Abstract
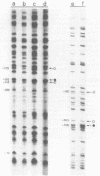
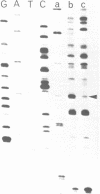
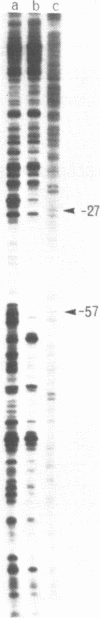
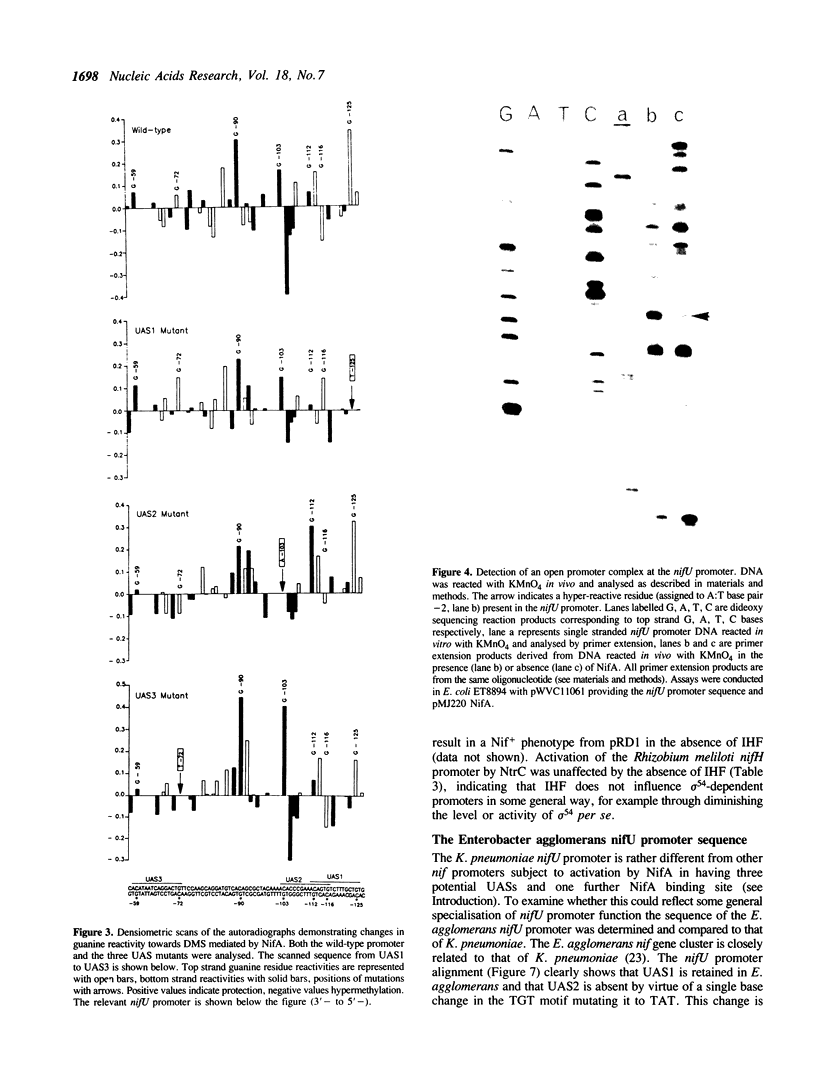
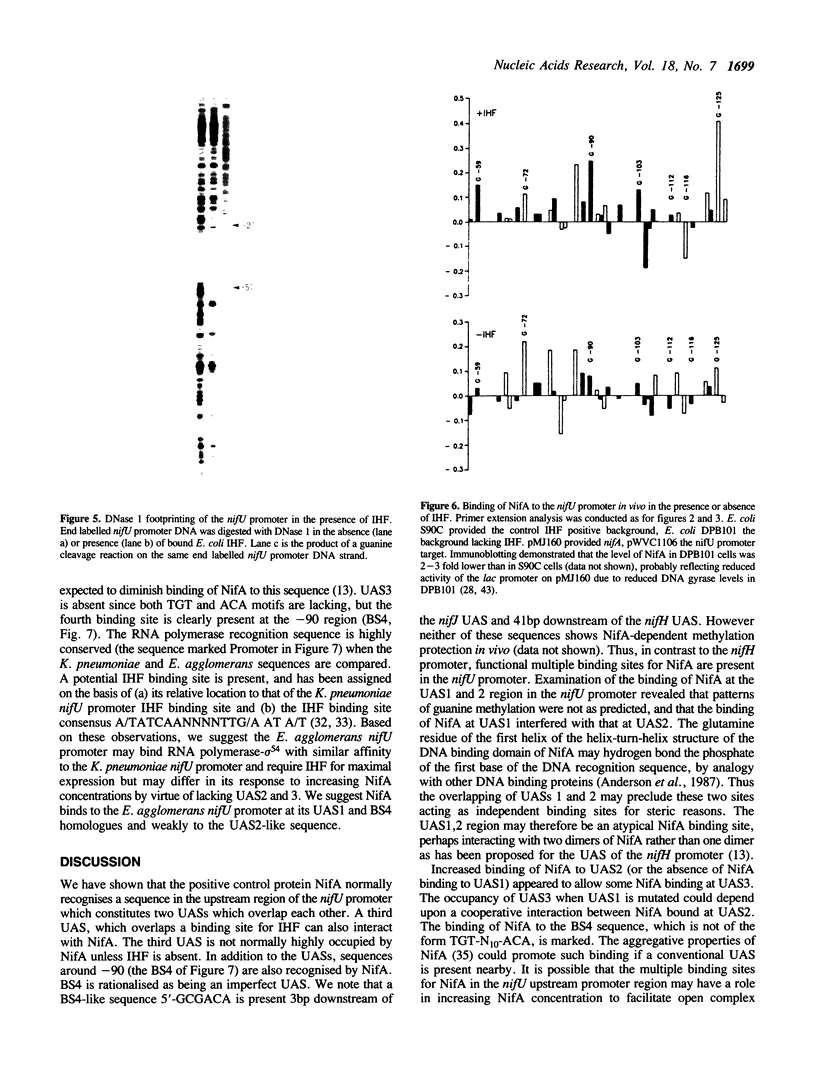
The Klebsiella pneumoniae nifU promoter is positively controlled by the NifA protein and requires a form of RNA polymerase holoenzyme containing the rpoN encoded sigma factor, sigma 54. Occupancy of the K. pneumoniae nifU promoter by NifA was examined using in vivo dimethyl sulphate footprinting. Three binding sites for NifA (Upstream Activator Sequences, UASs 1, 2 and 3) located at -125, -116 and -72 were identified which conform to the UAS consensus sequence TGT-N10-ACA. An additional NifA binding site was identified at position -90. The UASs located at -125 (UAS1) and -116 (UAS2) overlap and do not appear to bind NifA as independent sites. They may represent a NifA binding site interacting with two NifA dimers. UAS3 is located at -72, and abuts a binding site for integration host factor (IHF) and is not normally highly occupied by NifA. In the absence of IHF UAS3 showed increased occupancy by NifA. Mutational and footprinting analysis of the three UASs indicates (1) IHF and NifA can compete for binding and that this competition influences the level of expression from the nifU promoter (2) that UAS2 is a principle sequence of the UAS 1,2 region required for activation and (3) that none of the NifA binding sites interacts with NifA independently. In vivo KMnO4 footprinting demonstrated that NifA catalyses open complex formation at the nifU promoter. IHF was required for maximal expression from the nifU and nifH promoters in Escherichia coli, and for the establishment of a Nif+ phenotype in E. coli from the nif plasmid pRD1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. E., Ptashne M., Harrison S. C. Structure of the repressor-operator complex of bacteriophage 434. 1987 Apr 30-May 6Nature. 326(6116):846–852. doi: 10.1038/326846a0. [DOI] [PubMed] [Google Scholar]
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- Austin S., Henderson N., Dixon R. Requirements for transcriptional activation in vitro of the nitrogen-regulated glnA and nifLA promoters from Klebsiella pneumoniae: dependence on activator concentration. Mol Microbiol. 1987 Jul;1(1):92–100. doi: 10.1111/j.1365-2958.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Biek D. P., Cohen S. N. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: characterization of pSC101 mutants that replicate in the absence of IHF. J Bacteriol. 1989 Apr;171(4):2056–2065. doi: 10.1128/jb.171.4.2056-2065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D. P., Cohen S. N. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: characterization of pSC101 mutants that replicate in the absence of IHF. J Bacteriol. 1989 Apr;171(4):2056–2065. doi: 10.1128/jb.171.4.2056-2065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Buck M., Cannon W., Woodcock J. Mutational analysis of upstream sequences required for transcriptional activation of the Klebsiella pneumoniae nifH promoter. Nucleic Acids Res. 1987 Dec 10;15(23):9945–9956. doi: 10.1093/nar/15.23.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Cannon W., Woodcock J. Transcriptional activation of the Klebsiella pneumoniae nitrogenase promoter may involve DNA loop formation. Mol Microbiol. 1987 Sep;1(2):243–249. doi: 10.1111/j.1365-2958.1987.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Buck M. Deletion analysis of the Klebsiella pneumoniae nitrogenase promoter: importance of spacing between conserved sequences around positions -12 and -24 for activation by the nifA and ntrC (glnG) products. J Bacteriol. 1986 May;166(2):545–551. doi: 10.1128/jb.166.2.545-551.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Khan H., Dixon R. Site-directed mutagenesis of the Klebsiella pneumoniae nifL and nifH promoters and in vivo analysis of promoter activity. Nucleic Acids Res. 1985 Nov 11;13(21):7621–7638. doi: 10.1093/nar/13.21.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A., Drummond M. The effect on the function of the transcriptional activator NtrC from Klebsiella pneumoniae of mutations in the DNA-recognition helix. Nucleic Acids Res. 1988 May 11;16(9):4025–4039. doi: 10.1093/nar/16.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Henderson N. C., Austin S. DNA supercoiling and aerobic regulation of transcription from the Klebsiella pneumoniae nifLA promoter. Nucleic Acids Res. 1988 Nov 11;16(21):9933–9946. doi: 10.1093/nar/16.21.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Cannon F., Kondorosi A. Construction of a P plasmid carrying nitrogen fixation genes from Klebsiella pneumoniae. Nature. 1976 Mar 18;260(5548):268–271. doi: 10.1038/260268a0. [DOI] [PubMed] [Google Scholar]
- Drummond M., Whitty P., Wootton J. Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J. 1986 Feb;5(2):441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Menzel R., Mizuuchi K., O'Dea M. H., Friedman D. I. Regulation of DNA supercoiling in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):763–767. doi: 10.1101/sqb.1983.047.01.087. [DOI] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Huala E., Ausubel F. M. The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J Bacteriol. 1989 Jun;171(6):3354–3365. doi: 10.1128/jb.171.6.3354-3365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer R., Singh M., Klingmüller W. Identification and characterization of the nifH and nifJ promoter regions located on the nif-plasmid pEA3 of Enterobacter agglomerans 333. Gene. 1989 May 15;78(1):101–109. doi: 10.1016/0378-1119(89)90318-1. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil D. General method, using Mu-Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol. 1981 Apr;146(1):260–268. doi: 10.1128/jb.146.1.260-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin S. D., Austin S., Dixon R. A. The role of activator binding sites in transcriptional control of the divergently transcribed nifF and nifLA promoters from Klebsiella pneumoniae. Mol Microbiol. 1988 Jul;2(4):433–442. doi: 10.1111/j.1365-2958.1988.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Minchin S. D., Austin S., Dixon R. A. Transcriptional activation of the Klebsiella pneumoniae nifLA promoter by NTRC is face-of-the-helix dependent and the activator stabilizes the interaction of sigma 54-RNA polymerase with the promoter. EMBO J. 1989 Nov;8(11):3491–3499. doi: 10.1002/j.1460-2075.1989.tb08514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Morett E., Buck M. In vivo studies on the interaction of RNA polymerase-sigma 54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. The role of NifA in the formation of an open promoter complex. J Mol Biol. 1989 Nov 5;210(1):65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- Morett E., Buck M. NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nif promoters is a protein binding site. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9401–9405. doi: 10.1073/pnas.85.24.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett E., Cannon W., Buck M. The DNA-binding domain of the transcriptional activator protein NifA resides in its carboxy terminus, recognises the upstream activator sequences of nif promoters and can be separated from the positive control function of NifA. Nucleic Acids Res. 1988 Dec 23;16(24):11469–11488. doi: 10.1093/nar/16.24.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W., Merrick M. The nucleotide sequence of the nifM gene of Klebsiella pneumoniae and identification of a new nif gene: nifZ. Eur J Biochem. 1987 Dec 30;170(1-2):259–265. doi: 10.1111/j.1432-1033.1987.tb13694.x. [DOI] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Riedel G. E., Brown S. E., Ausubel F. M. Nitrogen fixation by Klebsiella pneumoniae is inhibited by certain multicopy hybrid nif plasmids. J Bacteriol. 1983 Jan;153(1):45–56. doi: 10.1128/jb.153.1.45-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Santero E., Hoover T., Keener J., Kustu S. In vitro activity of the nitrogen fixation regulatory protein NIFA. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7346–7350. doi: 10.1073/pnas.86.19.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzey B. Modulation of gene expression by drugs affecting deoxyribonucleic acid gyrase. J Bacteriol. 1979 Apr;138(1):40–47. doi: 10.1128/jb.138.1.40-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8934–8938. doi: 10.1073/pnas.85.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. C., Nash H. A. The interaction of E. coli IHF protein with its specific binding sites. Cell. 1989 Jun 2;57(5):869–880. doi: 10.1016/0092-8674(89)90801-5. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]