Abstract
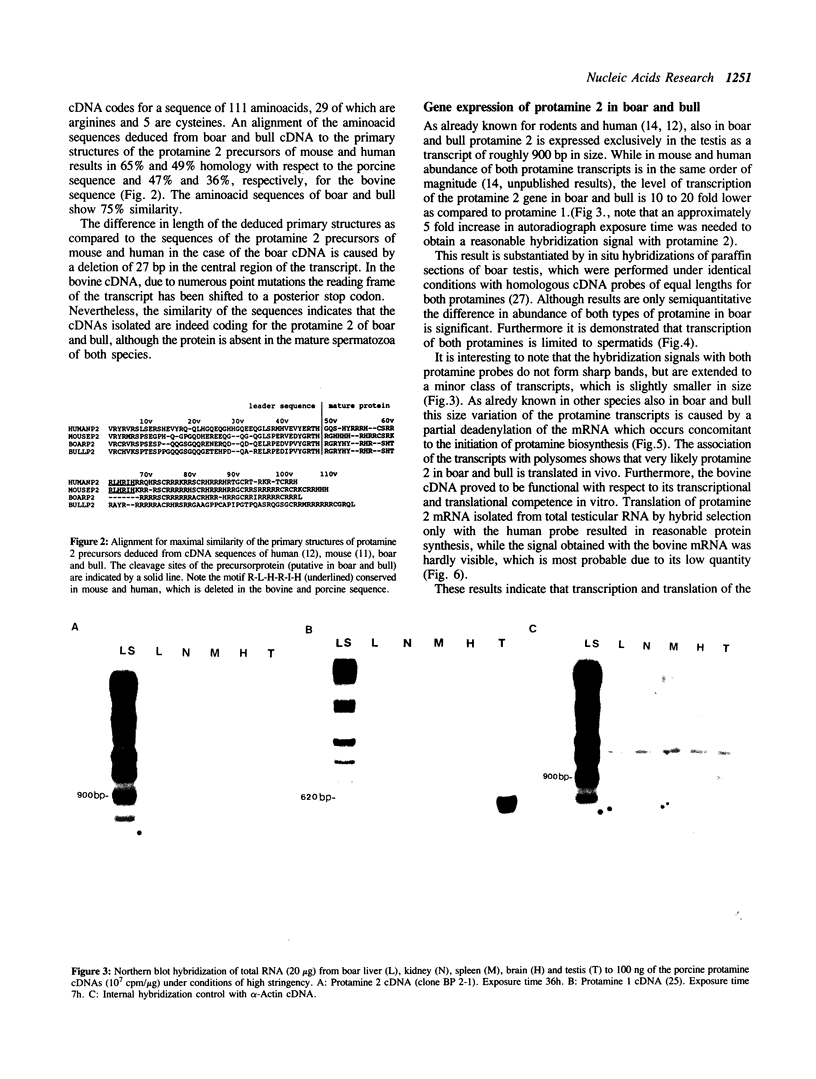
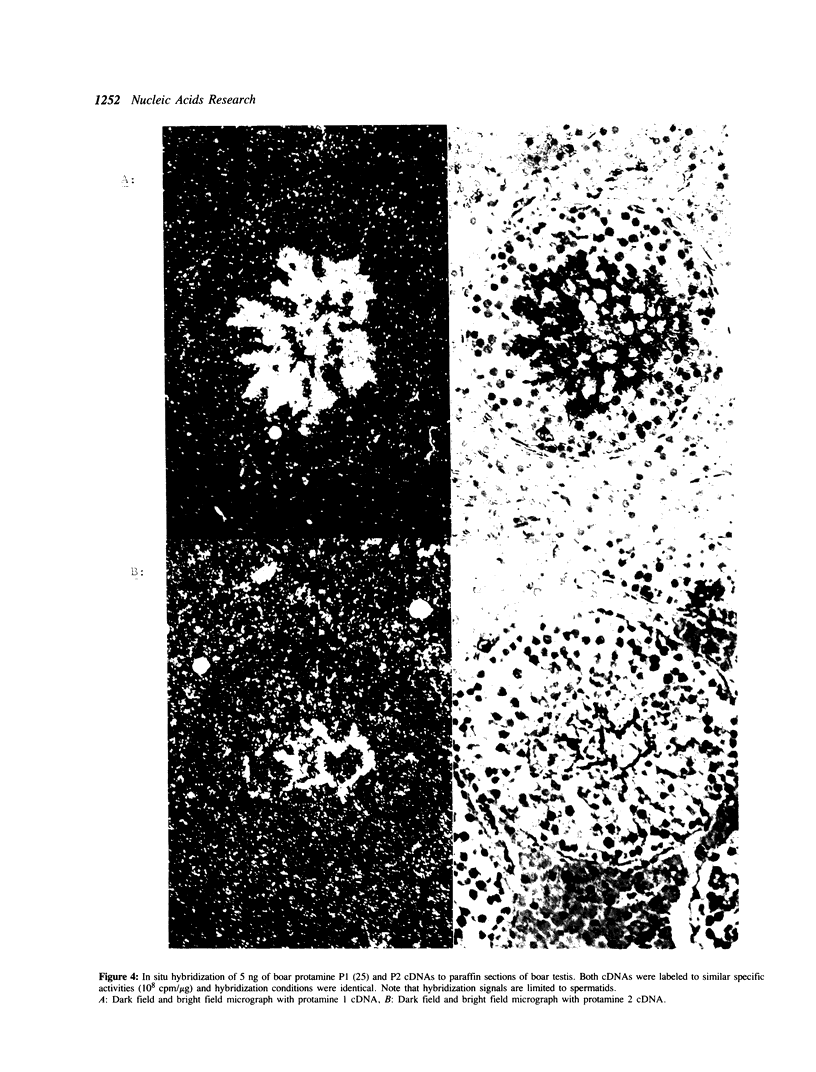
The nuclei of spermatozoa in all mammals examined so far contain P1 protamine. A second protamine variant, protamine P2, has to date been isolated only from human and murine spermatozoa where it represents the major fraction of basic nuclear protein. In order to elucidate the reason for this unusual distribution of the protamine variants among mammals we have investigated the expression of protamine P2 in boar and bull. It can be shown that also in these species protamine 2 is transcribed and translated on low levels. Various mutational events though have altered the primary structure of the protein: In boar, a deletion of 8 aminoacids has removed a sequence motif from the amino-terminus of the molecule, which highly probable is of functional relevance. The bovine sequence, as a consequence of numerous point mutations has accumulated neutral and hydrophobic aminoacids which reduce the affinity of the protamine 2 to DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adham I. M., Klemm U., Maier W. M., Hoyer-Fender S., Tsaousidou S., Engel W. Molecular cloning of preproacrosin and analysis of its expression pattern in spermatogenesis. Eur J Biochem. 1989 Jul 1;182(3):563–568. doi: 10.1111/j.1432-1033.1989.tb14864.x. [DOI] [PubMed] [Google Scholar]
- Ammer H., Henschen A., Lee C. H. Isolation and amino-acid sequence analysis of human sperm protamines P1 and P2. Occurrence of two forms of protamine P2. Biol Chem Hoppe Seyler. 1986 Jun;367(6):515–522. doi: 10.1515/bchm3.1986.367.1.515. [DOI] [PubMed] [Google Scholar]
- Ashikawa I., Kinosita K., Jr, Ikegami A., Tobita T. Changes of the DNA packaging mode during boar sperm maturation. Biochim Biophys Acta. 1987 Apr 29;908(3):263–267. doi: 10.1016/0167-4781(87)90106-0. [DOI] [PubMed] [Google Scholar]
- Balhorn R. A model for the structure of chromatin in mammalian sperm. J Cell Biol. 1982 May;93(2):298–305. doi: 10.1083/jcb.93.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhorn R., Gledhill B. L., Wyrobek A. J. Mouse sperm chromatin proteins: quantitative isolation and partial characterization. Biochemistry. 1977 Sep 6;16(18):4074–4080. doi: 10.1021/bi00637a021. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Weston S., Thomas C., Wyrobek A. J. DNA packaging in mouse spermatids. Synthesis of protamine variants and four transition proteins. Exp Cell Res. 1984 Feb;150(2):298–308. doi: 10.1016/0014-4827(84)90572-x. [DOI] [PubMed] [Google Scholar]
- Bedford J. M., Calvin H. I. The occurrence and possible functional significance of -S-S- crosslinks in sperm heads, with particular reference to eutherian mammals. J Exp Zool. 1974 May;188(2):137–155. doi: 10.1002/jez.1401880203. [DOI] [PubMed] [Google Scholar]
- Bower P. A., Yelick P. C., Hecht N. B. Both P1 and P2 protamine genes are expressed in mouse, hamster, and rat. Biol Reprod. 1987 Sep;37(2):479–488. doi: 10.1095/biolreprod37.2.479. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Peschon J. J., Behringer R. R., Brinster R. L., Palmiter R. D. Protamine 3'-untranslated sequences regulate temporal translational control and subcellular localization of growth hormone in spermatids of transgenic mice. Genes Dev. 1989 Jun;3(6):793–802. doi: 10.1101/gad.3.6.793. [DOI] [PubMed] [Google Scholar]
- Calvin H. I. Comparative analysis of the nuclear basic proteins in rat, human, guinea pig, mouse and rabbit spermatozoa. Biochim Biophys Acta. 1976 Jun 15;434(2):377–389. doi: 10.1016/0005-2795(76)90229-4. [DOI] [PubMed] [Google Scholar]
- Coelingh J. P., Monfoort C. H., Rozijn T. H., Leuven J. A., Schiphof R., Steyn-Parvé E. P., Braunitzer G., Schrank B., Ruhfus A. The complete amino acid sequence of the basic nuclear protein of bull spermatozoa. Biochim Biophys Acta. 1972 Nov 28;285(1):1–14. doi: 10.1016/0005-2795(72)90174-2. [DOI] [PubMed] [Google Scholar]
- Courtens J. L., Plöen L., Loir M. Immunocytochemical localization of protamine in the boar testis. J Reprod Fertil. 1988 Mar;82(2):635–643. doi: 10.1530/jrf.0.0820635. [DOI] [PubMed] [Google Scholar]
- Domenjoud L., Fronia C., Uhde F., Engel W. Sequence of human protamine 2 cDNA. Nucleic Acids Res. 1988 Aug 11;16(15):7733–7733. doi: 10.1093/nar/16.15.7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr Nuclear proteins in spermatogenesis. Comp Biochem Physiol B. 1986;83(3):495–500. doi: 10.1016/0305-0491(86)90285-3. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Yelick P. C., Liem H., Hecht N. B. Differential distribution of the P1 and P2 protamine gene sequences in eutherian and marsupial mammals and a monotreme. Gamete Res. 1988 Feb;19(2):169–175. doi: 10.1002/mrd.1120190207. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed Proc. 1978 Sep;37(11):2512–2516. [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kleene K. C. Poly(A) shortening accompanies the activation of translation of five mRNAs during spermiogenesis in the mouse. Development. 1989 Jun;106(2):367–373. doi: 10.1242/dev.106.2.367. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Schäfer U., Schäfer M. Cis-acting regions sufficient for spermatocyte-specific transcriptional and spermatid-specific translational control of the Drosophila melanogaster gene mst(3)gl-9. EMBO J. 1988 Feb;7(2):447–454. doi: 10.1002/j.1460-2075.1988.tb02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanneau M., Loir M. An electrophoretic investigation of mammalian spermatid-specific nuclear proteins. J Reprod Fertil. 1982 May;65(1):163–170. doi: 10.1530/jrf.0.0650163. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Bartels I., Engel W. Haploid expression of a protamine gene during bovine spermatogenesis. Biol Chem Hoppe Seyler. 1987 Jul;368(7):807–811. doi: 10.1515/bchm3.1987.368.2.807. [DOI] [PubMed] [Google Scholar]
- Maier W. M., Adham I., Klemm U., Engel W. The nucleotide sequence of a boar protamine 1 cDNA. Nucleic Acids Res. 1988 Dec 23;16(24):11826–11826. doi: 10.1093/nar/16.24.11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. J., Renaux B. S., Dixon G. H. Human sperm protamines. Amino-acid sequences of two forms of protamine P2. Eur J Biochem. 1986 Apr 1;156(1):5–8. doi: 10.1111/j.1432-1033.1986.tb09540.x. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puwaravutipanich T., Panyim S. The nuclear basic proteins of human testes and ejaculated spermatozoa. Exp Cell Res. 1975 Jan;90(1):153–158. doi: 10.1016/0014-4827(75)90368-7. [DOI] [PubMed] [Google Scholar]
- Rentrop M., Knapp B., Winter H., Schweizer J. Aminoalkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J. 1986 May;18(5):271–276. doi: 10.1007/BF01676237. [DOI] [PubMed] [Google Scholar]
- Tobita T., Nomoto M., Nakano M., Ando T. Isolation and characterization of nuclear basic protein (protamine) from boar spermatozoa. Biochim Biophys Acta. 1982 Oct 5;707(2):252–258. doi: 10.1016/0167-4838(82)90358-2. [DOI] [PubMed] [Google Scholar]
- Tobita T., Tanimoto T., Nakano M. The binding mode of a mammalian (boar) protamine to DNA. Biochem Int. 1988 Jan;16(1):163–173. [PubMed] [Google Scholar]
- Vanfleteren J. R., Van Bun S. M., Van Beeumen J. J. The primary structure of histone H2A from the nematode Caenorhabditis elegans. Biochem J. 1987 Apr 1;243(1):297–300. doi: 10.1042/bj2430297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Wagner K. G. The binding of protamines to DNA; role of protamine phosphorylation. Biophys Struct Mech. 1980;6(2):95–110. doi: 10.1007/BF00535747. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelick P. C., Balhorn R., Johnson P. A., Corzett M., Mazrimas J. A., Kleene K. C., Hecht N. B. Mouse protamine 2 is synthesized as a precursor whereas mouse protamine 1 is not. Mol Cell Biol. 1987 Jun;7(6):2173–2179. doi: 10.1128/mcb.7.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]