Abstract
Background
In flowering plants, the development of male reproductive organs is controlled precisely to achieve successful fertilization and reproduction. Despite the increasing knowledge of genes that contribute to anther development, the regulatory mechanisms controlling this process are still unclear.
Results
In this study, we analyzed the transcriptome profiles of early anthers of sterile mutants aborted microspores (ams) and found that 1,368 genes were differentially expressed in ams compared to wild type anthers, affecting metabolism, transportation, ubiquitination and stress response. Moreover, the lack of significant enrichment of potential AMS binding sites (E-box) in the promoters of differentially expressed genes suggests both direct and indirect regulation for AMS-dependent regulation of anther transcriptome involving other transcription factors. Combining ams transcriptome profiles with those of two other sterile mutants, spl/nzz and ems1/exs, expression of 3,058 genes were altered in at least one mutant. Our investigation of expression patterns of major transcription factor families, such as bHLH, MYB and MADS, suggested that some closely related homologs of known anther developmental genes might also have similar functions. Additionally, comparison of expression levels of genes in different organs suggested that anther-preferential genes could play important roles in anther development.
Conclusion
Analysis of ams anther transcriptome and its comparison with those of spl/nzz and ems1/exs anthers uncovered overlapping and distinct sets of regulated genes, including those encoding transcription factors and other proteins. These results support an expanded regulatory network for early anther development, providing a series of hypotheses for future experimentation.
Background
In flowering plants, male reproductive organs are called stamens, each of which consists of a filament and an anther [1]. Cells in the anther undergo meiosis to produce microspores, which further develop into mature pollen grains [2]. Therefore, anther development is critical to achieve pollen formation and subsequent success of fertilization [3-6]. According to morphological features, anther development can be grouped into two phases and then be further divided into 14 anther stages [5,7,8]. At the beginning of phase 1 (anther stages 1 to 8), the stamen primordium has 3 layers, L1-L3 from surface to interior. The L1 cells later become the epidermis and the L3 cells give rise to the vascular and connective tissues. Some of the L2 cells develop into archesporial cells which then divide into parietal cells and primary sporogenous cells. Additional cell division and differentiation in the L2-lineage establish a characteristic four-lobed structure at anther stage 5. Each lobe consists of central pollen mother cells surrounded by outer endothecium, middle layer and inner tapetum. Pollen mother cells undergo meiosis at stage 5-6, producing tetrads at stage 7. Dissolution of the tetrad callose wall releases microspores at stage 8. In phase 2, the microspores undergo mitosis and develop into mature pollen grains during stages 9-12. Meanwhile, pollen wall materials are deposited from both the microspores and the tapetum layer. After the degeneration of tapetum, the mature pollen is released and is able to start pollination.
Previous studies indicated that early anther development depends on transcriptional regulation and cell-cell communication [5,7-9]. The SPOROCYTELESS (SPL)/NOZZLE (NZZ) gene is one of the earliest genes that regulate anther cell fate determination [10,11]. SPL/NZZ is activated by AG, a C function gene in the ABC model [12-14]. SPL/NZZ is expressed as early as anther stage 2-5 and a mutation in SPL/NZZ leads to the failure of differentiation of parietal and sporogenous cells, and consequentially blocks the formation of anther wall and microsporocytes [15,16].
EXCESS MALE SPOROCYTES1 (EMS1) and TAPETUM DETERMINANT1 (TPD1) are also essential for male fertility with a later expression peak at stage 5 [17]. EMS1 is a leucine-rich repeat receptor-like protein kinase (LRR-RLKs) and TPD1 is likely its ligand [15,18,19]. In both ems1 and tpd1 mutants, anthers produce more microsporocytes at the expense of the tapetum, indicating that communication between adjacent cell layers determines the cell fate of archesporial cell progenies in order to form normal anther wall [17]. Besides EMS1 and TPD1, other cell-cell communication-related genes are also involved in anther development, such as SOMATIC EMBRYOGENESIS RECEPTORLIKE KINASES1/2 (SERK1/2), and RECEPTORLIKE PROTEIN KINASE2 (RPK2) [20,21].
Upon the formation of the anther lobes, DYSFUNCTIONAL TAPETUM1 (DYT1) and AMS, encoding two bHLH transcription factors, are required for tapetal functions at subsequent stages [22,23]. In dyt1, tapetum cells harbor enlarged vacuoles and reduced cytoplasm. The dyt1 meiocytes have comparatively thin callose walls, cannot complete cytokinesis and finally collapse. RNA in situ hybridization experiments showed that DYT1 reaches its peak expression at anther stage 5 to 6 [22]. AMS functions near the time of meiosis, slightly later than that of DYT1. In the ams mutant, the microsporocytes can complete meiosis but the tapetum cells prematurely collapse and microspores are degraded before the first pollen mitosis [23]. Beside these regulators, a large number of other genes are also expressed in the anther, and mutations in some of them lead to male sterility by affecting early anther cell formation, tapetum formation, meiosis or pollen maturation [5,7,16,24-28].
However, due to the functional redundancy of members of many gene families, the subtleties of the phenotypes of single-gene mutants, and possible early phenotypes that obscure anther function, forward genetics has limitations in uncovering anther gene functions [29]. Expression profiling has become increasingly informative and might circumvent the limitation of forward genetics. In recent years, global gene expression profiling by microarray has been used to detect floral gene expression and obtain clues for understanding reproductive development. However, most studies to investigate stamen expression profiles have been conducted by analyzing transcripts from the whole inflorescences of male sterile mutants [30-35], rather than the anther itself [32]. Little transcriptomic information about specific organs is currently available, especially for Arabidopsis whose male reproductive organs are quite tiny [32,33,36]. Thus the detection of anther-specific or preferential genes in mixed floral tissues might be hampered by the moderate detection sensitivity of microarray technology. As mentioned above, SPL, EMS1 and AMS have important functions at different stages of anther development, although they have temporal overlap of expression [10,17,22,23]. Therefore, analysis of their shared and distinct effects on the anther transcriptome can shed some light on gene regulatory networks [37-39].
To obtain more information on transcriptomes near the stage of meiosis, we collected anthers at stage 4 to 7 from ams mutants and wild-type Arabidopsis, even though it is time consuming and technically difficult to dissect developing anthers, because we wanted to identify the genes affected by the ams mutation that might be too diluted to detect using RNAs from whole-inflorescences. The ams transcriptome data and comparison with previous data from spl and ems1 anthers [32] provide detailed information on early anther development. Additionally, with known information of other floral organs in Arabidopsis, we identified genes that function during early anther stage around meiosis. We found that many transcription factor genes were preferentially expressed during early anther development, such as bHLH, MYB, and MADS. Closely related homologs were hypothesized to have either redundant or divergent functions according to phylogenic studies [40-42]. Moreover, further investigation of organ-specific transcriptome revealed the importance of both anther-specific and non-specific transcription factors in early anther development. We propose an expanded gene regulatory network that contributes to the precise regulation of temporal and spatial events during early anther development.
Results and discussion
Identification of genes regulated by AMS
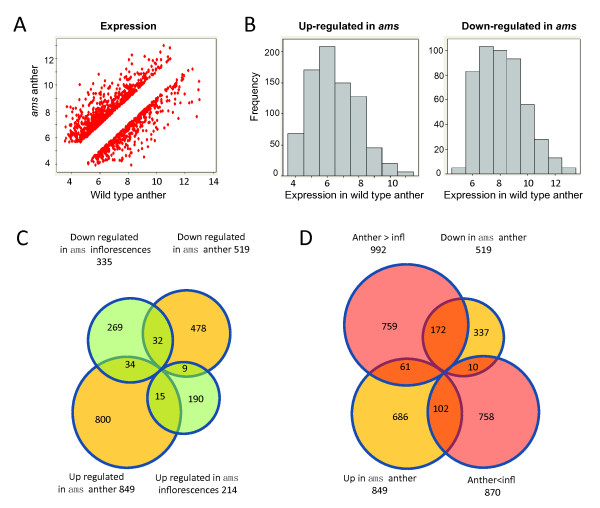
To characterize genes involved in tapetum development and function near the time of meiosis, we isolated total RNA of stage 4-7 anthers from wild-type and the ams mutant plants for Affymatrix ATH1 microarray analysis. We included three biological replicates for each genotype and the results are highly reproducible (with correlation coefficients higher than 0.96, Supplemental figure 1 in Additional file 1). We identified 1,368 genes that were differentially expressed in ams compared with wild-type anthers with at least 2-fold differences (P < 0.05) (Additional file 2) [32,43]. The scatter-plot of the 1,368 genes shows that they include genes expressed at different levels (Figure 1A,B, 1st sheet in Additional file 2); furthermore, genes with higher expression in ams than wild-type tend to have low wild type expression, whereas those with lower than normal expression in ams tend to be expressed at higher levels (Figure 1B).
Figure 1.
The expression of genes differentially expressed in ams anthers. (A) A comparison between transcriptome data from ams and wild type anthers. All expression data were converted to logarithm base 2 ratio. (B) A histogram of genes with elevated or reduced expression levels between ams and wild type anthers. The y-axis the frequency of expression and the x-axis is the log 2 ratio of expression signals. (C) A comparison between ams transcriptome data from anthers and inflorescences. (D) A comparison between differentially expressed genes in ams anthers and those in wild type inflorescences compared with wild type anthers.
Recently, Xu et al. reported totally 549 genes that are differentially expressed in ams floral buds compared with wild-type buds, at four different stages using two color arrays, including 134 genes that were differentially expressed near the time of meiosis (Additional file 2) [35]. Among the 1,368 genes identified in our study, 90 were also identified by Xu et al. in floral buds (Figure 1C). Because AMS is expressed from near anther stage 6 (meiosis) through the formation of microspores, our samples from early stage anthers allowed an examination of the early AMS function in regulating transcriptome and sensitive detection of expression shifts without dilution by other floral tissues, resulting in the identification of additional 1,278 genes (478 down- and 800 up-regulated in the ams anthers) with differential expression between wild-type and ams anthers (Figure 1C).
Nevertheless, our results and the previous study did both detect 90 genes that are significantly affected by the ams mutation (Additional file 2; Figure 1C) [35]. Some of these genes show the same direction in expression shifts between the two studies; however, others had the opposite directions (Figure 1C). Specifically, 34 genes with higher expression in the ams anther than the wild-type anther had reduced expression levels in the ams inflorescence compared with the wild-type inflorescence (Additional file 2); 9 genes showed the opposite trend. These differences might be due to the difference of sampling anther vs. flower bud that included later stages, although other possibilities cannot be ruled out. We observed more similar expression pattern between our anther transcriptome and the published flower bud transcriptome at meiosis stage (Additional file 2). 172 out of the 519 genes down-regulated in ams were expressed significantly higher in the wild type anther than the inflorescence, while 102 of the 849 up-regulated genes showed this pattern (P-value < 0.05, Figure 1D, Additional file 2), suggesting that preferential anther expression contributed to the difference between the two studies. It is also possible that the loss of AMS function might affect other aspects of flower development than anther development, although not revealed by phenotypic changes.
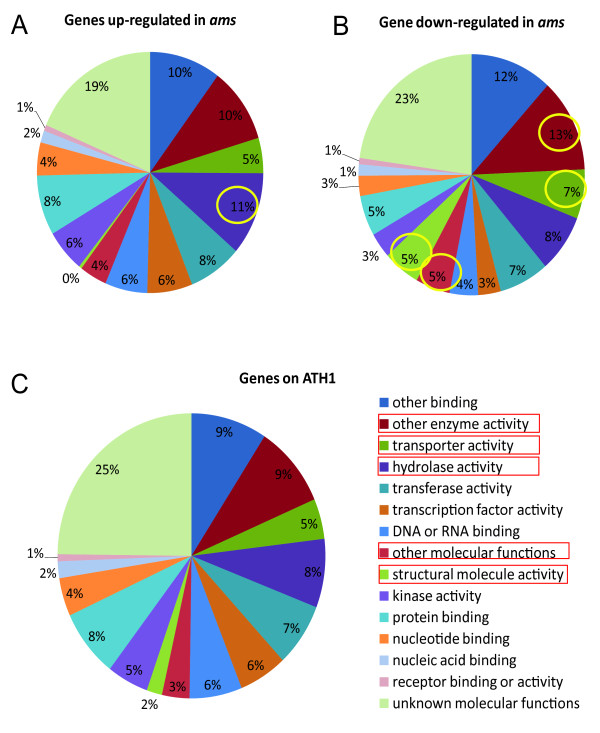
The GO categorization analysis of our anther transcriptome results showed that categories of enzymes, transporters, structural and other molecular proteins were over-represented in the genes with reduced expression, and hydrolases in those with elevated expression levels in ams compared with wild-type (Figure 2A-C, Additional file 3). To further investigate the putative functions of genes with different expression patterns in the ams anther from inflorescence, we then applied GO categorization to all the newly found differentially expressed genes in the ams anther. We found that some categories were enriched in those with reduced expression levels in the ams anther, such as structural molecules, transporters, oxidoreductases (supplemental figure 2 & 3 in Additional file 1). These categories are associated with metabolic activities that are very dynamic in tapetum, suggesting a positive role of AMS in regulating metabolic functions in the tapetum. Meanwhile, genes with ion binding, glycosyl-transferase and hydrolase activities were enriched among the genes activated in ams.
Figure 2.
A pie graph of GO categorization of genes differentially expressed in the ams mutant. (A-B) GO categorization of genes up- and down-regulated in ams, with enriched categories circled compared with all genes on ATH1. (C) GO categorization of all genes on the ATH1 chip.
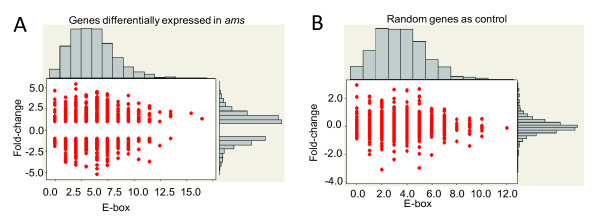
As a putative bHLH transcription factor, AMS has the ability to bind to the canonical bHLH binding site (E-box: CANNTG) in vitro and in vivo [35]. In order to find candidate AMS target genes, we searched E-box elements within 1 kb upstream sequences of genes with statistically significant differential expression between ams and wild-type anthers (Figure 3A &3B). We did not find statistically significant interaction between the number of E-boxes in the putative promoter regions and the fold change in gene expression (compared with randomly selected genes on the chip). It is possible that active AMS binding sites are located not just in the 1-kb regions being analyzed, but also in regions further upstream or even downstream of the coding region. It is also possible that a number of the genes affected in the ams anthers are indirectly regulated by AMS, hence not containing AMS-binding sites in their promoters.
Figure 3.
Marginal plot of fold changes of expression and number of E-box. (A) Genes differentially expressed in the ams anther compared with wild-type anther were selected (more than two fold changes with P < 0.05). The logarithm 2 ratio of fold-changes and the number of AMS binding sites (E-box) within 1 kb putative promoter sequence upstream of the start codon were plotted. (B) Randomly selected genes were plotted as control.
AMS affects genes with putative functions in phosphorylation, exocytosis, stress-response and ubiquitin-proteasome pathways during male reproduction
Both somatic and reproductive cells were evidently affected in the ams mutant anther, morphologically and transcriptomically [23,35]. Specifically, the ams inflorescence showed reduced expression of genes predicted to be involved in metabolism, such as lipid synthesis-related genes [35]. Our anther transcriptome data provided spatially more specific information for the expression patterns of metabolism-related genes (Supplemental figure 4 in Additional file 1) and showed that the expression levels of genes involved in cell wall formation, lipid synthesis and secondary metabolism were obviously altered in the ams anther, consistent with morphological defects.
Interestingly, 32 genes located on chloroplast DNA were reduced in expression in the ams anthers whereas starch and sucrose related genes were increased (Additional file 4, supplemental figure 4 in Additional file 1). In addition, more metabolism-related genes were found with shifted expression, especially glycosyl-transferase (P < 0.01, supplemental figure 5 in Additional file 1,). Besides, the expression levels of genes with putative regulatory functions were also changed, such as kinases and transcription factors (supplemental figure 6-8 in Additional file 1). Interestingly, most of the genes encoding kinases with expression shifts were activated in the ams mutant, suggesting a putative negative regulatory role of AMS (supplemental figure 4 in Additional file 1).
In addition, we found that genes likely involved in vesicular transport were up-regulated in ams, including genes encoding two SNARE proteins and others related to this process: 3 syntaxins, 3 myosin heavy chains and 2 clathrin proteins (Additional file 4, supplemental figure 9 in Additional file 1). Intracellular trafficking machinery such as SNARE complex is important in animal and plant development [44,45]; for example, one SNARE protein, SEC22, is preferentially expressed in the flower and essential for gametophyte development [46]. Other vesicular transport genes, such as AtVAM3 encoding a syntaxin-related protein, were shown to function in vacuolar assembly in Arabidopsis [47]. It is possible that the higher than normal expression of genes for vesicular transport contributes to the abnormally vacuolated tapetal cells observed in the ams anther [23].
We also found that the expression levels of stress-responsive genes were changed in ams (Additional file 4, Supplemental figure 10 in Additional file 1), especially the increased expression of 10 disease resistance genes and two genes encoding respiratory burst oxidases. These findings are consistent with recent studies showing that multiple abiotic stresses can lead to male sterility, such as extreme temperatures and drought [48-50]. In addition, some stress-inducible and/or hormone-related genes were also found with expression alteration, including RD22, an ABA-inducible gene responsive to dehydration; VSP1, a JA-inducible gene; EPS1, a gene possibly acting upstream of SA; CCR1, a cold inducible gene; four disease resistance genes encoding TIR-NBS-LRR class proteins; and three heat-shock genes, suggesting complex interactions between internal and external signals regulating anther development and/or functions [51].
Another regulatory pathway activated in ams is the ubiquitin-proteasome pathway (Additional file 4), with increased expression of genes encoding subunits of the E3 ubiquitin ligases (Supplemental figure 11 in Additional file 1) [52]. Previous studies demonstrated essential roles of the ubiquitin-proteasome pathway in embryogenesis, hormone signaling, light response, floral development, self-incompatibility, and senescence [48,52,53]. Our results suggested that this pathway may also be regulated by AMS. It is possible that AMS directly regulates the expression of some genes in the ubiquitin-proteasome pathway; alternatively, AMS could influence the expression of such genes indirectly either via AMS-target genes or possibly through the accumulation of damaged proteins which then induce the ubiquitin-proteasome pathway [54]. Further experiments are needed to test these hypotheses.
Anther-specific or preferential genes were over-represented among genes differentially expressed in the ams mutant
Differential expression patterns in vegetative and floral organs can provide clues about gene functions [43]. To find out the relationship between the gene expression shifts in the ams mutant and their expression preferences in different organs, we compared our data from wild-type anther with previous microarray data from roots, stems, leaves, seedlings, siliques and inflorescences. The same RNA extraction method and ATH1 platform were applied in both studies so the datasets should be comparable [43]. We defined as anther-specific (A-S) using these criteria: 1) the expression in anther is significantly higher than in any other tissue (with FDR < 0.05); 2) the expression is present in anther but not in any other tissues according to two alternative methods (see materials and methods for details and explanations) [43]. Using the presence call of the MAS5 algorithm identified 124 A-S genes, 76 of which had at least two fold difference; using expression level of 50 as threshold identified 172 A-S genes, 146 of which had at least two fold difference (those with two fold differences are marked with "*" in the second column in Additional file 5). Because both methods for calling "presence" have limitations, only the 43 genes detected by both methods were discussed as A-S gene (this rule also applied to the two groups described below).
Genes were defined as anther-preferential (A-P) if the expression in the anther is: 1) significantly higher than those in any other tissue with FDR < 0.05 (genes with more than 2 fold changes were marked with "*" in Additional file 5 and Additional file 3) present in anther according to the MAS5 algorithm or with expression level of at least 50. Therefore, A-P genes included A-S genes. In addition, those with statistically significantly higher expression levels in anther than in non-floral organs were called reproductive preferential (R-P) genes (Additional file 5, see material and methods for detail). We performed real-time PCR for 6 of these genes and the results were consistent (supplemental figure 12 in Additional file 1). In our result, 24 genes involved in male reproductive development were detected (Table 1). Consistent with previous studies, SPL was found in the A-P group and EMS1 in R-P group, while AMS as an A-S gene [10,17].
Table 1.
Expression of genes known as anther development related genes
| AGI | Name | wt | s/w | e/w | a/w | Function | References |
|---|---|---|---|---|---|---|---|
| A-S | |||||||
| AT2G16910 | AMS | 7.7 | -3.4 | -3.5 | 1.7 | tapetum dev. | Sorensen et al., 2003 |
| AT1G66170 | MMD1 | 5.5 | -1.8 | -1.8 | -0.1 | male meiosis | Alves-Ferreira et al., 2007 |
| AT1G01280 | CYP703A2 | 9.8 | -6.2 | -6.2 | -0.6 | pollen dev. and sporopollenin biosynthesis | Souza et al., 2009 |
| AT1G62940 | ACOS5 | 11.6 | -6.4 | -5.7 | -1.0 | ||
| AT3G11980 | MS2 | 10.6 | -6.1 | -5.9 | -0.6 | ||
| AT4G28395 | A7 | 9.6 | -4.1 | -3.9 | 0.5 | Rubinelli et al., 1998 | |
| A-P | |||||||
| AT2G17950 | WUSCHEL 1 | 7.7 | -2.5 | 0.4 | 0.5 | floral dev. | Ming et al., 2009 |
| AT4G27330 | NZZ/SPL | 9.3 | -3.9 | -0.5 | -0.5 | early anther formation | Ito et al., 2004 |
| AT5G14070 | ROXY2 | 9.1 | -3.2 | -1.7 | -0.2 | Xing et al., 2008 | |
| AT3G11440 | MYB65 | 8.9 | -2.2 | -0.5 | -0.4 | Millar et al., 2005 | |
| AT5G06100 | MYB33 | 7.6 | -1.1 | 0.1 | -0.4 | ||
| AT3G42960 | ATA1 | 11.9 | -7.4 | -6.7 | -1.7 | tapetum function | Lebel-Hardenack et al., 1997 |
| AT3G51590 | LTP12 | 10.4 | -6.1 | -5.2 | 0.9 | Ariizumi et al., 2002 | |
| AT3G28470 | MYB35 | 8.6 | -4.9 | -4.5 | 0.0 | Zhu et al., 2008 | |
| AT1G69500 | CYP704B1 | 11.5 | -7.4 | -7.0 | -2.0 | pollen dev. and sporopollenin biosynthesis | Souza et al., 2009 |
| AT4G34850 | LAP 5 | 11.4 | -6.3 | -5.8 | -1.6 | Dobritsa et al., 2010 | |
| AT4G35420 | DRL1 | 11.9 | -5.1 | -4.6 | -1.9 | Tang et al., 2009 | |
| AT5G62080 | MTG10 | 13.0 | -7.4 | -5.2 | -4.2 | Xing et al., 2007 | |
| AT3G22880 | DMC1 | 10.7 | -2.2 | -0.1 | 0.0 | male meiosis | Doutriaux et al., 1998 |
| AT3G15400 | ATA20 | 12.1 | -6.6 | -5.6 | 0.1 | pollen wall | Rubinelli et al., 1998 |
| R-P | |||||||
| AT5G20240 | PI | 11.8 | 0.4 | 0.0 | 0.2 | whorl specification | Li et al., 2008 |
| AT3G17010 | B3 | 8.7 | 0.9 | 0.4 | 0.3 | early anther formation | Gomez-Mena et al., 2005 |
| At5G07280 | EMS1 | 10.2 | -0.9 | -2.9 | 0.1 | Zhao et al., 2002 | |
| AT1G71830 | SERK1 | 8.0 | 0.8 | 0.1 | 0.1 | Albrecht et al., 2005 | |
"wt", wild-type; "s/w", fold change of the spl signals compared with wild-type; "e/w", of ems1; "a/w", of ams. A-S represents anther specific; A-P represents anther preferential (A-S excluded) and R-P represents reproductive preferential (A-S & A-P excluded). All expression values are log2 ratio. "dev" means "development" (Column sequence, abbreviation and the version of annotation are used as in tables and supplemental tables below.)
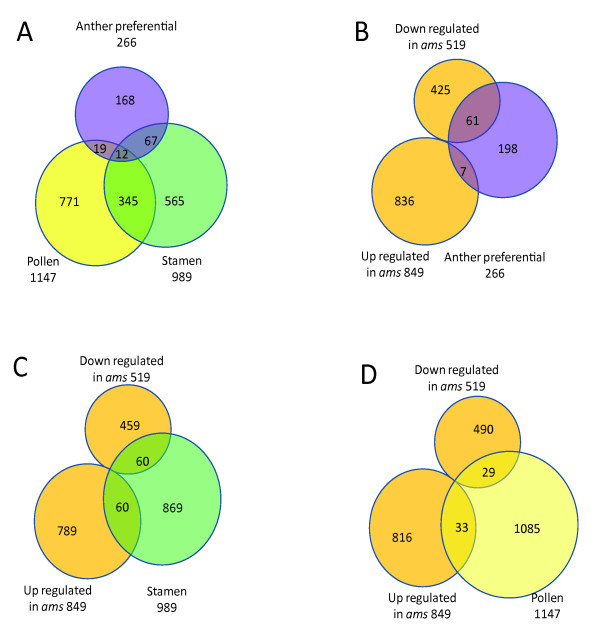
Recently, other studies were conducted to identify male reproductive development-related genes. Wellmer et al. identified genes expressed in stamen indirectly by comparing the inflorescence transcriptome of floral homeotic mutants lacking stamens with wild-type [30]. In another study, Honys et al. analyzed microspores/pollen from different stages and defined the male gametophytic transcriptome [33]. A comparison of our A-P genes with these two previous gene lists (defined as stamen and pollen, Additional file 6) revealed that only a small number of genes overlapped between the three male reproductive datasets (Figure 4A, Additional file 6). The differences in identified genes can be explained by the difference of samples used in different studies: our samples only included wild-type anthers at early stages (stage 4-7), whereas the pollen transcriptome data were from microspores and pollen at different stages; and stamen-specific genes was indirectly obtained by subtraction of mutant transcriptome from wild-type and genes in this list might function earlier during organ specification. The dramatic differences between different samples suggest strongly that gene activities alter dramatically between different developmental stages of male reproductive organs [33].
Figure 4.
Venn diagrams of microarray results and previous related study. (A) A comparison of anther preferential genes identified in our study with previously known pollen genes and stamen genes. (B-D) Comparisons between genes differentially expressed in the ams anther and those preferentially expressed in certain organ: anther preferential, stamen and pollen respectively.
We analyzed the GO categorization for possible enrichment of specific categories among the groups of differentially expressed genes (Additional file 3) and found that, among the 266 A-P genes, the over-represented GO categories were hydrolases, proteins with other binding activities, and other enzymes. No enrichment of other enzyme activity was detected in pollen-specific or stamen-specific datasets found previously [33], suggesting a specific expression profile of early anther development.
Among genes with differential expression in ams, the percentage of A-P genes (5%) is significantly higher than its percentage in the whole genome (1%) (Figure 4B, Additional file 2 and Additional file 3). The stamen-specific genes were also enriched among those differentially expressed in ams (9%) compared with whole genome data (5%) (Figure 4C &4D, Additional file 2). The results were consistent with our hypothesis that AMS regulates genes with important functions in male-reproductive organ where they have higher expression levels [1,8].
Genome-wide analysis of gene expression during early anther development by comparing anther transcriptomes of male sterile mutants, spl, ems1, and ams
Previous studies revealed essential roles of SPL and EMS1 in early anther development and ATH1 microarray data from anthers of these mutants at stage 4-6 were collected and analyzed [32]. To obtain a better overview of early anther development, we analyzed the anther transcriptome data from this study with those of spl and ems1 (detailed methods applied to all microarray data is described in experimental procedures). 1,813 and 802 genes were identified as differentially expressed in spl and ems1, respectively, contributing to a total of 3,058 genes that were differentially expressed by 2-fold or more between the wild-type anther and one or more of the spl, ems1 and ams mutant anthers (Additional file 7). Using the log2 values of the ratio of expression of the differentially expressed genes, hierarchical clustering was carried out to obtain heat-maps (Figure 5A). The patterns of spl and ems1 were similar whereas ams had a different pattern, consistent with the fact that the tapetum layer is absent in both spl and ems1 but is formed in the ams anther.
Figure 5.
Expression distribution of all genes differentially expressed and specific gene families. (A) Hierarchical clustering of genes differentially expressed in at least one mutant. (B-D) Heat-map of bHLH, MADS, MYB genes with putative or known function in male-reproductive development. The number indicate logarithm ratio of the fold change in mutant compared with wild-type anther. "w" represents wild-type anther and "s", "e" and "a" represents spl, ems1 and ams. Red color represents genes which have higher expression level in mutants and green indicates reduced expression.
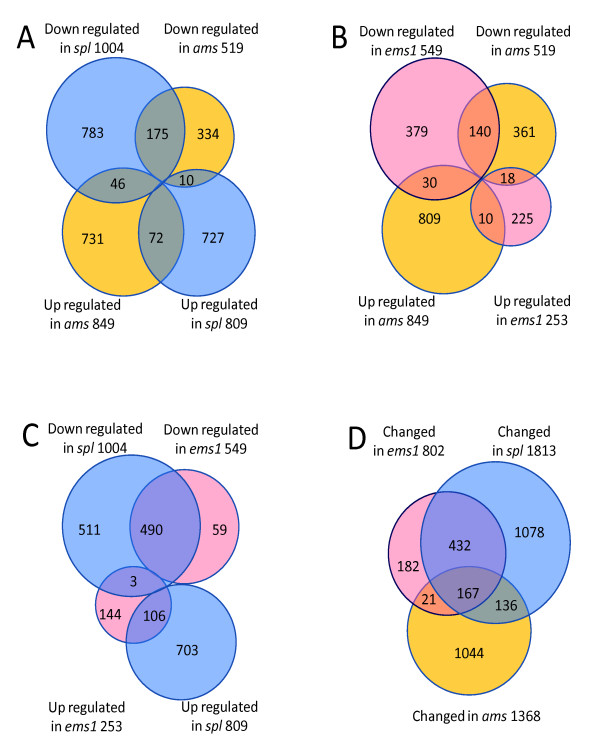
In addition, we compared the direction of differential gene expression by pair-wise comparison between different mutants, as shown in Venn diagrams (Figure 6A-D) and found that many more genes showed changes in the same direction in all three mutants than genes with changes in the opposite direction, suggesting that the three transcription factors had similar effects on some of the target genes. We also found that the non-overlapping (differentially expressed in one mutant, but not in either of the other two) percentage of differentially expressed genes in ams (76%) is larger than those in spl and ems1 (59% & 23%, respectively, Figure 6D), providing strong evidence at the transcriptome level that the AMS function was distinct from those of SPL and EMS1 and likely regulates late gene expression in anther development, consistent with other studies [23].
Figure 6.
Comparisons between transcriptome information from three mutants. (A-C) Genes differentially expressed in three mutants respectively compared with wild-type anther have been identified and then compared with each other pairwisely. (D) Comparison of genes differentially expressed in three mutants compared with wild-type.
Because the three mutants showed related but distinct phenotypes, we speculate that the functions of genes differentially expressed in these mutants might differ from each other. Thus we applied GO categorization of molecular function to genes up- or down-regulated in each mutant (in Additional file 3). First, genes annotated to have "other binding activities" and "other enzyme activities" were significantly enriched in categories with reduced expression in each mutant (P-value < 0.05), consistent with previous knowledge of dynamic metabolism in tapetum cells. In addition, genes encoding transcription factors and DNA binding proteins are enriched in categories with both up-and down-regulated genes in the spl mutant, suggesting that SPL control anther development at least in part by regulating genes encoding transcription factors. Furthermore, the ams mutant showed reduced expression of many genes encoding structural proteins, which mainly contribute to cell structural integrity, suggesting that AMS might activate these genes to promote maturation of tapetum cells.
SPL and EMS1 might control tapetum development by activating AMS-dependent gene expression
To gain a better understanding of genes that may function together in anther development, we divided the 3,058 genes into different clusters based on their expression patterns. Totally 136 genes had repressed expression in all three mutants (Additional file 7). Since tapetum cells are either absent or dysfunctional in the mutants, we expected that the expression of tapetum-related genes would reduce significantly. Previous studies indicated that tapetal cells were primarily involved in nutrition and material provision for pollen maturation [2]. Consistent with this notion, genes encoding enzymes in this group (21.8%) are obviously over-represented comparing with all genes on ATH1 chip (10.1%). Besides, genes belonging to the other binding category were also enriched in this group (16.4% V.s 9.9%, Additional file 3).
Among these genes, many of them are involved in biosynthesis of pollen wall-related compounds, such as lipids, lignin and flavonoids. A recent study showed that the loss of acyl-CoA synthetase, GhACS1 , which might be involved in biosynthesis and transfer of lipids, can lead to male sterility in cotton [55]. The expression levels of 8 Arabidopsis genes involved in the lipid metabolism pathway were significantly reduced in the three mutants, suggesting their potential roles in metabolism in tapetal cells (Table 2). Besides, we found that the expression levels of 30 genes involved in endomembrane system decreased in all mutants (Table 3). Recent studies in plants suggested that many endomembrane proteins might be involved in trafficking thus influencing signal transduction and development [56-58]. Based on the observation of tapetum defects in all three mutants [10,17,22], we speculate that genes sharing similar expression patterns might be important for maintaining the tapetum identity.
Table 2.
Genes significantly down-regulated in all three mutants are involved in metabolism of pollen wall formation, including lipid, pectin, lignin and exine
| AGI | wt | s/w | e/w | a/w | Function | Expression |
|---|---|---|---|---|---|---|
| lipid related | ||||||
| At5g61320 | 8.7 | -3.9 | -3.6 | -2.2 | cytochrome P450 - like protein | A-S |
| At5g08250 | 8.9 | -4.5 | -4.1 | -1.4 | A-P | |
| At1g06250 | 7.6 | -3.2 | -3.0 | -1.8 | lipase-like protein | |
| At5g62080 | 13 | -7.4 | -5.2 | -4.2 | lipid-transfer protein | |
| At3g07450 | 12.8 | -7.5 | -5.6 | -5.2 | ||
| At3g52130 | 12.9 | -7.4 | -6.0 | -3.3 | ||
| At5g07230 | 10.2 | -8.1 | -6.5 | -2.7 | ||
| At5g52160 | 10.7 | -6.3 | -6.2 | -1.7 | ||
| pectin | ||||||
| At3g24230 | 7.6 | -3.2 | -3.2 | -3.2 | pectate lyase | A-P |
| At4g22080 | 8.5 | -4.9 | -4.6 | -1.5 | ||
| At1g75790 | 10.2 | -5.8 | -5.5 | -1.9 | pectinesterase like protein | |
| At3g01270 | 6.2 | -1.2 | -1.2 | -1.1 | putative pectate lyase | R-P |
| At5g50030 | 6.5 | -1.3 | -1.1 | -1.4 | pectin methylesterase inhibitor | |
| lignin | ||||||
| At1g76470 | 10.3 | -4.8 | -5 | -2.3 | putative cinnamoyl-CoA reductase | A-P |
| At3g21230 | 7.7 | -3.1 | -3.1 | -1.8 | 4-coumarate-CoA ligase 2 | |
| exine | ||||||
| At3g13220 | 10 | -5.9 | -5.7 | -2.2 | WBC27 white-brown complex | A-S |
| At4g14080 | 12.5 | -8.1 | -7.1 | -3.8 | maternal effect embryo arrest 48 | A-P |
| At1g02050 | 11.6 | -4.6 | -4.7 | -1.9 | LESS ADHESIVE POLLEN 6 (LAP6) | |
All the expression values are log2 ratio.
Table 3.
Genes related to endomembrane system affected by SPL, EMS1 and AMS
| AGI | wt | s/w | e/w | a/w | Function | Expression |
|---|---|---|---|---|---|---|
| At5g24820 | 10 | -5.7 | -5.4 | -2.1 | cnd41 | A-S |
| At3g23770 | 9.9 | -5.2 | -5.3 | -2.7 | beta-1,3-glucanase, putative | |
| At1g28375 | 9.7 | -5.4 | -5.1 | -2.3 | ||
| At3g21620 | 7.2 | -2.7 | -2.5 | -2.1 | ||
| At4g30040 | 6.6 | -2.0 | -2.1 | -1.1 | cnd41 | |
| At2g24800 | 8.1 | -4.2 | -4.2 | -2.3 | peroxidase | A-P |
| At1g61070 | 10.2 | -6.1 | -5.7 | -1.1 | defensin | |
| At4g14080 | 12.5 | -8.1 | -7.1 | -3.8 | A6 anther-specific protein | |
| At1g75790 | 10.2 | -5.8 | -5.5 | -1.9 | pectinesterase like protein | |
| At4g20420 | 11.7 | -6.0 | -5.4 | -3.1 | tapetum-specific A3 | |
| At1g76470 | 10.3 | -4.8 | -5.0 | -2.3 | cinnamoyl-CoA reductase | |
| At2g21430 | 10.9 | -4.5 | -4.1 | -2.1 | cysteine proteinase | |
| At1g02640 | 11.4 | -2.0 | -2.1 | -1.2 | beta-xylosidase | |
| At1g04645 | 9.6 | -5.3 | -3.3 | -3.4 | ||
| At2g15120 | 8.2 | -4.1 | -3.4 | -4.3 | ||
| At4g20050 | 9.9 | -5.3 | -5.0 | -1.6 | ||
| At5g04820 | 8.8 | -1.6 | -2.2 | -1.2 | ||
| At4g29980 | 11.5 | -5.9 | -5.1 | -4.1 | ||
| At1g22015 | 10.0 | -4.1 | -3.8 | -2.7 | ||
| At1g28710 | 9.4 | -4.0 | -4.1 | -2.3 | ||
| At5g18290 | 9.2 | -2.3 | -2.3 | -1.7 | SIP1 | R-P |
| At4g32105 | 8.1 | -2.4 | -2.5 | -2.3 | ||
| At1g49490 | 6.6 | -1.7 | -1.9 | -2.0 | ||
| At1g32170 | 7.4 | -1.1 | -1.3 | -1.2 | endoxyloglucan transferase | |
| At4g16563 | 6.9 | -1.4 | -1.0 | -1.2 | nucleoid DNA-binding | |
| At5g50030 | 6.5 | -1.3 | -1.1 | -1.4 | pollen-specific protein | |
| At5g45880 | 7.1 | -1.3 | -1.0 | -1.3 | Ole e I | |
| At5g44380 | 5.9 | -1.2 | -1.2 | -1.7 | reticuline oxidase precursor | |
| At5g14300 | 5.8 | -1.4 | -1.3 | -1.3 | prohibitin - like protein | |
| At5g12940 | 8.3 | -2.0 | -2.1 | -1.1 | leucine rich repeat protein | |
| At5g09520 | 5.9 | -1.9 | -2.0 | -1.8 | surface protein PspC-related | |
| At3g06300 | 9.8 | -2.1 | -1.3 | -1.1 | 4-hydroxylase alpha subunit | |
| At1g60390 | 9.4 | -2.0 | -2.3 | -1.2 | polygalacturonase isoenzyme | |
| At3g05930 | 6.7 | -1.4 | -1.3 | -1.2 | germin-like protein | |
| At1g02790 | 6.2 | -2.0 | -1.7 | -1.5 | polygalacturonase | |
| At5g09730 | 8.2 | -3.8 | -2.7 | -1.9 | beta-xylosidase | |
| At5g51950 | 8.2 | -4.2 | -4.1 | -1.3 | mandelonitrile lyase | |
| At1g30760 | 7.4 | -3.5 | -3.3 | -3.1 | reticuline oxidase-like protein | |
| At1g22890 | 7.7 | -2.9 | -1.1 | -1.9 | ||
| At1g33055 | 7.8 | -1.9 | -1.9 | -2.5 | ||
| At1g49500 | 9.3 | -1.4 | -1.5 | -1.7 | ||
| At3g22640 | 7.6 | -3.0 | -1.6 | -3.1 | ||
| At4g15750 | 6.4 | -1.2 | -1.2 | -2.1 | ||
All the expression values are log2 ratio.
In addition, five genes for potential transcription factors were also found in this category (Table 4). Among them, At5g58610 and AGL25/At5g10140 are A-P genes. At5g58610 has a putative function in pathogen defense reaction, uncovering a possible factor in both anther development and external biotic stress response pathways [59,60]. AGL25, also known as FLC, is a repressor of flowering and its expression is epigenetically regulated [61]. However, its possible function in anther development is not known. Three others were AGL40/At4g36590, MYB80/At5g56110 and HAT9/At2g22800. AGL40 was found in the proliferative endosperm transcriptome and MYB80/At5g56110 in tapetum development [27]. These results suggested that normal tapetum functions might require multiple transcription factors preferentially expressed in the anther downstream of AMS.
Table 4.
Transcription factors in SEA-L and SE-L cluster with known or putative function in anther development
| Cluster | AGI | wt | s/w | e/w | a/w | Function | Expression |
|---|---|---|---|---|---|---|---|
| SEA-L | At5g58610 | 7.7 | -1.6 | -1 | -1.9 | PHD finger | A-P |
| At5g10140 | 8.1 | -3.2 | -3.1 | -1.8 | AGL25 | ||
| At4g36590 | 5.8 | -1.7 | -1.4 | -1.7 | AGL40 | R-P | |
| At2g22800 | 8.7 | -2.9 | -2.0 | -1.3 | HAT9 | ||
| At5g56110 | 7.5 | -1.6 | -1.5 | -1.8 | MYB 80/MYB103 | ||
| SE-L | At1g06170 | 9.6 | -5.9 | -4.6 | -0.8 | bHLH89 | A-P |
| At3g28470 | 8.6 | -4.9 | -4.5 | 0.0 | TDF1/MYB35 | ||
| At2g31210 | 7.1 | -3.4 | -2.9 | -1.1 | bHLH91 | ||
| At3g57370 | 7.3 | -2.8 | -2.8 | 0.8 | initiation factor IIB | R-P | |
| At1g77850 | 8.8 | -2.4 | -1.7 | -0.1 | auxin response factor | ||
| At2g28830 | 6.5 | -1.1 | -1.1 | 0.3 | transcription activator | ||
| At5g62320 | 8.6 | -3.3 | -3.4 | -0.3 | MYB99 | ||
| At4g09460 | 8.7 | -3.4 | -2.3 | 0.8 | MYB6 | ||
| At4g34680 | 9.3 | -2.6 | -1.2 | 0.3 | GATA 3 | ||
| At2g41630 | 10.6 | -1.1 | -1.4 | -0.7 | TFIIB | ||
| At3g10580 | 7.3 | -2.5 | -2.3 | 0.9 | MYB | ||
| At5g61590 | 9.7 | -2.3 | -1.6 | -0.3 | AtERF107 | ||
| At4g37790 | 8.1 | -1.9 | -1.8 | -0.8 | HAT22 | ||
| At4g34990 | 8.5 | -1.3 | -2.2 | -0.6 | MYB32 | ||
| At4g10920 | 8.0 | -1.9 | -1.8 | -0.5 | transcriptional co-activator | ||
| At1g66160 | 7.0 | -2.8 | -2.0 | -0.5 | PHOR1 like | ||
| At5g65790 | 7.5 | -3.7 | -3.2 | -0.1 | MYB68 | ||
| At4g34000 | 7.7 | -1.8 | -1.4 | -0.2 | OBF3 | ||
All the expression values are log2 ratio. SEA-L includes genes whose expression levels were reduced in all three mutants. SE- L include genes whose expression levels were reduced in both spl and ems1 mutants.
SPL and EMS1 can regulate early anther development by AMS-independent pathways
Moreover, 354 genes showed reduced expression in spl and ems1 but not in ams (Additional file 7), including the enrichment of the categories of hydrolase activity (15.5% vs. 8.4%), other binding activity (19.5% vs. 9.9%), and other enzyme activity (18.0% vs. 10.1%). Among the genes in this cluster, four genes: MS2, ACOS5, CYP703A2 and A7, were involved in sporopollenin monomer biosynthesis, the lack of which leads to male sterility (Table 1) [62,63]. Since these genes were not affected in the ams mutant, some lipid metabolic genes might be activated independent of AMS and they might exert functions earlier than AMS or in parallel to AMS [63,64].
Besides, several genes encoding putative transcription factors were found within this subset (Table 4). A-P genes with known functions, such as TDF1/At3g28470 (or MYB35) and bHLH89/At1g06170, were also identified in this category [24,32,35]. TDF1 is essential to the tapetum function controlling callose dissolution and acts downstream of SPL and upstream of AMS and MYB103 (Table 4) [24]. Our data also support the regulatory hierarchy of SPL-TDF1-AMS.
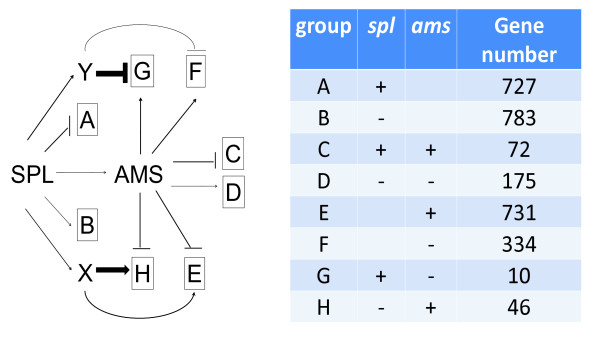
The expression of AMS is significantly reduced in spl, therefore we assumed that genes down-regulated in ams should have similar reduction in spl. Interestingly, we found that 56 genes showed opposite expression changes in spl and in ams compared with wild type anther, and even larger proportion (1,065 genes) only differentially expressed in ams (Figure 6A). Another gene with reduced expression in spl and ems1 mutants is DYT1, which encodes a bHLH protein similar to AMS [22]. It is possible that SPL might also regulate anther development through pathways independent of AMS, such as those requiring DYT1 function [22]. We speculate that SPL might activate other transcription factors that affect AMS-regulated genes in contrast to the function of AMS (represented by factors × and Y in Figure 7). The effects of AMS reduction in spl might be outweighed by the loss of × or Y; such regulatory interactions would explain the opposite expression changes in spl and ams. The identification and understanding of the proposed factors will require further investigations.
Figure 7.
AMS-dependent or -independent regulatory model during anther development. Genes with differential expression in the ams mutant is divided into eight groups A-H (plus represents for higher expression in mutant compared with wild-type and minus for lower). Negative regulation is shown by a T-bar and positive by an arrow, and stronger impact is shown by a thicker T-bar or arrow.
AMS-dependent and independent anther expression of genes encoding transcription factors
Expression of bHLH genes during anther development
Since many transcription factors have been found to play key roles in regulating anther development, we analyzed our anther transcriptome profiles by focusing on transcription factor gene families [5,28,65]. To identify additional candidate genes for anther development, we analyzed all 147 known bHLH genes in Arabidopsis (Additional file 8) [40]. For several clades according to the most recent phylogeny trees of bHLH family [40,66,67], including the clade that includes AMS, all or most members of the same clade were expressed similarly in the anther (Additional file 8), suggesting conserved functional roles in anther development. For example, bHLH91 and bHLH89 shared similar reductions in all three mutants, suggesting possible redundant functions in the anther (Figure 5B).
In other cases, the closely related homologs did not share similar expression patterns in mutant vs. wild type anthers (Additional file 8). For example, bHLH93 is a close homolog of AMS; but unlike AMS, it was preferentially expressed in the inflorescence compared with the anther. Also unlike AMS, it was elevated in expression in spl. It is possible that some compensatory mechanisms might act to increase transcription of bHLH93 when AMS is mutated (Figure 5B).
In addition, some bHLH genes with known functions in other organs showed increased expression in the spl mutant, suggesting that SPL acts to maintain the identity of male reproductive organ by reducing the expression of genes needed for other organs. For example, ZCW32 (bHLH31) controls petal formation and was activated in the spl anther [68,69], suggesting that SPL can promote the normal anther development at an early stage by repressing some genes normally expressed in nearby whorls.
Possible role of MADS-box genes in anther development
Genes of the MADS-box family have been extensively studied in Arabidopsis, because they were first identified as flower homeotic genes that determine floral organ and meristem identities [70,71]. Till now, more than one hundred MADS-box genes have been identified, 79 of which were found to be present in our anther microarray data but most were non-anther-specific (Additional file 7 & Additional file 8) [70]. Except for APETALA2 (AP2), majority of genes involved in the ABCDE model belong to the MADS family [71]. They are mostly inflorescence-preferential rather than anther-specific genes from the comparison of microarray data as described above. APETALA1 (AP1) is an A function gene controlling the first and second whorls and no expression shift was observed [72]. APETALA3 (AP3) and PISTILLATA (PI) are both B function genes, essential for the formation of petals and stamens [72-74]. Interestingly, their expression patterns were different. PI is an anther-preferential gene, but its expression level did not change in any mutant while AP3 was obviously up-regulated in spl, suggesting that AP3 is regulated more tightly than PI during anther development . AG, the C class gene controlling both stamen and carpel identities, shared similar expression patterns in the anther with AP3 [13], supporting a role of AG in anther development after the specification of stamen identity (Figure 5C).
Moreover, D class genes, including STK/AT4g09960, SHP1/At3g58780 and SHP2/At2g42830 , are important for ovule development [75,76]. Although the expression of D class genes was relatively low, we observed increased expression of SHP1 in the ams mutant, suggesting a possible negative regulatory role of AMS in ovule development. On the other hand, E class genes, SEP1, SEP2, SEP3 and SEP4, which are homologs that have redundant functions, had different expression pattern in the anthers. SEP1 and SEP2 were activated in spl and ams, whereas SEP3 and SEP4 did not change much (Figure 5C).
Beside the ABCDE genes, some other MADS genes were also expressed in the anther (Additional file 8). The expression levels of known flowering-time related genes (FLM, AGL15, AGL18 and AGL20) [77-79] were reduced in spl and ems1 slightly. FUL involved in fruit development [80] was up-regulated in the spl and ems1 mutants, suggesting negative roles of SPL and EMS1 in whorl 4. AGL80, important for central cell and endosperm formation in female gametophytes [81], was also reduced in all three mutants, suggesting a possible role in male gametophyte (Additional file 8).
Differential expression of MYB genes in three mutants
In addition to the bHLH and MADS-box families, other gene families are also involved in anther development. As the largest Arabidopsis transcription factor family, MYB genes play important roles in controlling many cellular processes, such as secondary metabolism, morphogenesis, and signal transduction (Additional file 8) [82]. Previous studies revealed a number of roles of MYB genes in early anther development (Figure 5D). For example, GAMYB in rice functions in anther development via GA signaling pathway [83]. In Arabidopsis, the GAMYB homologs MYB33 and MYB65 also share a redundant function regulating tapetum differentiation [22,27,84,85]. Our microarray results indicated that expression of MYB33 and MYB65 was reduced only in spl, not in the other two mutants, implying that the functions of MYB33 and MYB65 are independent of EMS1 or AMS.
In addition, MYB35/TDF1 and MYB80/MYB103 controlling callose dissolution and exine formation [27] were reduced in spl and ems1, and MYB80 was also down-regulated in ams, suggesting that it acts downstream of AMS. Moreover, the MYB99 and MYB101 genes that regulate phenylpropanoid metabolism [31] showed a similar expression pattern to that of MYB35/TDF1. MYB26/MS35 and MYB105 are closely related homologs; both were down-regulated in spl but up-regulated in ams. Previous study suggested that MYB26 is required for endothecium thickening and anther dehiscence [86]. RNA in situ hybridization revealed that MYB105 as well as MYB101 are expressed in late tapetum [86,87], consistent with our findings of the changes of their expression in the mutant anthers.
Expression of WRKY, bZIP, AP2/ERF and NAC genes
The WRKY family contains at least 72 members in Arabidopsis [42] and has diverse functions, such as abiotic and biotic stress response, hormone signaling pathway, immune response and development in plants [88]. However, it is not known whether WRKY genes are important for flower development. Here we compared the expression of all WRKY genes on the ATH1 chip and found that 29 of them were expressed in the anther (Additional file 8), with the highly similar WRKY2/At5g56270 and WRKY32/At4g30935 [88] being anther-preferential. Moreover, WRKY2 was down-regulated in spl, suggesting that it might function downstream of SPL in anther development.
We also analyzed bZIP, ERF and NAC families of transcription factors. Like the WRKY family, most genes in these families do not have known functions in reproductive development (Additional file 8). However, we found several of them were differentially expressed in the anthers of male sterile mutants, suggesting they are components of a complex transcriptional network regulating anther development.
Transcriptional regulatory network for anther development
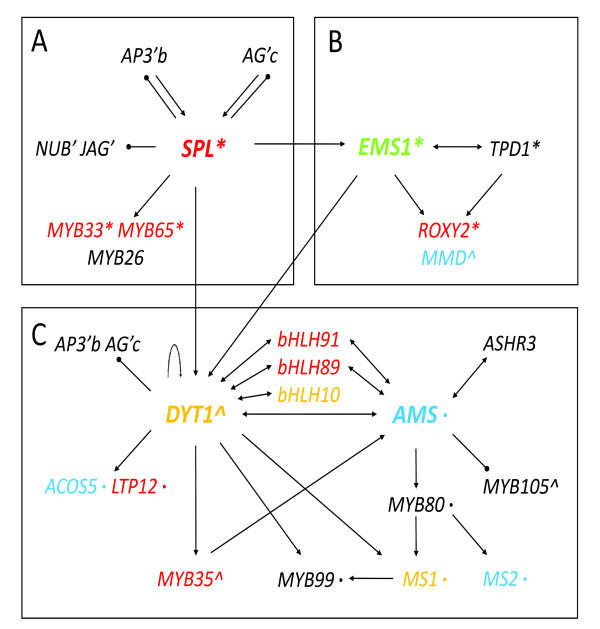
Genetic studies and our transcriptomic analyses reported here support an emerging transcriptional network (Figure 8). Previous molecular genetic studies showed that SPL up-regulates the expression of EMS1 and DYT1, which are upstream of AMS [22,23], as well as other genes encoding transcription factors shown to be important in anther development [8]. SPL also negatively regulates the expression of B and C function genes in the anther, as well as some genes that are normally expressed in petals and carpals, probably to prevent anther from developing traits of other floral organs. In addition, the key position of SPL in anther regulatory hierarchy as indicated by genetic studies is supported by its effects on the anther transcriptome (Figure 8A).
Figure 8.
Gene regulatory network of anther development during early stages. Gene regulation is represented by T-bars (negatively) and arrows (positively). The direct regulation confirmed by experiment is represented in bold line. Genes encoding proteins with interaction is represented by double arrows. Gene expression patterns in different tissues are shown by colors (blue for anther specific; red for anther-preferential; green for reproductive-preferential and yellow for genes not included in ATH1 chip). Gene function in tapetum formation is marked by an apostrophe; in pollen wall formation by an asterisk; in callose dissolution by double asterisks; in stamen and petal formation by the letter b; in stamen and carpel formation by the letter c.
EMS1 also positively regulates the expression of DYT1 [32]. EMS1 was shown to interact with its putative ligand TPD1 [18], thereby regulating genes essential for the differentiation of tapetum cells. In addition, some genes important for meiosis are also affected in the ems1 mutant. For example, the MMD and ROXY2 genes that are important in anther lobe formation and meiosis, respectively, were significantly reduced in ems1 (Figure 8B).
AMS was down-regulated in spl and ems1 according to the microarray data. Because DYT1 and AMS are related bHLH proteins, which are known to form homodimers or heterodimers with other bHLH proteins, we propose that they probably regulate the expression of different genes by forming different complex with other proteins. DYT1 is also a putative candidate that exerts opposite function as × and/or Y downstream of SPL in anther development by interact with different proteins (Figure 8C). This proposed transcriptional regulatory network of anther development is based on information from genetics, transcriptomics, and phylogenetics studies (Figure 8A-C). The hypothesized interactions, including the roles of some functionally redundant genes, could be tested by further experiments.
Conclusion
In this study, we identified genes whose expression was changed in spl, ems1 and ams at anther stage 4-7 and further categorized these genes according to their expression patterns. These genes might directly regulate some fundamental biological processes during anther development. In addition, both anther-specific and non-anther-specific genes are identified in anther development. Transcriptome analyses also showed AMS-dependent and -independent pathways. Careful analyses of transcriptome combined with genetic and phylogenetic information revealed an elaborate regulatory network during early anther development and expanded our understanding of the hierarchy of anther-development-related genes, especially transcription factors.
Methods
Plant materials
All the plants in this study were grown in soil under long day condition (16 h light/8 h dark) at constant 22°C. The wild-type in this paper refers to ecotype Landsberg erecta (L er). The mutants of spl, ems1 are of L er background as described [22,32], while the ams mutant is of Columbia background. We select 21-28 day old plant to collect anther at 4-7 stage as described previously [32].
Microarray experiment
Following the Affymetrix GeneChip Expression Analysis Overview described on the website [35], cRNA was synthesized for hybridization as described [32]. Hybridization, washing, staining, scanning and data collection were performed at the Genomics Core Facility, Pennsylvania State University, University Park.
Microarray analysis to identify differentially expressed genes in anther of mutants
Normalization was applied using Bioconductor package in R by RMA [43], and all expression values were converted to logarithms base 2. LIMMA library was then used to compare signals from mutant and wild-type anther. Only genes with more than two-fold changes were selected. To obtain more reliable result, we screened out genes with q-value (FDR) larger than 0.05, since q-value is more stringent than p-value of T-test based on previous study [89].
Similar data processing was performed with the microarray results from different organs. The microarray data from all organs in wild-type Arabidopsis were normalized together and converted to logarithms base 2 values. We defined genes as anther-specific if they met these criteria: 1) the expression in anther is significantly higher than in any other tissue with FDR < 0.05; 2) gene is present in anther but absent in any other tissues. We used two alternative methods to define whether a gene is present in a tissue. One of the methods was using the Affymetrix' MAS5 algorithm. This method uses a comparison of hybridization intensity with wild-type oligo set vs mismatched oligo set; sometimes similar levels of hybridization to both sets can actually be real expression, yet such results would lead to "absent" calls. Therefore, we also used a second method to define "presence", by using a threshold of 50 for expression value, previously determined on basis of analysis of variation among samples of the same tissue [32,43]. Both results are shown in Additional file 5.
For the anther-preferential genes, we used the criteria that the expression in anther is 1) present using both MAS and/or 50 cutoff; 2) significantly higher than in any other tissue with FDR < 0.05; 3) at least 2 fold more compared with any other tissues. The reproductive-preferential genes required the expression present and significantly higher in anther than only the vegetative organs using FDR < 0.05 and 2-fold changes.
Hierarchical clustering of co-expressed genes was performed by MeV 4.6 [86]. We used Euclidean distance metric to conduct this analysis. For the identification of the functions of the differentially expressed genes, the annotations of genes on ATH1 microarray chip were downloaded from Affymetrix website and we used the GO categorization function on TAIR website [71]. To verify whether one category is enriched compared with the whole genome, we applied hypergeometric test and only the categories with p-value less than 0.05 were called statistically enriched group [90].
Cis-regulatory element analysis
Possible promoter sequences of all genes on the microarray chip (1 kb upstream of the start codon) were obtained from TAIR website. The number of common bHLH binding site (E-box) was then counted. We then plotted the fold-changes of gene expression in ams against the numbers of their putative AMS binding sites using minitab [47]. The identification of cis-regulatory binding site was conducted by perl [46]. The binding motifs were obtained from Gene Regulation and PlantCARE [70].
Real-time PCR experiments
To test the reliability of our microarray hybridizations, six genes and one reference (ACT2, At3g18780) were studied using Quantitative Real-Time PCR. RNA extraction and Real-Time experiments followed the protocols described previously [91]. Triplicate reactions were performed for all tissues with "no reverse transcription" as a negative control. All primer information is provided in Additional file 5. Relative transcript quantities were calculated using the ΔΔCt method [92].
Abbreviations
AMS: Aborted microspores; AG: Agamous; AP2/ERF: APETALA2/ethylene response factor domain-containing transcription factor; bHLH: Basic helix-loop-helix; bZIP: Basic-leucine zipper; CCR1: Cinnamoyl coa reductase1; DYT1: Dysfunctional tapetum1; EMS1: Excess male sporocytes1; EPS1: Enhanced pseudomonas susceptibilty; EXO: Exocytosis; GO: Gene ontology consortium; MADS: MCM1-agamous-deficiens-SRF; MYB: Myeloblastosis-like gene; NAC: NAM/ATAF1/2/CUC2; RD22: Responsive to dessication22; PI: Pistillata; RPK2: Receptor-like protein kinase2; SEC: Secretion; SERK1/2: Somatic embryogenesis receptor-like kinases1/2; SNARE: Soluble NSF attachment protein receptor; SPL/NZZ: Sporocyteless/nozzle; TAIR: The arabidopsis information resource web site; TIR-NBS-LRR: Toll interleukine 1receptor-nucleotide binding site-leucine rich repeat domain;TPD1: Tapetum determinant1; VAM3: Vacuolar morphology; VSP1: Vegetative storage protein.
Authors' contributions
HM designed and supervised the research; BF performed tissue collection and RNA isolation; XM performed data analysis; XM and HM wrote the manuscript drafts; XM, BF and HM edited the manuscript; all authors approved the manuscript.
Supplementary Material
Figure S1. Correlation coefficients between signal intensities from wild-type and the ams anther replicates. Pearson's correlation coefficients were larger than 0.96 between pair of the biological replicates from the ams and wild type anther, indicating that the results were highly reproducible. Figures S2 & S3. GO annotation of genes up- and down-regulated in ams. GO categorization of genes differentially expressed in the ams mutant compared with wild type. The enriched groups were shown in different color with P-value provided. Figures S4-S11. The genes involved in different metabolic pathways that were activated or repressed in ams compared with wild type. Red color represents genes activated while green color represents genes repressed in ams compared with wild type. The overview of metabolism activities was shown in supplemental figure 4. Figure 5, 6, 7, 8-11 showed expression shifts of genes involved in secondary metabolism, regulatory pathways, receptor-like-kinase pathway, transcriptional regulation, protein trafficking, stress response, ubiquitin and autophagy dependent degradation pathway. Figure S12. Real-time PCR results consistent with microarray data. Six genes were verified using real-time PCR. The bars in blue represent the real-time RT-PCR results while red the microarray results. All the numbers shown in this figure are the fold changes of expression intensities in other tissues compared with anther. "infl" is the abbreviation of inflorescence.
Genes differentially expressed in anther and inflorescences from the ams mutant. This additional file contains information about genes differentially expressed in the ams anther and inflorescences compared with wild type. Column sequence, abbreviation and the version of annotation are as those used as in table 1 and all the other supplemental tables. All expression values are log2 ratio.
GO categorization of different clusters based on expression pattern. This additional file contains information about numbers of genes in each GO category. The enriched categories were highlighted in red color.
Genes differentially expressed in the ams mutant with putative function in exocytosis, transportation, ubiquitination and stress reaction. This additional file contains information about genes involved in different pathways with elevated expression levels in ams.
Genes defined as specifically or preferentially expressed in early anther or preferentially expressed in reproductive tissue. This additional file contains information about genes preferentially expressed in only anther or reproductive tissues compared with roots, stems, leaves, siliques.
Genes expressed in stamen, early anther and pollen. This additional file contains information about the expression levels of gene in different organs.
Genes differentially expressed in spl, ems1 or/and the ams mutants. This additional file contains information about the expression levels of gene differentially expressed in the three mutants.
Expression pattern of MADS, MYB, bHLH, WRKY, bZIP, AP2/ERF and NAC families. This additional file contains information about the expression levels of different gene families.
Contributor Information
Xuan Ma, Email: xxm108@psu.edu.
Baomin Feng, Email: baomin@berkeley.edu.
Hong Ma, Email: hongma@fudan.edu.cn.
Acknowledgements
We greatly appreciate the help of Dr. Craig Praul in performing microarray hybridizations. We thank Dr. Naomi Altman for discussion of statistic analysis. We also thank Ms. Jiong Wang for plant care. We appreciate the suggestion from Drs. Xiaofan Zhou, Zhenhai Zhang, Zhao Su on microarray data analysis. This work was supported by a US Department of Energy grant to H.M. and funds from Department Biology and the Huck Institutes of the Life Sciences, the Pennsylvania State University, and Fudan University.
References
- Ge X, Chang F, Ma H. Signaling and transcriptional control of reproductive development in Arabidopsis. Curr Biol. 2010;20:R988–997. doi: 10.1016/j.cub.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG. Stamen structure and function. Plant Cell. 2004;16(Suppl):46–60. doi: 10.1105/tpc.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. Male gametophyte development. Plant Cell. 1993;5:1265–1275. doi: 10.1105/tpc.5.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM. Anther development: basic principles and practical applications. Plant Cell. 1993;5:1217–1229. doi: 10.1105/tpc.5.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol. 2005;56:393–434. doi: 10.1146/annurev.arplant.55.031903.141717. [DOI] [PubMed] [Google Scholar]
- Ma H, Sundaresan V. Development of flowering plant gametophytes. Curr Top Dev Biol. 2010;91:379–412. doi: 10.1016/S0070-2153(10)91013-2. [DOI] [PubMed] [Google Scholar]
- Sanders P, Bui A, Weterings K, McIntire K, Hsu Y, Lee P, Truong M, Beals T, Goldberg R. Anther developmental defects in Arabidopsis thalianamale-sterile mutants. Sex Plant Reprod. 1999;11:297–322. doi: 10.1007/s004970050158. [DOI] [Google Scholar]
- Chang F, Wang Y, Wang S, Ma H. Molecular control of microsporogenesis in Arabidopsis. Curr Opin Plant Biol. 2011;14:66–73. doi: 10.1016/j.pbi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Coen E. Goethe and the ABC model of flower development. C R Acad Sci III. 2001;324:523–530. doi: 10.1016/S0764-4469(01)01321-X. [DOI] [PubMed] [Google Scholar]
- Yang WC, Ye D, Xu J, Sundaresan V. The SPOROCYTELESS gene of Arabidopsisis required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 1999;13:2108–2117. doi: 10.1101/gad.13.16.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K. Molecular analysis of NOZZL, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsisthaliana. Proc Natl Acad Sci USA. 1999;96:11664–11669. doi: 10.1073/pnas.96.20.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T. Molecular and genetic mechanisms of floral control. Plant Cell. 2004;16(Suppl):S1–17. doi: 10.1105/tpc.017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsishomeotic gene agamous resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- Ito T, Wellmer F, Yu H, Das P, Ito N, Alves-Ferreira M, Riechmann JL, Meyerowitz EM. The homeotic protein agamous controls microsporogenesis by regulation of sporocyteless. Nature. 2004;430:356–360. doi: 10.1038/nature02733. [DOI] [PubMed] [Google Scholar]
- Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, Sundaresan V, Ye D. TAPETUM DETERMINANTis required for cell specialization in the Arabidopsisanther. Plant Cell. 2003;15:2792–2804. doi: 10.1105/tpc.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hord CL, Chen C, Deyoung BJ, Clark SE, Ma H. The BAM1/BAM2 receptor-like kinases are important regulators of Arabidopsisearly anther development. Plant Cell. 2006;18:1667–1680. doi: 10.1105/tpc.105.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DZ, Wang GF, Speal B, Ma H. The EXCESS MICROSPOROCYTESgene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsisanther. Genes Dev. 2002;16:2021–2031. doi: 10.1101/gad.997902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Liu X, Owen HA, Zhao D. Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proc Natl Acad Sci USA. 2008;105:2220–2225. doi: 10.1073/pnas.0708795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Jiang LX, Puah CS, Xie LF, Zhang XQ, Chen LQ, Yang WC, Ye D. Overexpression of TAPETUM DETERMINANTalters the cell fates in the Arabidopsiscarpel and tapetum via genetic interaction with EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS. Plant Physiol. 2005;139:186–191. doi: 10.1104/pp.105.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S, Osakabe Y, Maruyama K, Ito T, Osakabe K, Sato T, Shinozaki K, Yamaguchi-Shinozaki K. Receptor-like protein kinase 2 (RPK 2) is a novel factor controlling anther development in Arabidopsisthaliana. Plant J. 2007;50:751–766. doi: 10.1111/j.1365-313X.2007.03083.x. [DOI] [PubMed] [Google Scholar]
- Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI. ArabidopsisSOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell. 2005;17:3350–3361. doi: 10.1105/tpc.105.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H. Regulation of Arabidopsistapetum development and function by DYSFUNCTIONAL TAPETUM(DYT) encoding a putative bHLH transcription factor. Development. 2006;133:3085–3095. doi: 10.1242/dev.02463. [DOI] [PubMed] [Google Scholar]
- Sorensen AM, Krober S, Unte US, Huijser P, Dekker K, Saedler H. The Arabidopsis aborted microspores(AM) gene encodes a MYC class transcription factor. Plant J. 2003;33:413–423. doi: 10.1046/j.1365-313X.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN. Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008;55:266–277. doi: 10.1111/j.1365-313X.2008.03500.x. [DOI] [PubMed] [Google Scholar]
- Boavida LC, Becker JD, Feijo JA. The making of gametes in higher plants. Int J Dev Biol. 2005;49:595–614. doi: 10.1387/ijdb.052019lb. [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ. The Arabidopsis MALE STERILITY1(MS) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J. 2001;28:27–39. doi: 10.1046/j.1365-313X.2001.01125.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H. et al. Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J. 2007;52:528–538. doi: 10.1111/j.1365-313X.2007.03254.x. [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Zhang DB. From Arabidopsisto rice: pathways in pollen development. J Exp Bot. 2009;60:1479–1492. doi: 10.1093/jxb/erp095. [DOI] [PubMed] [Google Scholar]
- Cutler S, McCourt P. Dude, where's my phenotype? Dealing with redundancy in signaling networks. Plant Physiol. 2005;138:558–559. doi: 10.1104/pp.104.900152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer F, Riechmann JL, Alves-Ferreira M, Meyerowitz EM. Genome-wide analysis of spatial gene expression in Arabidopsisflowers. Plant Cell. 2004;16:1314–1326. doi: 10.1105/tpc.021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Ferreira M, Wellmer F, Banhara A, Kumar V, Riechmann JL, Meyerowitz EM. Global expression profiling applied to the analysis of Arabidopsisstamen development. Plant Physiol. 2007;145:747–762. doi: 10.1104/pp.107.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeratne AJ, Zhang W, Sun Y, Liu W, Albert R, Zheng Z, Oppenheimer DG, Zhao D, Ma H. Differential gene expression in Arabidopsiswild-type and mutant anthers: insights into anther cell differentiation and regulatory networks. Plant J. 2007;52:14–29. doi: 10.1111/j.1365-313X.2007.03217.x. [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D. Comparative analysis of the Arabidopsispollen transcriptome. Plant Physiol. 2003;132:640–652. doi: 10.1104/pp.103.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YF, Tzeng JD, Liu MC, Yei FL, Chung MC, Wang CS. Identification of anther-specific/predominant genes regulated by gibberellin during development of lily anthers. J Plant Physiol. 2008;165:553–563. doi: 10.1016/j.jplph.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Xu J, Yang CY, Yuan Z, Zhang DS, Gondwe MY, Ding ZW, Liang WQ, Zhang DB, Wilson ZA. The aborted microspores regulatory network is required for postmeiotic male reproductive development in Arabidopsisthaliana. Plant Cell. 2010;22:91–107. doi: 10.1105/tpc.109.071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol. 2008;49:1429–1450. doi: 10.1093/pcp/pcn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal A, Elvitigala T, Ghosh B, Quatrano R. Arabidopsistranscriptome reveals control circuits regulating redox homeostasis and the role of an AP2 transcription factor. Plant Physiol. 2008;148:2050–2058. doi: 10.1104/pp.108.128488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzen WI, Wurtele ES. Regulon organization of Arabidopsis. BMC Plant Biol. 2008;8:99. doi: 10.1186/1471-2229-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Ogata Y, Shibata D. Approaches for extracting practical information from gene co-expression networks in plant biology. Plant Cell Physiol. 2007;48:381–390. doi: 10.1093/pcp/pcm013. [DOI] [PubMed] [Google Scholar]
- Li X, Duan X, Jiang H, Sun Y, Tang Y, Yuan Z, Guo J, Liang W, Chen L, Yin J. et al. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006;141:1167–1184. doi: 10.1104/pp.106.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus JT, Aquea F, Arce-Johnson P. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitisand Arabidopsisgenomes. BMC Plant Biol. 2008;8:83. doi: 10.1186/1471-2229-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Guo ZJ, Wang HH, Li J. The WRKY family of transcription factors in rice and Arabidopsisand their origins. DNA Res. 2005;12:9–26. doi: 10.1093/dnares/12.1.9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Feng B, Zhang Q, Zhang D, Altman N, Ma H. Genome-wide expression profiling and identification of gene activities during early flower development in Arabidopsis. Plant Mol Biol. 2005;58:401–419. doi: 10.1007/s11103-005-5434-6. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, de Wit H, Verhage M, Neher E, Sorensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330:502–505. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Jurgens G, El-Kasmi F, Pacher T, Strompen G, Stierhof YD, Muller LM, Koncz C, Mayer U. ArabidopsisSNARE protein SEC22 is essential for gametophyte development and maintenance of Golgi-stack integrity. Plant J. 2011;66:268–279. doi: 10.1111/j.1365-313X.2011.04487.x. [DOI] [PubMed] [Google Scholar]
- Sato MH, Nakamura N, Ohsumi Y, Kouchi H, Kondo M, HaraNishimura I, Nishimura M, Wada Y. The AtVAMencodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsisthaliana. J Biol Chem. 1997;272:24530–24535. doi: 10.1074/jbc.272.39.24530. [DOI] [PubMed] [Google Scholar]
- Chow B, McCourt P. Plant hormone receptors: perception is everything. Genes Dev. 2006;20:1998–2008. doi: 10.1101/gad.1432806. [DOI] [PubMed] [Google Scholar]
- Chen J. Characterization of the Arabidopsisthermosensitive mutant atts02reveals an important role for galactolipids in thermotolerance. Plant Cell Environ. 2006;29:1671–1671. doi: 10.1111/j.1365-3040.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Yang KZ, Xia C, Liu XL, Dou XY, Wang W, Chen LQ, Zhang XQ, Xie LF, He L, Ma X. et al. A mutation in THERMOSENSITIVE MALE STERILE1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis. Plant J. 2009;57:870–882. doi: 10.1111/j.1365-313X.2008.03732.x. [DOI] [PubMed] [Google Scholar]
- Peng J. Gibberellin and jasmonate crosstalk during stamen development. J Integr Plant Biol. 2009;51:1064–1070. doi: 10.1111/j.1744-7909.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- Moon J, Parry G, Estelle M. The ubiquitin-proteasome pathway and plant development. Plant Cell. 2004;16:3181–3195. doi: 10.1105/tpc.104.161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Sun P, Kao TH. S-RNase-based self-incompatibility in Petuniainflata. Ann Bot. 2010;108:637–646. doi: 10.1093/aob/mcq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk SJL, Timmers HTM. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- Wang XL, Li XB. The GhACS1gene encodes an acyl-CoA synthetase which is essential for normal microsporogenesis in early anther development of cotton. Plant J. 2009;57:473–486. doi: 10.1111/j.1365-313X.2008.03700.x. [DOI] [PubMed] [Google Scholar]
- Surpin M, Raikhel N. Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol. 2004;5:100–109. doi: 10.1038/nrm1311. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Bednarek SY, Raikhel NV. Membrane trafficking in plants: new discoveries and approaches. Curr Opin Plant Biol. 2004;7:701–707. doi: 10.1016/j.pbi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Jurgens G. Membrane trafficking in plants. Annu Rev Cell Dev Biol. 2004;20:481–504. doi: 10.1146/annurev.cellbio.20.082503.103057. [DOI] [PubMed] [Google Scholar]
- Libault M, Wan J, Czechowski T, Udvardi M, Stacey G. Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol Plant Microbe Interact. 2007;20:900–911. doi: 10.1094/MPMI-20-8-0900. [DOI] [PubMed] [Google Scholar]
- Ascencio-Ibanez JT, Sozzani R, Lee TJ, Chu TM, Wolfinger RD, Cella R, Hanley-Bowdoin L. Global analysis of Arabidopsisgene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 2008;148:436–454. doi: 10.1104/pp.108.121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien S, Fleury D, Mylne JS, Crevillen P, Inze D, Avramova Z, Dean C, Grossniklaus U. ARABIDOPSIS TRITHORAX1 dynamically regulates flowering locus C activation via histone 3 lysine 4 trimethylation. Plant Cell. 2008;20:580–588. doi: 10.1105/tpc.108.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinelli P, Hu Y, Ma H. Identification, sequence analysis and expression studies of novel anther-specific genes of Arabidopsisthaliana. Plant Mol Biol. 1998;37:607–619. doi: 10.1023/A:1005964431302. [DOI] [PubMed] [Google Scholar]
- de Azevedo Souza C, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, Haughn GW, Kombrink E, Douglas CJ. A novel fatty Acyl-CoA Synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell. 2009;21:507–525. doi: 10.1105/tpc.108.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A. The ArabidopsisMALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J. 1997;12:615–623. doi: 10.1046/j.1365-313X.1997.d01-8.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Dickinson HG. Cell-cell interactions during patterning of the Arabidopsisanther. Biochem Soc Trans. 2010;38:571–576. doi: 10.1042/BST0380571. [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsisbasic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PC, Martin C, Toledo-Ortiz G, Quail PH, Huq E, Heim MA, Jakoby M, Werber M, Weisshaar B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsisthaliana. Plant Cell. 2003;15:2497–2502. doi: 10.1105/tpc.151140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioudes F, Joly C, Szecsi J, Varaud E, Leroux J, Bellvert F, Bertrand C, Bendahmane M. Jasmonate controls late development stages of petal growth in Arabidopsis thaliana. Plant Journal. 2009;60:1070–1080. doi: 10.1111/j.1365-313X.2009.04023.x. [DOI] [PubMed] [Google Scholar]
- Szecsi J, Joly C, Bordji K, Varaud E, Cock JM, Dumas C, Bendahmane M. BIGPETALp, a bHLH transcription factor is involved in the control of Arabidopsispetal size. EMBO J. 2006;25:3912–3920. doi: 10.1038/sj.emboj.7601270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenicova L, de Folter S, Kieffer M, Horner DS, Favalli C, Busscher J, Cook HE, Ingram RM, Kater MM, Davies B. et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell. 2003;15:1538–1551. doi: 10.1105/tpc.011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. Encyclopedia of Life Sciences. 2.0. Chichester: John Wiley & Sons, Ltd; 2009. Regulatory genes in plant development: MADS. [Google Scholar]
- Irish VF, Sussex IM. Function of the apetala-1 gene during Arabidopsisfloral development. Plant Cell. 1990;2:741–753. doi: 10.1105/tpc.2.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM. Function and regulation of the Arabidopsisfloral homeotic gene PISTILLAT. Genes Dev. 1994;8:1548–1560. doi: 10.1101/gad.8.13.1548. [DOI] [PubMed] [Google Scholar]
- Jack T, Brockman LL, Meyerowitz EM. The homeotic gene APETALAof Arabidopsisthaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992;68:683–697. doi: 10.1016/0092-8674(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell. 2003;15:2603–2611. doi: 10.1105/tpc.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JD, Borevitz JO, Warthmann N, Trainer GT, Ecker JR, Chory J, Weigel D. Quantitative trait locus mapping and DNA array hybridization identify an FLM deletion as a cause for natural flowering-time variation. Proc Natl Acad Sci USA. 2005;102:2460–2465. doi: 10.1073/pnas.0409474102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han JH, Liou YC, Yu H. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development. 2008;135:1481–1491. doi: 10.1242/dev.020255. [DOI] [PubMed] [Google Scholar]
- Adamczyk BJ, Lehti-Shiu MD, Fernandez DE. The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J. 2007;50:1007–1019. doi: 10.1111/j.1365-313X.2007.03105.x. [DOI] [PubMed] [Google Scholar]
- Gu Q, Ferrandiz C, Yanofsky MF, Martienssen R. The FRUITFULMADS-box gene mediates cell differentiation during Arabidopsifruit development. Development. 1998;125:1509–1517. doi: 10.1242/dev.125.8.1509. [DOI] [PubMed] [Google Scholar]
- Portereiko MF, Lloyd A, Steffen JG, Punwani JA, Otsuga D, Drews GN. AGL80 is required for central cell and endosperm development in Arabidopsis. Plant Cell. 2006;18:1862–1872. doi: 10.1105/tpc.106.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000;3:423–434. doi: 10.1016/S1369-5266(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, Matsuoka M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009;21:1453–1472. doi: 10.1105/tpc.108.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Gubler F. The ArabidopsisGAMYB-like genes, MYB3and MYB6, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell. 2005;17:705–721. doi: 10.1105/tpc.104.027920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell. 2007;19:2776–2792. doi: 10.1105/tpc.107.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Xu Z, Song J, Conner K, Vizcay Barrena G, Wilson ZA. ArabidopsisMYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. Plant Cell. 2007;19:534–548. doi: 10.1105/tpc.106.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H. Disruption of Arabidopsisthaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J. 2003;34:519–528. doi: 10.1046/j.1365-313X.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genome-wide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Su Z. EasyGO: gene ontology-based annotation and functional enrichment analysis tool for agronomical species. BMC Genomics. 2007;8:246. doi: 10.1186/1471-2164-8-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn LM, Ma X, Altman NS, Zhang Q, Wall PK, Tian D, Gibas CJ, Gharaibeh R, Leebens-Mack JH, Depamphilis CW. et al. Comparative transcriptomics among floral organs of the basal eudicot Eschscholzia californica as reference for floral evolutionary developmental studies. Genome Biol. 2010;11:R101. doi: 10.1186/gb-2010-11-10-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlation coefficients between signal intensities from wild-type and the ams anther replicates. Pearson's correlation coefficients were larger than 0.96 between pair of the biological replicates from the ams and wild type anther, indicating that the results were highly reproducible. Figures S2 & S3. GO annotation of genes up- and down-regulated in ams. GO categorization of genes differentially expressed in the ams mutant compared with wild type. The enriched groups were shown in different color with P-value provided. Figures S4-S11. The genes involved in different metabolic pathways that were activated or repressed in ams compared with wild type. Red color represents genes activated while green color represents genes repressed in ams compared with wild type. The overview of metabolism activities was shown in supplemental figure 4. Figure 5, 6, 7, 8-11 showed expression shifts of genes involved in secondary metabolism, regulatory pathways, receptor-like-kinase pathway, transcriptional regulation, protein trafficking, stress response, ubiquitin and autophagy dependent degradation pathway. Figure S12. Real-time PCR results consistent with microarray data. Six genes were verified using real-time PCR. The bars in blue represent the real-time RT-PCR results while red the microarray results. All the numbers shown in this figure are the fold changes of expression intensities in other tissues compared with anther. "infl" is the abbreviation of inflorescence.
Genes differentially expressed in anther and inflorescences from the ams mutant. This additional file contains information about genes differentially expressed in the ams anther and inflorescences compared with wild type. Column sequence, abbreviation and the version of annotation are as those used as in table 1 and all the other supplemental tables. All expression values are log2 ratio.
GO categorization of different clusters based on expression pattern. This additional file contains information about numbers of genes in each GO category. The enriched categories were highlighted in red color.
Genes differentially expressed in the ams mutant with putative function in exocytosis, transportation, ubiquitination and stress reaction. This additional file contains information about genes involved in different pathways with elevated expression levels in ams.
Genes defined as specifically or preferentially expressed in early anther or preferentially expressed in reproductive tissue. This additional file contains information about genes preferentially expressed in only anther or reproductive tissues compared with roots, stems, leaves, siliques.
Genes expressed in stamen, early anther and pollen. This additional file contains information about the expression levels of gene in different organs.
Genes differentially expressed in spl, ems1 or/and the ams mutants. This additional file contains information about the expression levels of gene differentially expressed in the three mutants.
Expression pattern of MADS, MYB, bHLH, WRKY, bZIP, AP2/ERF and NAC families. This additional file contains information about the expression levels of different gene families.