SUMMARY
Regulatory networks orchestrated by key transcription factors (TFs) have been proposed to play a central role in the determination of stem-cell states. However, the master transcriptional regulators of adult stem cells are poorly understood. We have identified two TFs, Slug and Sox9, that act cooperatively to determine the mammary stem cell (MaSC) state. Inhibition of either Slug or Sox9 blocks MaSC activity in primary mammary epithelial cells. Conversely, transient coexpression of exogenous Slug and Sox9 suffices to convert differentiated luminal cells into MaSCs with long-term mammary gland-reconstituting ability. Slug and Sox9 induce MaSCs by activating distinct auto-regulatory gene expression programs. We also show that coexpression of Slug and Sox9 promotes the tumorigenic and metastasis-seeding abilities of human breast cancer cells and is associated with poor patient survival, providing direct evidence that human breast cancer stem cells are controlled by key regulators similar to those operating in normal murine MaSCs.
INTRODUCTION
The regulators that govern the adult stem cell (SC) state are poorly understood. Developmental studies have demonstrated that master transcription factors (TFs) play central roles in determining cellular states, among them the SC state (Halder et al., 1995; Tapscott et al., 1988). For example, in embryonic stem cells (ESCs), a set of core TFs, notably Oct4, Sox2 and Nanog, form an auto-regulatory network and act cooperatively to activate genes that maintain the ESC state and, at the same time, silence the expression of genes involved in lineage-specific differentiation (Boyer et al., 2005; Chen et al., 2008; Kim et al., 2008). Similarly organized transcriptional networks orchestrated by master TFs are likely to play a key role in the determination of tissue-specific SCs as well. However, the master TFs that control adult SC programs remain poorly defined, particularly in epithelial tissues.
The mammary gland represents a highly useful model system for studying the regulation of epithelial SCs, as it contains a small subpopulation of cells with robust SC activity. Thus, implantation of a single murine mammary stem cell (MaSC) into the murine mammary fat pad, which represents the stromal component of the normal mammary gland, is sufficient to generate an entire mammary ductal tree (Kordon and Smith, 1998; Shackleton et al., 2006; Stingl et al., 2006a). This in vivo regeneration assay makes the murine mammary gland a powerful experimental system for dissecting the regulatory mechanisms that control epithelial SCs and offers a stringent test of stemness. While recent advances have begun to delineate the mammary epithelial cell hierarchy and the cell-surface markers of MaSCs, the molecular determinants of MaSCs and derived epithelial lineages are still largely unknown (Stingl et al., 2006b; Visvader, 2009).
The epithelial-mesenchymal transition (EMT) is widely documented to play a key role in converting both normal and neoplastic epithelial cells into derivatives with a more mesenchymal phenotype. In the context of neoplasia, passage through an EMT results in the acquisition of cell-biological traits associated with high-grade malignancy, among them motility, invasiveness, and an increased resistance to apoptosis (Huber et al., 2005; Thiery et al., 2009). Recently, we and others uncovered an unanticipated link between the EMT and MaSCs (Mani et al., 2008; Morel et al., 2008). Thus, in addition to conferring malignant cell-biological traits, we found that forced passage of both normal and neoplastic mammary epithelial cells (MECs) through an EMT confers on the resulting cells many of the properties associated with MaSCs (Mani et al., 2008).
In fact, the conclusion that the EMT program drove the entrance of normal differentiated MECs into the MaSC state depended entirely on inference, specifically indirect evidence derived from examination of cell-surface markers. Since such markers identify a heterogeneous collection of cells, only a subset of which are bona fide MaSCs, this previous evidence did not provide direct indication of the acquisition of fully functional SC properties. As we describe here, our efforts to address this issue have led to the discovery of a novel genetic pathway that cooperates with the EMT to convert differentiated MECs into MaSCs. Interestingly, these pathways also appear to regulate the maintenance of human breast cancer SCs.
RESULTS
Expression of EMT-inducing transcription factors in mammary stem cells
The previously demonstrated connection between the EMT and certain MaSC properties suggested that TFs that are able to induce passage through an EMT program (EMT-TFs) could also serve as key regulators for conferring SC traits on differentiated MECs. We wished to extend this work by developing direct functional proof of the connection between passage through an EMT and the acquisition of SC traits in a normal epithelial tissue. To do so, we utilized primary murine mammary epithelial cells, as the murine mammary gland reconstitution assay offers a robust and stringent test of SC activity.
To begin, we used the CD49f and CD61 cell-surface antigen markers and fluorescence-activated cell sorting (FACS) to resolve distinct subpopulations of freshly isolated murine MECs; this allowed us to separate murine MECs into three subpopulations as effectively as the published CD29 and CD61 method (Asselin-Labat et al., 2007). These populations were CD49fhighCD61+ MaSC-enriched basal cells, CD49flowCD61+ luminal progenitors, and CD49flowCD61− differentiated luminal cells (Figure S1A). We confirmed that CD49fhighCD61+ cells were greatly enriched for MaSC activity using the cleared mammary fat pad transplantation assay (Figure S1B) and that CD49flowCD61+ cells were enriched in luminal progenitor activity using a Matrigel culture assay (Figure S1C).
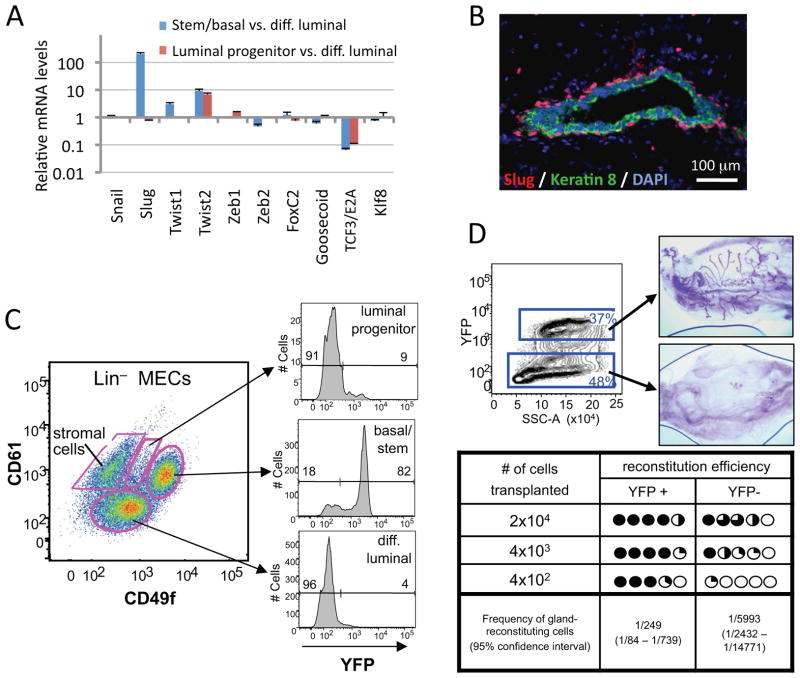
We proceeded to measure the expression in these MEC populations of various mRNAs encoding ten previously described EMT-TFs. Among this group of EMT-TFs, only Slug was highly over-expressed (~200-fold) in the MaSC-enriched basal population relative to other populations (Figure 1A). Consistent with the Slug expression, basal cells expressed high levels of mesenchymal markers and low levels of epithelial markers (Figure S1D). Analysis of published microarray data from various human MEC subpopulations confirmed that Slug was also the most highly expressed EMT-TF in the human MaSC-enriched basal cell population (Figure S1E) (Lim et al., 2010).
Figure 1. Slug is the major EMT-TF that is expressed in MaSCs.
(A) The mRNA levels of the EMT-TFs in MaSC-enriched basal (stem/basal) or luminal progenitor cells were compared to those of differentiated luminal cells (diff. luminal) by qRT-PCR in triplicate. GAPDH was used as a loading control.
(B) Slug protein expression in the mammary gland was analyzed by immunofluorescence on tissue sections. An anti-keratin 8 antibody was used to label the luminal cells.
(C) The expression of the Slug-YFP reporter in MaSC-enriched basal (basal/stem), luminal progenitor and differentiated luminal (diff. luminal) cells.
(D) The gland-reconstituting activities of Slug-YFP+ and Slug-YFP− MECs were measured by the limiting dilution analysis. Representative whole-mount images of carmine-stained fat pads (upper panel) and reconstitution efficiencies (lower panel) are shown. In the lower panel, each circle represents one transplanted fat pad, and the dark area of each circle represents the percentage of the fat pad that is occupied by reconstituted mammary ductal trees. P=1.6X10−5.
The data are represented as mean ± standard error of the mean (SEM). See also Figure S1.
The Slug protein was also specifically expressed in the nuclei of basal cells in the mammary epithelium, as determined by immunofluorescence (Figure 1B). This cell layer has been reported to contain virtually all of the MaSCs (Shackleton et al., 2006; Stingl et al., 2006a). However, we noted that a relatively high proportion of basal cells expressed Slug, suggesting that other basal cells, in addition to MaSCs, also express the Slug EMT-TF.
To demonstrate unequivocally that the MaSCs residing among the larger basal cell population do indeed express Slug, we generated a transgenic mouse line (Slug-YFP mice) in which expression of a yellow fluorescent protein (YFP) gene was driven by the endogenous Slug promoter. Consistent with the immunofluorescence data, only CD49fhighCD61+ basal cells expressed Slug-YFP, while luminal progenitors and differentiated luminal cells did not express this EMT-TF to a significant extent (Figure 1C). To examine whether MaSCs were enriched in Slug-YFP-positive MECs, we transplanted FACS-sorted YFP-positive and -negative MECs into cleared mammary fat pads at limiting dilutions. We calculated the frequency of mammary gland-reconstituting cells to be 1/250 in the Slug-YFP-positive cells versus 1/6000 in the Slug-YFP-negative cells (Figure 1D). These results demonstrated that Slug is strongly expressed in MaSCs. However, these correlative observations did not indicate whether Slug plays a functional role either in inducing the formation of MaSCs or maintaining their residence in the SC state.
Establishing an improved in vitro MaSC assay
To facilitate functional studies of MaSC regulators, we sought to establish a more robust in vitro MaSC assay by modifying existing Matrigel culture methods (Lim et al., 2009; Shackleton et al., 2006; Stingl et al., 2006a). Thus, we used a ROCK inhibitor to increase organoid-forming efficiency, because it has been shown to promote ESC seeding (Watanabe et al., 2007) and organoid formation by intestinal SCs (Sato et al., 2009). We also reduced Matrigel concentration to 5%, which did not affect organoid formation, but facilitated the retrieval of cells from already-formed organoids. In these 3-dimensional cultures, ~3% of MaSC-enriched basal cells formed solid organoids, whereas luminal progenitors formed acini with hollow lumina at high efficiencies (~13%), but rarely formed solid organoids (< 0.1%) (Figure S2A). This is consistent with previous observations that MaSCs have an ability to form solid organoids, whereas luminal progenitors form acinar structures in Matrigel cultures (Lim et al., 2009; Shackleton et al., 2006; Stingl et al., 2006a).
To demonstrate directly that the solid organoids indeed contained MaSCs, we examined whether organoids isolated from these cultures could reconstitute mammary ductal trees in vivo, a stringent test that had not been performed in previous studies using Matrigel culture assays. We generated secondary organoid cultures from isolated individual primary organoids and transplanted 25% of each culture into a cleared mammary fat pad. We found about 70% of such transplantations allowed reconstitution of full mammary ductal trees (Figure S2B). In addition, when recipient mice were impregnated, these reconstituted mammary ductal trees underwent robust alveologenesis, a differentiation process that gives rise to milk-secreting alveoli (Figure S2B). In contrast to solid organoids, the acini formed by luminal progenitor cells in the improved organoid culture could not reconstitute mammary ductal trees upon transplantation into cleared mammary fat pads (Figure S2C). These data showed that almost all solid organoids formed in the improved Matrigel culture contained functional MaSCs and that our Matrigel organoid assay could serve as a specific in vitro assay for the presence of MaSCs.
Effects of ectopic expression of Slug on MaSC activity in vivo
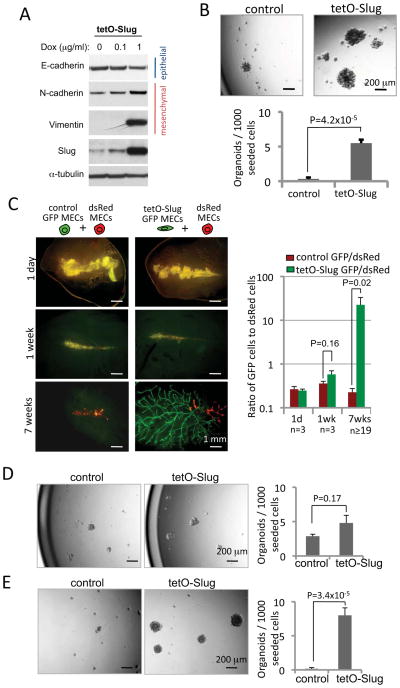
To examine a functional role for Slug in MaSC induction, we transiently expressed Slug in primary MECs by using a tetracycline-inducible lentiviral expression vector, which induced a robust EMT in primary MECs in monolayer cultures (Figure 2A and Figure S2D). We expressed Slug transiently in primary MECs in monolayer cultures for 7 days and then transferred these cells to organoid culture in the absence of doxycycline and thus in the absence of further ectopic Slug expression. We observed that MECs that had previously expressed Slug transiently generated 17 times more organoids than did control-vector-expressing MECs (Figure 2B).
Figure 2. The ectopic expression of Slug induces MaSC activity.
(A) The expression levels of EMT-associated proteins were determined by immunoblot. The primary MECs transduced with tetracycline-inducible Slug lentivirus were treated with the indicated concentration of doxycycline for 5 days.
(B) The organoid-forming efficiencies of the primary MECs transduced with the indicated vectors.
(C) The gland-reconstituting activity was measured using the competitive reconstitution assay. The left panel shows representative whole-mount fluorescence images of the mammary fat pads at the indicated time points post-injection. Right panel shows the ratios of GFP-expressing (either control vector or Slug) to dsRed-expressing cells, as measured by flow cytometry.
(D, E) The organoid-forming efficiencies of MaSC-enriched basal cells (D) or luminal progenitor cells (E) that were transduced with the indicated vectors.
The data are represented as mean ± SEM. See also Figure S2.
In addition, we measured Slug-induced formation of MaSCs, now using the more stringent in vivo cleared mammary fat pad reconstitution assay (DeOme et al., 1959). In a competitive reconstitution analysis, we expressed Slug or the control tetracycline-inducible vector in GFP-expressing primary MECs for 5 days in monolayer culture, and then implanted these cells (1x105) with an equal number of admixed dsRed-expressing MECs that had not been virally transduced into cleared mammary fat pads. The animals were treated with doxycycline for 7 additional days after the transplantation and then maintained on a doxycycline-free diet. We analyzed the implanted fat pads at different time points post-implantation in order to monitor the short-term and long-term effects of transient Slug expression on initial engraftment and subsequent ductal morphogenesis (see Figure S2E for a schematic description of this experiment).
At days 1 and 7 following implantation, the Slug and control-vector GFP-expressing cells contributed to engrafted murine MECs to comparable extents, indicating that a history of exposure to Slug had not affected the ability of MECs to survive the initial rigors of implantation. However, at 7 weeks post implantation, MECs that had experienced transient Slug exposure at the beginning of the experiment generated elaborate mammary ductal trees, while the control-vector-expressing cells formed only rudimentary structures (Figure 2C). The Slug-exposed MECs generated mammary ductal trees 35-fold more efficiently than did the control-vector-expressing cells, as measured by the ratio of GFP- to dsRed-expressing MECs engrafted in the fat pads (Figure 2C). Together, these results demonstrated that transient expression of Slug in unfractionated populations of MECs dramatically increased the representation of gland-repopulating MaSCs.
Slug induces MaSCs from luminal progenitors but not differentiated luminal cells
In the experiments described above, we reasoned that Slug exposure could function either by expanding a pre-existing population of MaSCs or by converting non-SCs into SCs. To distinguish between these two alternative mechanisms, we fractionated primary MECs into MaSC-enriched basal cells, luminal progenitors and differentiated luminal cells, as described in Figure S1A, and subsequently examined the effect of ectopic Slug expression on inducing MaSC activity in each of these three purified cell populations.
Transient expression of Slug in the MaSC-enriched basal cells for 7 days increased organoid-forming ability by less than 2-fold, suggesting that ectopic Slug expression only modestly increased the pool of MaSCs in a cell population that already contained significant numbers of endogenous MaSCs (Figure 2D). In contrast, similar transient expression of Slug in luminal progenitors led to a 50-fold increase in the representation of organoid-forming cells compared to vector-control cells treated in parallel, indicating that Slug could convert luminal progenitors into MaSCs (Figure 2E). However, transient Slug expression in the differentiated luminal cells failed to induce any organoid-forming cells whatsoever (Figure 3B). This indicated that expression of Slug on its own, while capable of inducing luminal progenitors to enter into the MaSC state, failed to induce differentiated luminal cells to do so.
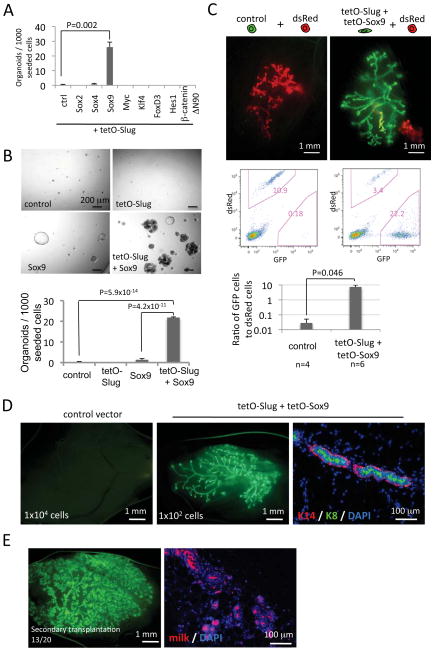
Figure 3. The cooperation of Sox9 with Slug in the formation of MaSCs.
(A) The screening for Slug cofactor(s) that are involved in the induction of organoid-forming cells. Differentiated luminal cells transduced with the indicated vectors were treated with doxycycline for 5 days in monolayer culture and then subjected to organoid culture without further doxycycline treatment.
(B) The organoid-forming efficiencies of differentiated luminal cells transduced with the indicated vectors and then treated as in (A).
(C) The gland-reconstituting activity of differentiated luminal cells transduced with the indicated vectors. The fat pads were analyzed 7 weeks post-injection by whole-mount analysis (top panel) and flow cytometry (middle panel). The relative MaSC activity was quantified as the ratio of GFP- to dsRed-positive cells (lower panel). The data are representative of three independent experiments.
(D) The mammary gland outgrowths generated by GFP-expressing differentiated luminal cells transduced with the indicated vectors. The cells were treated with doxycycline for 8 days in monolayer culture and then transplanted into cleared mammary fat pads at limiting dilutions. The fat pads were examined 3 months post-implantation by whole-mount imaging (left and middle panels) or immunofluorescence on tissue sections (right panel).
(E) Secondary transplantation generated by Slug/Sox9-exposed differentiated luminal cells. The mice were mated 4 weeks post-transplantation. The mammary fat pads around gestation day 18 were then analyzed by whole-mount fluorescent imaging (left) or immunofluorescence on tissue sections (right).
The data are represented as mean ± SEM. See also Figure S3.
Cooperation of Sox9 with Slug in the formation of mammary stem cells
We reasoned that the inability of Slug to induce the formation of MaSCs by differentiated luminal cells might be due to the fact that Slug required the cooperation of one or more additional factors to do so. To identify such cooperating factors, we selected eight TFs that had been implicated previously by others to play important roles in either embryonic or adult stem cell biology, or had been shown to cooperate with Slug in certain early developmental processes (Figure 3A) (Cheung et al., 2005; Pece et al., 2010; Takahashi and Yamanaka, 2006). We co-expressed each of these factors individually together with Slug in pre-sorted differentiated luminal cells to determine whether any of them could collaborate with Slug to induce organoids in the Matrigel culture assay.
Among these eight TFs, SRY (sex determining region Y)-box 9 (Sox9) was particularly effective in inducing, together with Slug, the formation of solid organoids by differentiated luminal cells. In contrast, the other co-expressed factors failed to collaborate with Slug to induce organoids (Figure 3A). Expression of Sox9 on its own in differentiated luminal cells had a far smaller effect in inducing organoid-forming cells relative to the effects of co-expressing Slug and Sox9 (Figure 3B). We concluded that Slug and Sox9 could collaborate to induce differentiated luminal cells to enter the MaSC state. Interestingly, the expression in differentiated luminal cells of Sox9 by itself led to the formation of acini with hollow lumina (Figure 3B and Figure S3B), which are indicative of luminal progenitor activity (Figure S2A) (Lim et al., 2009; Shackleton et al., 2006; Stingl et al., 2006a).
Our previous work had shown that the expression of other EMT-inducing TFs, such as Snail and Twist1, could induce stem-like cells in immortalized human MECs (Mani et al. 2008). We therefore tested whether these two EMT-TFs could also cooperate with Sox9 to induce MaSC formation in primary mouse MECs. Interestingly, while Snail could replace Slug to induce MaSCs in cooperation with Sox9, Twist1 failed to do so (Figure S3C). In fact, Twist1 also could not induce a robust EMT in monolayer cultures of primary mouse MECs, whereas Slug and Snail could indeed do so (Figure S3D). This inefficient EMT induction might well be the reason why Twist1 failed to induce MaSCs. These results suggested that potent EMT-TFs other than Slug, such as the related Snail TF, could potentially also cooperate with Sox9 to induce SC formation.
We further tested whether Slug and Sox9 could collaborate to convert differentiated luminal cells into MaSCs capable of reconstituting cleared mammary fat pads. To do so, we introduced tetracycline-inducible Slug and Sox9 expression vectors into sorted GFP-expressing differentiated luminal cells and induced the expression of both genes for 5 days in monolayer culture. The resulting cell populations were mixed with an equal number (1x105) of unsorted dsRed-expressing MECs and subjected to the in vivo competitive reconstitution assay without further doxycycline treatment. As anticipated, the control-vector-transduced differentiated luminal cells exhibited no reconstituting ability (Figure 3C). In contrast, the Slug/Sox9-exposed cells acquired robust gland-reconstituting activity, which was 7-fold higher than that of co-mixed unsorted dsRed-expressing primary MECs, which did contain a subpopulation of naturally arising, endogenous MaSCs (Figure 3C).
To directly measure the frequency of MaSCs, we transplanted the Slug/Sox9-exposed differentiated luminal cells into cleared mammary fat pads in limiting dilutions without admixing these cells with competing dsRed-labeled MECs. While control-vector-transduced cells failed to reconstitute, even when injected as an inoculum of 1x104 cells, the Slug/Sox9-exposed cells generated fully developed mammary ductal trees when as few as 100 of these cells were implanted (Figure 3D and Figure S3E). Immunofluorescence analyses showed that these ductal outgrowths were bilayer structures composed of cytokeratin 14- and α-smooth muscle actin-positive myoepithelial cells and cytokeratin 8-positive luminal cells (Figure 3D and S3F). These results showed once again that transient expression of Slug and Sox9 in differentiated luminal cells sufficed to convert them into bipotential MaSCs.
To examine whether MaSCs generated from differentiated luminal cells exhibited long-term reconstituting ability, we prepared small fragments (~1 mm) from primary mammary gland outgrowths formed by implanted, Slug/Sox9-exposed differentiated luminal cells; we then re-implanted these fragments into cleared mammary fat pads. We found that 13 out of 20 such transplantations generated full secondary gland reconstitution (Figure 3E). Furthermore, when the recipient mice were impregnated, these reconstituted mammary ductal trees generated large numbers of milk-secreting alveoli, indicating that the reconstituted mammary glands retained full differentiation potential (Figure 3E). These data demonstrated that transient Slug and Sox9 expression was sufficient to induce the formation of long-term repopulating MaSCs, and that such MaSCs were able to self-renew without the need of continuous expression of exogenous Slug and Sox9.
Effect of Sox9 on mammary stem cell induction in basal cells
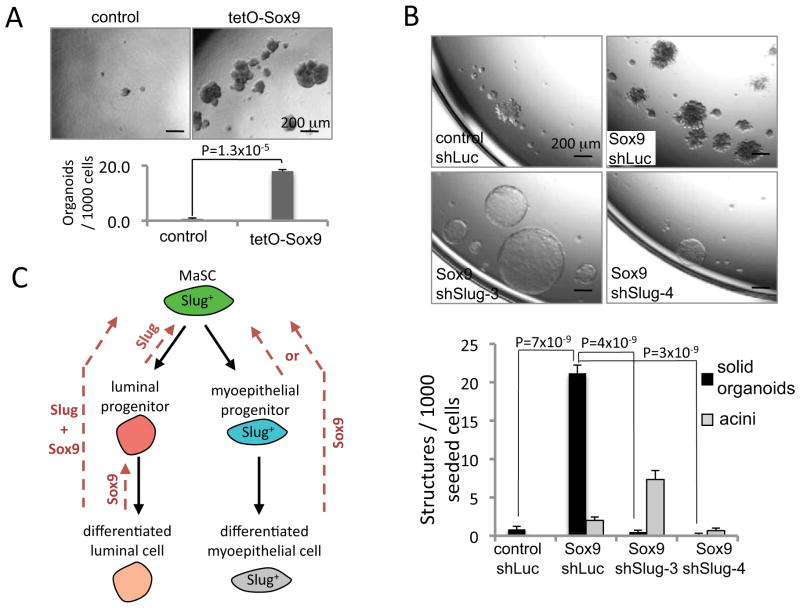
The above results suggested that Slug and Sox9, acting in concert, could function as master regulators of the MaSC state. If this were indeed the case, then these two TFs should be able to induce the formation of MaSCs in MECs prepared from various mammary epithelial lineages. In fact, as mentioned earlier, we found that the CD49fhighCD61+ basal cells, which contain both MaSCs and myoepithelial cells, already expressed significant levels of endogenous Slug and had mesenchymal attributes (Figure 1 and Figure S1D). This suggested that ectopic Sox9 expression on its own in these basal cells might suffice to induce MaSCs by acting together with the endogenously expressed Slug. Indeed, we detected a 26-fold increase in the number of organoid-forming cells when Sox9 was transiently overexpressed on its own in the basal cells for 5 days prior to organoid culture (Figure 4A).
Figure 4. The induction of MaSCs by Sox9 in basal cells.
(A) The organoid-forming efficiencies of basal cells transduced with the indicated vectors.
(B) The solid organoid- and acinus-forming efficiencies of basal cells transduced with the indicated cDNA and shRNA expression vectors. Cells were subjected to organoid culture 5 days post-infection. The shLuciferase (shLuc) shRNA was used as a control.
(C) A model showing the mammary epithelial hierarchy and the actions of the forced expression of Slug and Sox9 in various mammary epithelial lineages. The dashed lines indicate that the expression of the indicated factor(s) converts differentiated cells into stem or progenitor cells. Whether Sox9 expression converts differentiated myoepithelial cells or myoepithelial progenitors into MaSCs and/or expands a pre-existing MaSC population remains to be determined.
The data are represented as mean ± SEM.
To examine the requirement for endogenous Slug in the Sox9-mediated MaSC induction, we knocked down Slug in these basal cells concomitant with constitutive Sox9 overexpression. In this case, the induction of organoid-forming cells was completely abolished; in contrast, basal cells expressing Sox9 and a control shRNA readily formed organoids (Figure 4B). This revealed that ectopically expressed Sox9 could collaborate with endogenously expressed Slug to induce organoid formation in basal cells, and that both of these factors were required for the outgrowth of these structures. As the basal cell population contained naturally present, endogenous MaSCs, which cannot to be separated from myoepithelial cells with currently available markers, we could not distinguish whether in this case Sox9 converted myoepithelial cells into MaSCs or expanded a pre-existing MaSC population. Similarly, we could not distinguish whether Sox9 converted myoepithelial progenitors or differentiated myoepithelial cells into MaSCs.
Of note, when the endogenously expressed Slug was knocked down in the basal cells, Sox9 overexpression induced the formation of acinar structures instead of solid organoid structures, which were otherwise induced by Sox9 in unperturbed basal cells (Figure 4B). This indicated that the inhibitory effect of Slug knockdown on MaSC induction was not simply caused by non-specific cytostatic or cytotoxic effects, because the knockdown of Slug still permitted the robust outgrowth of acinar structures. This also suggested that, in the absence of endogenous Slug, ectopic Sox9 expression might cause trans-differentiation of basal cells into luminal progenitor cells, since formation of acinar structures is indicative of the activity of luminal progenitor cells (Lim et al., 2009; Stingl et al., 2006a). Taken together with the above findings that Sox9 overexpression alone in differentiated luminal cells induced acinus-forming cells (Figure S3B), these results suggested a role of Sox9 in luminal progenitor cells, in addition to its function in inducing the formation of MaSCs. This evidence allowed us, in turn, to propose the hierarchical scheme illustrated in Figure 4C, in which co-expression of Slug and Sox9 in either luminal or basal MECs sufficed to convert them to MaSCs.
Role of Slug and Sox9 in the maintenance of endogenous mammary stem cells
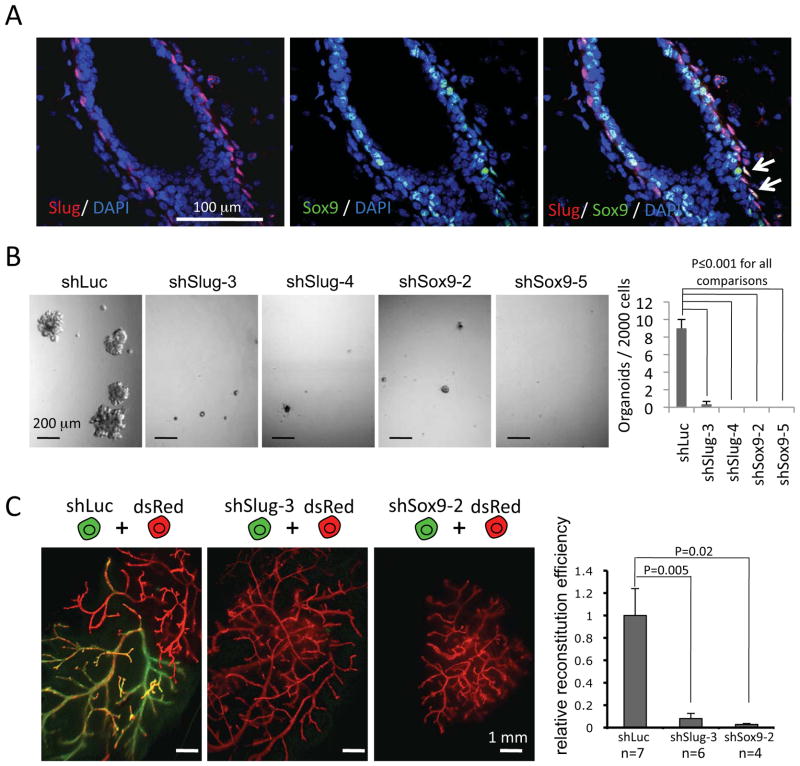
The combined effects of Slug and Sox9 on the induction of MaSC formation suggested that continued coexpression of these two TFs might also be required to maintain naturally arising MaSCs. Consistent with this notion, we detected a subset of basal cells in the murine mammary gland expressing high levels of both Slug and Sox9 by using immunofluorescence (Figure 5A) or single-molecule fluorescence in situ hybridization (Figure S4A), suggesting Slug and Sox9 were coexpressed by naturally arising MaSCs in vivo.
Figure 5. Slug and Sox9 are required for maintaining endogenous MaSCs.
(A) Confocal immunofluorescence analyses of mammary gland sections stained with rabbit anti-Slug and goat anti-Sox9 antibodies. The arrows point to Slug and Sox9 double-positive nuclei.
(B) The organoid-forming efficiencies of primary MECs transduced with the indicated shRNA vectors. Cells were subjected to organoid culture 4 days post-infection.
(C) The gland-reconstituting activity of primary MECs transduced with the indicated shRNA vectors. The shRNA vector-transduced and GFP-expressing primary MECs (1×105) were mixed with an equal number of dsRed-expressing MECs and then transplanted into cleared mammary fat pads. The ratio of GFP- to dsRed-positive cells was normalized against that of the shLuc control to obtain the relative reconstitution efficiency.
The data are represented as mean ± SEM. See also Figure S4.
To further examine the function of Slug and Sox9 in MaSCs, we knocked down the expression of either Slug or Sox9 in unsorted primary MECs with shRNAs (Figure S4B). The Slug shRNAs reduced the number of organoid-forming cells by more than 27 fold, while the Sox9 shRNAs reduced the number of organoid-forming MECs to an undetectable level (Figure 5B). In contrast, knockdown of either of these genes reduced the number of primary MECs in monolayer cultures by only 2- to 4-fold during the same period (Figure S4D), suggesting that the effect of Slug and Sox9 inhibition on MaSC activity in organoid culture was not primarily due to general inhibition of cell proliferation or survival. Furthermore, knockdown of Sox9 or Slug also blocked the in vivo gland-reconstituting activity of primary, unfractionated MECs, demonstrating that Slug and Sox9 are both required for the maintenance of the endogenous MaSC population (Figure 5C).
Distinct gene expression programs activated by Slug and Sox9
The above results demonstrated that Slug and Sox9 act cooperatively as master regulators of the formation and maintenance of MaSCs. To gain insight into how Slug and Sox9 succeed in doing so, we examined whether they promoted the EMT synergistically, in light of previous work that showed a connection between passage through an EMT and entrance into a SC-like state (Mani et al., 2008; Morel et al., 2008). We found, as shown earlier, that expression of Slug alone in these cells propagated in monolayer culture induced a robust EMT (Figure 6A and Figure S5A). In contrast, expressing Sox9 on its own had only a modest effect on EMT induction; and when co-expressed with Slug, Sox9 did not potentiate the EMT-inducing powers of Slug (Figure 6A and Figure S5A). This result suggested that while Slug may contribute to SC induction by inducing an EMT, Sox9 activates a complementary, ostensibly distinct cell-biological program that cooperates with the EMT program to enable entrance into the SC state.
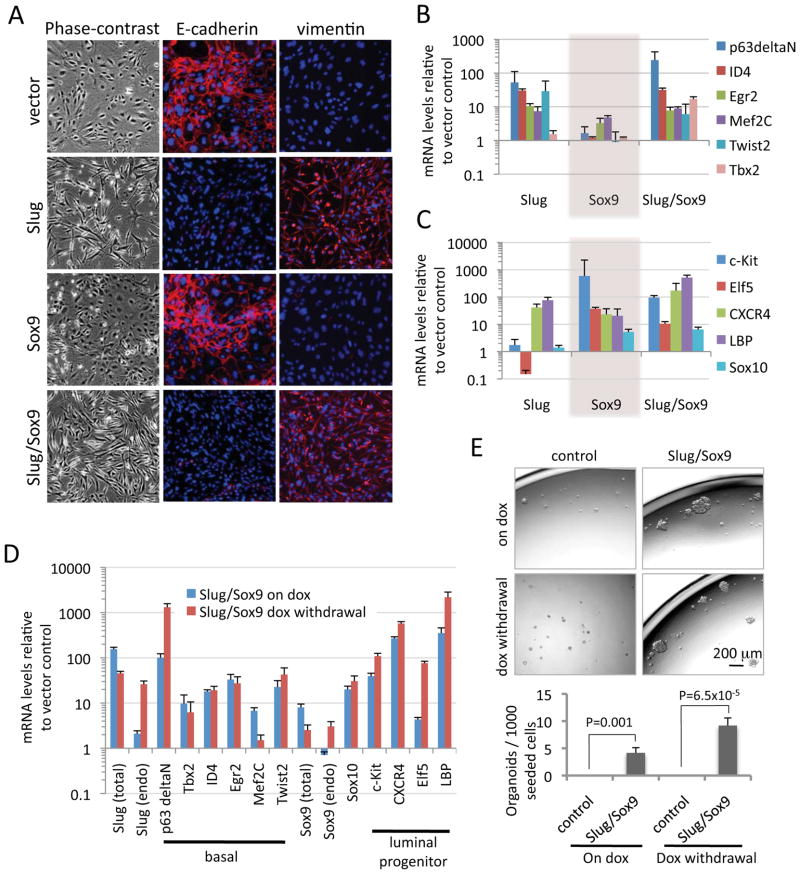
Figure 6. Slug and Sox9 activate distinct auto-regulatory gene expression programs.
(A) Phase-contrast and immunofluorescence images of differentiated luminal cells expressing the indicated vectors for 4 (phase-contrast) or 5 (immunofluorescence) days.
(B and C) The mRNA levels of basal cell TFs (B) and luminal progenitor genes (C) in differentiated luminal cells expressing the indicated vectors for 5 days, as measured by qRT-PCR. GAPDH was used as a loading control.
(D) The mRNA levels of various signature genes in tetracycline-inducible Slug and Sox9-transduced differentiated luminal cells after a 6-day doxycycline treatment (Slug/Sox9 on dox) or a 6-day doxycycline treatment plus a 6-day doxycycline withdrawal (Slug/Sox9 dox withdrawal). The mRNA levels were normalized to those of the control vector-transduced cells after a 6-day doxycycline treatment. Primers for amplifying protein-coding sequences were used to detect the total mRNA levels of Slug and Sox9 (total); and primers for amplifying the 5’UTRs were used to detect the endogenously expressed Slug and Sox9 mRNA (endo).
(E) The organoid-forming efficiencies of differentiated luminal cells transduced with the indicated vectors after a 5-day doxycycline treatment (on dox) or a 5-day treatment plus a 6-day withdrawal (dox withdrawal) in monolayer culture.
The data are represented as mean ± SEM. See also Figure S5.
Gene expression microarray analyses had previously identified signature genes of various mammary epithelial cell subpopulations in both human and mouse (Lim et al., 2010). We first validated the expression of these signature genes in the corresponding murine MEC subpopulations by using qRT-PCR (Figure S5B), then examined whether Slug and/or Sox9 regulate cellular states by altering the expression of these signature-associated genes. We found that expression of Slug in differentiated luminal cells upregulated expression of mRNAs encoding five of six basal cell-associated TFs by at least 7-fold (Figure 6B), consistent with a previously suggested role of Slug in maintaining basal-like phenotypes (Proia et al., 2011). In contrast to the behavior of Slug, forced Sox9 expression in differentiated luminal cells specifically upregulated the expression of genes associated with luminal progenitors. Thus, expression of all four luminal progenitor genes was increased by more than 20-fold in Sox9-expressing cells (Figure 6C). In addition, Sox9 induced Sox10 expression by more than 5-fold (Figure 6C). When Slug and Sox9 were co-expressed in differentiated luminal cells, the gene expression signatures of both basal cells and luminal progenitors were concomitantly upregulated (Figure 6B and C). These results reinforced and extended earlier findings that Slug and Sox9 regulated basal and luminal lineage programs, respectively. Moreover, these two programs appeared to contribute distinct sets of biological functions that are required, in aggregate, for MECs to enter into and reside stably within the MaSC state.
Because transient concomitant expression of Slug and Sox9 sufficed to induce entrance into the MaSC state, we asked whether the Slug- and Sox9-induced gene expression programs remained active in the resulting MaSCs even after the ectopically expressed Slug and Sox9 TFs had been turned off. Consequently, we transiently expressed tetracycline-inducible Slug and Sox9 in differentiated luminal cells for 6 days in monolayer culture and then turned off the expression of Slug and Sox9 for a subsequent 6 days via doxycycline withdrawal. At this time point, we confirmed that expression of the exogenous TFs had been successfully silenced (Figure 6D). However, endogenous basal and luminal progenitor signature genes remained highly upregulated (Figure 6D). Interestingly, expression of exogenous Slug and Sox9 led, in turn, to the induction of endogenously expressed EMT-TFs, including Twist2 and Slug itself, as well as endogenous Sox factors, including Sox9 and its close paralog Sox10 (Figure 6D and Figure S5C). Hence, the ectopically expressed Slug and Sox9 induced expression of their corresponding endogenous counterparts or paralogs, forming a self-reinforcing auto-regulatory network that contributed to maintenance of the SC program long after the exogenous factors had been silenced.
Consistent with the persistent activation of the SC gene expression program, the representation of SCs in Slug/Sox9-exposed cells in monolayer culture remained stable after exogenous Slug and Sox9 were turned off. Thus, six days after turning off the expression of exogenous Slug and Sox9, cells that had previously experienced these TFs exhibited a similar organoid-forming efficiency as they did when introduced into organoid culture immediately after halting expression of these exogenous TFs (Figure 6E).
We further tested whether continuous expression of endogenous Slug and Sox9 was required for maintaining the induced MaSCs. To do so, we transduced shRNA vectors directed against either Slug or Sox9 into differentiated luminal cells that had expressed tetracycline-inducible Slug and Sox9 for 6 days. These cells were then cultured in monolayer in the absence of doxycycline for additional 6 days before subjected to organoid culture. We found that knocking down either Slug or Sox9 reduced the numbers of organoids by more than 10-fold (Figure S5D). Hence, activation of endogenous Slug and Sox9 expression is necessary for the maintenance of MaSCs that are induced by transient ectopic expression of these factors.
We further examined the functional relevance of Sox9- and Slug-induced TFs, specifically Sox10 and Twist2, in MaSC induction, as measured by organoid culture. To do so, we knocked down either Sox10 or Twist2 together with expressing exogenous Slug and Sox9 in differentiated luminal cells. The inhibition of Sox10 led to more than 90% reduction in Slug/Sox9-induced organoid formation (Figure S5E), while the inhibition of Twist2 suppressed the organoid formation modestly (Figure S5F). Conversely, expressing Sox10 together with Slug could induce formation of organoids from differentiated luminal cells (Figure S5G), suggesting that Sox10 acted as a key downstream effector of Sox9 in the MaSC induction.
Roles of Slug and Sox9 in breast cancer stem cells
The identification of master regulators of the normal MaSC state in murine MECs, as described above, provided an opportunity to test whether a similar regulatory circuitry operates in human breast CSCs. We first examined whether Slug and Sox9 were required to maintain the tumor-initiating ability of usually aggressive MDA-MB-231 human breast cancer cells. These cells expressed significant levels of the Slug protein and a Sox9 isoform that was ~10 kDa smaller than the Sox9 protein expressed in normal human MECs (Figure S6A). The precise nature of this isoform is unknown.
We found that knockdown of either Slug or Sox9 greatly inhibited the tumor-initiating ability of MDA-MB-231 cells. The cells forced to express the shSox9 exhibited a greater than 70-fold lower tumor-initiating ability than did the control-vector-expressing cells, as calculated by limiting dilution analysis (Figure 7A and Figure S6B). Unlike the shSox9-expressing cells, however, the shSlug-expressing cells could form primary tumors at the same frequency as the control-shRNA-expressing cells, but the resulting tumors were 6-fold smaller upon Slug knockdown (Figure 7A). In contrast to their dramatic effects in vivo on tumor initiation and growth, shSox9 and shSlug had no adverse effect on the proliferation of MDA-MB-231 cells in monolayer culture (Figure S6C). These results demonstrated that Sox9 and, to a lesser extent, Slug are required for maintaining the robust tumorigenicity of MDA-MB-231 cells.
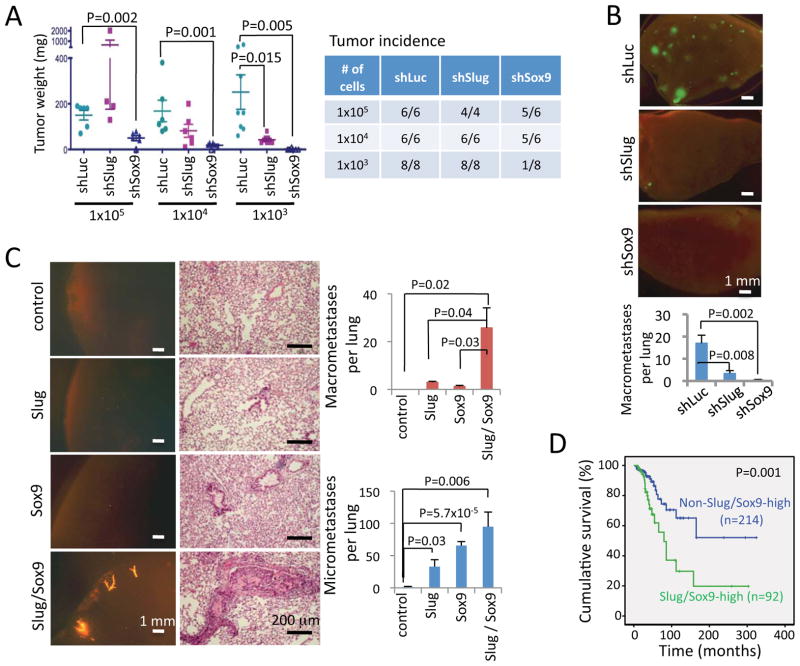
Figure 7. Slug and Sox9 act as regulators of breast CSCs.
(A) The tumor weight and incidence of MDA-MB-231 cells expressing the indicated shRNAs. Cells were injected subcutaneously at the indicated numbers. The tumor weight and incidence were determined 3 months post-injection. Each data point represents one tumor. The mean and SEM of each group are represented by horizontal and vertical bars. The table shows the tumor incidence.
(B) Lung metastases formed by MDA-MB-231 cells expressing the indicated shRNA vectors upon tail vein injection.
(C) Lung metastases formed by tdTomato-labeled MCF7ras cells that were transduced with the indicated vectors and injected orthotopically into the mammary fat pads. Whole-mount fluorescence lung images and the histology of the lung sections are shown. n=4 for each group. The data are representative of two independent experiments.
(D) The cumulative survival rate of human breast cancer patients with primary tumors expressing high levels of both Slug and Sox9 (Slug/Sox9-high) or tumors expressing either only one factor or neither factors at high levels (Non-Slug/Sox9-high).
The data are presented as mean ± SEM. See also Figure S6.
During the process of metastasis, tumor-initiating ability would appear to be critical for disseminated cancer cells to seed metastases (Nguyen et al., 2009; Valastyan and Weinberg, 2011). We therefore tested whether knocking down Slug and Sox9 also inhibited the experimental metastasis of MDA-MB-231 cells upon tail vein injection. Consistent with their effects in tumorigenesis, Slug knockdown inhibited the metastasis formation by MDA-MB-231 cells in the lungs by 5-fold, while Sox9 knockdown inhibited metastasis by more than 40-fold (Figure 7B).
We further tested whether Slug and Sox9 could function cooperatively to induce metastasis-seeding cells in the otherwise-non-metastatic MCF7ras human breast cancer cells. We implanted orthotopically MCF7ras cells transduced with inducible Slug, Sox9 or both TFs in NOD-SCID mice. The mice were then treated with doxycycline for two weeks. At this time point, Slug and Sox9 had induced a clear, although partial EMT in MCF7ras cells (Figure S6E). The animals were then kept on a doxycycline-free diet for ten weeks and examined for primary tumor growth and lung metastasis thereafter. The control MCF7ras cells generated virtually no detectable macroscopic metastases (macrometastases) and only a few microscopic metastases (micrometastases) per lung (Figure 7C). Transient expression of either Slug or Sox9 alone in the primary tumors generated numerous micrometastases per lung, but only led to a few macrometastases. However, when Slug and Sox9 were concomitantly expressed for two weeks in the primary tumors, the number of macrometastases dramatically increased, from virtually no macrometastases in control vector-transduced cells to ~26 macrometastases per lung in Slug and Sox9-coexpressed cells (Figure 7C). Hence, the coexpression of Slug and Sox9 induced macrometastasis-seeding cells in usually non-metastatic MCF7ras cells.
We sought to extend our findings with the two human breast cancer cell lines to clinical samples by examining the expression of Slug and Sox9 proteins in a panel of 306 clinically-defined human breast cancer samples on tissue microarray. We found 92 samples expressing high levels of both Slug and Sox9 and 214 samples expressing high levels of only one factor or neither factors (Figure S6G). The patients with primary tumors expressing high levels of both Slug and Sox9 had a significantly lower overall survival rate than the rest of patients (~20% vs. ~50% cumulative overall survival, P=0.001) (Figure 7D). These results showed that high expression levels of both Slug and Sox9 in human breast cancers are associated with poor patient outcomes, consistent with the effects of these two factors on promoting experimental tumorigenesis and metastasis.
DISCUSSION
The findings presented here identify for the first time that the actions of key transcriptional regulators suffice to convert differentiated epithelial cells into long-term repopulating epithelial SCs. More specifically, transient coexpression of Slug and Sox9 resulted in the conversion of differentiated luminal cells of the mammary gland into MaSCs that could, upon subsequent implantation, generate long-term reconstitution of mammary ductal trees. We found that Slug and Sox9 activated an endogenous auto-regulatory network that remained stable for many days in culture after silencing the ectopic Slug and Sox9 expression. This auto-regulatory program is involved in inducing and sustaining the SC state, suggesting that adult SCs, similar to ES cells, maintain their SC state via master regulator-mediated auto-regulatory networks (Boyer et al., 2005; Chen et al., 2008; Kim et al., 2008).
Our previous work and that of others suggested a link between the EMT and certain MaSC properties (Mani et al., 2008; Morel et al., 2008). However, it remained unclear whether the EMT creates fully functional MaSCs or only cells that exhibit many of the markers of SCs while not entering the full SC state, i.e., acquiring the functional properties of bona fide SCs. In the present work, we employed the mammary gland reconstitution assay as a stringent test of MaSC activity and observed that transient expression of the EMT-TF Slug induced MaSCs from the luminal progenitors but not from differentiated luminal cells. This suggests that while the EMT program is important for inducing entrance into the SC state, it is not sufficient on its own to induce this change in differentiated luminal cells. Instead, activation of an additional genetic program, in the present case through expression of Sox9, is required to work in concert with the EMT program to induce SCs.
Our observations have also yielded evidence that human CSCs and normal murine tissue-specific SCs share key transcriptional regulators, at least in the mammary gland. Although accumulating evidence has begun to support this notion (Dick, 2008; Lessard and Sauvageau, 2003; Lobo et al., 2007; Reya et al., 2001; Shimono et al., 2009), direct evidence has remained scarce thus far. Our results, in particular, have suggested that co-expression of Slug and Sox9 could confer upon dormant micrometastasis-forming cells extensive self-renewal ability, thereby allowing them to spawn macrometastases. Nonetheless, this notion requires further substantiation, since it is formally possible that Slug and Sox9 coexpression allows mobilization of a distinct population of macrometastasis-seeding cells that is not mobilized by expressing Slug or Sox9 alone or, alternatively, that the coexpression allows cancer cells to adapt more readily to the microenvironment of lung parenchyma. Future experiments are needed to resolve these possibilities.
The conversion of differentiated cells to SCs by transient Slug and Sox9 expression also demonstrated significant plasticity in the epithelial cell hierarchy. Recent studies using immortalized or cancer cell lines have shown that non-stem cells can de-differentiate to stem-like cells either spontaneously or under specific culture conditions (Chaffer et al., 2011; Gupta et al., 2011; Scheel et al., 2011). Here, using primary epithelial cells and stringent criteria for stem cells, we demonstrated that even differentiated primary cells could be converted to bona fide SCs, suggesting that residence in each of these states is maintained in a metastable fashion. Accordingly, we speculate that certain tissue or tumor microenvironmental signals may be able to transiently induce the expression of Slug and Sox9, thereby allowing de novo formation of SCs.
The paradigm identified here in MaSCs may apply to other epithelial SCs as well. In addition to their function in MaSCs described here, Slug and Sox9 act cooperatively in neural crest cell induction during embryonic development (Cheung et al., 2005). This suggests that Slug and Sox9 (and perhaps related TFs) may play a developmentally conserved role in regulating other types of SCs. Supporting this notion, Sox9 has been shown by others to be expressed in SCs or progenitor cells in multiple epithelial tissues, including the skin, intestine, liver and pancreas (Furuyama et al., 2011; Kopp et al., 2011; Nowak et al., 2008; van der Flier et al., 2009; Vidal et al., 2005). We therefore speculate that either Slug or other EMT-TFs cooperate with Sox9 in these other tissue-specific SCs to orchestrate the SC state. If this were proven to be correct, it would establish a common regulatory mechanism for various types of adult epithelial stem cells and lay the groundwork for the induced formation of a variety of epithelial SCs from their differentiated derivatives.
EXPERIMENTAL PROCEDURES
Mouse primary MEC isolation and FACS
Primary MECs were isolated from the mammary glands of 8- to 14-week-old virgin mice by collagenase, dispase and trypsin digestion. Various MEC subpopulations were FACS sorted after staining single cells with antibodies against EpCAM, CD61, CD49f and lineage-specific markers. The primary MECs were cultured in advanced DMEM/F12 (Invitrogen) supplemented with 2% calf serum, 10 ng/ml EGF, 10 ng/ml bFGF (Millipore), 4 μg/ml heparin (Sigma-Aldrich), 5 μM Y-27632 ROCK inhibitor (Tocris) and 0.5 μM Bio GSK-3 inhibitor (Tocris). More details are included in the Supplemental Information.
Matrigel organoid culture
MECs were dissociated into single cells and cultured with Epicult-B medium (Stem Cell Technology) containing 5% Matrigel, 5% heat-inactivated FBS, 10 ng/ml EGF, 20 ng/ml bFGF, 4 μg/ml heparin and 5 μM Y-27632. Cells were seeded at 1000–2000 per well in 96-well ultra-low attachment plates (Corning). All organoid cultures were performed in the absence of doxycycline. The number of organoids (> 100 μm in diameter) was counted 7–14 days after seeding.
Cleared mammary fat pad transplantation
Cell aliquots that were resuspended in 10 μl PBS containing 25% Matrigel were injected into the inguinal mammary fat pads of NOD-SCID mice, which had been cleared of endogenous mammary epithelium at 3 weeks of age. Details of the reconstitution analysis are included in the Supplemental Information.
Statistical analysis
All the data are presented as the mean ± standard errors of mean (SEM) except as otherwise specified. A Student’s t-test was used to calculate the P values except as otherwise specified. P<0.05 is considered significant.
Additional Procedures
The details of the mouse reagents, cell culture media, lentiviral vector cloning and infection, immunofluorescence, immunoblot, qRT-PCR, cleared mammary fat pad reconstitution analysis, tumor implantation, metastasis analysis, in vivo doxycycline treatment and analyses of the human clinical samples are included in the Supplemental Information.
Supplementary Material
Acknowledgments
We thank Scott Valastyan, Christina Scheel, Christine Chaffer and Richard Goldsby for critical review of the manuscript. We thank Kong Jie Kah and Dr. Tal Kafri for providing tetracycline-inducible lentiviral vectors and Dr. Rudolf Jaenisch for providing Rosa26-M2rtTA mice. This research was supported by grants from the Breast Cancer Research Foundation, National Cancer Institute Program P01-CA080111 and National Institute of Health (NIH) R01-CA078461. R.A.W. is an American Cancer Society and Ludwig Foundation professor. W.G. was supported by a Susan G. Komen for the Cure postdoctoral fellowship and a fellowship from the National Cancer Institute (F32CA144404). Z.K. was supported by a Department of Defense Breast Cancer Postdoctoral Fellowship. S.I. and A.v.O. were supported by the NIH/NCI Physical Sciences Oncology Center at MIT (U54CA143874).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21. doi: 10.1186/bcr2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, Gilmore H, Tung N, Naber SP, Schnitt S, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8:149–163. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006a doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Stingl J, Raouf A, Eirew P, Eaves CJ. Deciphering the mammary epithelial cell hierarchy. Cell Cycle. 2006b;5:1519–1522. doi: 10.4161/cc.5.14.2983. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.