Abstract
Background
Chitosan has gained considerable attentions as a biocompatible carrier to improve delivery of active agents. Application of this vehicle in the form of nanoparticle could profit advantages of nanotechnology to increase efficacy of active agents.
The purpose of this study was to provide detailed information about chitosan–glutathione (Cht-GSH)nanoparticles which are gaining popularity because of their high mucoadhesive and extended drug release properties.
Methods
Depolymerization of chitosan was carried out using sodium nitrite method.Glutathione was covalently attached to chitosan and the solubility of the resulting conjugates was evaluated. Nanoparticles were prepared by ionic gelation method and then the effect of glutathione immobilization on properties of nanoparticles was investigated.
Results
Thiolation efficiency was higher in lower molecular weight chitosan polymers compared to unmodified chitosan nanoparticles. Cht-GSH conjugates of the same molecular weight but with different degrees of thiolation had the same hydrodynamic diameter (995± nm) and surface charge (102± mV) as unmodified chitosan, but comprised of a denser network structure and lower concentration. Cht-GSH nanoparticles also exhibited greater mucoadhesive strength which was less affected by ionic strength and pH of the environment.
Conclusion
Thiolation improves the solubility of chitosan without any significant changes in size and charge of nanoparticles, but affects the nanogel structure.
Keywords: Thiolated chitosan, Glutathione, Nanoparticle, Mucoadhesion
INTRODUCTION
Chitosan and its derivatives are useful polymeric biomaterials that have found a number of applications in drug delivery. It has been shown that chitosan is biocompatible, biodegradable, nontoxic and has mucoadhesion properties by establishment of electrostatic interactions with sialic groups of mucin (1–3).
Thiolated derivatives of chitosan known as thiomers have been produced via immobilization of thiol groups on the primary amino groups of chitosan backbone. Thiolation of chitosan has also demonstrated to improve the mucoadhesive properties of chitosan through disulfide bonds with cysteine-rich domains of mucus glycoproteins. Permeation enhancement and antiprotease activity have also been observed with thiolated chitosan (4–6). Synthesis of different thiolated derivatives of chitosan including chitosan cysteine (7), chitosan-thiobutylamidine (8), chitosan-thioglycolic acid (9) and chitosan-glutathione conjugates (10) have been described. TripeptideGlutathione(L-y-glutamyl-L- cysteinyl- glycine) in its reduced form (GSH), is assumed to play a pivotal role in the opening of tight junctions of intestinal epithelia by interaction with and inhibition of protein tyrosine phosphatase (PTP) (11, 12) and its efficacy as the permeation enhancer for oral delivery of hydrophilic drugs has been reported. Glutathione which is present in its reduced (GSH) and oxidized (GSSG) form at the apical side of the intestinal mucosa, is also involved in the likely mechanism underlying the permeation enhancement of thiomers (13–15) and seems to be the multifunctional one among various thiolating agents.
In this study, chitosan-glutathione conjugates using chitosan polymers of different molecular weights were prepared for nanoparticle preparation. Cht-GSH conjugates of the same molecular weight but with different degrees of thiolation were used.
Nanoparticles were prepared by tripolyphosphate (TPP) ionic gelation of chitosan and its derivatives concerning their hydrodynamic diameter, zeta potential, TPP content and mucoadhesion were determined.
Material and Methods
Chitosan (medium molecular mass, degree of deacetylation about 96% Fluka Germany), L-Glutathione reduced form (GSH), 1-ethyl-3-(3-dimethyl amino-propyl) carbodiimide hydrochloride (EDAC), N-hydroxysuccinimide (NHS), sodium nitrite, basic fuchsin, mucin, periodic acid, sodium metabisulfite,glucose, sucrose, dextrose, sorbitol, mannitol. hydrochloric acid, glacial acetic acid, sodium hydroxide and potassium hydrogen phosphate were all purchased from Merck (Germany). Ellman's reagent, 5,5′-dithiobis (2-nitro benzoic acid), was obtained from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade. Deionized water was used throughout experiments.
Depolymerization of chitosan
Depolymerization of chitosan was carried out according to the method previously reported (16, 17). Briefly10 ml of sodium nitrite solution (0.3, 1, 2.5, 5 and 7 mg/ml) was added to the solution of chitosan (2% w/v) in acetic acid within one hour while stirring. A white-yellowish solid was obtained by raising the pH to 9. Filtrate was dialyzed against deionized water (2× 2l for 90 min and 1×2 l overnight). The product was lyophilized for further uses.
Measurement of the molecular weight
Molecular weight of the depolymerized chitosan (Cht) was determined by gel permeation chromatography (GPC).The lyophilized powder of depolymerized chitosan (3 mg/ml)in acetate buffer (pH 4.5) at flow rate of 5 ml/min and a PL Aquagel-OH mixed gel filtration column (300 mm×7.5 mm internal diameter, pore size 8 µm) from Agilent Technologies (Santa Clara, California) were used for this determination.
Synthesis and purification of chitosan-Glutathione conjugate
Covalent attachment of GSH to chitosan polymers of different molecular weights was carried out based on a method described previously (19). Briefly,pH of the solution of chitosan (1% w/v) was raised to 6.5 followed by addition of glutathione. To activate the carboxyl group of the glutathione for amidation reaction, 4.6 g of EDC and 2.8 g of NHS were added and pH was readjusted to 6.5 and the resulting solution left for 17 hrs at room temperature. The preparation was dialyzed twice against HCl containing 1% NaCl and then against HCl, for 8 hrs. The product was lyophilized for further uses. Table 1 summarizes factors that were changed in different experiments. Cht-GSH50L, Cht-GSH50M and Cht-GSH50H are addressed as lightly, mildly and highly thiolated chitosan, respectively.
Table 1.
Molecular weight of chitosan and amount of thiolating reagents in chitosan-glutathione conjugates.
| Cht-GSH type | Molecular weight of chitosan (kDa) | Chitosan (% w/v) | GSH (g) | EDC (g) | NHS (g) |
|---|---|---|---|---|---|
| Cht-GSH18 | 18 | 1 | 5 | 4.6 | 2.8 |
| Cht-GSH25 | 25 | 1 | 5 | 4.6 | 2.8 |
| Cht-GSH50M. | 50 | 1 | 5 | 4.6 | 2.8 |
| Cht-GSH115 | 115 | 1 | 5 | 4.6 | 2.8 |
| Cht-GSH300 | 300 | 1 | 5 | 4.6 | 2.8 |
| Cht-GSH50L | 50 | 1 | 2.5 | 2.3 | 1.4 |
| Cht-GSH50H | 50 | 1 | 10 | 9.2 | 5.6 |
Measurement of thiol group and disulfide content of Cht–GSH
Ellman's reagent was used to measure thiol content of Cht-GSH conjugates by the method reported previously (10). To the known amount of each conjugate in deionized water and phosphate buffer (pH 8.0) was added, 2 ml of freshly prepared (0.3 mg/ml) Ellman's reagent. The reaction was incubated for 2 hrs and the precipitate was collected by centrifugation at 24000 ×g for 5 min.
To determine oxidized thiol moieties available in form of disulfide bonds, NaBH4 was used to reduce disulfide bonds to free thiol groups. Quantification with Ellman's reagent gives the total amount of sulfhydryl groups fixed on the polymer either as reduced or oxidized forms.
To 3 ml of thiolated chitosan in tris (hydroxymethyl) amino methane buffer (pH 6.8) was added 3 ml of 2% NaBH4. The solution was left for one hour at room temperatureand and then treated with 300 µl of 0.3 mg/ml Ellman's reagent in 0.5 M phosphate buffer (pH 8.0). The reaction was allowed to proceed for 20 min at room temperature and then the absorbance of the sample was measured at 412 nm.
Solubility of Cht-GSH with different thiolation degrees
Solubility of Cht-GSH was studied by determination of pH50 value defined as the pH value when transmittance reaches to 50%. Polymers were dissolved in 2% acetic acid (2 mg/ml) and after adjustment of the PH were left at room temperature for 30 min. The corresponding transmittance was measured at 600 nm.
Preparation and characterization of nanoparticles
In situ gelation of Cht-GSH with polyanion TPP was undertaken for nanoparticle preparation by a method reported earlier (19). In brief, 3 ml of TPP (1 mg/ml) was mixed with 5 ml of Cht-GSH (1 mg/ml) solution (pH 5) while stirring. Nanoparticle suspension was lyophilized using 1% (w/v) of one of the cryoprotectants; glucose, sucrose, dextrose, sorbitol and mannitol.
Hydrodynamic mean diameter and zeta potential of nanoparticles were measured by dynamic light scattering and Laser Doppler Electrophoresis using Zetasizer (Nano-ZS, Malvern, UK)at wavelength of 633 nm at 25°C with an angle of detection of 90°.
Surface morphology of nanoparticles was observed by scanning electron microscopy (SEM)using a XL 30; (Philips, Eindhoven the Netherlands) instrument. One drop of nanoparticles was layered on the SEM stub and allowed to air dry at room temperature. The dried nanoparticles were then coated with gold metal using a sputter coater (SCD 005; Bal-Tec, Balzers, Switzerland).
Determination of remaining TPP
The amount of TPP not incorporated in nanoparticles was measured using a method developed from Murphy-Riley colorimetric method (20). The modified Murphy-Riley (MR) reagent consists of: 10 ml of H2SO4 (2.5 M), 4 ml molybdate (40 mg/ml), 5 ml ascorbic acid (60 mg/ml), 2 ml antimony (2 mg/ml) and 4 ml deionized water. Components were added in the proper order and the mixtures were swirled after each addition. Nanoparticle was centrifuged at 30,000 rpm for 20 min and then 800 of µl MR reagent and 4 ml water were added to 200 µl of the supernatant and the solution was left for 19 hrs at room temperature and in dark. The absorbance of the resulting molybdene blue was measured at 712 nm.
Adsorption of mucin by Cht-GSH conjugates and mucin assay
Mucoadhesion study was carried out by means of periodic acid/Schiff (PAS) colorimetric method (21, 22). To prepare Schiff reagent, 100 ml of fuchsin (1%) was mixed with 20 ml HCl (1 N). 0.1 g sodium metabisulfite for every 6 ml of Schiff reagent and left in dark over night.the solution was decolorized with charcoal and was filtered and kept at 4°C.
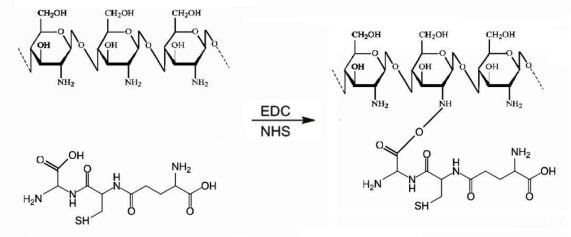
Figure 1.
Synthesis of chitosan-glutathione conjugate.
Oxidation of hydroxyl groups on mucin to aldehyde groups required for Schiff test was achieved by using freshly prepared periodic acid reagent consisting of 0.1 ml of 5% (w/v) periodic acid in 7 ml of 7% acetic acid (v/v).
RESULTS AND DISCUSSION
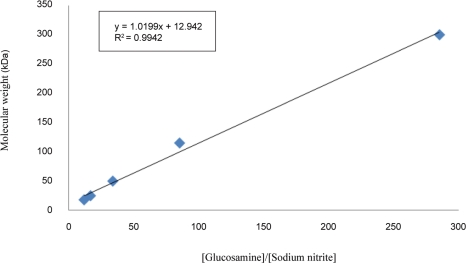
A plot of molecular weight of depolymerized chitosan versus the molar ratio of Glucosamine to sodium nitrite yielded a straight line, with square of the correlation coefficient equal to 0.9942 as shown in figure 2. Result of a similar study was also a linear correlation whose slope increased with molecular weight of parent chitosan. This linearity is in agreement with the reported depolymerization reaction mechanism (23) in which rate-limiting step is nitrosation of the unprotonated amine by nitrous acidium ion and rate of depolymerization is first order with respect to the concentration of nitrous acid (equations 1–4).
Figure 2.
Dependence of molecular weight of depolymerized chitosan on glucosamine/sodium nitrite molar ratio.
Then,
Defining Molecular weight as:
Confirms the linear correlation:
Where β represents the number of the broken glycosidic linkages; Rβ, the rate of breakage; Kβ, the apparent first-order constant for linkage breaking; βt, total number of linkages broken by time t; Wi, initial molecular weight; and Wt, molecular weight at time t.
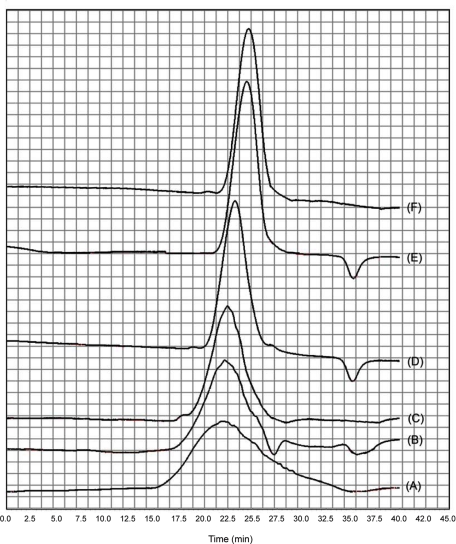
Furthermore, while GPC analysis of parent MMW chitosan showed a broad peak reflecting the presence of chitosan polymers of a wide range of molecular weight, depolymerization gave rise to a sharp GPC peak and thus a more homogenous chitosan product (Fig 3). This reduced polydispersity is attributed to the proportionality of the number of breaks to the length of the macromolecular chain i.e; the longer the chitosan chain, the higher the probability of its oxidative breakdown by sodium nitrite.
Figure 3.
GPC chromatogram of parent and depolymerized chitosan: (A) parent MMW chitosan; (B) 300 kDa chitosan; (C) 115 kDa chitosan; (D) 50 kDa chitosan; (E) 25 kDa chitosan (F) 18 kDa chitosan.
Table 2 recapitulates results of immobilization of glutathione on chitosan. Similar to the previous studies lower molecular weight of chitosan results in higher accessibility to thiolating agents and hereby a higher thiolation rate (10, 18, 26). Though both free thiol and disulfide contents showed a decreasing trend by increase in the molecular weight of chitosan, a comparison of the percentage values indicates a slightly lower inclusion of sulfurs into intra- and/or inter-molecular disulfide bonds in Cht-GSH conjugates of higher molecular weights. This is presumably due to higher density of thiol groups in low molecular weight chitosan; the consequent vicinity of thiol residues on shorter polymer chains renders them more susceptible to oxidation into disulfide linkages. These results differs from those reports (24) that disulfide percentage is higher in thiomers of higher molecular weight due to thiol group's being concentrated in the more accessible parts of the long chain and thus more probable to form disulfide bonds. This difference may stem from the difference in deacetylation degree (DD%) between parent chitosans of these two studies. Parent chitosan used in Bravo Osuna's (24) study had a DD% of 88 while parent chitosan with DD% of 97 was used in this study. Higher degree of deacetylation causes a stronger pulse repulsion resulting in a more extended conformation and hereby, causes thiol groups to be distributed more evenly along the polymer chain rather than being concentrated.
Table 2.
Charactristics of the thiolated chitosan.
| Cht-GSH type | Molecular weight of chitosan (kDa) | Functionalization of NH2 groups with GSH (%) | Reduced thiol (mol/g) (% with respect to total thiol) | Disulfide bond (mol/g) (%with respect to total thiol) |
|---|---|---|---|---|
| Cht-GSH18 | 18 | 17.8 | 191.4 (22.6%) | 321.3 (77.4%) |
| Cht-GSH25 | 25 | 17.1 | 188 (23.2%) | 316.4 (76.8%) |
| Cht-GSH50M | 50 | 16 | 182.4 (24.1%) | 298.6 (75.9%) |
| Cht-GSH115 | 115 | 14.1 | 171.2 (26.2%) | 246.8 (73.8%) |
| Cht-GSH300 | 300 | 9.5 | 151.1 (29.1%) | 177.2 (70.1%) |
| Cht-GSH50L | 50 | 6.8 | 72,3 (29.0%) | 154.9 (81.0%) |
| Cht-GSH50H | 50 | 31.2 | 337,1 (26.6%) | 466.6 (73.4%) |
Doubling and half-cutting-down on thiolating reagents, i.e. glutathione, EDC and NHS, as expected, resulted in 50 kDa Cht-GSH conjugates with significantly different amount of immobilized thiols.
Hydrodynamic diameter and zeta potential of nanoparticles showed no significant difference between unmodified and thiolated chitosans (Table 3). However, a comparison of ACN between different nanoparticle formulations revealed that ionic gelation produced lower concentration of nanoparticles in thiolated chitosan. Preparation of nanoparticle by using unmodified chitosan polymer led to an ACN of 23640 kilo count, while 10.9%, 30% and 41% decrease in ACN was detected in Cht-GSH50L, Cht-GSH50M and Cht-GSH50H, respectively.
Table 3.
Mean hydrodynamic diameter, zeta potential, incorporated TPP and ACN of nanoparticles made by chitosan polymers with different thiolation degrees.
| Cht-GSH type | Mean hydrodynamic diameter (nm) | Zeta Potential (mV) | Incorporated TPP (%) | ACN |
|---|---|---|---|---|
| Cht50 | 104 | 12.7 | 86 | 23640 |
| Cht-GSH50L | 99 | 13.2 | 78 | 21063 |
| Cht-GSH50M | 94 | 10.8 | 73 | 16548 |
| Cht-GSH50H | 107 | 11.5 | 67 | 13947 |
In addition, ionically gelated chitosan nanoparticles encounter the problem of non-redispersibility after freeze-drying. To ameliorate the damaging effects associated with lyophilization process, glucose, dextrose, sorbitol and mannitol were added as cryoprotective reagent to modulate the to-be-removed water. Among these cryoprotectants, mannitol showed no bettering effect on redispersion of nanoparticles. Carbohydrate cryoprotectants, i.e. glucose, sucrose and dextrose were satisfactory with regard to the stability of carbohydrate (chitosan) nanoparticles during freeze-drying. In the case of the sugar alcohol, sorbitol, an increase of 150 nm in diameter was observed upon redispersion. Being a non-reducing sugar and therefore not affecting thiol groups, sucrose was the cryoprotectant of choice for following studies.
The strength of crosslinking in ionically gelated chitosan nanoparticles was evaluated by measurement of TPP participating in nanoparticle network. The results illustrates that Glutathione-thiolation decreased incorporation of TPP in nanoparticles from 86% in unmodified chitosan to 78%, 73% and 67% respectively in lightly, mildly and highly thiolated chitosan. This significant decrease may be attributed to the observed lower ACN indicating lower number of nanogels requiring lower amount of TPP. It is assumed that disulfide bonds in thiolated chitosan give it a coiled structure less liable to interact with TPP in nucleation stage of nanoparticle preparation; formation of fewer nucleuses and a decrease in the number of prepared nanoparticles. However, drop in ACN is steeper than drop in TPP incorporation because of the growth stage of ionic gelation. Chitosan has a pKa value of ∼6.5 and amide bond formation between carboxylic group of glycine with amine groups of chitosan result in conjugated glutathione with pKa values of 2.5 (Glu-α-COOH), 9.2 (SH) and 9.5 (Glu-α-NH3+) (25). Therefore, glutathione conjugation intensifies the positivity of chitosan; thus, during the growth stage, more TPP molecules attend nanogels of Cht-GSH conjugates and result in a denser network structure of interpenetrating polymer chains crosslinked to each other by counterions which is important when studying drug release behavior of such thiolated formulations.
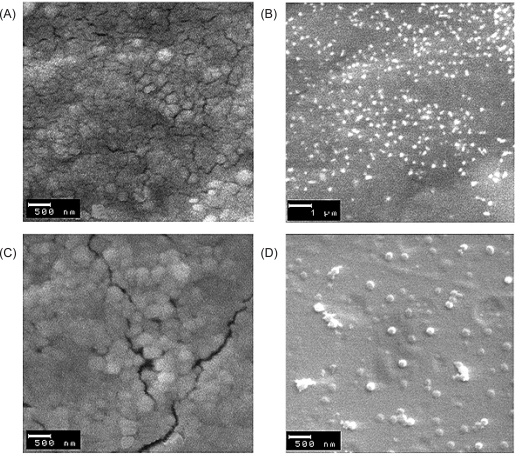
Morphological characteristics of the prepared nanoparticle formulations were studied by SEM (Fig. 4).SEM photographs showed semispherical nanoparticles with a regular size distribution for all thiolated and non-thiolated formulations. However, compared to hydrodynamic diameters measured by DLS recorded in table 3, nanoparticles observed in SEM images seemed of larger size and this discrepancy may result from electron bombardment during SEM imaging which is known to cause nanoparticles of a carbohydrate nature like chitosan to lose shape (26). It also may be related to different conditions of sample preparation for SEM and DLS.
Figure 4.
SEM micrographs of nanoparticles prepared from unmodified and thiolated chitosan: (A) unmodified chitosan; (B) lightly thiolated chitosan; (C) mildly thiolated chitosan; (D) highly thiolated chitosan.
Covalent binding of glutathione to chitosan increased its pH50 value. Compared to 50 kDa unmodified chitosan with pH50 value of 7.5, pH50 value in lightly and mildly thiolated chitosan were found to be 8.6 and 11.4, respectively. In highly thiolated chitosan, pH50 value could not be defined. It was expected as glutathione possesses a more hydrophilic nature than its amino and carboxylic groups conjugation to chitosan, improves its solubility reflected in its higher pH50 value.
Cationic polyelectrolyte nature of chitosan enables it to interact electrostatically with anionic substructures of the mucus. However, thiolated derivatives of chitosan have proved to improve its mucoadhesive properties by the formation of disulfide bonds between thiol groups of the thiomers and cysteine rich subdomains of glycoproteins in the mucus layer. The results of turbidimetric measurement confirmed expected increase in mucoadhesive properties in thiolated chitosan.
Moreover, mucoadhesion interactions were evaluated under different pHs and ionic strengths.
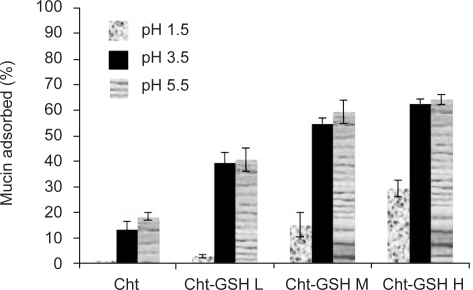
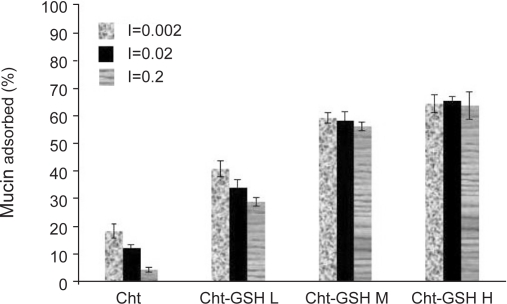
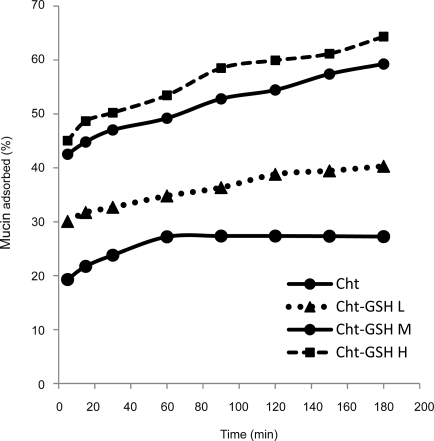
The effectof pH on the adsorption of mucin on Cht-GSH nanoparticles was studied at pH 1.2, (simulated gastric fluid), 3.5 and 5.5 (acetate buffer 2 mM). To study the effectof ionic strength, suitable amount of sodium chloride was added to adjust the ionic strength (I) to 0.2, 0.02 and 0.005 (acetate buffer, 2 mM, pH 5.5). In accord with earlier studies (27), experimental conditions of low pH and high ionic strength decrease the interaction between mucus and chitosan nanoparticles; the lower the pH of the medium, the higher the positive charge of chitosan but the lower the negative charge of mucin leads to the lowered strength of ionic interactions under such conditions. However, enfeebling effects of low pH and high ionic strength were mitigated in thiolated derivatives of chitosan (Figs 5, 6). This is due to the important role played by covalent disulfide bonds in mucoadhesivity of Cht-GSH nanoparticles and thus their lesser dependences on electrostatic interactions. However, when pH goes down to that of gastric fluid (pH 1.5), the strength of ionic interaction diminishes to such a great extent that causes a dramatic fall in mucoadhesion. Monitoring of the mucoadhesion progress over a 3 hrs course revealed that while the interaction between Cht nanoparticles and mucin comes to a standstill after 90 min, Cht-GSH nanoparticles continue to adsorb mucin over the 3 hrs period (Fig. 7).
Figure 5.
Effects of pH on mucin adsorption onto unmodified/thiolated chitosan nanoparticles.
Figure 6.
Effects of ionic strength on mucin adsorption onto unmodified/thiolated chitosan nanoparticles.
Figure 7.
Mucoadhesion progress with time (acetate buffer, 2 mM, pH 5.5).
CONCLUSION
Chitosan-glutathione conjugates of different thiolation degrees were synthesized. Ionically gelated nanoparticles were prepared from unmodified and thiolated polymers, characterized, and compared. Thiolation improves the solubility of chitosan without any significant alteration in size and charge of nanoparticles, but affects the nanogels structure. pH change along the gastrointestinal tract proved to have a much weaker influence on mucoadhesion of thiolated nanoparticles, Therefore, Cht-GSH seems to possess suitable characteristics for the preparation of nanoparticulate oral drug delivery system.
ACKNOWLEDGEMENTS
The authors would like to thank Nanotechnology Research Centre of Tehran University of Medical Sciences.
REFERENCES
- 1.Singla AK, Chawla M. Chitosan: Some pharmaceutical and biological aspects-An update. J Pharm Pharmacol. 2001;53:1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 2.Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. Pharmaceutical Science & Technology Today. 1998;1:246–253. [Google Scholar]
- 3.Moghaddam FA, Atyabi F, Dinarvand R. Preparation and in vitro evaluation of mucoadhesion and permeation enhancement of thiolated chitosan-pHEMA core-shell nanoparticles. Nanomedicine: Nanotechnology Biology and Medicine. 2009;5:208–215. doi: 10.1016/j.nano.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Atyabi F, Talaie F, Dinarvand R. Thiolated Chitosan nanoparticles as an oral delivery system for amikacin: In vitro and ex vivo evaluations. J Nanosci Nanotechnol. 2009;9:4593–4603. doi: 10.1166/jnn.2009.1090. [DOI] [PubMed] [Google Scholar]
- 5.Bernkop-Schnürch A, Kast CE, Guggi D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules thiomer/GSH systems. J Control Release. 2003;93:95–103. doi: 10.1016/j.jconrel.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bernkop-Schnürch A, Kast CE. Chemically modified chitosans as enzyme inhibitors. Advanced Drug Delivery Reviews. 2001;52:127–137. doi: 10.1016/s0169-409x(01)00196-x. [DOI] [PubMed] [Google Scholar]
- 7.Atyabi F, Talaie F, Dinarvand R. Thiolated chitosan nanoparticles as an oral delivery system for amikacin: In vitro and ex vivo evaluations. Journal of Nanoscience and Nanotechnology. 2009;9:4593–4603. doi: 10.1166/jnn.2009.1090. [DOI] [PubMed] [Google Scholar]
- 8.Bernkop-Schnürch A, Hornof M, Zoidl T. Thiolated polymers-Thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int J Pharm. 2003;260:229–237. doi: 10.1016/s0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 9.Bernkop-Schnürch A, Hopf TE. Synthesis and in vitro evaluation of chitosan-thioglycolic acid conjugates. Scientia Pharmaceutica. 2001;69:109–118. [Google Scholar]
- 10.Talaei F, Azizi E, Dinarvand R, Atyabi F. Thiolated chitosan nanoparticles as a delivery system for antisense therapy evaluation against EGFR in T47D breast cancer cells. International Journal of Nanomedicine. 2011;6:1963–1975. doi: 10.2147/IJN.S22731. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Barrett WC, DeGnore JP, König S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry (Mosc) 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 12.Rao RK, Li L, Baker RD, Baker SS, Gupta A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2000;279:332–340. doi: 10.1152/ajpgi.2000.279.2.G332. [DOI] [PubMed] [Google Scholar]
- 13.Clausen AE, Kast CE, Bernkop-Schnürch A. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm Res. 2002;19:602–608. doi: 10.1023/a:1015345827091. [DOI] [PubMed] [Google Scholar]
- 14.Yousefpour P, Atyabi F, Vasheghani-Farahani E, Mousavimovahedi AA, Dinarvand R. Targeted Delivery of Doxorubicin Utilizing Chitosan Nanoparticles Surface Functionalized with Anti Her2 Trastuzumab. International Journal of Nanomedicin. 2011;6:1977–1990. doi: 10.2147/IJN.S21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernkop-Schnürch A, Pinter Y, Guggi D, Kahlbacher H, Schöffmann G, Schuh M. The use of thiolated polymers as carrier matrix in oral peptide delivery-Proof of concept. J Control Release. 2005;106:26–33. doi: 10.1016/j.jconrel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Saremi S, Atyabi F, Akhlaghi SP, Ostad SN, Dinarvand R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: preparation, in vitro and ex vivo evaluation. International Journal of Nanomedicine. 2011;6:119–128. doi: 10.2147/IJN.S15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhlaghi SP, Saremi S, Ostad SN, Dinarvand R, Atyabi F. Discriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell lines. Nanomedicine: Nanotechnology Biology and Medicine. 2010;6:689–697. doi: 10.1016/j.nano.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Atyabi F, Moghaddam FA, Dinarvand R, Zohuriaan-Mehr MJ, Ponchel G. Thiolated chitosan coated poly hydroxyethyl methacrylate nanoparticles: Synthesis and characterization. Carbohydrate Polymers. 2008;74:59–67. [Google Scholar]
- 19.Talaei F, Azhdarzadeh M, Hashemi Nasel H, Moosavi M, Foroumadi AR, Dinarvand R, Atyabi F. Core shell methyl methacrylate chitosan nanoparticles: In vitro mucoadhesion and complement activation. Daru. 2011;19:257–265. [PMC free article] [PubMed] [Google Scholar]
- 20.Carter MR, Island E. Canada, Ottava: CRC Press; 2007. Soil sampling and methods of analysis. [Google Scholar]
- 21.Yousefpour P, Atyabi F, Vasheghani-Farahani E, Sakhtianchi R, Dinarvand R. Polyanionic carbohydrate doxorubicindextran nanocomplex as a delivery system for anticaner drugs: in vitro analysis and evaluations. International Journal of Nanomedicin. 2011;6:1487–1496. doi: 10.2147/IJN.S18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atyabi F, Majzoob S, Dorkoosh F, Sayyah M, Ponchel G. The impact of trimethyl chitosan on in vitro mucoadhesive properties of pectinate beads along different sections of gastrointestinal tract. Drug Development and Industrial Pharmacy. 2007;33:291–300. doi: 10.1080/03639040601085391. [DOI] [PubMed] [Google Scholar]
- 23.Allan GG, Peyron M. Molecular weight manipulation of chitosan I: Kinetics of depolymerization by nitrous acid. Carbohydr Res. 1995;277:257–272. doi: 10.1016/0008-6215(95)00207-a. [DOI] [PubMed] [Google Scholar]
- 24.Bravo-Osuna I, Ponchel G, Vauthier C. Tuning of shell and core characteristics of chitosan-decorated acrylic nanoparticles. Eur J Pharm Sci. 2007;30:143–154. doi: 10.1016/j.ejps.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Bourne GH. New York: Academic Press Inc; 1978. International Review of Cytology; p. 54. toxsci.Oxfordjournals.org. [Google Scholar]
- 26.Bilensoy E, Sarisozen C, Esendagli G, Dogan AL, Aktaş Y, Sen M, et al. Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. Int J Pharm. 2009;371:170–176. doi: 10.1016/j.ijpharm.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88. [Google Scholar]