Abstract
AIMS
As part of a larger study to estimate the global burden of disease and injury attributable to alcohol:
To evaluate the evidence for a causal impact of average volume of alcohol consumption and pattern of drinking on diseases and injuries;
To quantify relationships identified as causal based on published meta-analyses;
To separate the impact on mortality vs. morbidity where possible; and
To assess the impact of the quality of alcohol on burden of disease.
METHODS
Systematic literature reviews were used to identify alcohol-related diseases, birth complications and injuries using standard epidemiologic criteria to determine causality. The extent of the risk relations was taken from meta-analyses.
RESULTS
Evidence of a causal impact of average volume of alcohol consumption was found for the following major diseases: tuberculosis, mouth, nasopharynx, other pharynx and oropharynx cancer, oesophageal cancer, colon and rectum cancer, liver cancer, female breast cancer, diabetes mellitus, alcohol use disorders, unipolar depressive disorders, epilepsy, hypertensive heart disease, ischaemic heart disease (IHD), ischaemic and haemorrhagic stroke, conduction disorders and other dysrhythmias, lower respiratory infections (pneumonia), cirrhosis of the liver, preterm birth complications, foetal alcohol syndrome. Dose-response relationships could be quantified for all disease categories except for depressive disorders, with the relative risk increasing with increased level of alcohol consumption for most diseases. Both average volume and drinking pattern were causally linked to IHD, foetal alcohol syndrome, and unintentional and intentional injuries. For IHD, ischaemic stroke and diabetes mellitus beneficial effects were observed for patterns of light to moderate drinking without heavy drinking occasions (as defined by 60+ grams pure alcohol per day). For several disease and injury categories, the effects were stronger on mortality compared to morbidity. There was insufficient evidence to establish whether quality of alcohol had a major impact on disease burden.
CONCLUSIONS
Overall, these findings indicate that alcohol causally impacts many disease outcomes, both chronic and acute, and injuries. In addition, a pattern of heavy episodic drinking increases risk for some disease and all injury outcomes. Future studies need to address a number of methodological issues, especially the differential role of average volume versus drinking pattern, in order to obtain more accurate risk estimates and to better understand the nature of alcohol-disease relationships.
Keywords: Alcohol, average volume, patterns of drinking, injury, risk relation, mortality, morbidity, burden of disease
INTRODUCTION
The present paper can be seen as an update of Rehm and colleagues [1], addressing the direction, form and strength of the relationship of different dimensions of alcohol consumption to a whole range of chronic and infectious diseases and injuries. The relationship between alcohol consumption and health outcomes is complex and multidimensional. In the six years since the previous comprehensive overview [1], however, the field has been substantially transformed by an accumulation of new studies, partly by expansion into new areas of research, such as alcohol and infectious diseases, but particularly by the widespread use of systematic reviews and meta-analyses. While the general conclusions from previous research have been confirmed, some new relationships between alcohol consumption and disease/injury have also been identified and others have been clarified.
Figure 1 provides an overview of the conceptual model used for the current analysis of alcohol within the Global Burden of Disease and Injury (GBD) 2005 study. The GBD 2005 study is a comprehensive effort to estimate and analyse mortality and disability on a global level, including measuring the impact of major risk factors in a comparative fashion [2] In our conceptual model for the impact of alcohol consumption on disease morbidity and mortality, two separate but related dimensions of individual level drinking are hypothesized as exerting the main causal impact on burden of disease: overall volume of alcohol consumption and pattern of drinking. Volume, usually operationalized as the total absolute alcohol consumed over a time period, such as one year, has been the traditional measure of exposure in alcohol epidemiology [3], and has been causally linked to many International Classification of Diseases (ICD) codes following the seminal work of English and colleagues [4]. Specifically, more than 30 ICD-10 (version 10) three or four-digit codes include alcohol in their name or definition [5], indicating that alcohol consumption is a necessary cause (these are listed later in this paper in Table 1). In addition, alcohol has been identified as a sufficient component cause for over 200 ICD-10 three-digit disease codes (these are listed later in the paper in Tables 2 and 5). The sufficient component model states that a sufficient component cause consists of a number of components, none of which alone is sufficient for causing the disease. When all the components are present, the sufficient cause is formed. For each disease, different sufficient component causes may be relevant (for a more thorough definition of component causes see [6]). In epidemiological practice, researchers mostly focus on one component of the sufficient component cause, such as alcohol consumption, often with a counterfactual model, examining what proportion of a disease under consideration would disappear if the risk factor was absent, or what proportion would disappear when the population distribution of the risk factor would shift to level associated with lower harm [7]. This kind of model also underlies the calculation of attributable fractions (see below; [8]).
Figure 1.
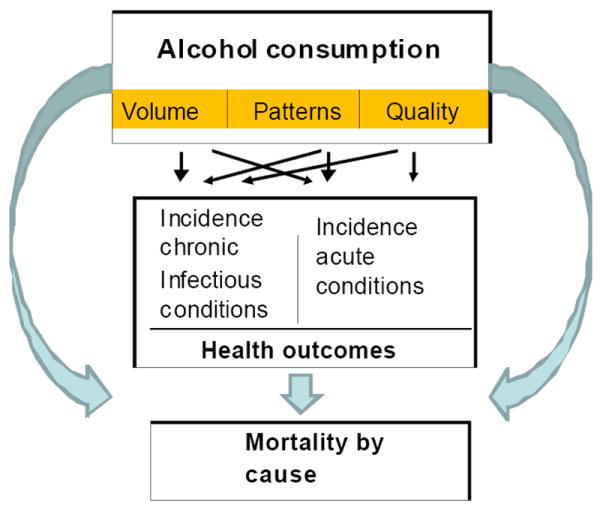
Causal model of alcohol consumption, intermediate mechanisms, and long-term consequences
Table 1.
Disease conditions which are by definition alcohol-attributable (AAF = 100%)
| ICD-10 | Disease |
|---|---|
| E24.4 | Alcohol-induced pseudo-Cushing’s syndrome |
| F10 | Mental and behavioural disorders due to use of alcohol |
| F10.0 | Acute intoxication |
| F10.1 | Harmful use |
| F10.2 | Dependence syndrome |
| F10.3 | Withdrawal state |
| F10.4 | Withdrawal state with delirium |
| F10.5 | Psychotic disorder |
| F10.6 | Amnesic syndrome |
| F10.7 | Residual and late-onset psychotic disorder |
| F10.8 | Other mental and behavioural disorders |
| F10.9 | Unspecified mental and behavioural disorder |
| G31.2 | Degeneration of nervous system due to alcohol |
| G62.1 | Alcoholic polyneuropathy |
| G72.1 | Alcoholic myopathy |
| I42.6 | Alcoholic cardiomyopathy |
| K29.2 | Alcoholic gastritis |
| K70 | Alcoholic liver disease |
| K70.0 | Alcoholic fatty liver |
| K70.1 | Alcoholic hepatitis |
| K70.2 | Alcoholic fibrosis and sclerosis of liver |
| K70.3 | Alcoholic cirrhosis of liver |
| K70.4 | Alcoholic hepatic failure |
| K70.9 | Alcoholic liver disease, unspecified |
| K85.2 | Alcohol-induced acute pancreatitis |
| K86.0 | Alcohol-induced chronic pancreatitis |
| O35.4 | Maternal care for (suspected) damage to foetus from alcohol |
| P04.3 | Foetus and newborn affected by maternal use of alcohol |
| Q86.0 | Foetal alcohol syndrome (dysmorphic) |
| R78.0 | Finding of alcohol in blood |
| T51 | Toxic effect of alcohol |
| T51.0 | Ethanol |
| T51.1 | Methanol |
| T51.8 | Other alcohols |
| T51.9 | Alcohol unspecified |
| X45 | Accidental poisoning by and exposure to alcohol |
| X65 | Intentional self-poisoning by and exposure to alcohol |
| Y15 | Poisoning by and exposure to alcohol, undetermined intent |
| Y90 | Evidence of alcohol involvement determined by blood alcohol level |
Note: ICD codes in italics represent sub-codes within a main code of classification.
Abbreviations: AAF - alcohol-attributable fraction; ICD - International Classification of Diseases
Table 2.
Disease conditions which are not wholly attributable to alcohol identified by various meta-analyses and reviews (at least one review has concluded that there is a relationship of the condition with alcohol)
| GBD category | N GBD 2005 code | ICD-10# | Reference to meta-analyses/systematic reviews | Effect |
|---|---|---|---|---|
| Tuberculosis | IA | A15-A19, B90 | [32;34;241] Technical meeting 2008 concluded sufficient evidence for a causal impact [32]. | Detrimental |
| Human Immunodeficiency Virus / Acquired Immune Deficiency Syndrome (HIV/AIDS) | IB | B20-B24 | [32;43;44;46;48] There is not sufficient evidence to conclude a causal impact on incidence, but there is sufficient evidence of causal impact on course of disease, which can in part be modelled. | Detrimental |
| Mouth, nasopharynx, other pharynx and oropharynx cancer | IIA1-IIA2b | C00-C13 | [4;9;16-20;242;243] Enough data to calculate RR for subcategories of disease, e.g. [68;244]. IARC confirmed cancers of the oral cavity and pharynx to be causally related to alcohol drinking in monograph meeting of February 2007 [59]. | Detrimental |
| Oesophagus cancehr | IIA3 | C15 | [4;9;16-20;68;242;245] IARC confirmed oesophagus cancer to be causally related to alcohol drinking in monograph meeting of February 2007 [59]. | Detrimental |
| Stomach cancer | IIA4 | C16 | [246] IARC concluded inadequate evidence that alcohol causes stomach cancer because of the inconsistency of the research evidence. Last IARC meeting concluded a possible relationship but confounding of smoking and dietary habits could not be excluded [59]. | Detrimental |
| Colon and rectum cancer | IIA5 | C18-C21 | [9;67-69;244;247] IARC newly included colorectal cancer as causally related to alcohol drinking in monograph meeting of February 2007 [59]. | Detrimental |
| Liver cancer | IIA6 | C22 | [4;9;16-20;68;244] IARC confirmed primary liver cancer as causally related to alcohol drinking in monograph meeting of February 2007 [59]. | Detrimental |
| Larynx cancer | IIA9 | C32 | [4;9;16-20;68;242-244;248;249] IARC confirmed larynx cancer to be causally related to alcohol drinking in monograph meeting of February 2007 [59]. | Detrimental |
| Trachea, bronchus and lung cancer | IIA10 | C33, C34 | Was excluded from the list of outcomes causally related to alcohol by [4]. This decision has not been revised by any further meta-analysis. Recent meta-analyses on alcohol and lung cancer found mixed results [244;250-252], but even when RRs were elevated for heavy consumption, confounding by smoking could not be fully excluded [250]. The last substantive reviews found no sufficient support for a causal relation [253;254]. Last IARC meeting concluded a possible relationship but confounding of smoking and dietary habits could not be excluded [59]. | Detrimental |
| Breast cancer (female) | IIA13 | C50 | [9;16-20;68;70-73;244;255-257] [4] concluded there was only limited evidence for causality, although they found a consistent relationship. Subsequent studies using the same criteria concluded that there was sufficient evidence of a relationship. IARC newly included breast cancer as causally related to alcohol drinking in monograph meeting of February 2007 [59]. | Detrimental |
| Other neoplasms | IIB | D00-D48 (except D09.9, D37.9, D38.6, D39.9, D40.9, D41.9, D48.9) | [9] Mixed category, no assessment of causality possible. | Detrimental |
| Diabetes mellitus | IIC | E10-E13 | [4;9;16;17;19;258-260] [4] found only inadequate evidence of causality. Subsequent studies using the same criteria for causality revised this decision (see also [261]). | Mainly beneficial |
| Alzheimer’s disease and other dementias | IIF1 | F01-F03, G30, G31 | [262-265] Overall, studies showed a beneficial effect with the beneficial effects on the ischaemic system being the main potential explanation [266]. However, studies show substantial heterogeneity and confounding and other explanations could not be ruled out [261;264;267]. | Mainly beneficial |
| Unipolar depressive disorders | IIE1 | F32, F33, F34.1 | [9] Available data is not sufficient for meta-analyses, for estimating RRs or AAFs. While there is agreement that some of the unipolar depressive disorders are caused by alcohol consumption (for a examination of causal criteria see [1]), the determination of the AAF was not possible, as it could not be determined which portion of the association between alcohol consumption and depressive disorders was caused by alcohol consumption, which portion by depression, and which portion by a third cause impacting both alcohol consumption and depression. | Detrimental |
| Epilepsy | IIF3 | G40, G41 | [4;9;16-20;89] Meta-analyses did not distinguish between primary epilepsy/unprovoked seizures and provoked seizures such as alcohol withdrawal induced seizures. We will conduct a new systematic review and meta-analysis consistent with GBD definition of primary epilepsy/unprovoked seizures. | Detrimental |
| Hypertensive heart disease | IIH2 | I11-I13 | [4;9;10;16-20;68] Because of lack of data for I11-I13 only, we included essential hypertension in our analyses (I10). | Detrimental, may depend on patterns of drinking for low volume |
| Ischaemic heart disease | IIH3 | I20-I25 | [4;9;16-20;68;108;113;268] Most recent meta-analysis [108] found heterogeneity among studies. Pattern of drinking must be included in analysis [9;115;116;118] to reduce this heterogeneity. | Beneficial or detrimental, depending on patterns of drinking for low to medium volume; detrimental effect for high volume |
| Cardiomyopathy | IIH6 | I42 | Not clear if alcohol has a relationship to cardiomyopathy over and beyond the subcategory of alcoholic cardiomyopathy I42.6. Systematic review is ongoing. | Detrimental |
| Conduction disorders and other dysrhythmias | IIH7 | I47-I48 | [4;9;16-20] Not yet included into prior CRAs yet, but as GBD 2005 has established a new code for dysrhythmias, the epidemiological assessment of underlying causality applies. | Detrimental |
| Heart failure (no GBD category) | No GBD code; the respective ICD codes will be redistributed in GBD 2005 | [4;9;16;17;19;20] This is an unspecific category with no identification of underlying pathology. Therefore, the relationship between average volume of consumption and outcome was usually not determined by meta-analyses, but indirectly from other circulatory diseases. | Detrimental | |
| Ischaemic stroke | IIH4a | I63-I67, I69.3 | [4;9;16-20;68;155;222;269;270] | Beneficial or detrimental dependent on patterns of drinking (similar to IHD) |
| Haemorrhagic and other non-ischaemic stroke | IIH4b | I60-I62, I69.0, I69.1, I69.2 | [4;9;16-20;68;155;160;222;269-271] | Mainly detrimental, except for low doses |
| Oesophageal varices (no GBD category) | No GBD code; the respective ICD codes will be redistributed in GBD 2005 | [4;9;16-20] Not based on meta-analyses. As most oesophageal varices are due to liver cirrhosis, the AAFs derived for unspecific liver cirrhosis are usually applied. No GBD category. | Detrimental | |
| Lower respiratory infections: pneumonia | IK1 | J09-J22, J85, P23 | Influenza and pneumonia had not been included into GBD so far. However, the transfer to GBD is only possible for disease categories with J10-18 as definition, as the epidemiological studies are usually restricted to these codes. Also, the impact of alcohol use is restricted to community-acquired pneumonia. | Detrimental |
| Cirrhosis of the liver | IIJ6 | K70, K73-K74 | [4;9;16-20;68] and the other meta-analyses included studies on all kinds of liver cirrhosis, included the ones defined as caused by alcohol by definition (see Table 1 above). | Detrimental |
| Gall bladder and bile duct disease | IIJ7 | K80-K83 | [4;16-20] Beneficial effect only found for K80 (cholelithiasis); the above references are limited to this. | Beneficial |
| Pancreatitis | IIJ8 | K85-K86 | [4;9;16-20;68] Usually determined by clinical case studies (but see [222]. There are sub-categories of alcohol-induced acute (K85.2) and chronic pancreatitis (K86.0). We are currently conducting a new meta-analysis which will include all forms of pancreatitis. | Detrimental |
| Other digestive diseases | IIJ9 | K20-K22, K28-K31, K38, K57-K63, K75.2, K75.3, K75.4, K76-K77, K90-K92 (except K92.0, K92.1, K92.2, K92.9) | [4;9;16-20] Detrimental effect of alcohol has only been found for K22.6 (gastro-oesophageal haemorrhage). AAFs for this category were not based on meta-analyses but clinically documented. The above references are limited to K22.6. | Detrimental |
| Psoriasis | IIL2 | L40-L41 | [4;9;16;17;19;20;184-186;189] Overall insufficient evidence on the causal impact of alcohol on the association with psoriasis. As this condition had been included in other analyses of alcohol on burden of disease, we included an overview on the evidence as part of the text. | Detrimental |
| Abortion | IIN1 | O00-O08 | [4;9;16;17;19;20] To be applied to the fraction of women with alcohol consumption during pregnancy. | Detrimental |
| Preterm birth complications | IM1 | P05-P07, P22, P25-P28, P77 | [4;9;16;17;19;20;272-276] To be applied to the fraction of women with alcohol consumption during pregnancy. [4] concluded that for all birth defects combined (ICD-10: Q00-Q99) there was inadequate evidence for a causation of alcohol during pregnancy. Other reviews, based on the same criteria for causality as [4] concluded sufficient evidence for a causal effect. | Detrimental |
The shaded rows indicate conditions for which the current analyses concluded sufficient evidence for a causal relationship and sufficient data on outcomes and risk relations to be included into the CRA 2005.
The ICD-10 code refers to the respective categories as defined by GBD 2005. These may include fewer codes than elsewhere, as GBD separately identifies ill-specified codes [so called garbage code(s)], which are to be redistributed to more meaningful codes based on specified procedures.
AAF - alcohol-attributable fraction; CRA - Comparative Risk Assessment; GBD - Global Burden of Disease and Injury; IARC – International Agency for Research on Cancer; ICD - International Classification of Diseases; IHD - ischaemic heart disease; RR – relative risk
Table 5.
GBD 2005 injury categories and alcohol
| N GBD 2005 code | Category | ICD-10 code |
|---|---|---|
| IIIA | Unintentional injuries | V01-V98, W00-W52, W65-W74, X00-X19, X34-X44, X46-X49, Y40-Y84, Y85.0, Y88 |
| IIIA1 | Transport injuries (including road traffic accidents) | V01-V98, Y85.0 |
| IIIA2 | Poisonings | X40-X44, X46-X49 |
| IIIA3 | Falls | W00-W19 |
| IIIA4 | Fires, heat, and hot substances | X00-X19 |
| IIIA5 | Drowning | W65-W74 |
| IIIA6 | Exposure to mechanical forces (including machinery accidents) | W20-W52 |
| IIIA7 | Natural disasters | X34-X39 |
| IIIA8 | Adverse effects of medical treatment | Y40-Y84, Y88 |
| IIIA9 | Injuries due to animal bites or contact with a marine animal | W53-W64, X20-X29 |
| IIIA10 | Other unintentional injuries | W75-W99, X30-X33, X50-X58 |
| IIIB | Intentional injuries | X60-Y09, Y35-Y36, Y87.0, Y87.1, Y89.0, Y89.1 |
| IIIB1 | Self-inflicted injuries | X60-X84, Y87.0 |
| IIIB2 | Interpersonal violence | X85-Y09, Y87.1 |
| IIIB3 | Collective violence | Y36, Y89.1 |
| IIIB4 | Legally sanctioned deaths | Y35, Y89.0 |
The shaded rows indicate conditions for which the current analyses concluded sufficient evidence for a causal relationship and sufficient data on outcomes and risk relations to be included into the CRA 2005.
The ICD-10 code refers to the respective categories as defined by GBD 2005. These may include fewer codes than elsewhere, as GBD separately identifies ill-specified codes [so called garbage code(s)], which are to be redistributed to more meaningful codes based on specified procedures.
Abbreviations: CRA - Comparative Risk Assessment; GBD - Global Burden of Disease and Injury; ICD - International Classification of Diseases
Pattern of drinking has often been linked to two main categories of disease outcome, injuries (both unintentional and intentional) and cardiovascular risk (mainly ischaemic heart disease -IHD; [1;9]). Within the context of the current Comparative Risk Assessment (CRA), pattern of drinking was mainly operationalised as the presence of heavy drinking occasions, defined as 60 or more grams of pure alcohol on one single occasion (corresponding to 5+ drinks in most countries; for details on prior definitions see [9;10]). Thus, in this paper, heavy alcohol consumption will be defined as 60+ g per occasion, unless otherwise noted.
The three main intermediate mechanisms between average volume of drinking, pattern of drinking and diseases have been identified: (1) toxic and beneficial biological effects of alcohol on organs and tissues; (2) intoxication; and (3) dependence [1]. In addition, the quality of alcoholic beverages may impact health outcomes and mortality (Figure 1), for instance via methanol or lead poisoning outbreaks. However, the latter pathway seems to be of less importance from a public health perspective [11;12] because the documented impact is much lower compared to the impact of volume and pattern (described below).
This paper reports the first stage of the process of deriving new estimates of alcohol’s role in the burden of mortality and disease, based on research completed as part of the ongoing GBD 2005 study [2]. This process includes evaluating the existing evidence for a causal impact of the dimensions of alcohol on different categories of disease as a first step, then identifying meta-analyses to quantify the corresponding dose-response relationships. As in previous estimates [13], alcohol consumption is but one of a number of risk factors for the GBD study, with the teams working on the different risk factors coordinating their work in the common frame of a CRA. The conceptual model shown in Figure 1 is different from previous models in that it separates the impact of alcohol consumption on morbidity from the impact on mortality. This carries the implication that wherever possible, we tried to find or conduct separate meta-analyses for morbidity and mortality.
Because the alcohol analyses in the CRA process are still proceeding, we indicate in the text the direction to be taken in those analyses that are not yet completed. To allow cross-referencing with other analyses, we also indicate in Tables 2 and 5 both the ICD-10 codes and the GBD 2005 codes for the disorders considered.
METHODS
A 6-step procedure was used for the systematic review and article-selection phases of this project: (1) disease case definition; (2) computer-assisted search for systematic reviews and existing meta-analyses; (3) decision about causality; (4) decision about whether a new meta-analysis is necessary; (5) selection of individual articles, including hand searches, for conducting new meta-analyses; (6) extraction of information about dose-response relationships from existing or new meta-analyses.
CASE DEFINITION (STEP 1)
Case definitions were always based on clinical descriptions related to ICD coding schemes and the case definitions in the GBD 2005 operations manual (http://www.globalburden.org/gbdops.html) except for some outcomes (injury, for example) where articles may not have included ICD-10 codes and instead relied on other diagnoses or descriptions which can be coordinated with the codes. Uncorroborated self-reports were excluded.
COMPUTER-ASSISTED SEARCH FOR SYSTEMATIC REVIEWS AND EXISTING META-ANALYSES ON ALCOHOL AND DISEASE (STEP 2)
A systematic review of the literature published between 1980 and the 4th week of January 2008 was completed using computer searches of the following databases: Ovid Medline, EMBASE, Web of Science (including Science Citation Index, Social Sciences Citation Index, and Arts and Humanities Citation Index), CINAHL, PubMed, Cochrane Database of Systematic Reviews, CABS (BIDS), WHOLIST, SIGLE, ETOH, Alcohol in Moderation (alcohol industry database), and Google Scholar. The searches were not restricted geographically or by language.
For each disease outcome, findings were extracted from all available systematic reviews and quantitative meta-analyses and added to the results already included in the prior review [1]. In addition, individual studies and reviews on biological pathways or underlying mechanisms were analysed as well.
SELECTION OF DISEASE CONDITIONS CAUSALLY RELATED TO ALCOHOL (STEP 3)
As described in the following sections, disease conditions related to alcohol can be grouped into three categories, reflecting the nature of the conditions and the form of etiologic influence of alcohol.
1. WHOLLY ALCOHOL-ATTRIBUTABLE HEALTH CONDITIONS
Wholly attributable conditions can be easily identified by the inclusion of “alcohol” or “alcoholic” in their names, or by an ICD definition which identifies alcohol consumption as a necessary cause. With regard to the attribution of alcohol-relatedness, these conditions are by definition wholly attributable to alcohol with an alcohol-attributable fraction (AAF) of 100%, where AAF denotes the proportion of a certain disease category which would not have occurred had there been no alcohol consumption [14].
2. CHRONIC AND INFECTIOUS DISEASE CONDITIONS WHERE ALCOHOL IS A COMPONENT CAUSE (I.E., WITH AAFS LOWER THAN 100%)
In the present analyses, sufficient evidence of causality was defined as meeting all of the following criteria: (1) evidence of an association (positive or negative) between alcohol consumption and the disease or injury; (2) chance, confounding variables and other bias can be ruled out with “reasonable confidence” as factors in this association; and (3) evidence of a plausible mediating process [4]. Reasonable confidence in the present analyses was operationalised using two criteria: first, that the meta-analyses showed effects significantly different from no relations; second, that there was no empirical evidence of confounders or biases that eliminated the relationship. The latter was usually tied to study design and methodology. Usual criteria for establishing causality in epidemiology [6;15] were applied, with the most weight placed on the following four criteria (for example see [1]):
Established experimental biological evidence of mediating processes or at least physiological plausibility (biological mechanisms);
Temporality (cause before effect);
Strength (effect size);
Consistency of the association (dose-response relationship) across different studies.
3. ACUTE CONDITIONS WHERE ALCOHOL IS A COMPONENT CAUSE (I.E., WITH AAFs LOWER THAN 100%)
With respect to acute conditions, most researchers have agreed that alcohol has a causal impact on almost all injury categories if the above criteria are applied [4;9;16-21]. Detailed reasoning could also be found in the prior review [1]. Thus, we will mainly identify and discuss the exceptions from this rule.
DECISION ABOUT NEW META-ANALYSES (STEP 4)
For all disease/injury categories where causality was considered likely, we looked for existing meta-analyses to quantify the dose-response relationship. Because alcohol consumption can have different curvatures with different diseases (linear, j-shape, exponential), we looked for meta-analyses that allowed second or third order polynomials or fractional polynomials instead of just fitting a linear relationship. In addition, we sought meta-analyses that separated morbidity from mortality, especially for disease and injury conditions with long survival time. If no such analyses could be found, our group conducted a meta-analysis for these conditions. The final decisions on causality were made based on knowledge of all the literature, including the articles underlying the new meta-analyses and the results of the meta-analyses.
SELECTION OF RELEVANT LITERATURE FOR CONDUCTING NEW META-ANALYSES TO DETERMINE DOSE-REPONSE RELATIONSHIPS (STEP 5)
The same databases as for step 2 were searched, with the timeframe extending until January 2009. For disease outcomes where we conducted new meta-analyses, we examined all published evidence on the relationship between alcohol and the disease outcome. For this step, a priori exclusion criteria included any of the following:
No measure of association between outcome and appropriate alcohol exposure;
Less than three levels of alcohol consumption reported;
Cross-sectional design;
Duplicate data already identified in the search;
Outcome data not specific to disease ICD codes;
Inability to acquire data (e.g. unavailable thesis or dissertation).
Further technical details for quantitative meta-analyses for specific disease outcomes are provided in Table 3 of the Results section.
Table 3.
Details of the quantitative meta-analyses from which estimates on dose-response relationships were extracted
| Disease | N articles from main search | Exclusion criteria& | N articles in analysis | % agreement for data abstraction | Total N | % male | Notes |
|---|---|---|---|---|---|---|---|
| Tuberculosis | 16,527 articles in a comprehensive private collection of scientific TB publications were screened; PubMed revealed 2,007 abstracts | 1, 3, 4, | 21 | Not reported | 166,893 (calculated from Table 1 of [34] | not reported | [34] |
| Incident HIV | 856 | 1, 3, 6 | 10 | N/A | 28,584 | 67 | This will not be included in the CRA because insufficient proof of causality! |
| Mouth, nasopharynx, other pharynx andoropharynx cancer | 58* | 1, 2, 3, 4, 5 | 15 | Discrepancies to include an article and in quality score assignment were resolved in conference. | 4,507 | not reported | [68] |
| Oesophagus cancer | 51* | 1, 2, 3, 4, 5 | 14 | Same as above | 3,233 | not reported | [68] |
| Colon cancer | 16* | 1, 2, 3, 4, 5 | 16 | Same as above | 5,360 | not reported | [68] |
| Rectum cancer | 49* | 1, 2, 3, 4, 5 | 6 | Same as above | 1,420 | not reported | [68] |
| Liver cancer | 43* | 1, 2, 3, 4, 5 | 10 | Same as above | 1,321 | not reported | [68] |
| Larynx cancer | 38* | 1, 2, 3, 4, 5 | 20 | Same as above | 3,789 | not reported | [68] |
| Breast cancer (female) | 65* | 1, 2, 3, 4 | 53 | Not reported | 153,582 | 0 | [71] |
| Diabetes mellitus | 1,615 | 1, 2, 3, 4, 6 | 20 | 91.1 | 437,447 | 33 | [277] |
| Epilepsy | 1,360 | 1, 2, 3,4 | 6 | N/A | 2,332 | 56 | [97] |
| Hypertensive disease | 1,194 | 1, 2, 3, 5 | 12 | 90.4 | 282,977 | 46 | [278] |
| Ischaemic heart disease (volume) | 196* | 1, 2, 3, 4, 5 | 51 | N/A | 2,109,607 (calculated from [108]); 66,118 cases | not reported | [108] New meta-analysis by Rehm and colleagues ongoing |
| Ischaemic heart disease (irregular heavy drinking occasions) | 734 | 1, 2, 3, 4, 5 | 14 | N/A | 55,677 | 91 | [279] |
| Conduction disorders and other dysrhythmias | 1,431 | 1, 2, 4, 6 | 8 | N/A | 11,5931 | 33 | New meta-analysis by Rehm and colleagues |
| Ischaemic stroke | 190 | 1, 2, 3, 4, 5 | 20 | 92.0 | 571,277 | 56 | New meta-analysis by Rehm and colleagues |
| Haemorrhagic and other non-ischaemic stroke | 143 | 1, 2, 3, 4, 5 | 16 | 92.0 | 536,685 | 55 | New meta-analysis by Rehm and colleagues |
| Lower respiratory infections: pneumonia | 1,580 | 1, 2, 3, 4, 5 | 5 | N/A | 112,100 | 27 | New meta-analysis by Rehm and colleagues |
| Cirrhosis of the liver | 3,238 | 1, 2, 3, 4, 5 | 17 | 95.6 | 1,477,887 | 38 | [170] |
| Pancreatitis | 2,712 | 1, 2, 3, 4, 5 | 6 | n/a | 146,517 | 46 | [171] |
| Preterm birth complications: low birth weight | 1342 | 1, 2, 3, 4, 5 | 19 | N/A | 277,300 | 0 | New meta-analysis by Rehm and colleagues |
| Injury | 323 | 1, 2, 3, 4, 5 | 28 | 94.2 | 177,300 | 57 | New meta-analysis by Rehm and colleagues |
Exclusion Criteria: 1) No measure of association between outcome and appropriate alcohol exposure; 2) Less than 3 levels of alcohol consumption; 3) Cross-sectional study; 4) Duplicate data already identified in the search; 5) Outcome data not specific to disease ICD codes; 6) Inability to acquire data (e.g. unavailable thesis dissertation)
The search criteria of Corrao et al [68;108] were narrower compared to most searches of our group, as our search was intended not only to elicit papers for a quantitative meta-analysis on dose-response relations, but also to give insight into other causal criteria (see text above)
Abbreviations: CRA - Comparative Risk Assessment; TB - tuberculosis
Hand search
All of the reference lists in pertinent existing reviews and meta-analyses were hand searched to identify any relevant studies that may have been missed in the main search. Each identified article was obtained, translated into English where necessary, and analysed.
EXTRACTION OF INFORMATION FROM ANALYSES (STEP 6)
The following information was extracted from existing meta-analyses or calculated in new meta-analyses:
Quantitative information on the number of underlying studies and dose-response relationship on average volume of alcohol consumption and the respective outcome.
Fit of fractional polynomial models to determine the dose-response relationship: this involved two main steps – (1) testing for heterogeneity, publication and other bias between the studies identified in the systematic review [22-26], and (2) generating curves. The latter employed a fixed or random-effects model (depending on results of the first step) of 1st and/or 2nd order polynomials to define the curve, with goodness-of-fit determined by decreases in overall deviance compared to linear respectively quadratic referent models [27].
In order to check for reliability in extracting data in our own meta-analyses, a sample of 15 articles were extracted by two independent reviewers. In cases where the systematic review resulted in less than 15 articles, all of the selected articles were independently reviewed by two reviewers. Table 3 shows the percent agreement between the two raters for each completed meta-analysis.
RESULTS
HEALTH CONDITIONS WHOLLY ATTRIBUTABLE TO ALCOHOL
Table 1 lists all conditions 100% attributable to alcohol, or, in other words, disease conditions which could only have occurred as a result of alcohol consumption. Global prevalence data do not exist for most of the wholly attributable conditions and thus these conditions were neither included in the GBD 2000 nor will they be included in the GBD 2005: the exceptions are alcohol use disorders (AUD) and foetal alcohol syndrome (FAS). Although relevant data on the mortality, morbidity and disability of other conditions of Table 1 may exist for specific countries [28], even when the information exists for a country, there are often problems in records of alcohol diagnoses. Specifically, in addition to usual problems with coding smaller categories, disease categories including alcohol in their name are often stigmatized, leading to under-recording and underestimates [29;30]. Because of these problems with data on alcohol conditions wholly attributable to alcohol, we used broader categories (e.g., all liver cirrhosis) in our calculations, and determined AAFs indirectly.
While these disease categories by definition involve some alcohol consumption, the specific impact of patterns of drinking is less clear, even for AUD, although a recent review identified a pattern of heavy drinking occasions as a potential cause of FAS [31].
CHRONIC AND INFECTIOUS DISEASE CONDITIONS WHERE ALCOHOL IS A COMPONENT CAUSE
Table 2 provides an overview of mainly chronic non-communicable and infectious disease conditions that are not wholly attributable to alcohol, but where at least one review has found significant relations to alcohol. The shaded rows in Table 2 indicate conditions for which the current analyses concluded sufficient evidence for a causal relationship and sufficient data on outcomes and risk relations to be included into the CRA 2005. Table 3 provides an overview of the technical details of the quantitative meta-analyses from which the information on dose-response relationships was extracted for these diseases.
In the following, we describe briefly the evidence of the relationship, including biological pathways, for those conditions which had sufficient evidence for causality and which have been proposed for inclusion in the GBD 2005 (discussed in order of their ICD-10 codes). In addition, we briefly describe two conditions where relatively strong and consistent associations were found, but we could not exclude confounding (for HIV/AIDS) or we did not find enough evidence for a biological pathway (psoriasis). The dose-response relationships for all conditions with a causal impact of alcohol and evidence for a differential effect for mortality versus morbidity are summarized in Table 4.
Table 4.
Quantitative dose-response relationships between alcohol consumption and causally impacted disease conditions
| Disease | Monotonic relationship | Threshold | Dose-response relationship RR (95% confidence interval) | Differential effect mortality vs. morbidity | RR for ex-drinker compared to lifetime abstainers |
|---|---|---|---|---|---|
| Tuberculosis | Not enough data to model dose-response relationship | Yes, modelled at 40g/day or diagnosis of alcoholism | After exclusion of small studies because of suspected publication bias from Lönnroth et al., 2008: 2.94 (1.89-4.59). [34] | Hypothesized [241] but not modelled | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Mouth, nasopharynx, other pharynx and oropharynx cancer | Yes | No | From Corrao et al. (2004) (based on 14 case-control & 1 cohort studies): 25 g/day: 1.86 (1.76–1.96); 50 g/day: 3.11 (2.85–3.39); 100 g/day: 6.45 (5.76–7.24). [68] | No data from any of the meta-analyses | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Oesophagus cancer | Yes | No | From Corrao et al. (2004) (based on 13 case-control & 1 cohort studies): 25 g/day: 1.39 (1.36–1.42); 50 g/day: 1.93 (1.85–2.00); 100 g/day: 3.59 (3.34–3.87). [68] | No data from any of the meta-analyses | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Colon and rectum cancer | Yes | No | From Corrao et al. (2004) colon: (based on 12 case-control & 4 cohort studies): 25 g/day: 1.05 (1.01–1.09); 50 g/day: 1.10 (1.03–1.18); 100 g/day: 1.21 (1.05–1.39); rectum: (based on 4 case-control & 2 cohort): 25 g/day: 1.09 (1.08–1.12); 50 g/day: 1.19 (1.14–1.24); 100 g/day: 1.42 (1.30–1.55). [68] | No data from any of the meta-analyses | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Liver cancer | Yes | No | From Corrao et al. (2004) (based on 8 case-control & 2 cohort studies): 25 g/day: 1.19 (1.12–1.27); 50 g/day: 1.40 (1.25–1.56); 100 g/day: 1.81 (1.50–2.19). [68] | No data from any of the meta-analyses | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Larynx cancer | Yes | No | From Corrao et al. (2004) (based on 20 case-control studies): 25 g/day: 1.43 (1.38–1.48); 50 g/day: 2.02 (1.89–2.16); 100 g/day: 3.86 (3.42 – 4.35). [68] | No data from any of the meta-analyses | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Breast cancer (female) | Yes | No | From Hamajima et al. (2002) (58 515 women with invasive breast cancer and 95 067 controls from 53 studies): 35–44 g/day: 1.32 (1.19 – 1.45); ≥45 g/day: 1.46 (1.33 – 1.61) as compared with abstainers. The RR of breast cancer increased by 7.1% (5.5–8.7%) for each additional 10 g/day intake of alcohol, i.e. for each extra unit or drink of alcohol consumed on a daily basis. [71] | No data from any of the meta-analyses | Not computed; CRA will use all-cause mortality RRs |
|
| |||||
| Diabetes mellitus | No | No | From Baliunas et al. (2009): | No data to establish comparison (almost all risk relations between alcohol consumption and diabetes were based on morbidity) | Men:1.18 (0.89-1.52); Women: 1.14 (0.99-1.31) |
| Men: nadir at 22 g/day: 0.87 (0.76-1.00); deleterious > 60 g/day: 1.01 (0.71-1.44); | |||||
| Women: nadir at 24 g/day: 0.60 (0.52-0.69); deleterious at>50 g/day: 1.02 (0.83-1.26). [277] | |||||
|
| |||||
| Epilepsy | Yes | No | From Samokhvalov et al. (in press): not enough data to model separate by sex: 25g/day: 1.37 (1.28-1.47); 50 g/day: 1.86 (1.62-2.13); 100 g/day: 3.44 (2.61-4.52). [97] | Not enough data to model | Not enough data to estimate. CRA will use all-cause mortality RRs. |
|
| |||||
| Hypertensive disease | Men: yes, women: no | No | From Taylor et al., (2009): | Insufficient data for mortality, so the relationship was only modelled for hypertension morbidly | Both gender: 0.94 (0.49-1.39) |
| Men: 25g/day: 1.25 (1.19-1.32); 50g/day: 1.62 (1.46-1.81); 100g/day: 2.64 (2.14-3.26). | |||||
| Women: <5 g/day: 0.82 (0.73-0.93); 25 g/day: 1.24 (0.87-1.77); 50g/day: 1.81 (1.13-2.90), 100g/day: 2.81 (1.56-5.05). [278] | |||||
|
| |||||
| Ischaemic heart disease | No | No | From Corrao et al. (2000): curvilinear relationship for average consumption; based on all 54 studies: nadir at 25 g/day: 0.75 (0.73-0.77); protective effect up to 90 g/day: 0.94 (0.90-1.00); harmful effect at 113 g/day: 1.08 (1.00-1.16); based on 28 high quality cohort studies: nadir at 20 g/day: 0.80 (0.78-0.83); protective effect up to 72 g/day: 0.96 (0.92-1.00); detrimental effect >89 g/day: 1.05 (1.00-1.11). [108] | Yes, stronger relationship with mortality | Men mortality: 1.21 (1.12-1.30); morbidity: 0.98 (0.89 -1.08) Women mortality 1.39 (1.17-1.66); morbidity 1.11 (0.94-1.32). |
| Bagnardi et al. (2008) estimated an RR for irregular heavy drinking patterns compared to abstainers of 1.10 (1.03-1.17). [118] | |||||
| From Roerecke & Rehm (submitted): Heavy drinking occasions (>60g per occasion) compared to non-heavy drinking occasions: 1.45 (1.24-1.70). [279] | |||||
|
| |||||
| Ischaemic stroke | No | No | From Reynolds et al. (2003): <12 g/day: 0.80 (0.67-0.96); 12-24 g/day: 0.72 (0.57-0.91); 24-60 g/day: 0.96 (0.79-1.18); >60 g/day: 1.69 (1.34-2.15). [155] | Men: no, women: yes | Strokes combined: men 1.33 (0.91-1.96); women 1.15 (0.71-1.92); both 1.22 (0.87-1.76) |
|
| |||||
| Haemorrhagic and other non-ischaemic stroke | Men: yes, women: no (only for morbidity) | No | From Reynolds et al. (2003): <12 g/day: 0.79 (0.60-1.05); 12-24 g/day: 0.98 (0.77-1.25); 24-60 g/day: 1.19 (0.80-1.79); >60 g/day: 2.18 (1.48-3.20). [155] | Men: no, women: yes | |
|
| |||||
| Conduction disorders and other dysrhythmias | Yes | Not clear; volumes of average consumption < 36g have RR of about 1, but based on few studies | From own meta-analysis (not enough data to model separate by sex): <24 g/day: 1.02 (0.94-1.12); 24-36 g/day: 1.13 (0.98-1.30); 36-48 g/day: 1.19 (1.03-1.37); 48+ g/day: 1.45 (1.24-1.69). | Not enough data to model | Not enough data to estimate. |
|
| |||||
| Lower respiratory infections: pneumonia | Yes | Not clear, as lower volumes of average consumption have RR of about 1; mechanism similar to TB | From own meta-analyses (not enough data to model separate by sex: 25g/day: 1.13 (1.03-1.24); 50 g/day: 1.27 (1.05-1.53); 100 g/day: 1.61 (1.10-2.35) | Not enough data to model | Not enough data to estimate. |
|
| |||||
| Cirrhosis of the liver | Yes | There are good indications for a threshold for morbidity as endpoint, but not for mortality [170] | From Rehm et al., (in press): mortality | Yes, higher risks for mortality [170] | Morbidity and mortality combined: Women: 6.50 (2.21-19.08); Men: 1.31 (0.67-2.57); Women only based on one study [280] |
| Men: 30g/day: 2.8 (2.3-3.4); 54g/day: 7.0 (5.8-8.0); >60 g/day: 14.0 (11.7-16.7); | |||||
| Women: 30g/day: 7.7 (6.3-9.5); 54g/day: 14.7 (11.0-19.6); >60 g/day: 22.7 (17.3-30.1); Morbidity | |||||
| Men: 30g/day: 0.7 (0.5-1.0); 54g/day: 2.3 (1.7-3.2); >60 g/day: 5.0 (3.9-6.4); | |||||
| Women: 30g/day: 2.4 (1.8-3.2); 54g/day: 5.9 (3.7-9.3); 60g/day: 6.1 (4.6-8.0). | |||||
| From categorical analysis [170] | |||||
|
| |||||
| Pancreatitis | Yes, but risk for lower doses of average volume of drinking may not be significantly different from risk of abstention | Yes, a threshold effect at about 48 g/day or 4 drinks average volume was found [171] | From Irving et al. (2009): not enough data to model separately by sex:; 25g/day: 1.10 (1.08-1.12); 50g/day: 1.46 (1.34-1.59); 100g/day: 4.50(3.22-6.31). [171] | Not enough data to model | Not enough data to estimate. CRA will use all-cause mortality RRs. |
|
| |||||
| Preterm birth complications | Yes | No | From Rehm et al. (2004): | Not relevant | Not meaningful, as only drinking during pregnancy can affect newborns |
| Men <40 g/day: 1.00; 40-60 g/day: 1.40; 60+g/day: 1.40; Women <20 g/day: 1.00; 20-40 g/day: 1.40; 40+ g/day: 1.40. [9] | |||||
|
| |||||
| Injury | Yes | No | From Corrao et al. (1999): 25 g/day: 1.4 (1.2–1.6); 50 g/day: 2.0 (1.5-2.6); 100 g/day: 4.0 (2.4-6.6) | Yes, higher risks for mortality | Not meaningful, as risk is mainly determined by drinking before injury |
| Corrao et al. (1999) was based on average volume in g/day. [222] | |||||
Abbreviations: CRA - Comparative Risk Assessment; RR – relative risk
TUBERCULOSIS (TB)
A technical meeting was hosted by the South African Medical Research Council and co-sponsored by the World Health Organization at Cape Town in July 2008 to review evidence about a potential causal impact of alcohol consumption on Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome (HIV/AIDS) and tuberculosis (TB). After reviewing the evidence from epidemiology, social sciences and immunology, the meeting concluded that there was sufficient evidence for a causal impact of alcohol on TB incidence and on worsening the disease [32].
It has long been known that alcohol has been associated with TB [33]. The association between heavy drinking and risk of TB incidence is both strong and consistent with a risk ratio of about 3 ([34]; see also Table 3). Heavy drinking in the underlying meta-analysis and most other studies in the TB area was defined as either drinking more than 40g pure alcohol per day or “alcoholism” [34]. Despite its strength, however, the causality of this association had not been established [35].
Our analyses identified two plausible pathways between heavy alcohol consumption and TB. First, heavy alcohol consumption affects the immune system, thus facilitating susceptibility to infection as well as conversion to active TB in infected individuals [36-38]. Second, alcohol use may lead to being in social environments which facilitate the spread of TB infection [39]. Other common cause factors (e.g., poverty) may impact the association, but this does not invalidate the conclusion that alcohol is one component in a sufficient component causal mechanism for incidence of TB [39].
Regarding the course of the disease, there is sufficient evidence indicating that heavy alcohol consumption disrupts medication intake regimens and negatively affects help-seeking and treatment processes, leading to worse treatment outcomes compared to abstinence [32;40]. This would indicate the necessity to derive separate RR estimates for alcohol and TB mortality, which should be higher than for morbidity if the above finding is true.
HUMAN IMMUNODEFICIENCY VIRUS / ACQUIRED IMMUNE DEFICIENCY SYNDROME (HIV/AIDS)
At the Cape Town meeting mentioned above, it was similarly concluded that there is conclusive evidence of a causal linkage between heavy drinking patterns and/or AUD and the worsening of the disease course for HIV/AIDS (for more detailed reasoning see [41;42]). While alcohol use is consistently associated with the prevalence and incidence of HIV [43-45], however, further research is needed to substantiate causality [46]. As indicated above, alcohol use, especially heavy use, weakens the immune system, thus creating a larger vulnerability for infections. However, in contrast to TB which can be acquired through essentially passive behavioural means, in order to become infected with HIV, there must be an additional active behavioural component, which in most cases involves engaging in unprotected sex. Although generalized alcohol use has been shown to be associated with overall reports of unprotected sex [47-49], when the relationship between alcohol consumption and unprotected sex is examined within event-level contexts (e.g., based on daily diary assessments), the relationship weakens, and in many cases, disappears [47;49-52]. These findings therefore suggest that alcohol consumption on its own may not be causally linked to the active behaviours that are required in order for HIV acquisition to take place. Rather, alcohol use may serve as a marker for other variables that potentially underlie the association between alcohol and unprotected sex, including personality characteristics such as sexual compulsivity [53] or sensation seeking [54;55], and/or psychiatric conditions such as anti-social personality disorder [56] Thus, sufficient evidence for causality of the impact of alcohol on HIV incidence could not be concluded (see also the reasoning in [45]). Without going into further details, the same kind of reasoning would hold for other sexually transmissible diseases.
However, there is enough evidence to conclude a causal impact of alcohol on the course of the disease [32], Similarly, as with TB, alcohol consumption was shown to disrupt HIV treatment, with markedly higher drop-outs and more treatment failures associated with this exposure [57]. There is also an indication of a dose-response relationship, where problem drinking or alcohol use disorders interfere more strongly than does alcohol exposure per se [57]. A conservative quantification of this impact is possible based on the effect of alcohol consumption on antiretroviral treatment adherence [57] combined with the impact of failed adherence on survival [58].
CANCER (IN GENERAL)
In February 2007, the Monograph Working Group of the International Agency for Research on Cancer (IARC) concluded that there was “sufficient evidence” for the carcinogenicity of ethanol in animals and classified alcoholic beverages as carcinogenic to humans. Specifically, the group confirmed or newly established the causal link between alcohol consumption and the following malignant neoplasm categories: oral cavity, pharynx, larynx, oesophagus, liver, colorectal, and female breast cancer ([59]; see Table 2). However, the working group also confirmed a lack of carcinogenicity of alcoholic beverages for renal-cell cancer and non-Hodgkin’s lymphoma. For stomach and lung cancer, the verdict was that carcinogenicity was possible but not established, while evidence on causality between alcohol consumption and risks of other types of cancer was sparse or inconsistent (these results are summarized in Table 2). All cancers showed evidence of a dose-response relationship (shown in Table 3).
The molecular and biochemical mechanisms by which chronic alcohol consumption leads to the development of cancers of various organs are not fully understood. It is suggested that these mechanisms differ by target organ and include polymorphisms in genes that encode enzymes responsible for ethanol metabolism (e.g., alcohol dehydrogenase, aldehyde dehydrogenase, and cytochrome P450 2E1), increased oestrogen concentration, and changes in folate metabolism and in DNA repair [60]. For the digestive tract cancers, especially those of the upper digestive tract, acetaldehyde, both from alcohol metabolism in the human body and ingested as a component of alcoholic beverages, has been recently highlighted as an important likely causal pathway [59;61-65].
Several of the cancers that were identified as alcohol-attributable in the latest review had already been included in prior studies of the alcohol-attributable disease burden, such as the 2000 or 2004 CRAs [10;66]. The following sections provide more details on colorectal and female breast cancers that had not consistently been included in prior analyses.
COLORECTAL CANCER
A causal relation between alcohol and colorectal cancer was established only recently by the IARC [59]. Several meta-analyses [67-69] observed a positive linear relation between alcohol consumption and colorectal cancer. The studies provide evidence for an increased relative risk (RR) of about 10-20%for colorectal cancer with regular consumption of about 50 grams of alcohol per day, compared with abstainers. This association is similar for both colon cancer and rectal cancer [67;69].
A potential mechanism is that alcohol may act through folate metabolism or synergistically with low folate intake (as low folate intake increases the risk of colorectal cancer); however the effects might be modest [60;69]. Moskal and colleagues [69] also suggested a genotoxic effect of acetaldehyde, a metabolite of alcohol, and genetic polymorphism as factors for enhancing the risk of colorectal cancer.
BREAST CANCER (FEMALE)
Many epidemiological studies have indicated a positive relation between alcohol consumption and incidence of breast cancer [68;70-72]. The risk exists even at a moderate level of alcohol drinking [73] and increases monotonically with the level of alcohol consumption [68;71]. Based on several epidemiological studies, each additional 10g (less than one standard drink in most countries) of alcohol per day is associated with an increase of 7% in the RR of breast cancer [71] or higher (an increase of 10% was estimated [72]). Hamajima and colleagues [71] estimated that about 4% of the female breast cancer cases in developed countries may be attributable to alcohol drinking.
The mechanism of association between alcohol and breast cancer may involve increased levels of oestrogen [60;74] or increased levels of plasma insulin-like growth factor (IGF) produced by the liver due to moderate consumption of alcohol ([75]; also see [70;72]).
DIABETES MELLITUS
Moderate alcohol consumption is associated with a reduced risk of type 2 diabetes. There are several possible biological mechanisms that may explain the observed relationship. Development of insulin resistance is a key factor in the pathogenesis of type 2 diabetes [76] and the risk reduction may be explained by an increase in insulin sensitivity after moderate alcohol consumption [77] which has been found in observational studies [78-80] as well as randomized controlled trials [81;82]. Alternatively, ethanol oxidation produces measurable downstream metabolites such as acetaldehyde and acetate [83] which may reduce the risk of type 2 diabetes. Moderate alcohol consumption is also known to increase high density lipoproteins (HDL) cholesterol concentrations [84], although at higher consumption levels, body weight, triglyceride concentration, and blood pressure may increase ([85;86]; see also IHD below). Another plausible protective mechanism is through the anti-inflammatory effect of alcohol [87;88]. However, whether moderate alcohol intake is itself a protective factor for diabetes or whether it is a marker for other healthy lifestyle choices is not certain. We decided to include diabetes into the CRA as causally impacted by alcohol consumption, but the overall level of evidence for this was considered as borderline between sufficient and limited.
EPILEPSY
Although it has long been known that alcohol withdrawal can lead to seizures [89], these provoked seizures have been excluded in the GBD 2005 definition of epilepsy. Consistent with the GBD 2005 definition, we used the definitions of epilepsy from the International League against Epilepsy and the International Bureau for Epilepsy, which describe epilepsy as a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures. The cited definition of epilepsy requires the occurrence of at least one epileptic seizure [90]. In line with this definition we used the studies which had unprovoked or other grand mal seizures as an outcome, in addition to those that used physician-diagnosed epilepsy as their outcome. We found a consistent relationship between alcohol consumption and increased risk of thus-defined epilepsy, especially for higher doses of alcohol [91-97].
The consistent dose-response relationship is rooted in plausible pathways. In particular, chronic alcohol consumption has diverse effects on the central nervous system, affecting its structure and functioning in different ways. There are several major theories explaining the effects of alcohol consumption on the development of epilepsy. One postulates a “kindling” effect [98]. According to this theory, repeated withdrawals, including natural withdrawal through sleep over the years, may lead to the gradual lowering of the epileptogenic threshold [98]. Epileptogenesis in heavy alcohol users may also be explained by cerebral atrophy [99]. Additional hypothesized causes of epilepsy in alcohol users include cerebrovascular infarctions, lesions, head traumas (from alcohol-attributable injuries), and changes in neurotransmitter systems and ionic imbalances leading to the onset of seizures [99-102]. Our analyses indicated that the strongest evidence was for the “kindling” hypothesis, for cerebral atrophy and for brain lesions each linking direct irreversible CNS changes to alcohol consumption, potentially leading to the onset of epilepsy or spontaneous seizures not immediately related to alcohol intake.
Additionally, it has been shown that heavy alcohol consumption may worsen the clinical course of existing epilepsy via increased clearance of antiepileptic drugs and non-compliance to treatment regimen [101].
HYPERTENSIVE DISEASE
The only relevant disease or cause of death in GBD for this category is hypertensive heart disease (see Table 2 for exact definition). As there were no studies on alcohol consumption and hypertensive heart disease fulfilling the inclusion criteria, we substitute the studies on alcohol consumption and a wider definition of hypertensive diseases including essential hypertension. The relationship between alcohol consumption and hypertensive disease is relatively complex. On the one hand, an overall consistently detrimental dose-response effect has been shown [68] for men, while moderate consumption of alcohol has, in some studies, shown to be protective for women. However, despite the consistent effect of chronic alcohol use on increasing blood pressure [103;104], the mechanism of action is unclear, although a number of theories about potential pathways have been proposed [104]. These include activation of the sympathetic nervous system to constrict blood vessels and increase the contractile force of the heart [105], or a possible role of sensitivity of baroreceptors in vessel walls that results in a diminished ability to regulate blood pressure via arterial contraction and relaxation [106]. The protective effect, seen only in moderate doses and among women, may be explained in the same way that alcohol is protective for IHD: by changing concentrations of high and low density lipoproteins (HDL, LDL, respectively) in the blood or reduction in platelet aggregation on vessel walls. The fact that it is not seen in men may be a result of higher rates of binge drinking in men resulting in an overall detrimental effect, but the literature is equivocal.
ISCHAEMIC HEART DISEASE
IHD is a major cause of death and disease burden around the world, and its impact is projected to increase in the future [107]. The relation of alcohol consumption to IHD is complex, with mechanisms for both beneficial and detrimental causal impact, depending on drinking pattern. Light to moderate regular alcohol consumption has been linked to reduced risk and severity for incidence of coronary events, with greater risk reduction for non-fatal events; however, most of the effect can be achieved with consumption of 12 g pure alcohol (about one standard drink in the US and many other countries) every other day, with a no benefits obtained for consumption of more than 20 g pure alcohol per day (less than 2 standard drinks) [108;109]; as IHD constituted the main beneficial effect in medical cohorts, the relationship between alcohol and total mortality showed about the same form [110]. The pattern of light to moderate regular alcohol consumption with no heavy drinking occasions also reduces the risk for recurrence after an IHD event [111] and among people with existing IHD risk factors, such as diabetes [112;113] or hypertension [114], whereas a drinking pattern that includes heavy drinking occasions, even when usual consumption is light or moderate, has been related to an increase in IHD risk [115-118]. Chronic heavy alcohol use, on the other hand, has been associated with adverse cardiac outcomes, not only IHD but also dilated cardiomyopathy or cardiac dysrhythmias (see below and [119]).
The epidemiological evidence that regular light to moderate alcohol consumption protects against IHD is strengthened by growing and, in some instances, substantial evidence concerning the biological mechanisms by which a protective effect could be mediated [84;120-123]. First, moderate alcohol intake has been clearly linked to favourable lipid profiles, especially an increase in HDL [84;124]. It has been estimated that as much as 40%-50% of the protective effect may be attributable to this mechanism [125;126]. Gene interactions may play a role in influencing HDL levels as well [127-129], but more work may be required to more fully explain this complex pathway [130].
Second, moderate alcohol intake favourably affects coagulation profiles [84], in particular through its effects on platelet aggregation [131] and fibrinolysis [132]. A meta-analysis of 42 published short-term trials confirmed the influence of alcohol both on serum lipid profiles and on blood-clotting factors [84]. Other mechanisms for the cardioprotective effect of light to moderate consumption have been discussed [117;129] but, based on current knowledge, appear to play less prominent roles in explaining the beneficial effects of regular light to moderate drinking.
Due to the complexity of processes leading to IHD and general limitations of observational studies, the protective effect of alcohol on IHD risk remains a highly debated topic. In many of the older studies on IHD risk, alcohol measurement failed to take into account variability of alcohol consumption over time by relying on one baseline measurement. In addition, a recent review by Fillmore and colleagues [133] suggested that the cardiac protection caused by alcohol might have been over-estimated. According to the authors, many studies had used contaminated abstainer groups by not excluding former drinkers from this group. Because former drinkers have a significantly different risk profile from true lifetime abstainers, risk estimates may have been inflated (the so called “sick quitter” effect; see [134]). This led Fillmore and colleagues to infer that regular light to moderate drinking might be a marker for good health among older people, but not a cause of it [133]. While this effect would seriously bias risk estimates when abstainers (including former drinkers with substantial prior alcohol consumption) are the reference category, three major points underline the cardioprotective effect despite this theoretical argument [135]. First, experimental evidence has confirmed mediating pathways for a cardioprotective effect of moderate regular drinking [84]. Second, studies where lifetime abstainers were separated from former drinkers confirmed a cardioprotective effect [136;137]. Third, a cardioprotective effect also has been reported among participants with existing IHD risk factors [111-114].
Nevertheless, classification of lifetime abstainers is indeed a difficult task and, when carefully measured at more than one point in time, a substantial fraction of “lifetime abstainers” reported alcohol consumption at other different time points [138]. But those who were drinkers of small quantities on rare occasions would not bias the results of the comparison group since there is no plausible biological pathway for an effect of such drinking [139]. The best comparison group for alcohol epidemiology with respect to chronic disease outcomes would thus be a combination of lifetime abstainers with those who never consumed alcohol in quantities which could have a biological impact [138].
The relationship between alcohol and IHD risk is further complicated by the fact that at least two dimensions of consumption have to be taken into account in determining IHD risk. Heavy drinking occasions, not adequately captured by measurement of average consumption, have been linked to adverse cardiovascular events for some time [108;117;140]. Unfortunately, most epidemiological studies on alcohol and IHD risk have used average consumption as the exposure measure, and it is only recently that studies have been conducted with methodologically rigorous assessment of IHD as an endpoint, and with control for average volume as a confounder and/or with lifetime abstention as reference (e.g. [141-144]). All of these recent studies found a protective effect for daily average light to moderate drinkers but no or detrimental effects for people whose drinking patterns included heavy drinking occasions (even if their usual pattern was moderate). A review by Agarwal [145] and a study conducted by Mukamal and colleagues [137] also found drinking patterns to be important to the risk of IHD for a given volume of alcohol consumption. The detrimental effects of heavy drinking occasions on IHD are consistent with the physiological mechanisms of increased clotting and a reduced threshold for ventricular fibrillation after heavy drinking occasions (see review [115]).
In summary, heavy drinking occasions are mainly associated with physiological mechanisms that increase the risk of sudden cardiac death and other cardiovascular outcomes, in contrast to the physiological mechanisms triggered by steady low to moderate consumption that are linked to favourable cardiac outcomes. The relationship between alcohol and IHD will thus be modelled based on two dimensions: average volume of consumption and patterns of drinking, operationalised by heavy drinking occasions.
DYSRHYTHMIAS
The association between alcohol consumption and cardiac rhythm disorders has been recognized for some time [146]. In two studies, alcohol was identified as a cause of new onset atrial fibrillation in 30 - 60% of patients [147;148]. Several mechanisms explaining the development of dysrhythmias due to alcohol consumption have been proposed, including direct alcohol cardiotoxicity, hyperadrenergic activity during drinking and withdrawal, impairment of vagal tone and increased intra-atrial conduction time [149].
Existing estimations of the association between alcohol consumption and the onset of cardiac dysrhythmias vary in different studies, including several large-scale prospective studies – the Framingham Heart Study [150], the Manitoba study [151], the Multifactor Primary Prevention Study [152] and others. The Framingham Heart Study revealed a low association with moderate alcohol consumption, but the association became significant among individuals consuming over 36 g pure alcohol per day (about three drinks) [150]. In the Copenhagen City Heart Study increased risk of atrial fibrillation was described for persons consuming more than 35 drinks per week [153]. A recent study by Planas and colleagues showed an increased risk of the reoccurrence of atrial fibrillation with moderate alcohol consumption [154].
Overall, existing studies show increased risk of development of dysrhythmias related to heavy alcohol consumption, whereas the effects of light to moderate alcohol consumption are inconclusive.
STROKE
The two most recent meta-analyses [68;155] indicate that alcohol use both reduces and increases the risk of stroke depending on the type of stroke, quantity of alcohol consumed, and drinking pattern. Both of these studies found a positive, almost linear relation between alcohol consumption and logarithmized RR of haemorrhagic stroke (corresponding to an exponential relationship between consumption and the RR of haemorrhagic stroke, when not logarithmized), but observed a curvilinear relationship between alcohol consumption and the logarithmized RR of ischaemic stroke. According to these meta-analyses, low to moderate alcohol consumption (1-2 drinks per day) seemed to have a protective effect on ischaemic stroke, and then the risk curve turned upwards.
The variation in the effect of alcohol consumption on different types of stroke may be ascribed to the difference in the causes of haemorrhagic and ischaemic stroke. Ischaemic stroke is caused by the blockage in a blood vessel in the brain, commonly arising from a blood clot formed somewhere else, and therefore has a similar aetiology to that of IHD (for biological pathways see IHD above). Consequently, a pattern of irregular heavy drinking occasions should also have the same detrimental effects as for IHD. Thus, combining fatal and non-fatal ischaemic strokes in a Finnish cohort study, Sundell and colleagues [156] reported a RR of 1.99 (95% CI: 1.39 to 2.87) for ischaemic stroke among participants whose drinking pattern included binge drinking occasions (defined as 6+ drinks in one setting for men or 4+ drinks for women, equivalent to 72 g and 48 g of pure alcohol respectively) compared to non-binge drinkers after adjusting for long-term average alcohol consumption, age and sex. An RR of 1.56 (95% CI: 1.06 to 2.31) was estimated, when hypertension, study area, smoking, diabetes, BMI, education, history of MI, and study year were taken into account as well.
Haemorrhagic stroke, on the other hand, is caused by a rupture of a blood vessel supplying the brain, thus releasing blood into the brain. Two major subtypes of haemorrhagic stroke can be distinguished: intracerebral haemorrhage (ICH) and subarachnoid haemorrhage (SAH). The impact of alcohol on both types of haemorrhagic stroke is high. For instance, in a study conducted in Germany, more than one third of men with ganglionic ICH were alcoholics [157]. Mechanisms by which alcohol could impact on ICH and SAH include hypertension [158]. However, the risk for ICH remains elevated even when alcohol-induced hypertension has been accounted for, due to alcohol-induced vasospasm [158]. For SAH, heavy drinking occasions seem to play a specific role [158]. The vasospastic mechanism discussed for ICH may also play a role with SAH. Finally, the same mechanisms leading to a detrimental effect of heavy alcohol consumption on IHD also come into play in explaining the effect of heavy alcohol consumption on haemorrhagic stroke [156].
Based on the above evidence, the relationship between alcohol consumption and stroke will be modelled separately for these two major types of stroke, as specified by the GBD categories. The epidemiologic evidence allows mainly for differentiation of these two main types (for further differentiation see [159] and [160]).
LOWER RESPIRATORY INFECTIONS: COMMUNITY ACQUIRED PNEUMONIA
Community acquired pneumonia (CAP), distinguished from hospital-acquired pneumonia because of its different aetiology, has been recognized as caused by heavy alcohol consumption since the seminal work of Rush in the late 18th century [33]. An association between CAP and alcohol intake has been found [161], and plausible biological pathways have been identified. As already indicated above (see section on TB), heavy alcohol consumption can cause alterations of the immune system, thereby increasing host susceptibility to CAP. Also, because of the sedative properties of alcohol which lead to diminished oropharyngeal tone, an increased risk of aspiration and diminished cough reflex and mucociliary clearance [38;162], alcohol intake can facilitate the development of CAP. A preliminary meta-analysis by our group indicated that the risk curve is relatively flat for lower consumption, and reaches a RR of 1.3 only at a consumption level of about 5 drinks per day (equivalent to 60 g pure alcohol per day). On the other hand, older studies showed that patients defined as “alcoholics” according to clinical symptoms showed an 8-fold increased risk for CAP [163;164]. Also, in a recent study in Russia, drinkers of more than 3 bottles of vodka per week showed an RR of 3.29 (95% CI 2.83–3.83) for men, and 3.42 (95% CI: 2.64–4.44) for women, both compared to drinkers of half a bottle of vodka weekly or less [165]
LIVER CIRRHOSIS
Liver cirrhosis has been linked to alcohol consumption in every review of alcohol-attributable disease, and the underlying mechanisms have been described previously in detail [166-168]. Thus, we will restrict our present discussion to the differential relationship of alcohol with liver cirrhosis mortality versus morbidity. Specifically, evidence suggests that alcohol consumption is more strongly linked to cirrhosis mortality than to morbidity because drinking, especially heavy drinking, has been shown to worsen existing liver disease considerably and to have detrimental effects on the immune system, thus negatively affecting the course of existing liver disease and increasing the chance of death [38;166;169]. Our own meta-analyses confirmed clearly, that for both men and women the impact of alcohol was stronger on liver cirrhosis mortality compared to morbidity [170].
PANCREATITIS
Alcohol consumption has been shown to be consistently associated with an increased risk of pancreatitis, and plausible biological mechanisms have been identified for the effect [see [171]]. In particular, the metabolites of alcohol, such as acetaldehyde and fatty acid ethyl esters, may initiate and/or enhance pancreatic injury. Alcohol may also attenuate or augment inflammatory cell activation, leading to fibrosis in the pancreas [38;172-175]. All these mechanisms come into place especially with heavy consumption. Thus, the meta-analysis of Irving and colleagues cited above found that, compared with non-drinkers, alcohol consumption of two or fewer drinks per day (<= 24 g pure alcohol/day) was almost identical to the risk of non-drinkers (RR=1.0, 95% CI: 0.8-1.2; P=0.887). Drinking 3 to 4 drinks was associated with only marginally significant higher risk than for abstention (RR=1.2, 95% CI: 1.0-1.5, P=0.059), but overall the dose-response relationship increased monotonically (see Table 3).
PSORIASIS
Psoriasis is a chronic, autoimmune, inflammatory, sometimes disfiguring, skin and joints disease, which has a high impact on health-related quality of life. Epidemiological studies from around the world have estimated the prevalence of psoriasis to be 0.6% to 4.8% [176]. Current evidence suggests that psoriasis is caused by the interaction of genetic-environmental factors and the immune system [177-179].
Medical literature for practitioners and patients describes alcohol as a psoriasis trigger. Most of the studies and reviews conducted in the past decades support a detrimental impact of alcohol consumption on psoriasis, especially in male patients [4;19;180-187]. In addition to the finding of a clear dose-response relationship [187-189], the prevalence of psoriasis among alcoholics was between two and ten times greater than in the general population [180;190-192]. In one recent study, between 17% and 30% of patients with psoriasis (depending on the measure of alcohol consumption) were classified as having difficulties with alcohol [193]. However, part of the association between alcohol and psoriasis could alternatively be attributed to higher alcohol consumption as a consequence of the disease [180;194]. Abstinence from alcohol was found to be related to psoriasis remission, whereas restarting alcohol consumption has been associated with the recurrence of psoriasis [195;196]. Alcohol consumption is also associated with less favourable responses to treatment and decreased compliance with the treatment of psoriasis [197;198].
The mechanism by which alcohol consumption affects psoriasis has not been fully clarified. Most researchers suggest that alcohol affects psoriasis mainly by adversely affecting the immune system, and thus predisposing drinkers to infections ([199-201], but see [202]). It has also been suggested that the stimulatory effect of ethanol and acetone (which exceeds its normal endogenous level in the blood of heavy drinkers) on keratinocytes, causing epidermal hyperproliferation, may be one of the reasons why psoriasis can be precipitated by alcohol use in genetically predisposed individuals [199-201]. In sum, consistent with [202], we conclude that there is a consistent association between alcohol consumption and psoriasis, but that the overall level of evidence for causality is insufficient, especially with respect to biological pathways.
PRETERM BIRTH COMPLICATIONS
Heavy ingestion of alcohol has been implicated in an increased risk of preterm birth [203-207]. However, some studies reported modest inverse associations between low levels of alcohol consumption and preterm delivery with low birth weight (e.g., [206;208]), although this may be a result of a higher prevalence of such drinking behaviour among women who are more socio-economically advantaged [203;204]. Further, the risks associated with different dimensions of drinking during different phases of pregnancy are unclear. For example, moderate amounts of alcohol increased the risk for preterm delivery during late pregnancy, but not during early pregnancy in one study [206], whereas another study found elevated risk of preterm birth complication also with drinking in early pregnancy [209].
The biological mechanism responsible for the association between alcohol use and preterm birth complications may be explained in terms of an enhanced prostaglandin level among drinkers, because a rise in prostaglandin level can cause preterm birth [210]. Progesterone hormone has been shown to suppress prostaglandin production [211-213], which is an important regulator of prostaglandin synthesis during parturition. Heavy alcohol exposure decreases the progesterone synthesis and level [214;215], hence resulting in failure to control the prostaglandin level and eventually leading to the onset of the physiological events associated with parturition [210;216].
The biological mechanisms through which maternal drinking affects foetal growth are not completely understood. However, acetaldehyde, the major metabolite of alcohol, affects the human placenta exposed to this toxic metabolite, consequently causing various problems such as foetal hypoxia, impaired cell proliferation, or delayed placental development [217].
ACUTE CONDITIONS WHERE ALCOHOL IS A COMPONENT CAUSE
As detailed in the previously published review based on the GBD 2000 project [1], alcohol consumption has been shown to have a causal impact on different forms of injury, both unintentional and intentional. In the present review, we focus on two additional issues: whether there is evidence of a causal relationship between alcohol and injury for all categories of injury, and how best to estimate AAFs for each injury outcome. Table 5 provides an overview of the GBD 2005 categories for injury.
For the shaded categories, causality has been established by the earlier review [1]. For fire injuries and related conditions, while alcohol has been established as a component cause and its contribution has been quantitatively estimated in some countries based on insurance or police records (e.g., [28]), global estimates seem to be impossible. For natural disasters, while it may certainly be true that intoxicated people may be more vulnerable when disaster strikes, this effect has never been estimated, and therefore, natural disasters are not part of the current CRA. Similar reasoning was used for injuries due to animal bites.
For the categories of adverse effects of medical treatment as well as war and other forms of collective violence, including legally sanctioned deaths, there is insufficient evidence for a causal impact of alcohol, though there is ample anecdotal evidence of alcohol intoxication as the source of “liquid courage” in collective violence [218] and as being used to amplify cruelty in wartime (e.g., [218;219]). Thus, Mueller [218] notes that the killing squads at Srebrenica were often shored up with generous quantities of liquor, as was typical for the wars in former Yugoslavia. In the Rwanda genocide, massacres were often committed by drunken militia bands, fortified with assorted drugs from pharmacies [220]. Likewise, there are reports documenting the intoxication involved in the purposive violence of football hooligan crowds [221].
The second issue is how best to estimate AAFs for injury categories? One way would be to apply the same procedure as with chronic disease categories, based on average volume of consumption with risk relations taken from the meta-analyses of Corrao and colleagues [222]. However, this procedure cannot take into consideration the cultural differences between societies in the relationship between alcohol consumption and injury, involving both the physical and social context of drinking and the proportion of heavy drinking occasions in the overall volume of drinking. Particularly for intentional injuries, cultural differences in the meaning of drinking are also involved [223]. For example, Rossow [224] found a considerably greater increase in homicide per litre of alcohol per capita consumption in Nordic countries compared to countries in Southern Europe.
To include this important effect of patterns of drinking (see also [31]), we will base the estimates of AAFs for injury categories on the number of heavy drinking occasions as estimated by survey, and the RR associated with each heavy drinking occasion. We have recently developed the methodology for estimating such AAFs [225;226]; however, this methodology only accounts for injury effects of drinking on the drinkers themselves. Further adjustments still need to be developed to estimate the overall effect of drinking on injury, including for example, the effect of drunk driving on innocent bystanders who did not consume alcohol.
QUALITY OF ALCOHOL
There are not many reviews about the effects of quality of alcohol, and the available reviews are all linked to unrecorded alcohol [12;227-229] for a definition of unrecorded see [229;230]. Despite evidence of methanol poisoning outbreaks and some problems with potentially harmful levels of other ingredients such as acetaldehyde [231], ethyl carbamate [232] and coumarin [230], overall there is no evidence of a large impact of unrecorded consumption over and above the effects of alcohol, per se [11;229]. However, even though research activity has increased in recent years, mainly in countries of the ex-Soviet Union and in Africa, there are still too few systematic large-scale investigations of quality of alcohol in different parts of the world to conclude definitively that quality of alcohol does or does not have a significant impact on health.
DISCUSSION
The present analyses are consistent with previous conclusions that the average volume of alcohol consumption is associated with increased risk for many disease outcomes. They also confirm the protective role of light to moderate drinking with certain diseases, and clarify that this protection only occurs if the pattern of average light-moderate drinking does not include episodes of heavy drinking. In addition, heavy drinking was found to add additional risk to that of average volume for certain disease and injury categories. While these findings provide a strong basis for establishing the importance of alcohol consumption for public health, there are a number of methodological issues and research gaps that prevent more precise estimates and a greater understanding of the relationship between alcohol consumption and disease/injury. In particular, future research needs to address the role of drinking pattern, use better measures of exposure and outcome, use stronger research designs and more representative samples.
First, it is likely that additional disease outcomes are influenced by a drinking pattern that includes episodes of heavy drinking. However, there is still relatively little focus on drinking patterns in medical epidemiological research to date, and many potential links have not yet been explored. For many chronic disease outcomes, no research has been done yet to examine the impact of other dimensions of alcohol consumption besides average volume. In addition, although there are now studies in which drinking patterns were explored, no standardised measure of drinking pattern has been adopted, thus making comparisons and pooling of studies difficult [31].
Second, in addition to a bias in the literature that does not allow a clear separation of the effects of drinking pattern from those of overall consumption, there are other measurement problems in alcohol epidemiology that affect the interpretation of findings. Typically, cohort studies have measured alcohol consumption at just one point or period in the respondent’s life. In particular, even though it has been argued and demonstrated convincingly that separating ex-drinkers from lifetime abstainers or very light drinkers is essential for identifying both beneficial and detrimental effects [134], many studies in medical epidemiology continue to fail to make this crucial distinction. Furthermore, aside from not being able to capture variability in consumption (see above), many of the largest cohorts used a single-item semi-quantitative food frequency questionnaire, which depicts either frequency or volume of consumption depending on the answer category. These and other problems have long been recognized [233-235], but are still overlooked in much of the relevant research. In addition, measurement of alcohol consumption is not always in line with theoretical assumptions. For example, studies postulating accumulated volume as causally relevant for the disease outcome assess average volume of alcohol at baseline only and do not include measures of alcohol consumption during the theoretically relevant time period (e.g. cumulated alcohol intake in the 6 years before the outcome as a relevant period for some cancers). Similarly, studies postulating blood alcohol concentration (BAC) as a causally relevant measure often measured usual alcohol consumption but not quantity of alcohol consumed at the specific occasion of incidence.
On the outcome side, outcomes such as chronic disease endpoints are measured poorly - often only by self-report - and there is often a lack of control for relevant non-substance use related confounders in many studies in alcohol epidemiology specifically (as distinct from general medical epidemiology). Clearly, alcohol epidemiology needs to improve dramatically in outcome measurement in order to obtain better estimates of the relationships between alcohol consumption and disease risks.
The effects of these problems for the final estimates of alcohol-attributable burden are not clear. Analyses for some European countries have shown that the volume indicator had about twice as much variance explained as the patterns indicator, independent of each other [236]. This is likely an underestimate, but we will only know the real numbers when there are studies combining better exposure measures of alcohol epidemiology with state-of-the-art outcome measures. Determining the relative importance of volume and patterns of drinking is not only crucial for understanding the aetiology of disease and injury, but also for tailoring prevention measures [237].
Third, a greater understanding of the relationship between alcohol consumption and adverse outcomes is hampered by the use of weak study designs. In particular, most of the underlying evidence, even if exposure and outcomes are measured properly, is based on non-experimental studies (e.g. case-control or cohort studies). There are limitations to such study designs, as is clearly indicated by the recent failures to confirm the protective effect of various antioxidants on mortality which had been found in observational studies. In contrast to the observational studies the intervention trials with placebo control groups showed no protective effect, and even some indication of a detrimental effect [238]. This is a limitation that is unlikely to be overcome, however, because long-term intervention trials with alcohol are problematic for a number of reasons, and long-term placebo control groups are impossible. Thus, plausible mechanisms from experimental and clinical studies remain an essential aspect of interpreting epidemiologic findings and such studies warrant further development. As has been argued before [46], one way to settle the question concerning a true causal impact of alcohol would be to conduct trials in the tradition of Kalichman and colleagues [239;240] with populations with a high risk of HIV infection, such as clients in clinics for sexually transmitted disease in countries with high HIV prevalence, and see if proven effective interventions for problem drinking do in fact lower the infection rate.
Fourth, the sampling used in many cohort studies is problematic for studying alcohol. Many cohort studies are restricted to established market economies, and cohorts are typically selected for ease of follow-up and the resulting high retention rate. As a result, many samples used for cohort studies are comprised of nurses, medical doctors, other health professionals, members of the American Cancer Society, or other middle to upper middle class professionals. While such cohorts may have enough variation in average volume of drinking for analyses, they are unlikely to have enough variation in terms of patterns of drinking and potential moderating lifestyle factors.
Finally, we would like to comment on the new systematic reviews and meta-analyses done by our group that are referenced in this paper. We believe that the current effort has been the most comprehensive and systematic to date to examine the relationships between alcohol consumption and disease/injury. Although we have systematically controlled for quality of the analyses by conducting inter-rater studies on random sub-samples, there will be errors. However, all the meta-analyses will be published in peer-reviewed journals including full specification of included articles for each disease/injury condition, the full methodology and the statistical model, so that all steps can be criticised and improved upon via the usual scientific procedures of replication and additional analyses.
In summary, there are limitations to our current ability to estimate the disease burden of alcohol. The CRA can only try to develop the best possible estimates given the current state of knowledge. It is likely that new confounders will be found for some of the relationships described above, and, as new evidence emerges, there will be additions to and deletions from the list of alcohol-related disease and injury categories. Nonetheless, these limitations cannot change the overall conclusion that alcohol is related to many disease outcomes, both chronic and acute, and causes a considerable part of the global burden of disease [66].
Acknowledgments
The authors are grateful to the journal Addiction for providing an opportunity to update an earlier article (Rehm, J., Room, R., Graham, K., Monteiro, M., Gmel, G., & Sempos, C.T. (2003). The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease - An overview. Addiction, 98(10), 1209-1228). NIAAA (contract # HHSN267200700041C “Alcohol- and Drug-Attributable Burden of Disease and Injury in the US” to the first author), the Global Burden of Disease and Injury 2005 Project, and the Centre for Addiction and Mental Health in Toronto, Canada provided financial and/or technical support for this study. In addition, support to CAMH for salary of scientists and infrastructure has been provided by the Ontario Ministry of Health and Long Term Care. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIAAA or NIH or those of the Ministry of Health and Long Term Care.
We would like to thank G. Gmel, B. Grant, R. Norman, P. Shuper and T. Vos for helpful comments on earlier versions of this text and for advising on the systematic reviews.
List of Abbreviations
- AAF
alcohol-attributable fraction
- AIDS
Acquired Immune Deficiency Syndrome
- AUD
alcohol use disorders
- BAC
blood alcohol concentration
- CAP
community acquired pneumonia
- CI
confidence interval
- CRA
Comparative Risk Assessment
- FAS
foetal alcohol syndrome
- GBD
Global Burden of Disease and Injury
- HDL
high density lipoproteins
- HIV
Human Immunodeficiency Virus
- IARC
International Agency for Research on Cancer
- ICD
International Classification of Diseases
- ICH
intracerebral haemorrhage
- IGF
insulin-like growth factor
- IHD
ischaemic heart disease
- LDL
low density lipoproteins
- MI
myocardial infarction
- RR
relative risk
- SAH
subarachnoid haemorrhage
- TB
tuberculosis
Footnotes
Conflict of interest: none
References
- 1.Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos C. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease - an overview. Addiction. 2003;98:1209–28. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD, Black R, Mathers CD, Shibuya K, Ezzati M, et al. Global burden of disease 2005: call for collaborators. Lancet. 2007;370:109–10. doi: 10.1016/S0140-6736(07)61064-2. [DOI] [PubMed] [Google Scholar]
- 3.Bruun K, Edwards G, Lumio M, Majeka K, Pan L, Popham RE, et al. Alcohol control policies in public health perspective. Helsinki, Finland: Finnish Foundation for Alcohol Studies; 1975. [Google Scholar]
- 4.English D, Holman C, Milne E, Winter M, Hulse G, Codde G, et al. The quantification of drug caused morbidity and mortality in Australia 1995. Canberra, Australia: Commonwealth Department of Human Services and Health; 1995. [Google Scholar]
- 5.WHO. International classification of diseases and related health problems, 10th revision (version for 2007) Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 6.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3. PA, USA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 7.Parascandola M, Weed DL. Causation in epidemiology. J Epidemiol Community Health. 2001;55:905–12. doi: 10.1136/jech.55.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter SD. Prevention of multifactorial disease. Am J Epidemiol. 1980;112:409–16. doi: 10.1093/oxfordjournals.aje.a113007. [DOI] [PubMed] [Google Scholar]
- 9.Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, et al. Alcohol Use. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Vol. 1. Geneva: WHO; 2004. pp. 959–1109. [Google Scholar]
- 10.Rehm J, Rehn N, Room R, Monteiro M, Gmel G, Jernigan D, et al. The global distribution of average volume of alcohol consumption and patterns of drinking. Eur Addict Res. 2003;9:147–56. doi: 10.1159/000072221. [DOI] [PubMed] [Google Scholar]
- 11.Lachenmeier DW, Rehm J. Unrecorded alcohol: a threat to public health? Addiction. 2009;104:875–7. doi: 10.1111/j.1360-0443.2009.02587.x. [DOI] [PubMed] [Google Scholar]
- 12.Lachenmeier DW, Rehm J, Gmel G. Surrogate alcohol: what do we know and where do we go? Alcohol Clin Exp Res. 2007;31:1613–24. doi: 10.1111/j.1530-0277.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- 13.Ezzati M, Lopez AD, Rodgers AD, Vander Horn S, Murray CJL Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55:508–14. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 16.Single E, Robson L, Xie X, Rehm J, Moore R, Choi B, et al. The costs of substance abuse in Canada. Ottawa: Canadian Centre on Substance Abuse; 1996. [Google Scholar]
- 17.Single E, Robson L, Rehm J, Xie X. Morbidity and mortality attributable to alcohol, tobacco, and illicit drug use in Canada. Am J Public Health. 1999;89:385–90. doi: 10.2105/ajph.89.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjögren H, Eriksson A, Brostrom G, Ahlm K. Quantification of alcohol-related mortality in Sweden. Alcohol Alcohol. 2000;35:601–11. doi: 10.1093/alcalc/35.6.601. [DOI] [PubMed] [Google Scholar]
- 19.Gutjahr E, Gmel G, Rehm J. The relation between average alcohol consumption and disease: an overview. Eur Addict Res. 2001;7:117–27. doi: 10.1159/000050729. [DOI] [PubMed] [Google Scholar]
- 20.Ridolfo B, Stevenson C. The quantification of drug-caused mortality and morbidity in Australia 1998. Canberra: Australian Institute of Health and Welfare; 2001. [Google Scholar]
- 21.Gmel G, Rehm J. Harmful alcohol use. Alcohol Res Health. 2003;27:52–62. [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 24.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval SJ, Tweedie RL. Trim and fill: A simple funnel plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:276–84. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 26.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 27.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modeling. Applied Statistics. 1994;43:429–67. [Google Scholar]
- 28.Rehm J, Patra J, Popova S. Alcohol-attributable mortality and potential years of life lost in Canada 2001: Implications for prevention and policy. Addiction. 2006;101:373–84. doi: 10.1111/j.1360-0443.2005.01338.x. [DOI] [PubMed] [Google Scholar]
- 29.Zaridze D, Maximovitch D, Lazarev A, Igitov V, Boroda A, Boreham J, et al. Alcohol poisoning is a main determinant of recent mortality trends in Russia: evidence from a detailed analysis of mortality statistics and autopsies. Int J Epidemiol. 2009;38:143–53. doi: 10.1093/ije/dyn160. [DOI] [PubMed] [Google Scholar]
- 30.Rehm J. Commentary: alcohol poisoning in Russia: implications for monitoring and comparative risk factor assessment. Int J Epidemiol. 2009;38:154–5. doi: 10.1093/ije/dyn209. [DOI] [PubMed] [Google Scholar]
- 31.Gmel G, Kuntsche E, Rehm J. Risky single occasion drinking: a binge is not a binge is not a binge. Addiction. doi: 10.1111/j.1360-0443.2010.03167.x. In press. [DOI] [PubMed] [Google Scholar]
- 32.Parry CDH, Rehm JR, Poznyak V, Room R. Alcohol and infectious diseases: are there causal linkages? Addiction. 2009;104:331–2. doi: 10.1111/j.1360-0443.2008.02500.x. [DOI] [PubMed] [Google Scholar]
- 33.Rush B. An inquiry into the effects of ardent spirits upon the human body and mind: With an account of the means of preventing, and of the remedies for curing them. 8. Exeter, N.H: Richardson; 1785. Reprint. [Google Scholar]
- 34.Lönnroth K, Williams B, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rieder HL. Epidemiologic basis of tuberculosis control. Paris: International Union Against Tuberculosis and Lung Disease; 1999. [Google Scholar]
- 36.Szabo G. Alcohol’s contribution to compromised immunity. Alcohol Health Res World. 1997;21:30–41. [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman H, Pros S, Klein TW. Addictive drugs and their relationships with infectious diseases. FEMS. 2006;47:330–42. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 38.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–32. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry CD, Lönnroth K, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. doi: 10.1186/1471-2458-9-450. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakubowiak WM, Bogorodskaya EM, Borisov ES, Danilova DI, Kourbatova EK. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]
- 41.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto S, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–7. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 42.Neuman MG, Monteiro M, Rehm J. Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Substance Use Misuse. 2006;41:1395–463. doi: 10.1080/10826080600846235. [DOI] [PubMed] [Google Scholar]
- 43.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34:856–63. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 44.Baliunas D, Rehm J, Irving H. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Toronto, Canada: Centre for Addiction and Mental Health; 2008. Report to be published in International Journal of Public Health. [DOI] [PubMed] [Google Scholar]
- 45.Shuper PA, Joharchi N, Irving H, Rehm J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: review and meta-analysis. AIDS Behav. 2009 doi: 10.1007/s10461-009-9589-z. Epub ahead of print July 18. [DOI] [PubMed] [Google Scholar]
- 46.Rehm J, Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N. Causal considerations on alcohol and HIV/AIDS - a systematic review. Toronto, ON: CAMH; 2009. [DOI] [PubMed] [Google Scholar]
- 47.Halpern-Felsher BL, Millstein SG, Ellen JM. Relationship of alcohol use and risky sexual behavior: a review and analysis of findings. J Adolesc Health. 1996;19:331–6. doi: 10.1016/S1054-139X(96)00024-9. [DOI] [PubMed] [Google Scholar]
- 48.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8:141–51. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- 49.Leigh BC, Stall R. Substance use and risky sexual behavior for exposure to HIV. Issues in methodology, interpretation, and prevention. Am Psychol. 1993;48:1035–45. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leigh BC. Alcohol and condom use: a meta-analysis of event-level studies. Sex Transm Dis. 2002;29:476–82. doi: 10.1097/00007435-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Weinhardt L, Carey M. Does alcohol lead to sexual risk behavior? Findings from event-level research. Annu Rev Sex Res. 2000;11:125–57. [PMC free article] [PubMed] [Google Scholar]
- 52.Woolf SE, Maisto SA. Alcohol use and risk of HIV infection among men who have sex with men. AIDS Behav. 2009;13:757–82. doi: 10.1007/s10461-007-9354-0. [DOI] [PubMed] [Google Scholar]
- 53.Kalichman SC, Rompa D. Sexual sensation seeking and sexual compulsivity scales reliability, validity, and predicting HIV risk behavior. J Pers Assess. 1995;65:586–601. doi: 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- 54.Kalichman SC, Heckman T, Kelly JA. Sensation seeking, substance use, and HIV-AIDS risk behavior: directional relationships among gay men. Arch Sex Behav. 1996;25:141–54. doi: 10.1007/BF02437933. [DOI] [PubMed] [Google Scholar]
- 55.Zuckerman M. Biological expression and biological bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- 56.Golding M, Perkins DO. Personality disorder in HIV infection. International Review of Psychiatry. 1996;8:253–8. [Google Scholar]
- 57.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181b18b6e. epub ahead of print Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rehm J, Anderson P, Kanteres F, Parry CD, Samokhvalov AV, Patra J. Alcohol, social development and infectious disease. Toronto, ON: Centre for Addiction and Mental Health; 2009. [Google Scholar]
- 59.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–3. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 60.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 61.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–47. [PMC free article] [PubMed] [Google Scholar]
- 62.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Reviews Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 63.Lachenmeier DW, Sohnius EM. The role of acetaldehyde outside ethanol metabolism in the carcinogenicity of alcoholic beverages: evidence from a large chemical survey. Food Chem Toxicol. 2008;46:2903–11. doi: 10.1016/j.fct.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Lachenmeier DW, Kanteres F, Rehm J. Carcinogenicity of acetaldehyde in alcoholic beverages: risk assessment outside ethanol metabolism. Addiction. 2009;104:533–50. doi: 10.1111/j.1360-0443.2009.02516.x. [DOI] [PubMed] [Google Scholar]
- 65.Salaspuro M. Acetaldehyde: a cumulative carcinogen in humans. Addiction. 2009;104:551–3. doi: 10.1111/j.1360-0443.2009.02546.x. [DOI] [PubMed] [Google Scholar]
- 66.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 67.Cho E, Smith-Warner S, Ritz J, van den Brandt P, Colditz G, Folsom A. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–13. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 68.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–9. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 69.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a doseresponse meta-analysis of published cohort studies. Int J Cancer. 2007;120:664–71. doi: 10.1002/ijc.22299. [DOI] [PubMed] [Google Scholar]
- 70.Singletary K, Gapstur S. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–51. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 71.Hamajima N, Hirose K, Tajima K, Rohan T, Calle E, Heath C, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Key J, Hodgson S, Omar RZ, Jensen TK, Thomson SG, Boobis AR, et al. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17:759–70. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 73.Singletary SE. Rating the risk factors for breast cancer. Annals of Surgery. 2003;237:474–82. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: re-analysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 75.Yu H, Berkel H. Do insulin-like growth factors mediate the effect of alcohol on breast cancer risk? Medical Hypotheses. 1999;52:491–6. doi: 10.1054/mehy.1998.0828. [DOI] [PubMed] [Google Scholar]
- 76.Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108:9S–14S. doi: 10.1016/s0002-9343(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 77.Hendriks HFJ. Moderate alcohol consumption and insulin sensitivity: observations and possible mechanisms. Ann Epidemiol. 2007;17:S40–S42. [Google Scholar]
- 78.Mayer EJ, Newman B, Quesenberry CP, Jr, Friedman GD, Selby JV. Alcohol consumption and insulin concentrations. Role of insulin in associations of alcohol intake with high-density lipoprotein cholestrol and triglycerides. Circulation. 1993;88:2190–7. doi: 10.1161/01.cir.88.5.2190. [DOI] [PubMed] [Google Scholar]
- 79.Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, et al. Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study) BMJ. 1996;313:1040–4. doi: 10.1136/bmj.313.7064.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazarus R, Sparrow D, Weiss ST. Alcohol intake and insulin levels. The Normative Aging Study. Am J Epidemiol. 1997;145:909–16. doi: 10.1093/oxfordjournals.aje.a009050. [DOI] [PubMed] [Google Scholar]
- 81.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287:2559–62. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 82.Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, et al. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care. 2004;27:184–9. doi: 10.2337/diacare.27.1.184. [DOI] [PubMed] [Google Scholar]
- 83.Sarkola T, Iles MR, Kohlenberg-Mueller K, Eriksson CJ. Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: effect of 4-methylpyrazole. Alcohol Clin Exp Res. 2002;26:239–45. [PubMed] [Google Scholar]
- 84.Rimm E, Williams P, Fosher K, Criqui M, Stampfer M. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;19:1523–8. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr. 2003;77:1312–7. doi: 10.1093/ajcn/77.5.1312. [DOI] [PubMed] [Google Scholar]
- 86.Wannamethee SG, Camargo CA, jr, Manson JE, Willett WC, Rimm EB. Alcohol drinking patterns and risk of type 2 diabetes mellitus among younger women. Arch Intern Med. 2003;163:1329–36. doi: 10.1001/archinte.163.11.1329. [DOI] [PubMed] [Google Scholar]
- 87.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–7. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 88.Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels: a randomized, diet-controlled intervention study. Eur J Clin Nutr. 2002;56:1130–6. doi: 10.1038/sj.ejcn.1601459. [DOI] [PubMed] [Google Scholar]
- 89.Hillbom M, Pieninkeronen I, Leone M. Seizures in alcohol-dependent patients. CNS Drugs. 2003;17:1013–30. doi: 10.2165/00023210-200317140-00002. [DOI] [PubMed] [Google Scholar]
- 90.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 91.Ogunniyi A, Osuntokun BO, Bademosi O, Adeuja AOG, Schoenberg BS. Risk factors for epilepsy: case-control study in Nigerians. Epilepsia. 1987;28:280–5. doi: 10.1111/j.1528-1157.1987.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 92.Ng SKC, Hauser AW, Brust JCM, Susser M. Alcohol consumption and withdrawal in new-onset seizures. N Engl J Med. 1988;319:666–73. doi: 10.1056/NEJM198809153191102. [DOI] [PubMed] [Google Scholar]
- 93.Leone M, Bottacchi E, Gionco M, Nardozza V, Sironi L. Alcohol and epileptic seizures: a case control study. Alcologia. 1994;6:215–9. [Google Scholar]
- 94.Leone M, Bottachi E, Beghi E, Morgando E, Mutani R, Amedeo G, et al. Alcohol use is a risk factor for a first generalized tonic-clonic seizure. Neurology. 1997;48:614–20. doi: 10.1212/wnl.48.3.614. [DOI] [PubMed] [Google Scholar]
- 95.Leone M, Bottacchi E, Beghi E, Morgando E, Mutani R, Cremo R, et al. Risk factors for a first generalized tonic-clonic seizure in adult life. Neurol Sci. 2002;23:99–106. doi: 10.1007/s100720200034. [DOI] [PubMed] [Google Scholar]
- 96.Zeng J, Zhen H, Mao-Sheng H, Mei-Hua J. A case-control study on the risk factors and other socio-psychological factors of epilepsy. Chin J Epidemiol. 2003;24:116–8. [PubMed] [Google Scholar]
- 97.Samokhvalov AV, Irving H, Mohapatra S, Rehm J. Alcohol consumption, unprovoked seizures and epilepsy: a systematic review and meta-analysis. Epilepsia. doi: 10.1111/j.1528-1167.2009.02426.x. In press. [DOI] [PubMed] [Google Scholar]
- 98.Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- 99.Dam AM, Fuglsang-Frederikse A, Svarre-Olsen U, Dam M. Late-onset epilepsy: etiologies, types of seizure, and value of clinical investigation, EEG, and computerized tomography scan. Epilepsia. 1985;26:227–31. doi: 10.1111/j.1528-1157.1985.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 100.Freedland ES, McMicken DB. Alcohol-related seizures, part I: pathophysiology, differencial diagnosis and evaluation. J Emerg Med. 1993;11:463–73. doi: 10.1016/0736-4679(93)90251-2. [DOI] [PubMed] [Google Scholar]
- 101.Rathlev NK, Ulrich AS, Delanty N, D’Onofrio D, D’Onofrio G. Alcohol-related seizures. J Emerg Med. 2006;31:157–63. doi: 10.1016/j.jemermed.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 102.Barclay GA, Barvour J, Stewart S, Day CP, Gilvarry E. Adverse physical effects of alcohol misuse. Advances in Psychiatric Treatment. 2008;14:139–51. [Google Scholar]
- 103.Beilin LJ, Puddey IB. Alcohol and hypertension. Clin Exp Hypertens A. 1992;14:119–38. doi: 10.3109/10641969209036176. [DOI] [PubMed] [Google Scholar]
- 104.Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacological Research. 2007;55:237–472. doi: 10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 105.Randin D, Vollenweider P, Tappy L, Jequier E, Nicod P, Scherrer U. Suppression of alcohol-induced hypertension by dexamethasone. N Engl J Med. 1995;332:1733–7. doi: 10.1056/NEJM199506293322601. [DOI] [PubMed] [Google Scholar]
- 106.el-Mas MM, Abdel-Rahman AA. Direct evidence for selective involvement of aortic baroreceptors in ethanol-induced impairment of baroreflex control of heart rate. J Pharmacol Exp Ther. 1993;264:1198–205. [PubMed] [Google Scholar]
- 107.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–23. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 109.Criqui M. Alcohol and the heart: Implications of present epidemiologic knowledge. Contemp Drug Probl. 1994;21:125–42. [Google Scholar]
- 110.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 111.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Prior alcohol consumption and mortality following acute myocardial infarction. JAMA. 2001;285:1965–70. doi: 10.1001/jama.285.15.1965. [DOI] [PubMed] [Google Scholar]
- 112.Tanasescu M, Hu FB, Willett WC, Stampfer MJ, Rimm EB. Alcohol consumption and risk of coronary heart disease among men with type 2 diabetes mellitus. J Am Coll Cardiol. 2001;38:1836–42. doi: 10.1016/s0735-1097(01)01655-2. [DOI] [PubMed] [Google Scholar]
- 113.Koppes LLJ, Dekker JM, Hendricks HF, Bouter LM, Heine RJ. Meta-analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49:648–52. doi: 10.1007/s00125-005-0127-x. [DOI] [PubMed] [Google Scholar]
- 114.Beulens JW, Rimm EB, Stampfer MJ, Ascherio A, Hendriks HF, Mukamal KJ. Alcohol consumption and risk of coronary heart disease in men with hypertension. Ann Intern Med. 2007;146:10–9. doi: 10.7326/0003-4819-146-1-200701020-00004. [DOI] [PubMed] [Google Scholar]
- 115.McKee M, Britton A. The positive relationship between alcohol and heart disease in Eastern Europe: potential physiological mechanisms. J R Soc Med. 1998;91:402–7. doi: 10.1177/014107689809100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Puddey IB, Rakic V, Dimmitt SB, Beilin LJ. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors - A review. Addiction. 1999;94:649–63. doi: 10.1046/j.1360-0443.1999.9456493.x. [DOI] [PubMed] [Google Scholar]
- 117.Rehm J, Sempos C, Trevisan M. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease - a review. J Cardiovasc Risk. 2003;10:15–20. doi: 10.1097/01.hjr.0000051961.68260.30. [DOI] [PubMed] [Google Scholar]
- 118.Bagnardi V, Zatonski W, Scotti L, La Vecchia C, Corrao G. Does drinking pattern modify the effect of alcohol on the risk of coronary heart disease? Evidence from a meta-analysis. J Epidemiol Community Health. 2008;62:615–9. doi: 10.1136/jech.2007.065607. [DOI] [PubMed] [Google Scholar]
- 119.Zakhari S. Alcohol and the cardiovascular system: molecular mechanisms for beneficial and harmful action. Alcohol Health Res World. 1997;21:21–9. [PMC free article] [PubMed] [Google Scholar]
- 120.Renaud SC, Criqui MH, Farchi G, Veenstra J. Alcohol drinking and coronary heart disease. In: Verschuren PM, editor. Health issues related to alcohol consumption. Washington, DC: International Life Sciences Institute; 1993. [Google Scholar]
- 121.Rankin J. Biological mechanisms at moderate levels of alcohol consumption that may affect coronary heart disease. Contemp Drug Probl. 1994;21:45–57. [Google Scholar]
- 122.Svärdsudd K. Moderate alcohol consumption and cardiovascular disease: is there evidence for a preventive effect? Alcohol Clin Exp Res. 1998;22:307S–14S. doi: 10.1097/00000374-199807001-00005. [DOI] [PubMed] [Google Scholar]
- 123.Mukamal K, Rimm E. Alcohol’s effects on the risk for coronary heart disease. Alcohol Res Health. 2001;25:255–61. [PMC free article] [PubMed] [Google Scholar]
- 124.Baraona E, Lieber C. Alcohol and lipids. In: Galanter M, editor. Recent developments in alcoholism, vol 14: The consequences of alcoholism. New York: Plenum Press; 1998. pp. 97–134. [DOI] [PubMed] [Google Scholar]
- 125.Criqui MH, Cowan LD, Tyroler JA, Bangdiwala S, Heiss G, Wallace RB, et al. Lipoprotein medicators for the effects of alcohol consumption and cigarette smoking on cardiovascular mortality. Results from the Lipid Research Clinics follow-up study. Am J Epidemiol. 1987;126:629–37. doi: 10.1093/oxfordjournals.aje.a114702. [DOI] [PubMed] [Google Scholar]
- 126.Criqui M, Ringel B. Does diet or alcohol explain the French paradox? The Lancet. 1994;344:1719–23. doi: 10.1016/s0140-6736(94)92883-5. [DOI] [PubMed] [Google Scholar]
- 127.Hines LM, Stampfer MJ, Ma J, Gaziano JM, Ridker PM, Hankinson SE, et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Engl J Med. 2001;344:549–55. doi: 10.1056/NEJM200102223440802. [DOI] [PubMed] [Google Scholar]
- 128.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system - research challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–24. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 129.Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, et al. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–19. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suh I, Shaten BJ, Cutler JA, Kuller LH. Alcohol use and mortality from coronary heart disease: the role of high-density lipoprotein cholesterol. The Multiple Risk Factor Intervention Trial Research Group. Ann Intern Med. 1992;116:881–7. doi: 10.7326/0003-4819-116-11-881. [DOI] [PubMed] [Google Scholar]
- 131.McKenzie C, Eisenberg P. Alcohol, coagulation, and arterial thrombosis. In: Zakhari S, Wassef M, editors. Alcohol and the cardiovascular system. Bethesda, MD: National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1996. pp. 413–439. [Google Scholar]
- 132.Reeder V, Aikens M, Li X, Booyse F. Alcohol and the fibrinolytic system. In: Zakhari S, Wassef M, editors. Alcohol and the cardiovascular system. Bethesda, MD: National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1996. pp. 391–411. [Google Scholar]
- 133.Fillmore KM, Kerr WC, Stockwell T, Chikritzhs T, Bostrom A. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies. Addict Res Theory. 2006;14:101–32. doi: 10.1016/j.annepidem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 134.Shaper A, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2:1267–73. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 135.Rimm EB, Moats C. Alcohol and coronary heart disease: drinking patterns and mediators of effect. Ann Epidemiol. 2007;17:S3–S7. [Google Scholar]
- 136.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers, and nondrinkers. Am J Cardiol. 1990;66:1237–42. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 137.Mukamal K, Conigrave K, Mittleman M, Camargo C, Stampfer M, Willett W, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 138.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008;168:866–71. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rehm J. On the limitations of observational studies. Addict Res Theory. 2007;15:20–2. [Google Scholar]
- 140.Poikolainen K. Inebriation and mortality. Int J Epidemiol. 1983;12:151–5. doi: 10.1093/ije/12.2.151. [DOI] [PubMed] [Google Scholar]
- 141.McElduff P, Dobson A. How much alcohol and how often? Population based case-control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314:1159–64. doi: 10.1136/bmj.314.7088.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Trevisan M, Ram M, Hovey K, Russel M, Freudenheim J, Muti P, et al. Alcohol drinking patterns and myocardial infarction. Am J Epidemiol. 2001;153:S97. [Google Scholar]
- 143.Trevisan M, Schisterman E, Mennotti A, Farchi G, Conti S. Drinking pattern and mortality: The Italian risk factor and life expectancy pooling project. Ann Epidemiol. 2001;11:312–9. doi: 10.1016/s1047-2797(00)00183-6. [DOI] [PubMed] [Google Scholar]
- 144.Murray R, Connett J, Tyas S, Bond R, Ekuma O, Silversides C, et al. Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: is there a U-shaped function? Am J Epidemiol. 2002;155:242–8. doi: 10.1093/aje/155.3.242. [DOI] [PubMed] [Google Scholar]
- 145.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Review. Alcohol Alcohol. 2002;37:409–15. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]
- 146.Balbão CEB, de Paola AAV, Fenelon G. Effects of alcohol on atrial fibrillation. Ther Adv Cardiovasc Dis. 2009;3:53–63. doi: 10.1177/1753944708096380. [DOI] [PubMed] [Google Scholar]
- 147.Lowenstein SR, Gbow P, Cramer J, Olivia PB, Ratner K. The role of alcohol in new-onset atrial fibrillation. Arch Intern Med. 1983;143:1882–5. [PubMed] [Google Scholar]
- 148.Rich EC, Siebold C, Campion B. Alcohol-related acute atrial fibrillation, a case-control study and review of 40 patients. Arch Intern Med. 1985;145:830–3. doi: 10.1001/archinte.145.5.830. [DOI] [PubMed] [Google Scholar]
- 149.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of ‘not-so-lone atrial fibrillation’. Europace. 2008;10:668–73. doi: 10.1093/europace/eun124. [DOI] [PubMed] [Google Scholar]
- 150.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 151.Krahn AD, Manfreda J, Tate RB, Mathewson FAL, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 152.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382–9. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 153.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women - the Copenhagen City Heart Study. Circulation. 2005;112:1736–42. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 154.Planas F, Romero-Menor C, Vasquez-Oliva G, Poblet T, Navarro-López F. Historia natural y factores de riesgo de recurrecnia de la fibrilación auricular primaria (Registro FAP) Revista Española de Cardiología. 2006;59:1106–12. [PubMed] [Google Scholar]
- 155.Reynolds K, Lewis B, Nolen J, Kinney G, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–88. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- 156.Sundell L, Salomaa V, Vartiainen E, Poikolainen K, Laatikainen T. Increased stroke risk is related to a binge drinking habit. Stroke. 2008;39:3179–84. doi: 10.1161/STROKEAHA.108.520817. [DOI] [PubMed] [Google Scholar]
- 157.Schütz H, Bödeker RH, Damian M, Krack P, Dorndorf W. Age-related spontaneous intracerebral hematoma in a German community. Stroke. 1990;21:1412–8. doi: 10.1161/01.str.21.10.1412. [DOI] [PubMed] [Google Scholar]
- 158.Dulli DA, Geraghty MC. Alcohol and stroke. In: Preedy VR, Watson RR, editors. Comprehensive handbook of alcohol related pathology. Vol. 2. London, UK: Elsevier Academic Press; 2005. pp. 627–646. [Google Scholar]
- 159.Rehm J, Taylor B. Alcohol consumption, stroke and public health - an overlooked relation? Addiction. 2006;101:1679–81. doi: 10.1111/j.1360-0443.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 160.Feigin VL, Rinkel GJE, Lawes CMM, Algra A, Bennett DA, Gijn JV, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–80. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 161.Koivula IH. Epidemiology of community-acquired pneumonia. In: Marrie TJ, editor. Community-acquired pneumonia. New York: Kluwer Academic Publishers; 2001. pp. 13–27. [Google Scholar]
- 162.Nelson S, Kolls JK. Alcohol, host defence and society. Nature Reviews Immunology. 2002;2:205–9. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 163.Fernández-Solá J, Junqué A, Estruch R, Monforte R, Torres A, Urbano-Márquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–54. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 164.Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–20. doi: 10.1016/0002-9343(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 165.Zaridze D, Brennan P, Boreham J, Boroda A, Karpov R, Lazarev A, et al. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet. 2009;373:2201–14. doi: 10.1016/S0140-6736(09)61034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lieber CS. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. N Engl J Med. 1988;319:1639–50. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- 167.Bautista AP. Liver injury during alcohol use and withdrawal. In: Preedy VR, Watson RR, editors. Comprehensive handbook of alcohol related pathology. London, UK: Elsevier Academic Press; 2005. pp. 491–500. [Google Scholar]
- 168.Becker U. Epidemiology and risk factors in alcohol liver disease. In: Preedy VR, Watson RR, editors. Comprehensive handbook of alcohol related pathology. London, UK: Elsevier Academic Press; 2005. pp. 467–480. [Google Scholar]
- 169.Neuman M. Cytokines - central factors in alcoholic liver disease. Alcohol Res Health. 2003;27:307–16. [PMC free article] [PubMed] [Google Scholar]
- 170.Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a risk factor for liver cirrhosis - a systematic review and meta-analysis. Drug Alcohol Rev. doi: 10.1111/j.1465-3362.2009.00153.x. In press. [DOI] [PubMed] [Google Scholar]
- 171.Irving HM, Samokhvalov A, Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP. 2009;10:387–92. [PMC free article] [PubMed] [Google Scholar]
- 172.Lerch MM, Albrecht E, Ruthenburger M, Mayerle J, Halangk W, Kruger B. Pathophysiology of alcohol-induced pancreatitis. Pancreas. 2003;27:291–6. doi: 10.1097/00006676-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 173.Szabo G, Mandrekar P, Oak S, Mayerle J. Effect of ethanol on inflammatory responses: implications for pancreatitis. Pancreatology. 2007;7:115–23. doi: 10.1159/000104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vonlaufen A, Wilson JS, Pirola RC, Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health. 2007;30:48–54. [PMC free article] [PubMed] [Google Scholar]
- 175.Tattersall SJ, Apte MV, Wilson JS. A fire inside: current concepts in chronic pancreatitis. J Intern Med. 2008;38:592–8. doi: 10.1111/j.1445-5994.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 176.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 177.Bowcock AM. Understanding the pathogenesis of psoriasis, psoriatic arthritis, and autoimmunity via a fusion of molecular genetics and immunology. Immunol Res. 2005;32:45–56. doi: 10.1385/IR:32:1-3:045. [DOI] [PubMed] [Google Scholar]
- 178.Gottlieb SL, Gilleaudeau P, Johnson R, Estes L, Woodworth TG, Gottlieb AB, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442–7. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- 179.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–26. doi: 10.1067/mjd.2002.120568. [DOI] [PubMed] [Google Scholar]
- 180.Poikolainen K, Reunala T, Karvonen J, Lauharanta J, Karkkainen P. Alcohol intake: a risk factor for psoriasis in young and middle-aged men? BMJ. 1990;300:780–3. doi: 10.1136/bmj.300.6727.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Higgins EM, du Vivier AW. Cutaneous disease and alcohol misuse. Br Med Bull. 1994;50:85–98. doi: 10.1093/oxfordjournals.bmb.a072887. [DOI] [PubMed] [Google Scholar]
- 182.Smith KE, Fenske NA. Cutaneous manifestations of alcohol abuse. J Am Acad Dermatol. 2000;43:1–16. doi: 10.1067/mjd.2000.104512. [DOI] [PubMed] [Google Scholar]
- 183.Kostovic K, Lipozencic J. Skin diseases in alcoholics. Acta Dermatovenerol Croat. 2004;12:181–90. [PubMed] [Google Scholar]
- 184.Behnam SM, Behnam SE, Koo JY. Alcohol as a risk factor for plaque-type psoriasis. Cutis. 2005;76:181–5. [PubMed] [Google Scholar]
- 185.Schäfer T. Epidemiology of psoriasis. Review and the German perspective. Dermatology. 2006;212:327–37. doi: 10.1159/000092283. [DOI] [PubMed] [Google Scholar]
- 186.Dika E, Bardazzi F, Balestri R, Maibach HI. Environmental factors and psoriasis. Curr Probl Dermatol. 2007;35:118–35. doi: 10.1159/000106419. [DOI] [PubMed] [Google Scholar]
- 187.Meyer N, Viraben R, Paul C. Addictions and psoriasis: an example of the dermatologist’s implication in preventive medicine? Ann Dermatol Venereol. 2008;135:S259–S262. doi: 10.1016/S0151-9638(08)70545-3. [DOI] [PubMed] [Google Scholar]
- 188.Schäfer T, Nienhaus A, Haupt G, Vieluf D, Nagel S, Berger J, et al. Befunde der hautuntersuchung und allergologischen diagnostik. In: Behörde für Arbeit Gesundheit, Soziales der Freien, Hansestadt Hamburg., editors. Epidemiologisches Untersuchungsprogramm Bille-Siedlung. Frankfurt am Main: Peter Lang Verlag; 1997. pp. 158–194. [Google Scholar]
- 189.Naldi L. Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:121–8. doi: 10.2174/1568010043343958. [DOI] [PubMed] [Google Scholar]
- 190.Lindegard B. Diseases associated with psoriasis in a general population of 159 200 middle-aged, urban, native Swedes. Dermatologica. 1986;172:298–304. doi: 10.1159/000249365. [DOI] [PubMed] [Google Scholar]
- 191.Evstaff’ev VV, Levin MM. Dermatologic pathology in chronic alcoholics. Vestn Dermatol Venerol. 1989;8:72–4. [PubMed] [Google Scholar]
- 192.Higgins EM, Peters TJ, du Vivier AW. Smoking, drinking and psoriasis. Br J Dermatol. 1993;129:749–50. doi: 10.1111/j.1365-2133.1993.tb03349.x. [DOI] [PubMed] [Google Scholar]
- 193.Ginsburg IH, Link BG. Psychosocial consequences of rejection and stigma feelings in psoriatic patients. Int J Dermatol. 1993;32:587–91. doi: 10.1111/j.1365-4362.1993.tb05031.x. [DOI] [PubMed] [Google Scholar]
- 194.Kirby B, Richards HL, Manson DL, Fortune DG, Main CJ, Griffiths CEM. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138–40. doi: 10.1111/j.1365-2133.2007.08299.x. [DOI] [PubMed] [Google Scholar]
- 195.Vincenti GE, Blunden SM. Psoriasis and alcohol abuse. J R Army Med Corps. 1987;133:77–8. doi: 10.1136/jramc-133-02-02. [DOI] [PubMed] [Google Scholar]
- 196.Bolognia JL, Lorizzo JL, Rapini RP. Psoriasis: Dermatology. 2. St. Louis, MO: Mosby Elsevier; 2008. [Google Scholar]
- 197.Gupta MA, Schork NJ, Gupta AK, Ellis CN. Alcohol intake and treatment responsiveness of psoriasis: a prospective study. J Am Acad Dermatol. 1993;28:730–2. doi: 10.1016/0190-9622(93)70101-x. [DOI] [PubMed] [Google Scholar]
- 198.Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408–14. doi: 10.1001/archderm.140.4.408. [DOI] [PubMed] [Google Scholar]
- 199.Cohen AD, Halevy S. Alcohol intake, immune response, and the skin. Clin Dermatol. 1999;17:411–2. doi: 10.1016/s0738-081x(99)00025-5. [DOI] [PubMed] [Google Scholar]
- 200.Higgins EM, du Vivier AW. Alcohol abuse and treatment resistance in skin disease. J Am Acad Dermatol. 1994;30:1048. doi: 10.1016/s0190-9622(09)80167-9. [DOI] [PubMed] [Google Scholar]
- 201.Farkas A, Kemeny L, Szell M, Dobozy A, Bata-Csorgo Z. Ethanol and acetone stimulate the proliferation of HaCaT keratinocytes: the possible role of alcohol in exacerbating psoriasis. Arch Dermatol Res. 2003;295:56–62. doi: 10.1007/s00403-003-0399-2. [DOI] [PubMed] [Google Scholar]
- 202.Wolf R, Wolf D, Ruocco V. Alcohol intake and psoriasis. Clin Dermatol. 1999;17:423–30. doi: 10.1016/s0738-081x(99)00028-0. [DOI] [PubMed] [Google Scholar]
- 203.Albertsen K, Andersen AN, Olsen J, Gronbaek M. Alcohol consumption during pregnancy and the risk of preterm delivery. Am J Epidemiol. 2004;159:155–61. doi: 10.1093/aje/kwh034. [DOI] [PubMed] [Google Scholar]
- 204.Kesmodel U, Olsen SF, Secher NJ. Does alcohol increase the risk of preterm delivery? Epidemiol. 2000;11:512–8. doi: 10.1097/00001648-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 205.Larroque B, Kaminski M, Lelong N, Subtil D, Dehaene P. Effects on birth weight of alcohol and caffeine consumption during pregnancy. Am J Epidemiol. 1993;137:941–50. doi: 10.1093/oxfordjournals.aje.a116764. [DOI] [PubMed] [Google Scholar]
- 206.Lundsberg LS, Bracken MB, Saftlas AF. Low-to-moderate gestational alcohol use and intrauterine growth retardation, low birthweight, and preterm delivery. Ann Epidemiol. 1997;7:498–508. doi: 10.1016/s1047-2797(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 207.Parazzini F, Chatenoud L, Surace M, Tozzi L, Salerio B, Bettoni G, et al. Moderate alcohol drinking and risk of preterm birth. Eur J Clin Nutr. 2003;57:1345–9. doi: 10.1038/sj.ejcn.1601690. [DOI] [PubMed] [Google Scholar]
- 208.McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and prematurity. Am J Public Health. 1992;82:87–90. doi: 10.2105/ajph.82.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Little RE, Asker RL, Sampson PD, Renwick JH. Fetal growth and moderate drinking in early pregnancy. Am J Epidemiol. 1986;123:270–8. doi: 10.1093/oxfordjournals.aje.a114235. [DOI] [PubMed] [Google Scholar]
- 210.Cook JL, Randal CL. Ethanol and parturition: a role for prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 1998;58:135–42. doi: 10.1016/s0952-3278(98)90153-3. [DOI] [PubMed] [Google Scholar]
- 211.Chaud M, Faletti A, Beron de Estrada M, Gimeno AL, Gimeno MA. Synthesis and release of prostaglandins D2 and E2 by rat uterine tissue throughout the sex cycle. Effects of 17-beta-estradiol and progesterone. Prostaglandins Leukot Essent Fatty Acids. 1994;51:47–50. doi: 10.1016/0952-3278(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 212.Mitchell SN, Smith SK. The effect of progesterone and human interferon alpha-2 on the release of PGF2 alpha and PGE from epithelial cells of human proliferative endometrium. Prostaglandins. 1992;44:457–70. doi: 10.1016/0090-6980(92)90140-o. [DOI] [PubMed] [Google Scholar]
- 213.Pakrasi PL, Cheng HC, Dey SK. Prostaglandins in the uterus: modulation by steroid hormones. Prostaglandins. 1983;26:991–1009. doi: 10.1016/0090-6980(83)90160-0. [DOI] [PubMed] [Google Scholar]
- 214.Ahluwalia B, Smith D, Adeyiga O, Akbasak B, Rajguru S. Ethanol decreases progesterone synthesis in human placental cells: mechanism of ethanol effect. Alcohol. 1992;9:395–401. doi: 10.1016/0741-8329(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 215.Wimalasena J. Ethanol has direct effects on human choriocarcinoma cell steroid hormone secretion. Alcohol Clin Exp Res. 1994;18:369–74. doi: 10.1111/j.1530-0277.1994.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 216.Challis J, Olson D. Parturition. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 2177–2216. [Google Scholar]
- 217.Abel EL. Consumption of alcohol during pregnancy: a review of effects on growth and development of offspring. Human Biology. 1982;54:421–53. [PubMed] [Google Scholar]
- 218.Mueller J. The banality of “ethnic war”. Int Secur. 2000;25:42–70. [Google Scholar]
- 219.Moskalewicz J. Monopolization of the alcohol arena by the state. Contemp Drug Probl. 1985;12:117–28. [Google Scholar]
- 220.Gourevitch P. We wish to inform you that tomorrow we will be killed with our families. New York: Farrar, Straus, & Giroux; 1998. [Google Scholar]
- 221.Buford B. Among the thugs. London & New York: Secker & Warburg and W.W.Norton; 1991. [Google Scholar]
- 222.Corrao G, Bagnardi V, Zambon A, Arico S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction. 1999;94:1551–73. doi: 10.1046/j.1360-0443.1999.9410155111.x. [DOI] [PubMed] [Google Scholar]
- 223.Room R. Intoxication and bad behaviour: understanding cultural differences in the link. Soc Sci Med. 2001;53:189–98. doi: 10.1016/s0277-9536(00)00330-0. [DOI] [PubMed] [Google Scholar]
- 224.Rossow I. Alcohol and homicide: cross-cultural comparison of the relationship in 14 European countries. Addiction. 2001;96:77–92. doi: 10.1080/09652140020021198. [DOI] [PubMed] [Google Scholar]
- 225.Rehm J, Room R, Taylor B. Method for moderation: measuring lifetime risk of alcohol-attributable mortality as a basis for drinking guidelines. Int J Methods Psychiatr Res. 2008;17:141–51. doi: 10.1002/mpr.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Taylor B, Rehm J, Room R, Patra J, Bondy S. Determination of lifetime injury mortality risk in Canada in 2002 by drinking amount per occasion and number of occasions. Am J Epidemiol. 2008;168:1119–25. doi: 10.1093/aje/kwn215. [DOI] [PubMed] [Google Scholar]
- 227.Haworth A, Simpson R. Issues in unrecorded alcohol beverage production and consumption. New York: Brunner-Routledge; 2004. Moonshine Markets. [Google Scholar]
- 228.ICAP. Review 3. Noncommercial alcohol in three regions. Washington DC: International Center for Alcohol Policies (ICAP); 2008. [Google Scholar]
- 229.Rehm J, Kanteres F, Lachenmeier DW. Unrecorded consumption, quality of alcohol and health consequences. Drug Alcohol Review. doi: 10.1111/j.1465-3362.2009.00140.x. Accepted. [DOI] [PubMed] [Google Scholar]
- 230.Lachenmeier DW, Sarsh B, Rehm J. The composition of alcohol products from markets in Lithuania and Hungary, and potential health consequences: a pilot study. Alcohol Alcohol. 2009;44:93–102. doi: 10.1093/alcalc/agn095. [DOI] [PubMed] [Google Scholar]
- 231.Kanteres F, Lachenmeier DW, Rehm J. Alcohol in Mayan Guatemala: consumption, distribution, production, and composition of Cuxa. Addiction. 2009;104:752–259. doi: 10.1111/j.1360-0443.2009.02507.x. [DOI] [PubMed] [Google Scholar]
- 232.Lachenmeier DW, Kuballa T, Lima MCP, Nóbrega ICC, Kerr-Corrêa F, Kanteres F, et al. Ethyl carbamate analysis in German fruit spirits and Brazilian sugarcane spirits (cachaça): improved sample cleanup with automated parallel evaporation. Deutsche Lebensmittel-Rundschau. 2009;105:507–12. [Google Scholar]
- 233.Knupfer G. Drinking for health: the daily light drinker fiction. Br J Addict. 1987;82:547–55. doi: 10.1111/j.1360-0443.1987.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 234.Dawson DA, Room R. Towards agreement on ways to measure and report drinking patterns and alcohol-related problems in adult general population surveys: Skarpö Conference Overview. J Subst Abuse. 2000;12:1–21. doi: 10.1016/s0899-3289(00)00037-7. [DOI] [PubMed] [Google Scholar]
- 235.Greenfield TK, Kerr WC. Alcohol measurement methodology in epidemiology: recent advances and opportunities. Addiction. 2008;103:1082–99. doi: 10.1111/j.1360-0443.2008.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rehm J, Sulkowska U, Manczuk M, Boffetta P, Powles J, Popova S, et al. Alcohol accounts for a high proportion of premature mortality in central and eastern Europe. Int J Epidemiol. 2007;36:458–67. doi: 10.1093/ije/dyl294. [DOI] [PubMed] [Google Scholar]
- 237.Stockwell T, Single E, Hawks D, Rehm J. Sharpening the focus of alcohol policy from aggregate consumption to harm and risk reduction. Addiction Research. 1997;5:1–9. [Google Scholar]
- 238.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008;16:CD007176. doi: 10.1002/14651858.CD007176. [DOI] [PubMed] [Google Scholar]
- 239.Kalichman SC, Simbayi LC, Vermaak R, Cain D, Jooste S, Peltzer K. HIV/AIDS risk reduction counseling for alcohol using sexually transmitted infections clinic patients in Cape Town South Africa. J Acquir Immune Defic Syndr. 2007;44:594–600. doi: 10.1097/QAI.0b013e3180415e07. [DOI] [PubMed] [Google Scholar]
- 240.Kalichmann SC, Simbayi LC, Vermaak R, Cain D, Smith G, Mthebu J, et al. Randomized trial of a community-based alcohol-related HIV risk-reduction intervention for men and women in Cape Town South Africa. Ann Behav Med. 2008;36:270–9. doi: 10.1007/s12160-008-9067-2. [DOI] [PubMed] [Google Scholar]
- 241.Rehm J, Samokhvalov AV, Popova S, Neuman MG, Room R, Parry C, et al. A systematic review and meta-analysis. Toronto, ON: CAMH; 2009. Alcohol consumption, alcohol use disorders and incidence and disease course of tuberculosis (TB) - is there a causal connection? [Google Scholar]
- 242.Zeka A, Gore R, Kriebel D. Effects of alcohol and tobacco on aerodigestive cancer risks: a meta-regression analysis. Cancer Causes Control. 2003;14:897–906. doi: 10.1023/b:caco.0000003854.34221.a8. [DOI] [PubMed] [Google Scholar]
- 243.Purdue MP, Hashibe M, Berthiller J, La Vecchia C, Dal Maso L, Herrero R, et al. Type of alcoholic beverage and risk of head and neck cancer. A pooled analysis within the INHANCE Consortium Meta-analysis. Am J Epidemiol. 2009;169:132–42. doi: 10.1093/aje/kwn306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health. 2001;25:263–70. [PMC free article] [PubMed] [Google Scholar]
- 245.Lewis SJ, Smith DG. Alcohol, ALDH2, and esophageal cancer: a meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev. 2005;14:1967–71. doi: 10.1158/1055-9965.EPI-05-0196. [DOI] [PubMed] [Google Scholar]
- 246.Shimazu T, Tsuji I, Inoue M, Wakai K, Nagata C, Mizoue T, et al. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2008;38:8–25. doi: 10.1093/jjco/hym152. [DOI] [PubMed] [Google Scholar]
- 247.Mizoue T, Inoue M, Wakai K, Nagata C, Shimazu T, Tsuji I, et al. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Meta-analysis. Am J Epidemiol. 2008;167:1397–406. doi: 10.1093/aje/kwn073. [DOI] [PubMed] [Google Scholar]
- 248.Altieri A, Garavello W, Boseti C, Gallus S, Vecchia CL. Alcohol consumption and risk of laryngeal cancer. Oral Oncology. 2005;41:956–65. doi: 10.1016/j.oraloncology.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 249.La Vecchia C, Zhang ZF, Altieri A. Alcohol and laryngeal cancer: an update. Eur J Cancer Prev. 2008;17:116–24. doi: 10.1097/CEJ.0b013e3282b6fd40. [DOI] [PubMed] [Google Scholar]
- 250.Korte JE, Brennan P, Henley SJ, Boffetta P. Dose-specific metaanalysis and sensitivity analysis of the relation between alcohol consumption and lung cancer risk. Am J Epidemiol. 2002;155:496–506. doi: 10.1093/aje/155.6.496. [DOI] [PubMed] [Google Scholar]
- 251.Freudenheim JL, Ritz J, Smith-Warner SA, Albanes D, Bandera EV, van den Brandt PA, et al. Alcohol consumption and risk of lung cancer: a pooled analysis of cohort studies. Am J Clin Nutr. 2005;82:657–67. doi: 10.1093/ajcn.82.3.657. [DOI] [PubMed] [Google Scholar]
- 252.Chao C. Associations between beer, wine, and liquor consumption and lung cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16:2436–47. doi: 10.1158/1055-9965.EPI-07-0386. [DOI] [PubMed] [Google Scholar]
- 253.Bandera E, Freudenheim J, Vena J. Alcohol and lung cancer: a review of the epidemiologic evidence. Cancer Epidemiol Biomarkers Prev. 2001;10:813–21. [PubMed] [Google Scholar]
- 254.Wakai K, Nagata C, Mizoue T, Nishino Y, Tsuji I, Inoue M, et al. Alcohol drinking and lung cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2007;37:168–74. doi: 10.1093/jjco/hyl146. [DOI] [PubMed] [Google Scholar]
- 255.Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt P, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA. 1998;279:535–40. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 256.Ellison R, Zhang Y, McLennan C, Rothman K. Exploring the relation of alcohol consumption to risk of breast cancer. Am J Epidemiol. 2001;154:740–7. doi: 10.1093/aje/154.8.740. [DOI] [PubMed] [Google Scholar]
- 257.Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status. A meta-analysis of epidemiological studies. Int J Cancer. 2008;122:1832–41. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 258.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med. 2004;140:211–9. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- 259.Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes meta-analysis of epidemiological studies indicates a u-shaped relationship. Diabetologia. 2005;48:1051–4. doi: 10.1007/s00125-005-1768-5. [DOI] [PubMed] [Google Scholar]
- 260.Koppes LLJ, Bouter LM, Dekker JM, Heine RJ, Hendricks HFJ. Moderate alcohol consumption lowers the risk of type 2 diabetes. A meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–25. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 261.Ashley M, Rehm J, Bondy S, Single E, Rankin J. Beyond ischemic heart disease: are there other health benefits from drinking alcohol? Contemp Drug Probl. 2000;27:735–77. [Google Scholar]
- 262.Graves AB, van Duijn CM, Chandra V, Fratiglioni L, Heyman A, Jorm AF, et al. Alcohol and tobacco consumption as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20:S48–S57. doi: 10.1093/ije/20.supplement_2.s48. [DOI] [PubMed] [Google Scholar]
- 263.Letenneur L, Larrieu S, Barberger-Gateau P. Alcohol and tobacco consumption as risk factors of dementia: a review of epidemiological studies. Biomed Pharmacother. 2004;58:95–9. doi: 10.1016/j.biopha.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 264.Letenneur L. Moderate alcohol consumption and risk of developing dementia in the elderly: the contribution of propsective studies. Ann Epidemiol. 2007;17:S43–S45. [Google Scholar]
- 265.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age and Ageing. 2008;37:505–12. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- 266.Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Santamato A, et al. Vascular risk factors, alcohol intake, and cognitive decline. J Nutr Health Aging. 2008;12:376–81. doi: 10.1007/BF02982669. [DOI] [PubMed] [Google Scholar]
- 267.Tyas SL. Alcohol use and the risk of developing Alzheimer’s disease. Alcohol Res Health. 2001;25:299–306. [PMC free article] [PubMed] [Google Scholar]
- 268.Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, de Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation. 2002;105:2836–44. doi: 10.1161/01.cir.0000018653.19696.01. [DOI] [PubMed] [Google Scholar]
- 269.Camargo CA. Moderate alcohol consumption and stroke: the epidemiologic evidence. Stroke. 1989;20:1611–26. doi: 10.1161/01.str.20.12.1611. [DOI] [PubMed] [Google Scholar]
- 270.Mazzaglia G, Britton R, Altmann DR, Chenet L. Exploring the relationship between alcohol consumption and non-fatal or fatal stroke: a systematic review. Addiction. 2001;96:1743–56. doi: 10.1046/j.1360-0443.2001.961217434.x. [DOI] [PubMed] [Google Scholar]
- 271.Teunissen LL, Rinkel GJE, Algra A, van Gijn J. Risk factors for subarachnoid hemorrhage: a systematic review. Stroke. 1996;27:544–9. doi: 10.1161/01.str.27.3.544. [DOI] [PubMed] [Google Scholar]
- 272.Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG. 2007;114:243–52. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 273.Henderson J, Kesmodel U, Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J Epidemiol Community Health. 2007;61:1069–73. doi: 10.1136/jech.2006.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Odendaal HJ, Steyn DW, Elliott A, Burd L. Combined effects of cigarette smoking and alcohol consumption on perinatal outcome. Gynecol Obstet Invest. 2009;67:1–8. doi: 10.1159/000150597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Abel EL, Hannigan JH. ‘J-shaped’ relationship between drinking during pregnancy and birth weight: reanalysis of prospective epidemiological data. Alcohol Alcohol. 1995;30:345–55. [PubMed] [Google Scholar]
- 276.Zuckerman BS, Hingson R. Alcohol consumption during pregnancy: a critical review. Developmental Medicine and Child Neurology. 1986;28:649–54. doi: 10.1111/j.1469-8749.1986.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 277.Baliunas D, Taylor B, Irving H, Roerecke M, Patra J, Mohapatra S, et al. Alcohol as a risk factor for type 2 diabetes - a systematic review and meta-analysis. Diabetes Care. 2009;32:2123–32. doi: 10.2337/dc09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, et al. Alcohol and hypertension: gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction. 2009 doi: 10.1111/j.1360-0443.2009.02694.x. Epub ahead of print October 5. [DOI] [PubMed] [Google Scholar]
- 279.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Public Health. doi: 10.1093/aje/kwp451. Submitted. [DOI] [PubMed] [Google Scholar]
- 280.Klatsky AL, Friedman GD, Armstrong MA, Kipp H. Wine, liquor, beer, and mortality. Am J Epidemiol. 2003;158:585–95. doi: 10.1093/aje/kwg184. [DOI] [PubMed] [Google Scholar]


