Abstract
Objective
Test the clinical efficacy of a web-based intervention designed to increase patient self-efficacy to perform headache self-management activities and symptom management strategies; and reduce migraine-related psychological distress.
Background
In spite of their demonstrated efficacy, behavioral interventions are used infrequently as an adjunct in medical treatment of migraine. Little clinical attention is paid to the behavioral factors that can help manage migraine more effectively, improve the quality of care, and improve quality of life. Access to evidenced-based, tailored, behavioral treatment is limited for many people with migraine.
Design
The study is a parallel group design with two conditions, (1) an experimental group exposed to the web intervention, and (2) a no-treatment control group that was not exposed to the intervention. Assessments for both groups were conducted at baseline (T1), 1-month (T2), 3-months (T3), and 6-months (T4).
Results
Compared to controls, participants in the experimental group reported significantly: increased headache self-efficacy, increased use of relaxation, increased use of social support, decreased pain catastrophizing, decreased depression, and decreased stress. The hypothesis that the intervention would reduce pain could not be tested.
Conclusions
Demonstrated increases in self-efficacy to perform headache self-management, increased use of positive symptom management strategies, and reported decreased migraine-related depression and stress, suggest that the intervention may be a useful behavioral adjunct to a comprehensive medical approach to managing migraine.
Keywords: migraine, e-Health, self-management, psychosocial, coping, self-efficacy
BACKGROUND
Migraine affects almost 12% of the population in the U.S., with a gender-based prevalence of 17.1% in women and 5.6% in men. However, the real incidence of migraine is believed to be much higher since migraine is often undiagnosed (1). The incidence of migraine is highest from ages 30 to 39, meaning its greatest impact is during peak productive years (2). Migraine is the most common pain condition cited by individuals losing productive time at work (3). Migraine takes a significant toll on the quality of life for people with migraine and their family members. Chronic migraine pain negatively influences daily functioning, emotions, and roles in social and family contexts (1). Concomitant psychiatric disorders, including depression and anxiety, often accompany migraine and add significant morbidity. For example, major depression occurs in 16% of people without migraine, but in 37% of people with migraine without aura and in 49.4% of people with migraine with aura (4). The majority of people with migraine are in great need of education regarding the efficacy of behavioral self-management, the use of over-the-counter medication, the advantages of prescription medications, and the benefits of physician referral (5). Disease specific patient education and the provision of behavioral support for people with migraine occur infrequently in clinical practice or occur in a non-systematic manner. Even when patient education and behavioral support are provided, large variations exist in how it is delivered. (6).
Behavioral approaches based on the theory that biological, psychological, and environmental factors all play significant roles in migraine, offer an approach to treatment that can enhance pharmacotherapy. Behavioral interventions generally fall into three broad categories: (1) behavioral skills training; (2) cognitive techniques; and (3) relaxation or stress management. Beliefs about pain, fears of harm, avoidance responses, attributions of the origins of pain, expectations of treatment, self-efficacy, and coping are some of the factors that influence behavioral responses to migraine pain, and are key targets of psychologically-based treatments (7). Three critically important psychological targets of intervention are: (1) anxiety and fear, (2) pain catastrophizing, and (3) environmental factors.
Behavioral interventions also include a focus on the prevention of headaches, increasing feelings of control over headaches, reducing the severity of headaches and headache disability, trigger avoidance, lifestyle modification, and appropriate use of medications. Over three decades of research have shown the utility of a number of behavioral treatments, primarily relaxation training and cognitive behavioral therapy (8; 9).
There are many barriers that interfere with helping people with migraine manage their headache pain more effectively, including negative attitudes and timidity about prescribing analgesics (10), misconceptions about the nature of migraine, medications, and medication side-effects (5), and communication problems between patients and providers (11). While pharmacologic interventions are readily available for both the acute and preventive treatment of migraine, and are used to some degree by most migraine sufferers, they are not always effective, well-tolerated, or preferred by the patient (12). While several comprehensive reviews of behavioral treatments have been published (9; 13), many providers (and patients) remain unaware of the effectiveness of behavioral interventions for migraine (12). In addition, people with chronic migraine are often less mobile (14), limiting their ability to attend face-to-face groups and educational programs that provide self-management and emotional support.
Unfortunately, the availability of behavioral self-management programs has been limited by factors such as low rates of physician referral, a lack of trained clinicians to teach patients self-management skills, and the fact that these programs are not readily available or affordable to many people with migraine. The Internet offers a viable way to deliver behavioral self-management support to assist patients in managing a wide variety of conditions (15; 16;17;18;19;20; 21). While online programs to help people with migraine are a logical way to overcome existing barriers, there has been little research that has focused on demonstrating the efficacy of these approaches. The present study tested a web-based intervention called painACTION. The intervention was developed in accordance with the self-management education model developed by Lorig and colleagues at Stanford University (22), which has been successfully applied to a wide range of conditions (23). The intervention incorporates Cognitive Behavior Therapy (CBT) and self-management principles to teach people with migraine “how to” apply practical self-management skills, techniques, and strategies to motivate and support participant engagement in active pain self-management behaviors. The most unique aspect of painACTION is the use of a custom “recommendation engine,” a personalized information filtering technology, to identify and deliver highly targeted content recommendations that address users’ priorities and needs.
The intervention includes components focused specifically on migraine, in addition to components focused on general behavioral pain management. The intervention contains five core components, dealing with: (1) migraine specific knowledge (e.g., factual medical information such as phases of the migraine cycle, understanding aura, migraine and the menstrual cycle, and medication overuse headaches); (2) migraine self-management skills (e.g., how to keep a headache diary, recognizing and managing headache triggers, relaxation and biofeedback, and managing migraine-related nausea); (3) emotional coping (e.g., reducing migraine-related anxiety, managing negative thinking, increasing social support, and controlling catastrophizing); (4) communication skills (e.g., describing headache pain, talking with family and co-workers about migraine, effective patient-provider communication); and (5) medication safety (e.g., alcohol and medication, understanding medication side-effects, and medication storage and disposal).
The content of the intervention was delivered in a variety of formats, including: (1) Lessons - interactive instruction for learning practical pain self-management skills and strategies, and how to apply them to solve problems; (2) Tools – visual and graphic interactive learning experiences that allow users to actively manipulate information to construct knowledge and learn to solve problems; (3) Self-assessments – structured sets of questions that help users to reflect on and learn what skills and knowledge they have, where their relative strengths and deficits are, and how to identify what behaviors to target for change; (4) User Generated Content (“Shared Knowledge”) –practical advice, strategies, and real-life examples gathered from other site users and presented via text, audio, and video.
Overall, the intervention aims to help people with migraine participate more fully in collaborative decision-making with health professionals; improve self-efficacy to manage headache pain, reduce negative thinking and emotional distress; understand goal setting and strategies to promote health behavior change; and learn skills to prevent headaches and relieve headache symptoms.
The intervention fits closely with the most recent definition of “Internet-supported therapeutic interventions” because of: (1) the inclusion of structured behavior change components intended to modify users’ cognitions and behavior; (2) its use of more than one multimedia format; (3) a high level of interactivity; and (4) the provision of tailored feedback to users (24). The site is non-promotional, and presents unbiased content that has been developed and reviewed by a multi-disciplinary team of behavioral and pain management experts, including physicians, psychologists, nurses, pharmacists, social workers, physical therapists, and other allied health professionals. We are not aware of any other tailored Internet-based interventions for migraine that have been the subject of a large-scale, randomized clinical trial.
This study tested the hypotheses that, relative to a control condition receiving no intervention (but maintaining their routine migraine care), participants exposed to the painACTION website would report: (1) reduced frequency and severity of headaches; (2) increased self-efficacy to perform headache self-management; (3) increased use of positive coping strategies; (4) increased internal locus of control; (5) reduced migraine-related emotional distress (e.g., stress, anxiety, and depression); and (6) reduced negative cognition (e.g., catastrophizing). Based on recommendations by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) (25), we also hypothesized clinically significant changes in physical and emotional functioning, global rating of improvement, and pain intensity.
METHODS
Participants
A total of 213 people with chronic migraine headache were recruited through several methods: (1) website postings (National Headache Foundation and the American Chronic Pain Association); (2) electronic newsletter announcements (PainEDU.org, Alliance of State Pain Initiatives, and the American Chronic Pain Association); (3) 22 neurology practices in a geographically diverse group of 16 states distributed informational flyers to patients; and (4) postings to social networking/community sites (Facebook and Craigslist).
Volunteers were eligible for the study if: (1) they were between ages 18–65; (2) they met ICHD-II diagnostic criteria for migraine, with or without aura; (3) their migraines had been present for at least one year; (4) they experienced at least 48 continuous hours of freedom from headache or migraine per month; (5) their age of onset of migraine was less than 50 years; (6) they were able to provide informed consent; (7) they had a migraine at least twice a month; (8) they had daily Internet access with email; and (9) they completed the Daily Headache Record for a minimum of 5 out of 14 days during a “run-in” period. Exclusion criteria were: (1) endorsement of questions indicating that migraine may be indicative of a progressive disease; (2) presence of fibromyalgia or epilepsy; (3) experiencing non-migraine headaches more than six days per month; (4) participants completing less than four entries during the “run-in” period (explained below); and (5) non-English speaking.
To validate whether participants met ICHD-II criteria for migraine, all volunteers were first asked: “Has a doctor diagnosed you with migraine?” Those responding “no” were screened out. Volunteers who responded “yes” were screened in, and were then asked through a structured interview conducted by the Research Coordinator, a migraine-specific set of questions corresponding to ICHD criteria. If the Research Coordinator had any questions about the volunteer’s responses, these were reviewed on a case-by-case basis with a board certified physician and pain management specialist, before a volunteer was included or excluded from the study. Volunteers who met ICHD-II criteria we included in the study and those not meeting criteria were screened out.
Participants were paid $25 for completing each assessment during the study (baseline, 1-month, 3-month, and 6-month follow ups). The study protocol and all recruitment materials were reviewed and approved on March 12, 2008 by an independent institutional review board (New England Institutional Review Board, Wellesley, MA). Participant recruitment began on Oct 24, 2008, and the collection of follow-up data ended on October 21, 2009.
Procedures
“Run-in” Period
All participants first completed a “run-in period” during which they completed the Daily Headache Record (described below) for two weeks to establish their baseline level of headache severity and duration. Participants were required to make at least 4 entries in the log over the two-week period. The average number of entries completed by all participants was 10.6 over the two-week period.
Prior to beginning the study, research staff identified three factors that could present a problematic source of confounding: gender, headache intensity as measured by the Daily Headache Record (low/high), and self-reported headache frequency (<5 migraines per month vs. >=5 headaches per month). The cut-off of less than five headaches per month was chosen because it reflects a headache frequency of one or less migraine headaches per week. Study staff created a randomization table that contained eight blocks (all combinations of the balancing factors; i.e., high pain/<5 headaches per month/male, low pain/>=5 headaches per month/female). Within each block, a random number table was used to generate experimental/control assignments. After finishing the two week “run-in” period, participants were matched to a block according to their demographic characteristics, and sequentially assigned to either the experimental or control condition.
Experimental Group
Participants were provided with a user name and password to access the site, and asked to complete a minimum of eight 20-minute sessions on the site over a four-week intervention period (two per week), and a minimum of five 20-minute follow-up sessions during the follow-up period (one per month). However, participants were given the freedom to spend as much time on the site as they wished. Instructions were provided via a structured “session checklist” which included a list of tasks for the participant to complete during each of the required sessions. These tasks included a minimum set of requirements for participants to complete migraine-specific content, such as completing self-assessments (e.g., Self-efficacy to Manage Migraines), taking lessons (e.g., How to Keep A Headache Diary), using interactive tools (e.g., Phases of Migrane tool), reading articles (e.g., Caffeine and Migraines), and using the pain tracker (daily tracking of migraine pain, triggers, and medications). In order to facilitate completion of the follow-up sessions, participants were e-mailed session checklists one-, two-, three-, four-, and five-weeks post-intervention, with instructions to complete each follow-up session within one week. Experimental group participants were asked to maintain their routine migraine care and self-management efforts during the study.
Because recent reviews of web-based behavioural intervention have recommended using several means to measure participant use of online interventions (26), a number of methods were employed to monitor and measure participant use of the website. Participants were asked to track the activities they completed and time spent during each session on a separate secure website using a unique identification number. In this way participant activity was continuously monitored on a daily basis by the research team. In addition, the research team was able to monitor each participant’s activity (session date and minutes spent) through a review of server logs which captured individual user’s unique identification numbers. During the study period, participants who did not complete sessions as scheduled, or spent insufficient time on a session, were sent email reminders to log in and complete their sessions or remaining time.
Control Group
The control condition for this study was treatment as usual, that is participants were only asked to maintain their routine migraine care and self-management efforts. While the “gold standard” in study design is a double-blind, placebo controlled study, it has been noted by various investigators in the migraine field (27, 28) that when testing a behavioral intervention, a “behavioral placebo” is often impossible to create, and double-blinding is not possible. Therefore, we chose to assign control participants to “treatment as usual” so that the Hawthorne effect (improvement due to being studied and given attention from researchers) will be controlled for, as will a history effect where some participants may improve over time regardless of the intervention given. The study hypothesis was that participants in both groups will improve, but participants in the experimental group who receive essentially the control intervention (treatment as usual) plus exposure to painACTION will improve to a greater extent, especially on measures of self-efficacy to manage headaches and locus of control. All participants in the control condition were offered access to the painACTION intervention once the study was completed.
Measures
Based on the recommendations made by the IMMPACT group (26), we included clinically validated measures of pain intensity, physical functioning, emotional functioning, and a global rating of perceived improvement. In addition, we included validated measures of pain coping, headache locus of control, cognitive functioning (catastrophizing), and self-efficacy to perform headache self-management. Specifically, the following measures were used:
Demographic Questionnaire
A demographic questionnaire gathered information on each participant’s age, gender, race, marital status, education, household size, job type, and health status (including common risk-factors for migraine). This information was gathered only at the baseline assessment.
Daily Headache Record (29)
This form records headache duration (number of hours) and severity on a 4-point scale (0= no headache; 1= mild headache pain, allowing normal activity; 2= moderate headache pain, disturbing but not prohibiting normal activity; 3= severe headache pain, normal activity was discontinued, bed rest may be necessary). Participants in the control and experimental conditions completed this measure for 14 days during the run-in period.
Migraine Disability Assessment Questionnaire (MIDAS) (30)
The 5-item questionnaire measures migraine disability, frequency and severity. The measure has high internal consistency and test-retest reliability. The MIDAS exhibited construct validity, since MIDAS scores in migraine sufferers were substantially higher than in people with non-migraine headache.
Chronic Pain Coping Inventory-42 (CPCI-42) (31; 32)
The CPCI-42 is a 42-item self-report measure that assesses the frequency of use of behavioral and cognitive coping strategies on eight subscales: Guarding, Resting, Asking for Assistance, Relaxation, Task Persistence, Exercise/Stretching, Seeking Social Support, and Coping Self-Statements. The CPCI-42 demonstrated very high correlations between the original and abbreviated CPCI scales, as well as comparable internal consistency, test-retest stability, and validity coefficients.
Headache Management Self-Efficacy Scale (HSES) (33)
The HSES is a 25-item measure of self-efficacy to manage headaches. The questionnaire is formatted such that the respondents identify items that they believe trigger their headaches, and then, for each item endorsed as a trigger, they are asked, “How confident are you that you can take action to prevent a headache in the following situations?” Respondents answer from 1 (very confident) to 5 (no confidence). Sample trigger situations are “When you drink red wine,” “When you’re arguing with someone,” and “When there is a problem you can’t solve.” Test-retest reliability during a 3-week period was r =.67.
Pain Catastrophizing Scale (PCS) (34)
The PCS assesses three areas of pain catastrophizing: rumination, magnification, and helplessness. The scale consists of 13 items rated from 0–4 (0 = not at all, 4 = all the time). The measure has demonstrated criterion-related, concurrent, and discriminant validity in community samples.
Headache-Specific Locus of Control (HSLC) (35)
The HSLC is a 33-item measure which assesses the extent to which individuals believe headache factors can be controlled internally. It has 3 subscales: healthcare professional’s locus of control (i.e., I usually recover from a headache when I get proper medical help), internal locus of control (i.e., When I drive myself too hard I get headaches), and chance locus of control (i.e., I’m likely to get headaches no matter what I do). The subscales have a high internal consistency with alphas ranging from .84 to .88. Test-retest reliability over a 3-week period was .75 for the internal subscale, .78 for the health professional subscale, and .72 for the chance subscale. Scores on the chance locus of control subscale positively correlated with headache frequency (.24), depression (.27), physical symptoms (.28), disability (.23), and catastrophizing (.44).
Depression Anxiety Stress Scales (DASS-21) (36)
The DASS is a 21-item questionnaire that distinguishes symptoms of depression, anxiety, and stress, factors that are known to be associated with migraines. The psychometric properties of the DASS have been initially tested by its authors (36) and then subsequently confirmed by others (37; 38; 39). The DASS yields 3 subscale scores: depression, anxiety, and stress.
Patient Global Impression of Change (PGIC) (40)
The PGIC is a single-item measure of global improvement with treatment on which respondents use a seven-point scale (“very much improved” to “very much worse”) to report perceived improvement with an intervention. The PGIC is widely used in chronic pain research (41; 42).
Power and Sample Size Considerations
This study was powered for a conservative small effect size, since both groups were expected to improve to some degree. Using a Bonferonni correction to account for two primary measures (alpha=.025), for four assessments and two experimental groups, an N of 168 produces 80% power for a small time*intervention interaction effect (d =0.16). For efficacy studies involving repeated measures analyses, the most important power to maximize is that of the interaction effect, as this effect distinguishes differential change over time between the two intervention groups. To account for possible attrition of 20%, we aimed to recruit 210 people with migraine to participate (210 less 20%=168). Power calculations were carried out using G*Power version 3.1.2 (Institut fur Experimentelle Psychologie, Dusseldorf, Germany).
Statistical Analysis
Data analysis was carried out in the following steps: (1) computed descriptive statistics for all demographic variables and tested for differences in demographics between conditions (experimental vs. control) at baseline, (2) tested for mean differences between conditions over time on each primary outcome) using linear mixed modeling (LMM), also known as mixed-effect regression modeling (MMRM), (3) tested for differential website effects based on participant recruitment source, age, and gender using LMM, (4) tested whether spending more time on the website resulted in a greater treatment effect using LMM, (5) compared the study outcomes to IMMPACT criteria (26), and (6) conducted a “completer” analysis to test whether the intervention effect was different for participants who completed all four assessments, compared to those who did not A mixed model approach was used because of its ability to handle missing data and to model co-variation using flexible covariance structures among repeated measures (an unstructured residual variance-covariance structure was employed for all analyses). LMM has been shown to provide better control of bias and Type I error in cases of incomplete or missing data, compared to other methods such as “last observation carried forward” (LOCF) approaches (43;44). The statistical focus was on the two-way interaction effect, treatment-BY-time, as this effect tests whether or not the website was more effective than the control condition over time. Significant two-way interactions were followed up by appropriate contrasts.
The study was originally powered for two primary outcomes; however, due to a data management error, these data are not available for analysis. Considering the secondary outcomes as primary and using the suggested Bonferroni correction, when the original power analysis was conducted with two primary outcomes in mind, could result in an inflation of the Type II error rate. As such, level of significance was set at α=.05 for each analysis. To maintain an alpha of .05 for each statistical test, a Bonferroni correction was applied to all post-hoc contrasts; p-values reported for post-hoc comparisons have been Bonferroni-corrected unless otherwise noted. All analyses were run using SAS 9.2 software (SAS Institute Inc., Cary, North Carolina, USA). All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
RESULTS
Sample
Participants
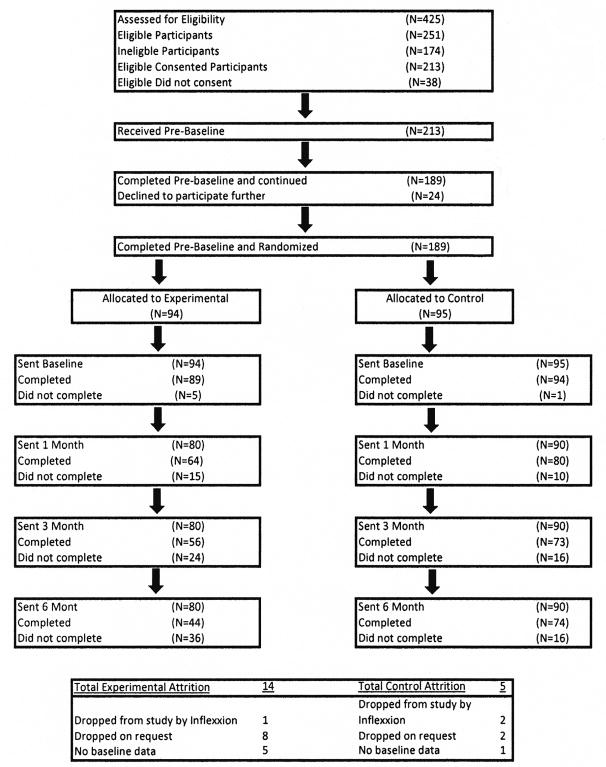
Four hundred and twenty-five volunteers were screened for eligibility, which resulted in 213 participants being consented and enrolled in the two-week run-in period. During this period, 24 participants dropped out or failed to complete the minimum number of pain ratings required, resulting in 189 participants who were randomized into the experimental (n=95) and control (n=94) conditions. Of these participants, four (three control and one experimental) dropped out following the run-in period, resulting in a final study sample of 185 participants (92 experimental group participants and 93 control group participants). Follow-up assessment completion was less than 100% for both the experimental and control groups: 80% of experimental participants completed post-assessment, 70% completed the three-month assessment, and 55% completed the six-month assessment, compared to the control group (89%, 82%, and 82%, respectively). Differential participant attrition is further described in the Discussion section. No adverse events were reported by participants during the study.
Participant Characteristics
Of the 185 participants included in the analysis, 123 were recruited through website postings (National Headache Foundation and the American Chronic Pain Association), 26 through newsletter announcements (PainEDU.org and the American Chronic Pain Association), 29 through advertisements in medical offices, and 7 through postings to social networking/online community sites (Facebook and Craigslist). Eighty-nine percent of the sample was female, and 87.5% were white; of the 23 non-white participants, 9 reported their race as African American, 4 identified as Hispanic/Latino, and 4 identified as Asian American. The mean age of the sample was 42.6 (SD=11.5) years, with participants’ ages ranging from 20 to 66 years old. Sixty percent of the enrolled participants reported their employment status as “full time,” 14.4% as “part-time,” 5% as “unemployed,” 2.2% as “disabled,” 3.9% as “retired,” 4.4% as “students,” and 10.0% “homemakers.” More than half of the sample reported their annual household income as greater than $50,000. Participants’ mean pre-baseline headache severity was 2.11 (SD=0.61), with a mean duration of 2.47 hours per day (SD=1.44). One hundred twenty-five participants (69.4% of the sample; 62.6% of the experimental group and 76.4% of controls) reported using opioid pain medication at the beginning of the study. No significant differences in demographics were found between the control and experimental groups. Descriptive statistics for the study sample are presented in Table 1.
Table 1.
Demographic characteristics for 185 study participants
| Total n=185* |
Control n=92 |
Experimental n=93 |
|
|---|---|---|---|
| Age | 42.62(11.50) | 41.91(11.53) | 43.32(11.49) |
| Gender | |||
| Male | 20(10.81) | 10(10.87) | 10(10.75) |
| Female | 165(89.19) | 82(89.13) | 83(89.25) |
| Race | |||
| White/Non-Hispanic | 161(87.50) | 81(88.04) | 80(86.96) |
| Black/African-American | 9(4.89) | 5(5.43) | 4(4.35) |
| Asian American | 4(2.17) | 0(0.00) | 4(4.35) |
| Native Hawaiian/Other Pacific Islander | 1(0.54) | 1(1.09) | 0(0.00) |
| Hispanic/Latino | 4(2.17) | 3(3.26) | 1(1.09) |
| Other | 5(2.72) | 2(2.17) | 3(3.26) |
| Marital Status | |||
| Single (never married) | 32(17.78) | 15(16.48) | 17(19.10) |
| Married | 119(66.11) | 62(68.13) | 57(64.04) |
| Separated | 3(1.67) | 1(1.10) | 2(2.25) |
| Widowed | 1(0.56) | 1(1.10) | 0(0.00) |
| Divorced | 13(7.22) | 4(4.40) | 9(10.11) |
| Remarried | 2(1.11) | 2(2.20) | 0(0.00) |
| Living with partner | 10(5.56) | 6(6.59) | 4(4.49) |
| Income | |||
| <$24,999 | 11(6.11) | 5(5.49) | 6(6.74) |
| $25,000–49,999 | 28(15.56) | 18(19.78) | 10(11.24) |
| $50,000–74,999 | 31(17.22) | 15(16.48) | 16(17.98) |
| $75,000–99,999 | 49(27.22) | 23(25.27) | 26(29.21) |
| $100,000–149,999 | 24(13.33) | 12(13.19) | 12(13.48) |
| $150,000–199,999 | 12(6.67) | 6(6.59) | 6(6.74) |
| >$200,000 | 9(5.00) | 5(5.49) | 4(4.49) |
| No Answer | 16(8.89) | 7(7.69) | 9(10.11) |
| Employment | |||
| Full-time | 108(60.00) | 60(65.93) | 48(53.93) |
| Part-time | 26(14.44) | 9(9.89) | 17(19.10) |
| Unemployed | 9(5.00) | 3(3.30) | 6(6.74) |
| Disability | 4(2.22) | 1(1.10) | 3(3.37) |
| Homemaker | 18(10.00) | 11(12.09) | 7(7.87) |
| Retired | 7(3.89) | 3(3.30) | 4(4.49) |
| Student | 8(4.44) | 4(4.40) | 4(4.49) |
| Education | |||
| <11th Grade | 1(0.56) | 0(0.00) | 1(1.12) |
| HS or GED | 31(17.22) | 18(19.78) | 13(14.61) |
| 2 years of college/AA degree/Technical school training | 40(22.22) | 12(13.19) | 28(31.46) |
| College graduate (BA or BS) | 61(33.89) | 33(36.26) | 28(31.46) |
| Master’s degree | 36(20.00) | 22(24.18) | 14(15.73) |
| Doctoral/Medical/Law degree | 11(6.11) | 6(6.59) | 5(5.62) |
| Opioid Users | |||
| Yes | 125(69.44) | 57(62.64) | 68(76.40) |
| No | 55(30.56) | 34(37.36) | 21(23.60) |
| Average Baseline Headache Severity | 2.11(0.61) | 2.13(0.64) | 2.10(0.57) |
| Average Baseline Headache Duration | 2.47(1.44) | 2.46(1.48) | 2.48(1.41) |
5 Participants (1 control and 4 experimental) were missing data on marital status, income, employment, education, and opioid use.
In accordance with the CONSORT group (45) the flow of participants through the study is documented in Figure 1. Because the study was designed with an intent-to-treat approach, the goal was to follow as many participants as possible, regardless of their completion of the interventions.
Figure 1.
Primary Analyses: Treatment Effects
LMMs were run to ascertain whether experimental participants, as compared to control participants, evidenced a significantly greater mean change over time on: (1) psychological distress; (2) use of positive coping strategies; (3) self-efficacy; (4) pain catastrophizing; (5) migraine-related disability, (6) locus of control, and (7) patient global impression of change. The results of these analyses are presented below. Further details can be found in Table 2.
Table 2.
Least-squares means and standard errors for all primary outcome measures over time for 185 participants
| Control | Experimental | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline n=91 |
Post n=87 |
3-month n=73 |
6-month n=74 |
Baseline n=89 |
Post n=68 |
3-month n=51 |
6-month n=46 |
|
| Chronic Pain Coping Inventory | ||||||||
| Assistance | 1.43(0.17) | 1.42(0.18) | 1.62(0.18) | 1.58(0.20) | 1.57(0.17) | 1.81(0.19) | 1.74(0.21) | 1.66(0.23) |
| Coping | 2.19(0.22) | 2.09(0.21) | 2.09(0.22) | 1.99(0.24) | 2.38(0.22) | 2.91(0.23) | 2.92(0.25) | 2.82(0.28) |
| Exercise | 1.66(0.17) | 1.61(0.19) | 2.01(0.21) | 2.00(0.22) | 1.55(0.17)a | 2.44(0.21)a | 2.48(0.24) | 2.16(0.26) |
| Persistence | 2.46(0.17) | 2.33(0.18) | 2.28(0.21) | 2.08(0.19) | 2.07(0.18)ab | 2.66(0.20)a | 2.80(0.24)b | 2.25(0.23) |
| Resting | 2.36(0.18) | 2.11(0.17) | 2.17(0.18) | 2.18(0.21) | 2.76(0.18) | 2.96(0.19) | 3.13(0.21) | 2.55(0.24) |
| Relaxation | 1.36(0.15) | 1.47(0.17) | 1.60(0.19) | 1.47(0.20) | 1.46(0.15)abc | 2.30(0.18)a | 2.45(0.21)b | 2.18(0.22)c |
| Social | 1.67(0.18) | 1.25(0.18) | 1.56(0.20) | 1.46(0.20) | 1.70(0.18)a | 2.29(0.20)a | 2.24(0.22) | 2.11(0.23) |
| Guarding | 1.14(0.14) | 1.05(0.14) | 1.19(0.15) | 1.38(0.17) | 1.33(0.14) | 1.28(0.15) | 1.41(0.17) | 1.16(0.20) |
| Depression/Anxiety/Stress Scale | ||||||||
| Anxiety | 19.52(0.83) | 19.85(0.76) | 19.57(0.79) | 19.39(0.85) | 20.42(0.83) | 18.89(0.80) | 18.54(0.86) | 18.66(0.99) |
| Depression | 21.38(0.97) | 21.44(0.90) | 21.40(0.84) | 22.03(1.02) | 22.77(0.98)ab | 20.39(0.97) | 18.63(0.94)a | 19.67(1.21)b |
| Stress | 26.68(1.07) | 26.66(0.96) | 26.69(0.96) | 25.21(1.08) | 26.95(1.08)ab | 24.10(1.02)a | 23.09(1.06)b | 22.79(1.28) |
| Headache Self-Efficacy Scale | ||||||||
| Total Score | 108.80(2.08) | 112.20(2.12) | 114.74(2.60) | 116.19(2.50) | 103.21(2.10)abc | 120.16(2.27)a | 122.12(2.86)b | 121.45(2.85)c |
| Headache-Specific Locus of Control | ||||||||
| Chance | 35.26(0.98) | 36.61(1.01) | 36.63(1.05) | 36.18(1.03) | 33.74(0.99)a | 36.41(1.07) | 37.08(1.17) | 38.97(1.17)a |
| Health Care | 34.55(0.81) | 33.25(0.79) | 34.29(0.85) | 33.26(0.88) | 33.93(0.82) | 32.09(0.84) | 32.72(0.93) | 31.40(1.01) |
| Professional Internal | 35.29(0.99) | 36.08(0.91) | 36.92(1.02) | 36.85(1.00) | 35.27(0.99) | 38.20(0.96) | 37.72(1.12) | 36.89(1.11) |
| MIDAS | ||||||||
| Total Score | 51.60(4.74) | 46.04(4.80) | 42.54(5.25) | 39.45(4.94) | 48.66(4.78) | 42.48(5.08) | 42.17(5.79) | 36.05(5.75) |
| Medication Safety Knowledge1 | ||||||||
| Total Correct | 6.13(0.19) | 6.30(0.19) | -- | 6.49(0.18) | 6.27(0.17) | 6.10(0.20) | -- | 5.88(0.22) |
| Pain Catastrophizing Scale | ||||||||
| Helplessness | 13.14(0.68) | 12.67(0.62) | 11.66(0.66) | 11.56(0.60) | 15.01(0.68)abc | 11.99(0.66)a | 11.10(0.74)b | 10.31(0.70)c |
| Magnification | 6.02(0.30) | 5.81(0.29) | 5.34(0.28) | 5.27(0.29) | 6.50(0.31)a | 5.44(0.31) | 4.63(0.32)a | 4.72(0.34) |
| Rumination | 11.09(0.49) | 10.25(0.47) | 9.44(0.49) | 9.83(0.51) | 11.93(0.50)ab | 9.71(0.51) | 8.34(0.57)a | 7.61(0.60)b |
| Total | 30.25(1.35) | 28.70(1.26) | 26.41(1.34) | 26.66(1.29) | 33.44(1.36)abc | 27.13(1.34)a | 24.12(1.50)b | 22.62(1.50)c |
| Patient Global Assessment of Change | ||||||||
| Improvement | -- | 3.56(0.09) | 3.48(0.11) | 3.34(0.12) | -- | 3.03(0.10) | 2.60(0.13) | 2.49(0.15) |
Analysis limited to participants currently taking opioid medications (control n=57; experimental n=68; total n=125).
Shared subscripts on one line indicate significantly different post-hoc contrasts (Bonferroni-adjusted alpha=.0167)
Changes from baseline to immediately post intervention were examined in light of IMMPACT criteria. Details of these comparisons are presented below, and are further outlined in Table 2.
Psychological Distress
A significant effect of treatment-BY-time was noted for two subscales of the DASS: depression (F3,179=4.86, p=0.0028) and stress (F3,179=4.07, p=0.0079). Post hoc tests revealed that participants who used the website reported a significantly greater decrease in depression, as compared to the control condition, from baseline to 3-month follow-up (t=3.66, p=0.0009) and baseline to 6-month follow-up (t=2.50, p=0.0399); participants exposed to the website also reported a significantly greater decrease in stress, as compared to the control condition, from baseline to post-intervention (t=2.57, p=0.0324) and from baseline to 3-month follow-up (t=3.23, p=0.0045). There was no significant difference between conditions over time on the anxiety subscale of the DASS. Experimental participants reported a 12.5% decrease in depression from baseline to immediately post-intervention, while controls reported no change; similarly, experimental participants reported a 7.7% decrease in anxiety and a 4.1% decrease in stress during the same time period, compared to a 2.4% increase in anxiety and a 0.7% decrease in stress for control participants.
Pain Catastrophizing
LMMs were run to test for a treatment-BY-time effect for all subscales of the PCS, including helplessness (F3,179=5.29, p=0.0016), magnification (F3,179=3.23, p=0.0238), rumination (F3,179=5.17, p=0.0019), and total score (F3,179=5.63, p=0.0010). Participants exposed to the website reported a greater decrease in feelings of helplessness from baseline to post-intervention (t=3.59, p=0.0012), baseline to 3-month follow-up (t=2.70, p=0.0228), and from baseline to 6-month follow-up (t=3.35, p=0.0030); they also reported a greater decrease in magnification of their pain from baseline to 3-month follow-up (t=2.92, p=0.0120), as well as a greater decrease in rumination from baseline to 3-month follow-up (t=2.53, p=0.0366) and from baseline to 6-month follow-up (t=3.88, p=0.0003). Participants exposed to the webs ite had a greater overall reduction in their pain catastrophizing from baseline to post-intervention (t=3.34, p=0.0030), baseline to 3-month follow-up (t=2.98, p=0.0099), and baseline to 6-month follow-up (t=3.80, p=0.0006), compared to control participants.
Positive Coping Strategies
A significant treatment-BY-time effect was noted for four subscales of the CPCI: exercise (F3,179=7.18, p<0.001), persistence (F3,179=4.04, p=0.0083), relaxation (F3,179=6.53, p=0.0003), and use of social support (F3,179=5.55, p=0.0012). Participants exposed to the website reported a greater increase in relaxation from baseline to post-intervention (t=−3.99, p<0.001), baseline to 3-month assessment (t=−3.30, p=0.0036), and baseline to 6-month assessment (t=−2.69, p=0.0237), as well as a greater increase in task persistence from baseline to post-intervention (t=−3.02, p=0.0087) and from baseline to 3-month follow-up (t=−3.03, p=0.0084), a greater increase in exercising from baseline to immediately post-intervention (t=−4.19, p<0.001), and greater increase in use of social supports from baseline to post-intervention (t=−4.06, p<0.001), relative to control participants.
Self-Efficacy
Results from LMM revealed a significant treatment-BY-time effect for self-efficacy (F3,179=10.99, p<0.0001): participants exposed to the website reported greater increases in self-efficacy from baseline to post-intervention (t=−5.36, p<0.0010), baseline to 3-month follow-up (t=−4.40, p<.0010), and baseline to 6-month follow-up (t=−3.47, p=0.0018), relative to control participants.
Migraine-related Disability
LMMs were run to test for a treatment-BY-time effect on migraine-related disability; no significant effect of treatment over time was noted. Experimental and control participants reported similar reductions in disability (12.8% decrease and 13.0% decrease, respectively) from baseline to immediately post intervention.
Locus of Control
A significant treatment-BY-time effect was noted for the chance subscale of the head-specific locus-of control scale (F3,179=3.59, p=0.0149); unexpectedly, participants who used the website has a greater increase in reporting that their headache problems and relief were influenced primarily by chance, from baseline to 6-month follow-up (t=−3.16, p=0.0018), relative to the control group.
Patient Global Impression of Change
A LMM was run to test for a significant effect of treatment on participants’ global impression of change. Significant effects of both treatment (F1,159=33.58, p<0.01) and time (F 2,159=8.30, p<0.01) were noted; compared to the control group, participants who used the website reported a greater average improvement in their condition at post (t=3.78, p<0.01), 3-month follow-up (t=5.23, p<0.01), and 6-month follow-up (t=4.32, p<0.01). Comparing participants who used the website to controls revealed that 70.6% of experimental participants rated their condition as at least minimally improved immediately post-intervention and none of the experimental participants indicated that their condition had worsened. By contrast, only 36.8% of controls rated their condition as at least minimally improved, while 57.5% reported no change in their condition and 4.6% reported that their condition had worsened since the beginning of the study.
Moderation Analyses
LMMs were also run to test for differential effects of treatment depending on participant recruitment source (online vs. clinic), age and gender. Participant recruitment source, age, and gender were not found to be important moderators of the effect of the treatment over time.
Dose Analyses
Effects of Dose over Time
In order to test whether more time spent on the intervention website resulted in a greater treatment effect, we divided the treatment group into low dose and high dose groups based on a sample median split of the number of minutes spent on the website. Participants were classified as “low dose” if they spend 153 minutes or less on the website during the course of the study, and “high dose” if they spent more than 153 minutes. All primary analyses were repeated for each outcome with the new “condition” variable consisting of three levels: (1) control, (2) low dose experimental, and (3) high dose experimental. The results of these analyses are presented below
Self-Efficacy
Results from LMM revealed a significant treatment-BY-time effect for the Headache Self Efficacy Scale (F6,178=7.13, p<0.0001). Compared to controls, participants who used the site the most reported greater increases in feelings of self-efficacy from baseline to post-intervention (t=5.41, p<0.0010), 3-month assessment (t=4.53, p<0.0010, and 6-month assessment (t=4.64, p<0.0010), and participants in the low dose group reported greater increases in self-efficacy from baseline to 6-month assessment (t=3.00, p=0.0279). When comparing the low and high dose group, it was noted that high-dose participants reported significantly greater increases in self-efficacy compared to low-dose participants, from baseline to post-intervention (t=3.17, p=0.0162).
Further dose analyses were carried out on all primary outcome measures; no other significant differences were noted between high-dose and low-dose participants, or between low-dose participants and controls.
Completer Analyses
Additional analyses were conducted to test whether there was a different intervention effect for participants who completed all study assessments (completers) compared to those who did not (non-completers). For all but two outcomes, no difference in treatment effect was noted for completers compared to non-completers. For the headache self-efficacy outcome, a significant difference in change between experimentals and controls was noted from baseline to post for the completers (M=19.74, SE=3.08, t177=6.41, p<.0001) but not for non-completers (M=5.39, SE=4.18, t177=1.29, p=.1989); this difference was significant (t177=2.76, p=.0063). In addition, when examining the headache-specific locus of control related to healthcare providers outcome, results showed that in completers, the experimental group had a negative mean change from baseline to post, relative to controls (M=-2.76, SE=1.04, t177=−2.65, p=.0089), while in non-completers, the experimental group mean increased (M=3.29, SE=1.42, t177=2.33, p=.0212); this difference was also significant (t177=−3.44, p=.0007). No other significant differences were noted between completers and non-completers.
DISCUSSION
The findings described above provide evidence that participants who used painACTION experienced significantly reduced pain catastrophizing, depression and stress, and increased headache self-efficacy, use of relaxation strategies, and use of social support, compared to control participants in the migraine headache population that was studied. Comparisons with the IMMPACT criteria (25) indicated differences between the experimental and control groups in ratings of depression, anxiety, stress and global ratings of improvement that appear to be clinically significant. The hypothesis that the intervention would reduce frequency and severity of headaches could not be tested due to a technical problem that resulted in a loss of this data.
Overall, the results of this study are very consistent with the outcomes of other research examining behavioral interventions to improve behavioral self-management of migraine in terms of: (1) improvements found in cognitive and emotional aspects of headache management (46); (2) improvements in locus of control and coping skills as mediators of psychological distress (47); (3) the association between increased self-efficacy and increased use of psychological coping strategies to prevent and manage headache episodes and reduce anxiety (48); and importantly (4) the apparent efficacy of delivering effective behavioral self-management interventions via the Internet (49). In addition, the goals of the study and the intervention are well aligned with expert recommendations for addressing critical needs of behavioral focused interventions for migraine (50).
The findings that suggest that the effect of the intervention may be moderated by level of website exposure (dosage) are less easy to fit into the existing literature. The effects of dosage in online interventions is not well understood (26). It is unclear whether participants experience a better intervention effect because they are exposed to the website more, or because participants who have a positive intervention experience elect to spend more time using the website; the fact that a dose effect was noted only for the self-efficacy finding may suggest that the latter scenario provides a better explanation, but further investigation into the effects of dosage in online interventions is needed.
These findings are important to physicians and other clinical professionals treating people with migraine for several reasons. First, the finding that psychological distress (stress, depression, and anxiety) was reduced is important because research has shown that reducing the impact of negative emotions is a key factor in helping people with migraine gain a sense of control (47). Increasing the belief that one has control over pain is also an important outcome, as increased feelings of control are significantly associated with decreased beliefs about pain-related disability (51). Managing negative cognitions, such as catastrophizing, may be one of the most important areas to address, as it is an important predictor of quality of life for people with migraine headache (52).
Second, the intervention tested addresses several factors that all play a key role in activating pain self-management behavior - disease modification, motivational models of pain self-management, and self-efficacy. Experts suggest that early diagnosis of migraine and treatment, or disease modification, may minimize long-term problems and improve the quality of life for people with migraine (53). “Early treatment” for migraine does not necessarily apply to the purely medical aspects of migraine care. A psychologically-focused disease modification approach, like painACTION, that targets critical psychological issues related to trigger avoidance, symptom control, and the appropriate use of preventive medications (among others), has the potential to elicit significant disease modifying effects. Because a person’s commitment to self-managing pain may mediate their success in doing so (54), the concept of motivation is linked to success in managing pain. Motivation to engage in self-management behaviors predicts the performance of self-management behaviors and coping efforts (55). Because of the relationship between motivation, self-efficacy and coping, it makes intuitive sense that online self-management interventions, which directly addresses all of these elements, would lead to significant changes self-efficacy and coping (readiness and motivation were not measured).
Third, the interventional approach is closely aligned with expert recommendations and guidelines for addressing critical needs of behavioral interventions for migraine in the following ways: (1) providing a viable means of increasing access to an empirically-validated treatment for those for whom behavioral treatment is not readily available; (2) directly addressing the issue of medication adherence and medication safety; (3) helping providers achieve the important aim of integrating a behavioral self-management approach into everyday clinical practice; and (4) specifically targeting important co-morbid conditions of depression, stress, anxiety, and sleep disturbance (50;56)
Fourth, the program provides clinicians with a framework for offering patients a highly consistent approach to behavioral self-management that is designed to enhance patient-provider collaboration, and extends self-management support beyond the boundaries of a time-limited, office-based, clinical encounter. The scientifically validated approach has the potential to be a powerful complimentary treatment when integrated with front-line medical/pharmacological headache management therapies. While there is a tendency to refer only the most difficult to manage patients for behavioral services (50) the outcomes of this study support the idea that even patients with less complicated clinical presentations may benefit greatly from a behavioral approach integrated with medical management.
Fifth, the web-based nature of the program makes it a viable means of increasing access to a trusted, empirically-validated treatment for those for whom behavioral treatment is not readily available. The current health care environment demands that efficacious interventions reach a broader audience, especially those that do not place additional burdens on front-line providers of medical care. In this way the program helps meet a critical public health need by making behavioral support available in a more timely way for larger numbers of patients.
Finally, the goals of painACTION are consistent with the United States Headache Consortium’s guidelines on the suitability and use of a non-pharmacologic migraine treatment (57), which state that behavioral interventions may be especially useful for people who: (1) prefer non-pharmacologic treatments; (2) cannot tolerate pharmacologic treatment; (3) have contraindications for pharmacologic treatment; (4) are not responsive to pharmacologic treatment; (5) are pregnant, planning to become pregnant, or nursing; (6) have a history of pharmacologic use that aggravates headache problems; and/or (7) show deficits in emotional-and stress-coping.
Study Limitations
The absence of pain severity and frequency data limits the conclusions that may be drawn from this study with respect to the potential alleviation of migraine pain severity and headache frequency. The results of this trial do suggest that the intervention produced substantial benefits to participants in terms of coping skills and improved psychological function, but the loss of migraine pain and severity data is a serious limitation. Further, because the study was originally powered with two primary outcomes in mind, the loss of these outcomes may raise concerns about the potential for inflation of Type I error rates. Faced with the choice between (1) considering the original secondary outcomes as primary and performing a Bonferroni correction on all main effects, or (2) presenting the findings uncorrected, it was decided that the first option would result in a concomitant inflation of Type II error, given the original power analysis. Readers are invited to consider the results of the study, and the exact, unadjusted p-values, with these limitations in mind.
A second limitation arises from the gender and racial/ethnic distribution of the study sample, which contains more women and fewer non-White participants than the general population of people with migraine. Although differences exist between the study sample and the general population of migraine sufferers, the demographic distribution of the study sample is similar to the general population of individuals who seek psychosocial support interventions online; that is, predominantly White, female, and college educated (58).
The higher attrition rate of experimental participants (80% completion immediately post-intervention, 55% at the end of six-month follow-up) relative to controls (89% completion immediately post-intervention, 82% at the end of six-month follow-up) represents a third limitation; this differential attrition rate raises questions about whether participants dropped out of the study for reasons relating to either the exposure (website) or the outcome (improvement or worsening of migraine or related symptoms). However, the results of the completer analysis may alleviate some of these concerns. For the large majority of study outcomes, no differences were noted between participants who completed all study visits and those who did not, with the exception of the self-efficacy finding and the headache-specific locus of control finding related to health care providers. Of these, the latter finding, in which a different direction of effect was noted for experimental participants compared to controls, suggests that differences may exist between completers and non-completers; however, given that this difference in direction of effect was noted for only one outcome, it is suggested that the completer effect does not present a major threat to validity.
While data detailing participants’ reasons for discontinuing participation were not collected, it was possible to examine experimental participants’ ongoing usage of the website with respect to their self-reported satisfaction. In order to do this, a series of sub-analyses were carried out as follows: (1) length of time a participant remained in the study was calculated as days from post-assessment to last site use, and (2) linear regression analyses were performed to test whether participants’ experience in the study immediately post-intervention (measured as overall satisfaction, as well as by the primary outcome measures) predicted the number of days they continued to use the website. No significant associations were noted between participant status and discontinuing use of the website. It seems likely that other explanations, such as the greater participant burden of using the website compared to the control condition, may adequately explain the observed differential attrition rate. However, it should be noted that high attrition rates are not unusual in web-based headache trials (49).
CONCLUSIONS
Engaging patients in active self-management is an important component of comprehensive migraine treatment. Self-management support helps the patient develop problem-solving skills, increase feelings of self-efficacy, and helps patients learn to apply knowledge and skills to real-life situations. The integration of behavioral support in the medical care of migraine is essential in helping people with migraine to manage their condition more effectively, avoid disease progression, increase adherence with medical management plans, improve function and overall quality of life, and reduce the high cost of migraine and migraine-related disability to individuals and society.
Results of this study indicate that participants in the experimental condition demonstrated increased self-efficacy to perform headache self-management activities, increased their use of positive symptom management strategies, and reported decreased migraine-related depression and stress to a significantly greater degree than control group participants. This improvement in participants’ abilities to manage their headache symptoms indicates that online self-management programs can be an important element of a comprehensive disease management approach, and may be an effective adjunct to standard medically-based chronic pain care. The inclusion of non-medically treated and medically treated people with migraine suggests that the program has the potential to benefit a broad range of adults with migraine headache. These findings are consistent with those reported by Chiauzzi et al (2010), and suggests that the painACTION intervention may be a reliable and effective way to deliver self-management support to people with a variety of chronic pain types.
Because many people with migraine may have limited access to expert behavioral and lifestyle-change support, or are reluctant to seek mental health services, medical providers who refer their patients to online self-management programs can help meet a critical public health need by making behavioral support available in a more timely way for larger numbers of migraine patients. Further studies are needed to establish which patient groups may derive the greatest benefit from online intervention, in addition to elucidating the most salient mechanisms of change, and to better understand why certain outcomes seem more amenable to change through this mode on clinical intervention.
Table 3.
Comparison of study results with IMMPACT criteria
| Experimental | Control | ||
|---|---|---|---|
| Physical Functioning1 | MIDAS | 12.8% decrease | 13.0% decrease |
| Emotional Functioning1 | DASS Depression | 12.5% decrease | 0.0% change |
| DASS Anxiety | 7.7% decrease | 2.4% increase | |
| DASS Stress | 4.1% decrease | 0.7% decrease | |
| Global Rating of Improvement2 | Very Much Improved | 1(1.5) | 2(2.3) |
| Much Improved | 16(23.5) | 10(11.5) | |
| Minimally Improved | 31(45.6) | 20(23.0) | |
| No Change | 20(29.4) | 50(57.5) | |
| Minimally Worse | 0(0.0) | 4(4.6) | |
| Much Worse | 0(0.0) | 0(0.0) | |
| Very Much Worse | 0(0.0) | 1(1.2) | |
| Pain Intensity | Not Measured | -- | -- |
Calculated as: [(Baseline Group Average − Post-Intervention Group Average)/Baseline Group Average]*100
Participants who endorsed each category, given as n(%)
Acknowledgments
Financial Support: Grant support was received from the National Institutes of Health (NIH), the National Institute of Drug Abuse (NIDA No. R44DA023539-02). The funders had no part in designing the study, the collection of data and its analysis, or in the decision to complete or write this manuscript.
Footnotes
Conflicts of Interest: All of the authors are employees of Inflexxion, Inc, Newton, MA
References
- 1.Lipton RB, Bigal ME, Amatniek JC, Stewart WF. Tools for diagnosing migraine and measuring its severity. Headache. 2004;44:387–398. doi: 10.1111/j.1526-4610.2004.04089.x. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF On behalf of the AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. J Am Med Assoc. 2003;290 (18):2243–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 4.Breslau N, Schultz LR, Stewart WF, Lipton RB, Lucia VC, Welch KMA. Headache and Major Depression: Is the association specific to migraine? Neurology. 2000;54(2):308–313. doi: 10.1212/wnl.54.2.308. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel R, Dortch M, Cady R, et al. Migraine headache misconceptions: barriers to effective care. Pharmacotherapy. 2004a;24(5):638–48. doi: 10.1592/phco.24.6.638.34751. [DOI] [PubMed] [Google Scholar]
- 6.Adelman JU, Adelman LC, Freeman MC, Von Seggern RL, Drake J. Cost considerations of acute migraine treatment. Headache. 2004;44(3):271–85. doi: 10.1111/j.1526-4610.2004.04060.x. [DOI] [PubMed] [Google Scholar]
- 7.Turk DC, Okifuji A. Psychological factors in chronic pain: Evolution and revolution. J Consult Clin Psychol. 2002;70:678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 8.Andrasik F. Behavioral treatment approaches to chronic headache. J Neurol Sci. 2003;24(2):80–85. doi: 10.1007/s100720300048. [DOI] [PubMed] [Google Scholar]
- 9.Holroyd KA. Assessment and psychological management of recurrent headache disorders. J Consult Clin Psychol. 2002;70:656–77. doi: 10.1037//0022-006x.70.3.656. [DOI] [PubMed] [Google Scholar]
- 10.Glajchen M. Chronic pain: Treatment barriers and strategies for clinical practice. J Am Board Fam Pract. 2001;14:178–183. [PubMed] [Google Scholar]
- 11.McDonald DD, Laporta M, Meadows-Oliver M. Nurses’ response to pain communication from patients: A post-test experimental study [Electronic Version] Intl Jl Nurs. 2006 doi: 10.1016/j.ijnurstu.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson R, Penzien D, McCrory DC, et al. Behavioral therapies for migraine. Cochrane Database Syst Rev. 2003;2 [Google Scholar]
- 13.Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback. 2002;27(2):163–81. doi: 10.1023/a:1016247811416. [DOI] [PubMed] [Google Scholar]
- 14.White R, Dorman SM. Receiving social support online: implications for health education. Health Educ Res. 2001;16(6):693–707. doi: 10.1093/her/16.6.693. [DOI] [PubMed] [Google Scholar]
- 15.Wantland DJ, Portillo CJ, Holzemer WL, et al. The effectiveness of Web-based vs. non-Web-based interventions: A Meta-analysis of behavioral change outcomes. J Med Internet Res. 2004;6(4) doi: 10.2196/jmir.6.4.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayliss MS, Dewey JE, Dunlap I, et al. As study of the feasibility of Internet administration of a computerized health survey: the headache impact test (HIT) Qual Life Res. 2003;12(8):953–961. doi: 10.1023/a:1026167214355. [DOI] [PubMed] [Google Scholar]
- 17.Artinian NT, Harden JK, Kronenberg MW, et al. Pilot study of a Web-based compliance monitoring device for patients with congestive heart failure. Heart Lung. 2003;32:226–33. doi: 10.1016/s0147-9563(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 18.Birchley D, Pullan R, DeFriend D. Patient attitudes to the Internet and analysis of the potential role of a dedicated colorectal website -a prospective study. Ann R Coll Surg Engl. 2003;85:398–401. doi: 10.1308/003588403322520771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croft DR, Peterson MW. An evaluation of the quality and contents of asthma education on the World Wide Web. Chest. 2002;121:1301–7. doi: 10.1378/chest.121.4.1301. [DOI] [PubMed] [Google Scholar]
- 20.Bichakjian CK, Schwartz JL, Wang TS, Hall JM, Johnson TM, Biermann JS. Melanoma information on the Internet: often incomplete - a public health opportunity? J Clin Oncol. 2002;20:134–41. doi: 10.1200/JCO.2002.20.1.134. [DOI] [PubMed] [Google Scholar]
- 21.Andersson G, Kaldo V. Internet-based cognitive behavioral therapy for tinnitus. J Clin Psychol. 2004;60:171–178. doi: 10.1002/jclp.10243. [DOI] [PubMed] [Google Scholar]
- 22.Lorig KR, Ritter P, Stewart A, et al. Chronic disease self- management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Holman Halsted, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public health reports (Washington, DC: 1974) 2004;119(3):239–43. doi: 10.1016/j.phr.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barak A, Klein B, Proudfoot JG. Defining Internet-supported therapeutic interventions. Ann Bev Med. 2009;38(1):4–17. doi: 10.1007/s12160-009-9130-7. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin, Turk, Wyrwich, et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Danaher BG, Seeley JR. Methodological issues in research on Web-based behavioral interventions. Ann Behav Med. 2009;38(1):28–39. doi: 10.1007/s12160-009-9129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rains JC, Penzien DB, McCrory DC, Gray RN. Behavioral headache treatment: history, review of the empirical literature, and methodological critique. Headache. 2005 May;45(Supplement 2):S92–109. doi: 10.1111/j.1526-4610.2005.4502003.x. [DOI] [PubMed] [Google Scholar]
- 28.Holroyd, Drew Behavioural Approaches to the Treatment of Migraine. Semin Neurol. 2006;28(2):199–207. doi: 10.1055/s-2006-939920. [DOI] [PubMed] [Google Scholar]
- 29.Subcommittee IHSCT. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20:765–786. doi: 10.1046/j.1468-2982.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 30.Stewart WF, Lipton RB, Kolodner K, et al. Reliability of the Migraine Disability Assessment (MIDAS) score in a population-based sample of headache sufferers. Cephalalgia. 1999;19:107–14. doi: 10.1046/j.1468-2982.1999.019002107.x. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Turner JA, Romano JM, Strom SE. The chronic pain coping inventory: development and preliminary validation. Pain. 1995;60(2):203–16. doi: 10.1016/0304-3959(94)00118-X. [DOI] [PubMed] [Google Scholar]
- 32.Romano JM, Jensen MP, Turner JA. The chronic pain coping inventory-42: reliability and validity. Pain. 2003;104(1–2):65–73. doi: 10.1016/s0304-3959(02)00466-9. [DOI] [PubMed] [Google Scholar]
- 33.Martin NJ, Holroyd KA, Rockicki LA. The Headache Self-Efficacy Scale: Adaptation to recurrent headaches. Headache. 1993;33:244–248. doi: 10.1111/j.1526-4610.1993.hed3305244.x. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Asses. 1995;7(4):524–532. [Google Scholar]
- 35.Martin NJ, Holroyd KA, Penzien DB. The Headache-Specific Locus of Control Scale: Adaptation to recurrent headaches. Headache. 1990;30:729–734. doi: 10.1111/j.1526-4610.1990.hed3011729.x. [DOI] [PubMed] [Google Scholar]
- 36.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2. Sydney: Psychology Foundation; 1995. [Google Scholar]
- 37.Brown TA, Korotitsch W, Chorpita BF, Barlow D. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther. 1997;35:79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 38.Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item version of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. 1998;10:176–181. [Google Scholar]
- 39.Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): Normative data and latent structure in a large non-clinical sample. Br J Clin Psychol. 2003;42:111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- 40.Guy W. DHEW Publication No ADM 76–338. Washington: US Government Printing Office; 1976. ECDEU assessment manual for psychopharmacology. [Google Scholar]
- 41.Dunkl PR, Taylor AG, McConnell GG, Alfano AP, Conaway MR. Responsiveness of fibromyalgia clinical trial outcome measures. J Rheumatol. 2000;27:2683–91. [PubMed] [Google Scholar]
- 42.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 43.Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effect models. Journal of Biopharmaceutical Statistics. 2001;11(1&2):9–21. doi: 10.1081/BIP-100104194. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui OH, Hung HMJ, O’Neill R. MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. Journal of Biopharmaceutical Statistics. 2009;19:227–246. doi: 10.1080/10543400802609797. [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15):1987–91. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 46.Smith, Nicholson, Banks Migraine Education Improves Quality of Life in a Primary Care Setting. Headache. 2010;50:600–612. doi: 10.1111/j.1526-4610.2010.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heath RL, Saliba M, Mahmassani O, et al. Locus of control moderates the relationship between headache pain and depression. J Headache Pain. 2008;9(5):301–8. doi: 10.1007/s10194-008-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.French DJ, Holroyd KA, Pinell C, et al. Perceived self-efficacy and headache related disability. Headache. 2000;40:647–56. doi: 10.1046/j.1526-4610.2000.040008647.x. [DOI] [PubMed] [Google Scholar]
- 49.Devineni, Blanchard A randomized controlled trial of an internet-based treatment for chronic headache. Behav Res Ther. 2005;43:277–292. doi: 10.1016/j.brat.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Penzien DB, Rains JC, Lipchik GL, et al. Future directions in behavioral headache research: applications for an evolving health care environment. Headache. 2005;45(5):526–34. doi: 10.1111/j.1526-4610.2005.05105.x. [DOI] [PubMed] [Google Scholar]
- 51.Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655–662. doi: 10.1037//0022-006x.69.4.655. [DOI] [PubMed] [Google Scholar]
- 52.Holroyd KA, Drew JB, Cottrell CK, et al. Impaired functioning and quality of life in severe migraine: the role of catastrophizing and associated symptoms. Cephalalgia. 2007;27(10):1156–65. doi: 10.1111/j.1468-2982.2007.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loder E, Biondi D. Disease modification in migraine: a concept that has come of age? Headache. 2003;43(2):135–43. doi: 10.1046/j.1526-4610.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 54.Kerns R, Rosenberg R. Predicting responses to self management treatments for chronic pain: Application of the pain stages of change model. Pain. 2000;84:49–55. doi: 10.1016/S0304-3959(99)00184-0. [DOI] [PubMed] [Google Scholar]
- 55.Jensen MP, Neilson WR, Kerns RD. Toward the development of a motivational model of pain self management. J Pain. 2003;4:477–492. doi: 10.1016/s1526-5900(03)00779-x. [DOI] [PubMed] [Google Scholar]
- 56.Penzien DB, Rains JC, Lipchik GL, Creer TL. Behavioral interventions for tension-type headache: Overview of current therapies and recommendation for a self-management model for chronic headache. Curr Pain Headache Rep. 2004;8:489–499. doi: 10.1007/s11916-004-0072-2. [DOI] [PubMed] [Google Scholar]
- 57.Campbell JK, Penzien DB, Wall EM. Evidence-based guidelines for migraine headache: behavioral and physical treatments. [Accessed June 23, 2010];US Headache Consortium. 2000 Available at: http://www.aan.com/professionals/practice/pdfs/gl0089.pdf.
- 58.Fox Jones. The Social Life of Health Information. 2009 http://www.pewinternet.org/~/media//Files/Reports/2009/PIP_Health_2009.pdf.