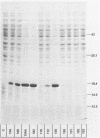
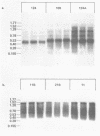
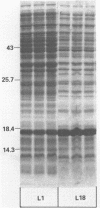
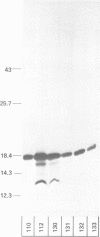
Abstract
Many heterologous genes when cloned into bacterial expression vectors are poorly expressed because of an inefficient ribosome binding site (RBS). We have constructed a plasmid which expresses human gamma-interferon (gamma-IF), where the level of expression is limited by the RBS. Expression was increased by placing the gamma-IF sequence immediately downstream of a small translated sequence. The production of gamma-IF was dependent upon the efficiency of translation of this upstream cistron and could be increased to very high levels. The same upstream cistron would greatly improve the expression of gamma-IF in a plasmid where the RBS was very poor due to inhibitory secondary structure at the 5' end of its mRNA. However, it would not improve the efficiency of a poor RBS containing a weak Shine-Dalgarno sequence. The general utility of the two-cistron expression strategy to diagnose a weak RBS is discussed.
Full text
PDF


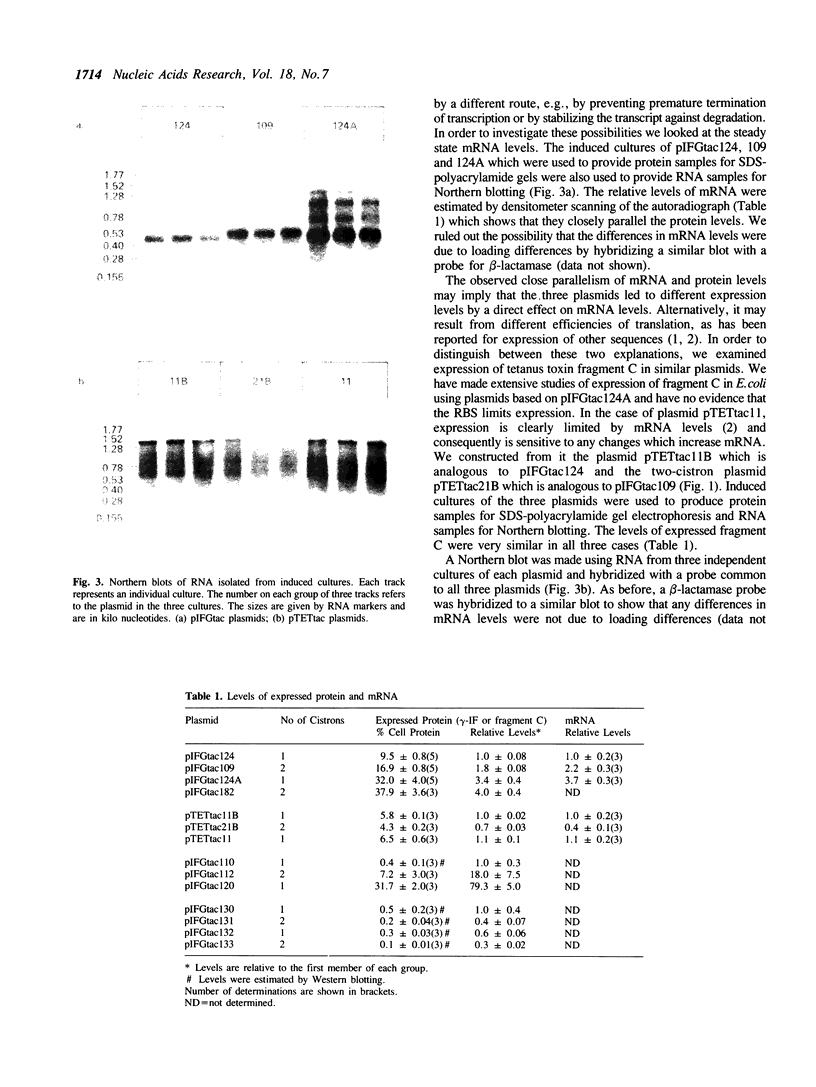
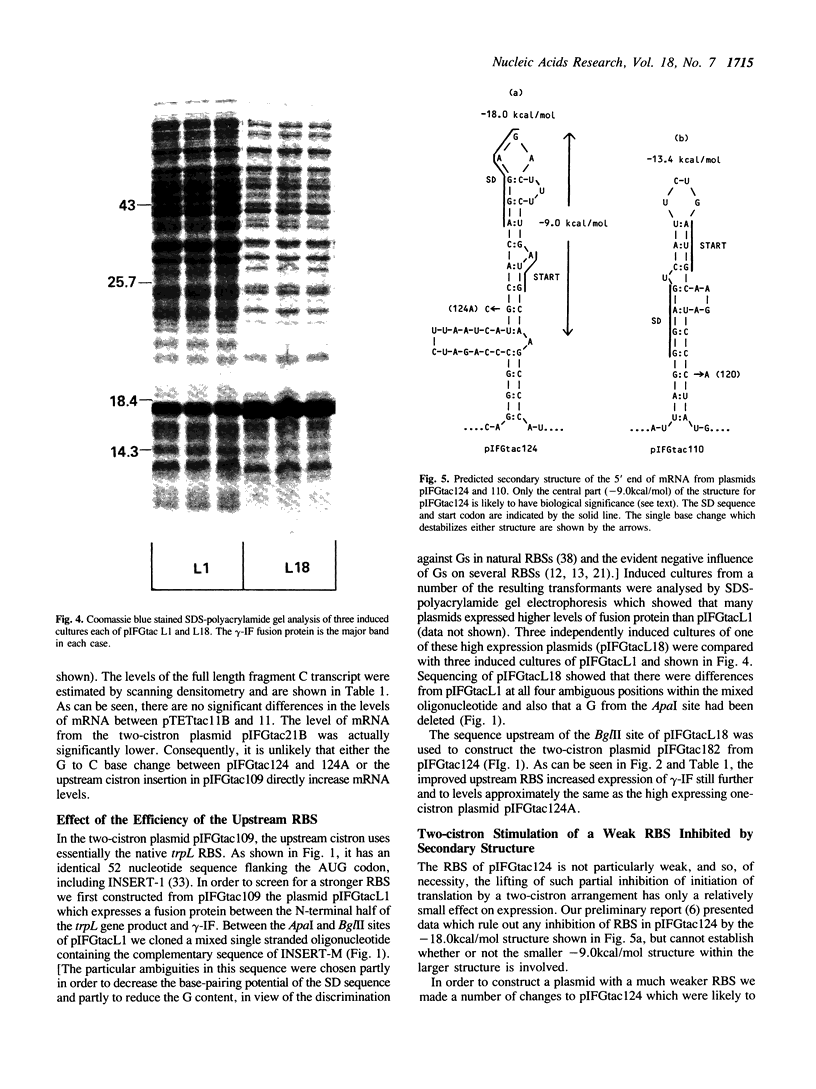
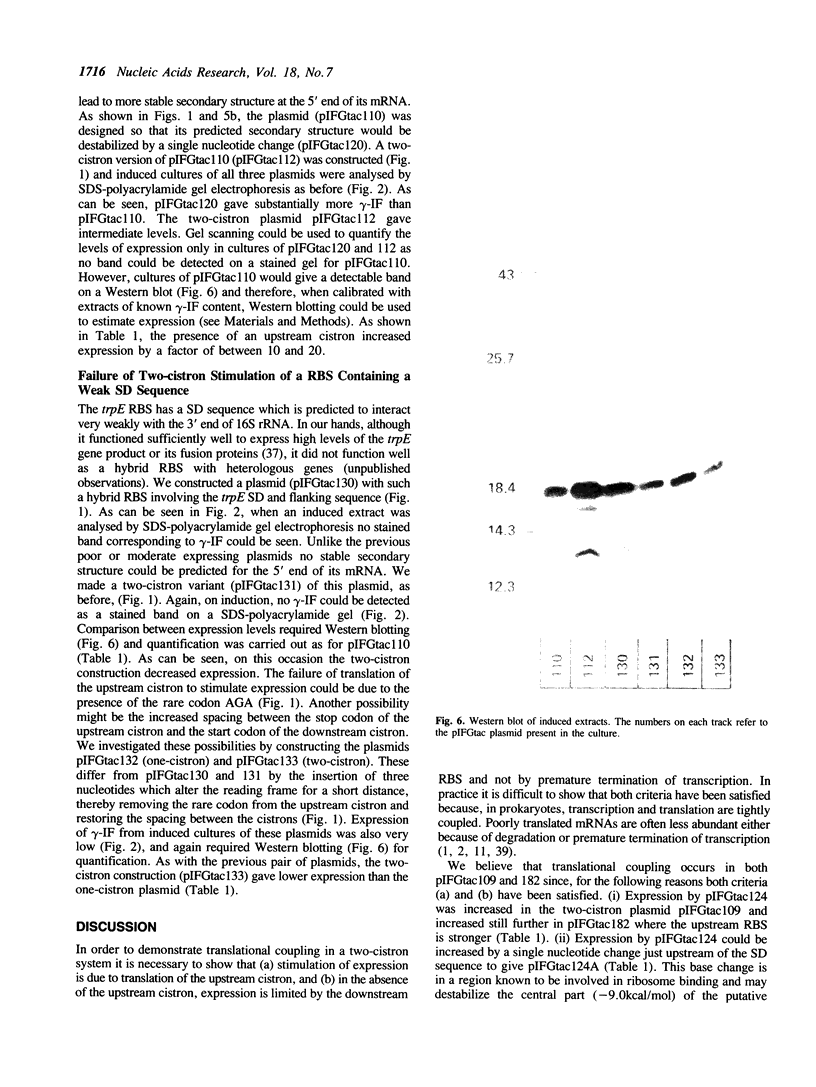


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhin M. R., van Duin J. Translational regulation of the lysis gene in RNA bacteriophage fr requires a UUG initiation codon. Mol Gen Genet. 1989 Jul;218(1):137–142. doi: 10.1007/BF00330576. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus P. W., Mihaly G. W., Morgan D. J., Smallwood R. A. Oxygen dependence of omeprazole clearance and sulfone and sulfide metabolite formation in the isolated perfused rat liver. J Pharmacol Exp Ther. 1989 Sep;250(3):1043–1047. [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Cho K. O., Yanofsky C. Sequence changes preceding a Shine-Dalgarno region influence trpE mRNA translation and decay. J Mol Biol. 1988 Nov 5;204(1):51–60. doi: 10.1016/0022-2836(88)90598-0. [DOI] [PubMed] [Google Scholar]
- Das A., Yanofsky C. A ribosome binding site sequence is necessary for efficient expression of the distal gene of a translationally-coupled gene pair. Nucleic Acids Res. 1984 Jun 11;12(11):4757–4768. doi: 10.1093/nar/12.11.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Steege D. A. Translation of phage f1 gene VII occurs from an inherently defective initiation site made functional by coupling. J Mol Biol. 1989 Jul 20;208(2):233–244. doi: 10.1016/0022-2836(89)90385-9. [DOI] [PubMed] [Google Scholar]
- Looman A. C., Bodlaender J., de Gruyter M., Vogelaar A., van Knippenberg P. H. Secondary structure as primary determinant of the efficiency of ribosomal binding sites in Escherichia coli. Nucleic Acids Res. 1986 Jul 11;14(13):5481–5497. doi: 10.1093/nar/14.13.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoff A. J., Oxer M. D., Romanos M. A., Fairweather N. F., Ballantine S. Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 1989 Dec 25;17(24):10191–10202. doi: 10.1093/nar/17.24.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoff A., Parry N., Dicken L. Translational fusions with fragments of the trpE gene improve the expression of a poorly expressed heterologous gene in Escherichia coli. J Gen Microbiol. 1989 Jan;135(1):11–24. doi: 10.1099/00221287-135-1-11. [DOI] [PubMed] [Google Scholar]
- Matteucci M. D., Heyneker H. L. Targeted random mutagenesis: the use of ambiguously synthesized oligonucleotides to mutagenize sequences immediately 5' of an ATG initiation codon. Nucleic Acids Res. 1983 May 25;11(10):3113–3121. doi: 10.1093/nar/11.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Munson L. M., Stormo G. D., Niece R. L., Reznikoff W. S. lacZ translation initiation mutations. J Mol Biol. 1984 Aug 25;177(4):663–683. doi: 10.1016/0022-2836(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Nishi T., Fujita T., Nishi-Takaoka C., Saito A., Matsumoto T., Sato M., Oka T., Itoh S., Yip Y. K., Vilcek J. Cloning and expression of a novel variant of human interferon-gamma cDNA. J Biochem. 1985 Jan;97(1):153–159. doi: 10.1093/oxfordjournals.jbchem.a135039. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder B., McCarthy J. E. The role of bases upstream of the Shine-Dalgarno region and in the coding sequence in the control of gene expression in Escherichia coli: translation and stability of mRNAs in vivo. Gene. 1989 May 15;78(1):59–72. doi: 10.1016/0378-1119(89)90314-4. [DOI] [PubMed] [Google Scholar]
- Schmidt B. F., Berkhout B., Overbeek G. P., van Strien A., van Duin J. Determination of the RNA secondary structure that regulates lysis gene expression in bacteriophage MS2. J Mol Biol. 1987 Jun 5;195(3):505–516. doi: 10.1016/0022-2836(87)90179-3. [DOI] [PubMed] [Google Scholar]
- Schoner B. E., Belagaje R. M., Schoner R. G. Translation of a synthetic two-cistron mRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8506–8510. doi: 10.1073/pnas.83.22.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner B. E., Hsiung H. M., Belagaje R. M., Mayne N. G., Schoner R. G. Role of mRNA translational efficiency in bovine growth hormone expression in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5403–5407. doi: 10.1073/pnas.81.17.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Shepard H. M., Yelverton E., Goeddel D. V. Increased synthesis in E. coli of fibroblast and leukocyte interferons through alterations in ribosome binding sites. DNA. 1982;1(2):125–131. doi: 10.1089/dna.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons G., Remaut E., Allet B., Devos R., Fiers W. High-level expression of human interferon gamma in Escherichia coli under control of the pL promoter of bacteriophage lambda. Gene. 1984 Apr;28(1):55–64. doi: 10.1016/0378-1119(84)90087-8. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Sor F., Bolotin-Fukuhara M., Nomura M. Mutational alterations of translational coupling in the L11 ribosomal protein operon of Escherichia coli. J Bacteriol. 1987 Aug;169(8):3495–3507. doi: 10.1128/jb.169.8.3495-3507.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanjaard R. A., van Dijk M. C., Turion A. J., van Duin J. Expression of the rat interferon-alpha 1 gene in Escherichia coli controlled by the secondary structure of the translation-initiation region. Gene. 1989 Aug 15;80(2):345–351. doi: 10.1016/0378-1119(89)90298-9. [DOI] [PubMed] [Google Scholar]
- Spanjaard R. A., van Duin J. Translational reinitiation in the presence and absence of a Shine and Dalgarno sequence. Nucleic Acids Res. 1989 Jul 25;17(14):5501–5507. doi: 10.1093/nar/17.14.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanssens P., Remaut E., Fiers W. Alterations upstream from the Shine-Dalgarno region and their effect on bacterial gene expression. Gene. 1985;36(3):211–223. doi: 10.1016/0378-1119(85)90176-3. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Remaut E., Fiers W. Inefficient translation initiation causes premature transcription termination in the lacZ gene. Cell. 1986 Mar 14;44(5):711–718. doi: 10.1016/0092-8674(86)90837-8. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutiphong J., Johansen H., Sathe G., Rosenberg G. S., Shatzman A. Selection of mutations that increase alpha 1-antitrypsin gene expression in Escherichia coli. Mol Biol Med. 1987 Oct;4(5):307–322. [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. Feedback regulation of ribosomal protein synthesis in E. coli: localization of the mRNA target sites for repressor action of ribosomal protein L1. Cell. 1981 Apr;24(1):243–249. doi: 10.1016/0092-8674(81)90520-1. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]