Abstract
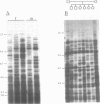
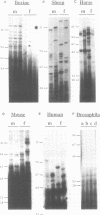
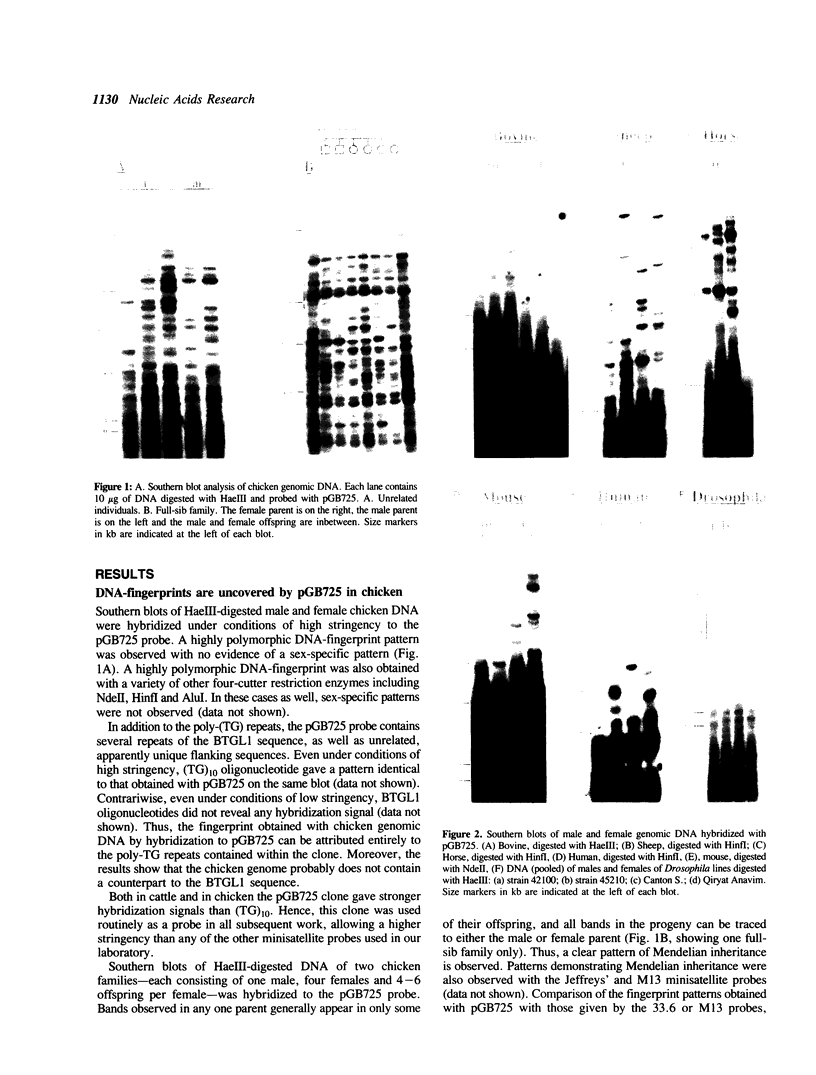
Southern blots of genomic DNA from a variety of species digested by restriction endonucleases having a four-bp specificity, were probed with a bovine genomic clone consisting of seven tandem poly-TG stretches separated by a 29bp linker sequence. Highly variable DNA 'fingerprint' patterns were obtained in chicken, sheep, and horse, moderately variable DNA 'fingerprints' in mouse and man, and a monomorphic pattern in Drosophila. In chicken, horse and man a (TG)10 synthetic oligonucleotide probe gave results identical to those given by the bovine probe. Furthermore, in chicken the DNA fingerprint variation showed typical Mendelian inheritance and differed from the fingerprints obtained with Jeffreys 33.6 and M13 minisatellite probes. Thus, for a variety of vertebrate species, poly-TG-containing probes can uncover useful genetic variation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomquist B. T., Shortridge R. D., Schneuwly S., Perdew M., Montell C., Steller H., Rubin G., Pak W. L. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988 Aug 26;54(5):723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Braaten D. C., Thomas J. R., Little R. D., Dickson K. R., Goldberg I., Schlessinger D., Ciccodicola A., D'Urso M. Locations and contexts of sequences that hybridize to poly(dG-dT).(dC-dA) in mammalian ribosomal DNAs and two X-linked genes. Nucleic Acids Res. 1988 Feb 11;16(3):865–881. doi: 10.1093/nar/16.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard W., Zachau H. G. Simple DNA sequences and dispersed repetitive elements in the vicinity of mouse immunoglobulin K light chain genes. J Mol Biol. 1983 Oct 25;170(2):567–573. doi: 10.1016/s0022-2836(83)80161-2. [DOI] [PubMed] [Google Scholar]
- Hallerman E. M., Nave A., Kashi Y., Holzer Z., Soller M., Beckmann J. S. Restriction fragment length polymorphisms in dairy and beef cattle at the growth hormone and prolactin loci. Anim Genet. 1987;18(3):213–222. doi: 10.1111/j.1365-2052.1987.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hill W. G. Rates of change in quantitative traits from fixation of new mutations. Proc Natl Acad Sci U S A. 1982 Jan;79(1):142–145. doi: 10.1073/pnas.79.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H., Dahlberg J. E. Topology and formation of triple-stranded H-DNA. Science. 1989 Mar 24;243(4898):1571–1576. doi: 10.1126/science.2648571. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Lynch M. The rate of polygenic mutation. Genet Res. 1988 Apr;51(2):137–148. doi: 10.1017/s0016672300024150. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Lowenhaupt K., Rich A., Nordheim A. (dC-dA)n.(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J. 1987 Jun;6(6):1781–1789. doi: 10.1002/j.1460-2075.1987.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada I., Beal M. P., Shen C. K., Chapman B., Wilson A. C., Schmid C. Intergenic DNA sequences flanking the pseudo alpha globin genes of human and chimpanzee. Nucleic Acids Res. 1983 Nov 25;11(22):8087–8101. doi: 10.1093/nar/11.22.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Trifonov E. N. The multiple codes of nucleotide sequences. Bull Math Biol. 1989;51(4):417–432. doi: 10.1007/BF02460081. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Zischler H., Nanda I., Schäfer R., Schmid M., Epplen J. T. Digoxigenated oligonucleotide probes specific for simple repeats in DNA fingerprinting and hybridization in situ. Hum Genet. 1989 Jun;82(3):227–233. doi: 10.1007/BF00291160. [DOI] [PubMed] [Google Scholar]