Emerging evidence over the past decade has shown that systemic, hepatic or adipose tissue inflammation can be one of the crucial mechanisms in the development of obesity-associated insulin resistance, T2DM and related diseases (18). Diabetes, the metabolic syndrome, and obesity are metabolic diseases which based on recent demonstration are associated and characterized by low-grade systemic inflammation (19–22). By regulating gut microbiota innate immunity may influence metabolism and the development of obesity. Recently we have shown a strong relationship between metabolic syndrome and Toll-like-receptor (TLR5). TLR5 is a transmembrane protein that is highly expressed in intestinal mucosa and that recognizes bacterial flagellin. Loss of TLR5 (T5KO) resulted in an alteration in the gut microbiota which is responsible for low grade inflammatory signaling (7). These mice were 20% greater in weight than their WT counterparts and had all the features of insulin resistance and resultant metabolic syndrome. This phenotype was worsened when the T5KO mice were fed a high fat diet. There were no differences in energy harvesting in these mice as assessed by bomb calorimetry and short chain fatty acids. Treatment of the T5KO mice with antibiotics for three months reversed the metabolic syndrome. Finally transferring of gut microbiota from T5KO to wild-type germ-free mice resulted in development of most features of metabolic syndrome to the recipients (7). Comparison of the composition of microbiota in WT and T5KO mice demonstrated a significantly different species composition between the two groups of mice. The conclusion from this series of experiments was that loss of TLR5 resulted in low grade inflammation leading to insulin resistance and subsequent hyperphagia and the development of the metabolic syndrome. Microbiota that initially colonize the human gut may be one of the factors that determines the ultimate metabolic phenotype of an individual.
Another study linking obesity and inflammation relates to Toll like receptor 4 and its ligand Lipopolysaccharide. Cani et al, demonstrated another mechanism linking the gut microbiota to the development of obesity. They hypothesized that bacterial lipopolysaccharide (LPS) which is an important structural component of Gram-negative bacteria cell walls, acts as a triggering factor linking systemic inflammation to high-fat diet-induced metabolic syndrome (23). They found that mice injected with LPS showed increased weight gain and insulin resistance without affecting the energy intake (23). High plasma LPS levels could result from an increased production of endotoxin due to in the gut microbiota (24). High-fat feeding significantly changes gut microbiota composition (24–26), specifically resulting in a decreased number of Bifidobacteria, a group of bacteria which has been shown to reduce intestinal LPS levels in mice and to improve the mucosal barrier function (25, 27, 28). In addition, it has been shown that higher levels of serum free fatty acids (FFAs) play a role in intestinal inflammation and activate pro-inflammatory pathways (29, 30). Further, FFAs activate Toll-like receptor 4 (TLR4) signaling in adipocytes and macrophages, and can induce metabolic inflammation (31).
In another study examining the effects of high fat diet, de al Serre et al, demonstrated that high-fat diets induces changes in the gut microbiota, but it is the development of inflammation that is associated with the appearance of hyperphagia and obesity phenotype. The high-fat diet led to alteration in microbial flora and resultant changes in intestinal alkaline phosphatase and TLR4 expression. In particular, the obesity prone rats had increased expression of TLR4, increased ileal inflammation, altered tight junctions, increased epithelial permeability and plasma LPS levels. It was postulated that increased serum LPS levels could then influence insulin resistance leading to the metabolic syndrome (32). Overall, although the relative role of these mechanisms in promoting gut inflammation, and consequently metabolic disease, in human disease remain undefined, such mechanisms are plausible contributors to metabolic disease.
Gut microbiota regulation of the Brain-Gut axis
The hypothalamus and the brain stem are sites of central regulation of appetite. Satiety signals include various hormones, neuropeptides as well as the constant bidirectional communication between the gut and the brain called the brain-gut axis. The brain-gut axis is a multi-component conceptual model describing the communication pathways connecting the brain with gut, enteric nervous system and the immune system (33). The impact of gut microbiota on behavior and central nervous system (CNS) function is a new research area (34), and the idea that microbiota may signal beyond the gut to the brain is supported by some studies that showed the gut microbiota could activate vagal sensory neurons and regions of the brain associated with central autonomic network (35–38). Gut microbiota, through developmental programming, a process whereby an environmental factor impact on structure and function of an organ, could affect other organs functionality (8, 39, 40). Recently a study demonstrated modulation of brain development by gut microbiota (41). Thus, one can postulate that the host microbiota can influence the development of the central regulation of appetite and satiety. The brain receives signals from gastrointestinal tract through sensory nerves, a classical example being, gastric vagal simulation or balloon distension induced satiety (42). The vagal afferent pathway is a major neural pathway conveying information from gastrointestinal luminal contents to the brain and thereby influencing gastrointestinal motility and feeding behavior. It has been shown that systemic endotoxemia can lead to changes in neuronal function including vagal afferent neurons (43–45). In a model of chronic infection with Helicobacter pylori, altered feeding behavior was noted two months after eradication of infection, implying a role for gastric inflammation regulating feeding behavior (46). In addition to gut microbiota regulating feeding behavior, the brain can in turn influence the colonization of the gut. The hypothalamic–pituitary–adrenal (HPA) axis is the central neuorendocrine pathway in man and activation of this axis takes place in response to a variety of physical and psychological stressors. Studies in germ free mice have demonstrated an exaggerated stress response that can be reversed with fecal recolonization (Sudo et al). In animal models of stress differences in gut microbiota have been noted (47). Studies on gut microbiota regulation of satiety through the brain-gut axis are ongoing and more research is needed to understand the exact mechanism of this pathway of microbiota regulation of feeding behavior.
Manipulating the gut microbiome- Therapeutic Strategies for Obesity
Alteration of gut microbiota through administration of probiotics and prebiotics can modulate weight gain (48). Cani et al found that in the presence of prebiotic oligofructose, there was an increase in Bifiobacterium spp. Further there was a reduced impact of high fat diet induced metabolic endotoxemia and inflammatory disorders. This was achieved by preventing increased gut permeability through increased synthesis of GLP2 (49, 50). GLP2 is associated with intestinal growth and adaptation. In another study administration of prebiotics (inulin and oligofructose) resulted in increase in the lactobaccilus species and bifidobacterium species numbers and in addition there was an increase in satiety hormones GLP-1 and PYY(51). Reduction in the load of microbiota with broad spectrum antibiotics can prevent diet-induced obesity through reduction in adipose tissue inflammation, oxidative stress, and macrophage infiltration in high-fat diet–fed mice. This supports the idea of modulation of gut microbiota as a therapeutic strategy (25, 52).
Future Directions
Further insights into the human microbial community can help us understand the generation of varied metabolic phenotypes. The human microbiota is shaped by the diet that is consumed (53), the host innate and adaptive immunity, host genetics and other factors influencing its environment. Gnotobiotic mice can help us understand the role of specific microbiota in influencing metabolic phenotypes. Kau et al report new strategies to transplant human gut microbiota to gnotobiotic mice(54). Using these models, the effects of diet, physical activity, stress, innate and adaptive immunity on microbiota can be carefully assessed and seem to play an interrelated role in regulating weight gain. Ongoing studies involving transfer of human microbiota from specific communities around the world to identify metabolic phenotype patterns are being performed (55). These “humanized” gnotobiotic animals can be important tools for understanding the role of diet and genetics in regulating obesity. Lastly, placebo-controlled trials have been initiated in humans to examine if microbiota transplants can improve metabolic health (56).
Summary
In summary with the rapidly spreading obesity epidemic new strategies for the prevention and treatment are critical. Understanding the role of gut microbiota in regulating metabolism can provide new targets for therapy. Microbiota can influence weight gain by enhancing energy extraction, creating a smoldering inflammatory response and by enhancing food intake through stimulation of the brain gut axis. Future studies to identify specific bacterial species or populations associated with a lean or obese phenotype can help in directing obesity therapy by altering the human microbiome.
Figure 1.
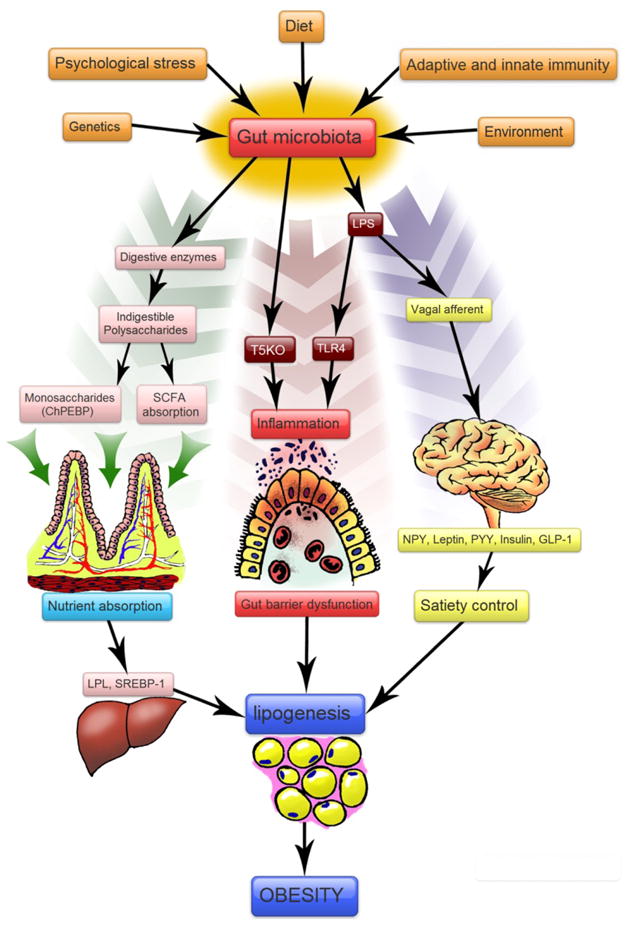
Schematic diagram of proposed mechanism of gut microbiota regulation of obesity. A variety of factors shape the human gut microbiota. The microbiota in turn can influence metabolic pathways by modulating energy extraction, inflammation and satiety, leading to the development of obesity. Abbreviations: SCFA: Short chain fatty acids; SREBP-1: Sterol Regulatory Element Binding protein-1; ChREBP:Carbohydrate Response Element Binding Protein; NPY: Neuropeptide Y; PYY: Peptide YY; GLP-1: Glucagon Like peptide-1; TLR4: Toll-like receptor 4, T5KO: TLR5 Knock out; LPS: Lipopolysaccharide
Acknowledgments
This work was supported by the grants NIH-RO1 (AG) and NIH-RO1 DK080684 and VA MERIT award (S.S.).
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 4.Raoult D. Obesity pandemics and the modification of digestive bacterial flora. Eur J Clin Microbiol Infect Dis. 2008;27:631–634. doi: 10.1007/s10096-008-0490-x. [DOI] [PubMed] [Google Scholar]
- 5.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gutmicrobiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 12.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 13.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 14.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 16.Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. The American journal of clinical nutrition. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira AV, Mario EG, Porto LC, Andrade SP, Botion LM. High-carbohydrate diet selectively induces tumor necrosis factor-alpha production in mice liver. Inflammation. 2011;34:139–145. doi: 10.1007/s10753-010-9217-0. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 22.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 23.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 24.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- 28.Ruan X, Shi H, Xia G, et al. Encapsulated Bifidobacteria reduced bacterial translocation in rats following hemorrhagic shock and resuscitation. Nutrition. 2007;23:754–761. doi: 10.1016/j.nut.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 31.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones MP, Dilley JB, Drossman D, Crowell MD. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil. 2006;18:91–103. doi: 10.1111/j.1365-2982.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 34.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 35.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Bjorkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS One. 2009;4:e6958. doi: 10.1371/journal.pone.0006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claus SP, Tsang TM, Wang Y, et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. doi: 10.1038/msb.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 2000;16:866–873. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 43.de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown R, Imran SA, Wilkinson M. Lipopolysaccharide (LPS) stimulates adipokine and socs3 gene expression in mouse brain and pituitary gland in vivo, and in N-1 hypothalamic neurons in vitro. Journal of neuroimmunology. 2009;209:96–103. doi: 10.1016/j.jneuroim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Autonomic neuroscience: basic & clinical. 2005;120:104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Bercik P, Verdu EF, Foster JA, et al. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. American journal of physiology Regulatory, integrative and comparative physiology. 2009;296:R587–594. doi: 10.1152/ajpregu.90752.2008. [DOI] [PubMed] [Google Scholar]
- 47.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Developmental psychobiology. 1999;35:146–155. [PubMed] [Google Scholar]
- 48.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nature reviews Endocrinology. 2011 doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 49.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 51.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. The British journal of nutrition. 2011:1–13. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Membrez M, Blancher F, Jaquet M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 53.Muegge BD, Kuczynski J, Knights D, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny andwithin humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodman AL, Kallstrom G, Faith JJ, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vrieze AFH, Serlie MJ, Ackermansz MTGM, Dallinga-ThiAKG E, van Nood JFW, Bartelsman RO, Zoetendal E, deVos WMJBL, Hoekstra MN. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome. Diabetologia (supplement) 2011 (Abstract, ESAD) [Google Scholar]


