Abstract
Different theories have been put forward during the last decade to explain the functional meaning of sleep and dreaming in humans. In the present paper, a new theory is presented which, while taking advantage of these earlier theories, introduces the following new and original aspects:
• Circadian rhythms relevant to various organs of the body affect the reciprocal interactions which operate to maintain constancy of the internal milieu and thereby also affect the sleep/wakefulness cycle. Particular attention is given to the constancy of natraemia and osmolarity and to the permissive role that the evolution of renal function has had for the evolution of the central nervous system and its integrative actions.
• The resetting of neuro-endocrine controls at the onset of wakefulness leads to the acquisition of new information and its integration within previously stored memories. This point is dealt with in relation to Moore-Ede’s proposal for the existence of a ’predictive homeostasis’.
• The concept of ‘psychic homeostasis’ is introduced and is considered as one of the most important states since it is aimed at the well-being, or eudemonia, of the human psyche. Sleep and dreaming in humans are discussed as important functions for the maintenance of a newly proposed composite state: that of ‘predictive psychic homeostasis’.
On the basis of these assumptions, and in accordance with the available neurobiological data, the present paper puts forward the novel hypothesis that sleep and dreaming play important functions in humans by compensating for psychic allostatic overloads. Hence, both consolatory dreams and disturbing nightmares can be part of the vis medicatrix naturae, the natural healing power, in this case, the state of eudemonia.
Keywords: sleep and dream theories, predictive psychic homeostasis, evolutionary tinkering, homeostasis of internal milieu, circadian rhythms of peripheral organs
Introduction
General premises
It is suggested that a basic distinction should be made between sleep and dreaming, even though these two phenomena usually coincide and are functionally correlated. This distinction is particularly relevant in view of a new theory of sleep1 in which it is suggested that the homeostatic regulation of sleep is not at the level of the whole organism but occurs in any recently active brain region. In particular, a sleep-like state can exist in cortical neuronal assemblies, or columns. Hence, these cortical columns of the brain might be the minimal units which manifest a sleep-like state. In agreement with such a view, it has been shown1 that not only do the cortical columns enter a sleep-like state after a period of intense activity but that their sleep-like state may also favor the entry into a similar state of those cortical columns to which they are connected. This serial entry into sleep may depend on both wiring and volume transmission communication modes: that is, via neural projections and synaptic contacts (Wiring Transmission; WT), and via diffusion of chemical and electrical signals within the extra-cellular space of the brain (Volume Transmission; VT).2,3 The interplay between WT and VT may have a role not only in the pattern of oscillation between different functional states within a cortical column, but also in the cross-talk between cortical columns. Thus, as discussed by Kruger and collaborators,1 several substances act as sleep promoters not only at the subcortical level but also at the cortical level by a local changing of the electrical properties of neurons and thereby altering their input–output relationships.
It should be underlined that although these data support the hypothesis that sleep can be a property of local neuronal assemblies, they do not deny the fundamental importance of a central coordinator of sleep/waking states, such as a timing, or clock mechanism. This latter is evident in the suprachiasmatic nucleus, which provides a “standard time” not only for neuronal assemblies but also for all peripheral tissues.4 Thus, although we shall discuss the relevance of peripheral circadian clocks affecting the integrative functions of entire brain systems via neural afferences, endocrine signals and chemico-physical parameters of the internal milieu, it should also be considered that this ‘extended orchestra’ has a ‘central conductor’.
Phenomenological aspects of sleep in mammals
The sleep of mammalian species can be broadly classified into two distinct types: non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. The latter is characterized not only by periodic bursts of rapid eye movement but also by the occurrence of electrical waves in the pons (P), the lateral geniculate nuclei of the thalamus (G), and the occipital cortex (O) – the so-called ‘PGO waves’.5 In primates and cats, NREM sleep has been subdivided into 4 stages (1–4) corresponding, in that order, to increasing depth of sleep.6 As NREM sleep progresses, pulses of EEG activity begin to slow in frequency. In particular, throughout stage-2 NREM, there is the presence of pulsed electrical events, including K-complexes (large electrical sharp waves in the EEG), and sleep spindles (short synchronized bursts of EEG electrical activity in the 11–15 Hz range).7 The deepest stages of NREM, stages 3 and 4, are often grouped together under the term “slow wave sleep” (SWS), characterized by the occurrence of low frequency waves (0.5–4 Hz), representing an expression of underlying mass synchrony of electrical discharges in the cortex.
In humans, NREM and REM sleep alternate with a “cycle” of about 90 min throughout the night. However, early in the night, stages 3 and 4 of NREM predominate, whereas stage-2 NREM and REM sleep prevail in the latter half of the night. During REM sleep, EEG waves are associated with oscillatory activity in the theta band range (4–7 Hz) together with more rapid 30–80 Hz synchronous activity in the gamma range of the EEG.8,9
The NREM and REM stages of sleep have been shown to be associated with different patterns of neurochemical activation of the Central Nervous System (CNS). Compared with the wakeful state, in NREM sleep, subcortical cholinergic systems in the brainstem and forebrain become markedly less active,10,11 whereas during REM sleep cholinergic systems become as active, or even more active, compared with wakefulness.12,13 By contrast, during REM sleep, the firing rates of serotonergic raphe neurons and noradrenergic locus coeruleus neurons are strongly inhibited, resulting in a brain state largely devoid of aminergic modulation (for a review see ref. 14) and dominated by acetylcholine. (For the early characterization of the role of 5-Hydroxytryptamine and noradrenergic neurons in the sleep-wakefulness cycle, see especially refs. 15-17). Hence, when cholinergic REM-‘on’ cells are activated, aminergic REM-‘off’ cell-groups are inhibited, and vice versa. Summing up, regulation of the REM/NREM sleep cycle is governed by two major ensembles of transmitter-identified neurons that act in an antagonistic fashion to turn ‘on’ or turn ‘off’ REM sleep.18-20
Activation patterns of entire brain areas can be revealed by neuroimaging techniques (for review see ref. 21). These have revealed a markedly different pattern of brain area activation/deactivation associated with NREM and REM sleep. Deactivation has been observed during NREM SWS in the brainstem, thalamic, basal ganglia, prefrontal, and temporal lobe regions. By contrast, during REM sleep, marked activity has been reported in the pontine tegmentum, thalamic nuclei, occipital cortex, mediobasal prefrontal lobes, together with limbic regions such as the amygdala, hippocampus and anterior cingulate cortex.22
On the functional meaning of sleep and dreams
The findings reported above give a rather detailed description of CNS phenomena associated with sleep. However, the ultimate meaning of sleep, and even more of dreams, has not been clarified.23 Dreams during either REM and NREM sleep in humans seem to simulate opposing types of social interactions. It has been reported that dreamer-initiated aggressive social interactions are more characteristic of REM than of NREM sleep, and that dreamer-initiated friendliness is more characteristic of NREM than of REM sleep.23
Studies based on comparative physiology (and, hence, based on a comparative analysis of the structure of the brain and sleep architectures in different species) have not given clear-cut answers to the possible functional meanings of sleep. Such studies, by evaluating brain differences in different species and the specific tasks for which each species is specialized, could in principle offer important hints about the basic mechanisms to which sleep, as a whole-brain function, is supposed to contribute. However, although several interesting data have been obtained, no general consensus has been reached on the functional meaning of sleep. Indeed, there are important articles which undermine the notion of there being any functional explanation. This is because of the inter-specific variation in both the total time spent asleep and in the relative proportions of time spent in REM and NREM sleep. For example, as pointed out by Capellini,24 there are no significant correlations between the time spent in specific sleep states (NREM or REM), brain size, metabolic rate, and developmental variables. In other words, functional explanations for inter-specific variation in sleep durations are unsustainable, and hypotheses that invoke energy conservation, cognition and development as being the drivers of sleep variations between different species should be abandoned.24
Recently, it has been proposed that ecological variables are the main determinants of sleep duration and sleep architecture across species. Hence, sleep can be seen as an acquired, or adaptive, state for the suppression of activity at times of day that a) have maximal predator risk, b) provide minimal opportunity for the efficient accomplishment of vital needs, and c) enable an increase of overall bodily efficiency by decreasing muscle tone and both brain and body metabolism during periods of inactivity.25
On the basis of these assumptions, it is understandable that, in each species, sleep might have somewhat different features and functions. Hence, sleep may have its own multi-factorial set of variables interconnected with the total wakefulness/sleep cycle, as well as having a characteristic structure which relates to the ecological variables encountered by the species in question.
Summing up at this point
Sleep/wakefulness cycling appears to be a complex cyclical and hierarchical phenomenon of the CNS which operates, at a high level, upon the circadian rhythms of peripheral organs. However, to qualify this conclusion somewhat, the reciprocal interactions between brain and peripheral organs in the sleep/wakefulness cycle have also to be considered. In the present paper, it is suggested that sleep serves many functional needs which, nevertheless, have a common motif: to restore the chemico-physical parameters of the internal environment, and to reset the optimal functional conditions of the peripheral apparatuses involved in maintaining homeostasis (Fig. 1, see also below). In addition, during sleep, an updating takes place, on the basis of the wakefulness experience, of the control mechanisms of the neuro-endocrine systems, and of those which are ‘intrinsic’ to some other physiological systems (see below for the renal apparatus).
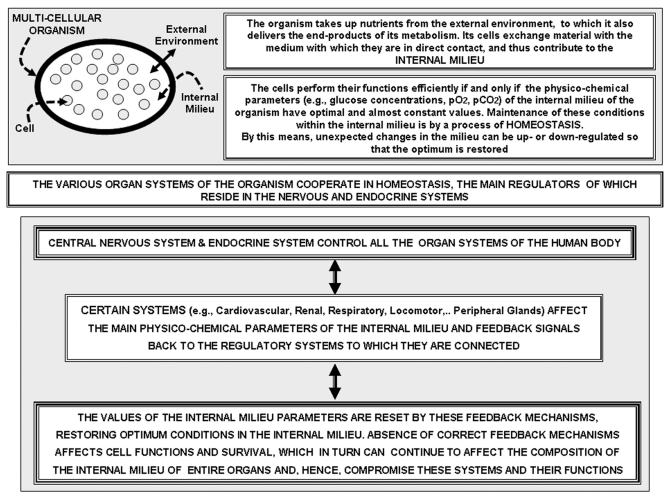
Figure 1.
Upper Panel: Schematic representation of the concept of the Internal Milieu (that is, the medium in contact with the cells of a multi-cellular organism), and of the concept of homeostasis. Lower Panel: box representation of some main logical steps involved in the homeostatic process and of the relevance of feed-back signals, that is, of signals which inform the control systems of the 'load error' (discrepancy between the observed value of a physico-chemico parameter and its optimal value) which needs to be compensated for in order to restore optimal parameters. This can be accomplished by changes in the performance of relevant organ systems.
The mentioned updating of control mechanisms can be viewed in the light of Moore-Ede’s proposal for the existence of ‘‘predictive homeostasis’’, i.e., of anticipatory adaptations of homeostatic reactions to future probable challenges.26 In order to carry out an efficient updating process based on the wakefulness experience, the neuronal networks should be in an ‘off-line’ condition. In particular, sleep updating of the control mechanisms should be considered not only in the light of classical homeostasis – that it is, to correct physiological or patho-physiological changes which have already occurred (‘‘reactive’’ homeostasis according to Moore-Ede classification) – but updating should also admit a ‘predictive’ component based on circadian rhythms and on experiences during wakefulness, especially those which hint at probable new challenges. This type of ‘predictive homeostasis’ can operate also at the peripheral organ level, as mentioned above and discussed below.
The order of the sections which follow (sections 1 – 4) is in accordance with the above-mentioned fundamental motifs which drive sleep. Hence, the themes to be discussed are as follows:
1. In connection with the possible role of sleep in resetting neuro-endocrine controls and in tuning autonomic responses to stress stimuli, the hypothesis is advanced that sleep may also tune the range of Heart Rate Variability (HRV) and, hence, lead to an increased capability of coping with stressors.27-29 As a matter of fact, subjects showing high levels of HRV perform better under stressful conditions. Contrariwise, reductions in HRV have been found in depression, anxiety and post-traumatic stress disorders.30,31 An interesting pathological case is the association between Post-Traumatic-Stress-Disorders (PTSDs), alterations in the autonomic control and endocrine secretion patterns by the anterior pituitary, and sleep/dream disturbances.32-35
2. The special role of sleep for the homeostatic control of the ion composition of the internal environment, in particular of osmolarity, will be emphasized. As proposed by Homer Smith in his fascinating book, ‘From the Fish to the Philosopher’,36 the evolution of the kidney is seen as crucial for the evolution of the CNS by allowing a precise maintenance of a constant internal environment as a prerequisite for proper organ functioning in general, and for the human brain in particular.37,38 The sleep period could allow resetting of the volume, ionic composition, and osmolarity of the extra-cellular fluid, especially that of the CNS, given that the kidney modulates these variables. As mentioned above, the existence of circadian rhythms in peripheral organs, which could affect the sleep/wakefulness cycle, should also be considered. These ‘peripheral rhythms’ might be thought of as being of a lower hierarchical order and thus subordinate to the circadian rhythm imposed by the brain.
3. The possible role of sleep in memory-trace formation and consolidation, thus allowing an updating of the control mechanisms on the basis of experiences during wakefulness, will be examined. Sleep has been postulated as a ‘cleansing procedure’, erasing memory traces of disturbing influences which may cause maladaptive responses.39 In this context, the effect of PTSDs on memories of fearful states, and of disturbances to sleep architecture, will also be discussed in relation to the role of sleep in the consolidation of fear memories in the amygdala.
4. The hypothesis will be introduced that the role of sleep and dreaming is functional, and is aimed at the maintenance of the composite state of ‘predictive psychic homeostasis’ (PPH). As mentioned above, the PPH concept can be deduced from the concept of ‘predictive homeostasis’ of the internal milieu, and from the concept of ‘psychic homeostasis’. They are merged within the unitary frame of PPH thanks to Jacob’s concept of ‘evolutionary tinkering’40,41. In fact, it will be suggested that even if one (or more than one) mechanism lies behind the wakefulness/sleep cycle in various species, including humans, then this mechanism might have been deployed differently in different species as a result of evolutionary tinkering. In particular, human sleep and dreams may have been tinkered in order to fulfil a new and important function: namely, that of psychic homeostasis, which, in predictive mode, leads to eudemonia.
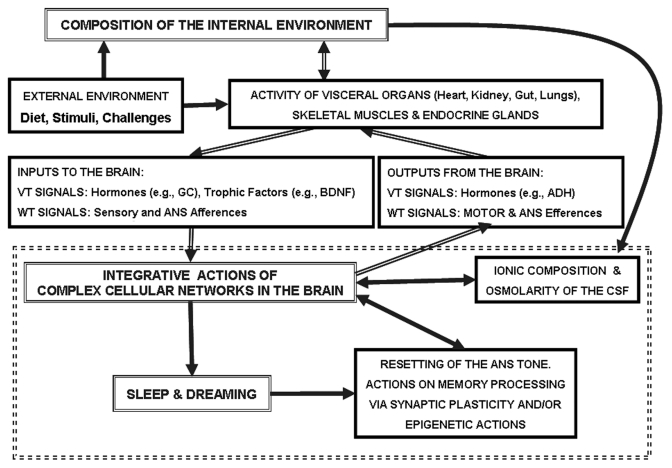
The scheme in Figure 2 summarizes the main aspects to be discussed in the present article. The two main players are shown in double-framed boxes where they are labeled as ‘composition of the internal environment’ and ‘integrative actions of the complex cellular networks in the brain’. One of the assumptions of the present paper is that sleep should be investigated in the light of the inter-relationships between these two entities, as indicated by the double-line arrows in the scheme. As indicated above, these inter-relationships can be different from species to species. This means that the same concept of homeostasis can assume particular connotations in different species (as ‘exaptations’)42 including humans where, in accordance with evolutionary forces, a new complex sphere – that of the psyche – has appeared. This last aspect points to one of the main topics for exploration in the future: the fundamental role of PPH for well-being, or eudemonia, of the human psyche.
Figure 2.
Schematic representation of the main functional connections between the composition of the internal environment and the integrative actions of the complex cellular networks in the brain, as they relate to sleep and dreaming.
1. POSSIBLE ROLE OF SLEEP IN RESETTING THE NEURO-ENDOCRINE CONTROLS
Several findings demonstrate the reciprocal relationships between the brain and the peripheral organs. These are based on observations concerning the bidirectional connections mediated by endocrine signals43,44 in relation to PTSDs.44
1A. Sleep and HPA hormone secretion
As indicated in the scheme of Figure 2, the main endocrine output of the brain is the pituitary gland. Activity of this gland has a profound effect not only on the secretions from peripheral endocrine glands but also on organs and tissues as different as kidney (ADH; Renin-Angiotensin45), heart (GH46), skeletal muscle (GH47). The level of hypothalamic–pituitary–adrenocortical (HPA) hormones not only shows a circadian pattern which depends on the sleep/wakefulness cycle,48,49 but it also, in turn, affects this cycle. As might be expected, the administration of various HPA hormones and their antagonists brings about specific sleep-EEG changes in several species, including humans.50 Our review of data in the literature has focused on the connections between both brain and kidney, and brain and adrenal gland (especially cortisol secretion) in view of the two respective main functional attributes of these interconnections, respectively, natraemia and osmolarity of the internal environment, and the responses to stress.
The circadian pattern of HPA production affects also the renin-angiotensin-aldosterone system (RAAS) since adrenocorticotropic hormone is a common stimulus for cortisol and aldosterone release. In addition, cortisol release is suppressed by mineralo-corticoid receptor agonists; and angiotensin II releases both corticotrophin releasing hormone (CRH) and vasopressin from the hypothalamus. In particular, it has been shown that renin and aldosterone (ALDO) secretion are synchronized to the REM-NREM cycle.51 Moreover, during sleep the plasma renin activity (PRA) reaches its maximal levels. Actually, in young normal male subjects, PRA shows oscillations with a 90 min period and having a distinct association with the cycles of REM and NREM sleep periods,52 and with a particular increase during NREM sleep stages 3 and 4, and a decrease during REM sleep. These findings are in agreement with previous data53 which demonstrated that the sleep period displays high mean ALDO levels, pulse amplitude, and frequency that are reduced during waking periods. During sleep, ALDO pulses are mainly related to PRA oscillations, whereas they are mainly associated with cortisol pulses during waking periods. It is also important to underline that acute sleep-deprivation induces natriuresis and osmotic diuresis, leading to excess nocturnal urine production, especially in men.52
All these data support Homer Smith’s assumption36 of a central role being played by the homeostasis of the internal environment with respect to osmolarity (and ion composition). The data also support the hypothesis concerning the relevance of these parameters for a sleep/wakefulness cycle in relation to the evolution of CNS complexity.
As far as the effects of HPA hormones on ‘psychic homeostasis’ are concerned, several studies have characterized the circadian secretion of anterior pituitary hormones in stress and mood disorders. In particular, impaired sleep and enhanced stress hormone secretion are the hallmarks of stress-related disorders, including major depression. The central neuropeptide CRH is a key hormone that regulates humoral and behavioral adaptation to stress. Its prolonged hypersecretion is believed to play a key role in the development and course of depressive symptoms. CRH has profound actions on sleep/wakefulness, and it is well documented that CRH impairs sleep and enhances vigilance. In addition, CRH may promote REM sleep, whereas acute cortisol administration inhibits REM sleep50 and increases SWS and GH (probably due to feedback inhibition of CRH). Furthermore, in healthy humans, repeated intravenous (i.v.) injections of CRH, as well as continuous i.v. infusion of ACTH, produce reductions of SWS; and intracerebroventricular administration of CRH to rats results in enhanced wakefulness and suppression of non-REM sleep. Recently, the specific effects of central CRH overexpression on sleep were evaluated54 in conditional mouse mutants that overexpress CRH in a) the entire central nervous system (CRH-COE-Nes), or b) the forebrain only, including limbic structures (CRH-COE-Cam). Compared with wild-type or control mice, both homozygous CRH-COE-Nes and -Cam mice showed increased REM sleep. CRH hypersecretion in the forebrain therefore seems to drive REM sleep, supporting the notion that enhanced REM sleep may serve as biomarker for clinical conditions associated with enhanced CRH secretion.
1B. Sleep, ANS function and stress coping: focus on PTSDs
A strict functional link associates HPA hormones with ANS function55 and, accordingly, as mentioned above, not only HPA hormone secretions but also autonomic nervous activities change in correspondence with the four sleep stages. An interesting study has analyzed the possible correlation between EEG recordings and autonomic nervous activities, as evaluated by means of the Heart Rate Variability (HRVa). In fact, the frequency analysis of oscillation in R-R intervals of electrocardiography (ECG) indicate that the HRV is a marker of autonomic nervous activity since the low frequency component (LF; 0.04–0.15 Hz) mainly reflects both sympathetic and parasympathetic nervous activities, whereas the high frequency component (HF; > 0.15Hz) reflects parasympathetic nervous activities. Thus, the LF/HF ratio has widely been used as a relative marker of sympatho-vagal balance.57 Moreover, it is a well accepted notion that HRV is a non-invasive method capable of providing a quantitative evaluation of the sympathovagal interactions, an evaluation that is in line with cardiac function modulation.58-61
Autonomic activities, as assessed by HRV, show a circadian rhythm and specific changes in different sleep phases. In more detail, HRV shows an increase in HF components and a decrease in LF components during NREM stages, and the opposite changes occur during REM sleep. Thus, the sympathetic nervous system is activated during REM sleep, and the parasympathetic nervous system is activated during NREM sleep.57,62 According to Lechin et al.,63 these findings can be implemented to provide information on the ANS changes registered in normal and stressed subjects at diurnal periods as well as during nocturnal periods, paying particular attention to changes during the different sleep stages (SWS and REM). Thus, poor parasympathetic plus high adrenal sympathetic activities have been registered in stressed subjects, and these findings are consistent with the frequent waking periods they show during nocturnal sleep.
Heart rate variability reflects a measure of an individual’s capacity for parasympathetic inhibition of autonomic arousal in emotional expression and regulation64 and, accordingly, a reduced HRV has been demonstrated to give indications on the emotional tone of a subject (see scheme of Figure 3). In order to differentiate the neural substrates of vagal tone due to emotion, HF-HRV parameters have been correlated with measures of regional cerebral blood flow (rCBF) derived from positron emission tomography (PET) and 15O-water flux in healthy women during different emotional states.68 Correlation was found between emotion-specific rCBF and HF-HRV, particularly in the medial prefrontal cortex. Emotion-specific rCBF also correlated with HF-HRV in the caudate nucleus, peri-acqueductal gray and left mid-insula. In agreement with the interrelationships between HRV and emotion-specific modulatory effects in the activity of specific brain areas,65 alterations in HRV during sleep have been found in depressed patients,69-73 anxious patients, and in those diagnosed with PTSD, as well as in chronically stressed subjects.69-73
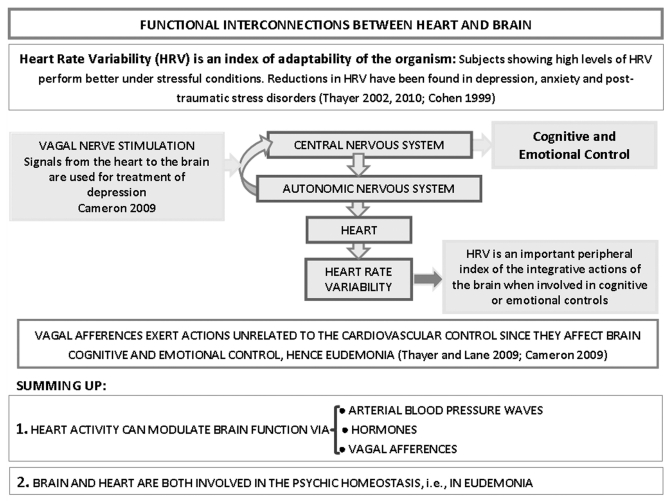
Figure 3.
Schematic representation of the multiple functional interconnections between heart and brain, according to Thayer and Lane.65 Special emphasis is given to the action of cardiac activity on brain integrative functions. It is worth noting that vagus nerve stimulation is employed for the treatment of neuropsychiatric disorders.66,67 For further details, see text.
The network of brain regions that controls appetitive (approach) and aversive (withdrawal) behaviors by regulation of visceromotor, neuro-endocrine, and behavioral responses is known as the central autonomic network (CAN). It includes the orbitofrontal and medial prefrontal cortices and the central nucleus of the amygdala, both of which project to the hypothalamic and brainstem autonomic nuclei, in the region where sympathetic and parasympathetic efferents to the heart originate.74 Depression is caused, at least, in part, by functional deficits in CAN, leading to autonomic dysregulation (hypersympathetic/hypovagal state) and to abnormalities of the hypothalamo-pituitary-adrenal axis and the hyperproduction of cortisol. These alterations in the neuro-endocrine controls cause reduced flexibility in responding to environmental demands and appropriate responsiveness.74 They also support the assumption that HRV is an important index for the diagnosis of mood disorders.56,75
A particular mood disorder is PTSD. It will be examined in some detail, not only because it is a special and well investigated pathological condition of chronic stress, which occurs following exposure to traumatic events, but also because it can be analyzed as a paradigmatic clinical case that supports our hypothesis on the relationship between overloads imposed upon the processes of psychic homeostasis and sleep/dreaming cycles. The epidemiological relevance of PTSDs can be understood by noting that not only are they observed after car accidents or sexual abuse, but also in approximately 20% of war-zone combat veterans. Such a syndrome is characterized by a re-experiencing of the trauma, which is commonly expressed in the form of chronic trauma-related nightmares, and of trauma-related intrusive thoughts during wakefulness.76 It should be noted that awakening from a nightmare is associated with a sympathetic arousal characteristic of a highly fearful state. This results in disturbed sleep in terms of both quality and quantity, and with consequent alterations in the ability to cope with daily life. PTSDs are thus characterized by intrusive re-experience, avoidance, and hyper-arousal reactions which persist for months and even years after the original exposure to the traumatic event ([DSM-IV], 1994). In addition, increasing attention has been paid to the repeated incorporation of emotionally charged waking episodes into sleep mentation. This is now a defining characteristic in the DSM-IV criteria for PTSD diagnosis.22 In fact, the sleep disruptions which occur following a trauma may constitute a specific symptom involved in the patho-physiology of chronic PTSD and its poor clinical outlook.77,78
In agreement with the already mentioned interconnections between sleep, the HPA axis and the ANS, it has been shown that, besides the sleep disturbance presented in PTSD, there are also alterations in both HRV and HPA hormone secretion, indicating a significantly increased sympathetic autonomic tone.79,80 Alterations of REM sleep (shorter average duration) have been observed to be associated with alterations in the circadian cortisol secretion and of HRV.81
Furthermore, studies using induced states of psychological stress to stimulate the HPA axis have shown a related and exaggerated cortisol response; this is also found in PTSD.82,83 As a matter of fact, acute psycho-physiological stress was associated with decreased levels of parasympathetic modulation during NREM and REM sleep, and also with increased levels of sympatho-vagal balance, were noted during NREM sleep. Moreover, whereas the parasympathetic modulation increased across successive NREM cycles in the control group, these increases were blunted in the stress group, remaining essentially unchanged across successive NREM periods. Higher levels of sympatho-vagal balance during NREM sleep were associated with poorer sleep maintenance and decreased delta activity.81 Under basal conditions, however, PTSD generally has been associated with lower levels of cortisol (for a detailed analysis see ref. 83).
In the light of our proposal of a possible role of sleep and dreams in psychic homeostasis, it should be noted that nightmares are a common feature of PTSD and that they are frequently resistant to treatment (see Section 4.). Two emerging treatments for nightmares are pharmacotherapy with prazosin and psychotherapy using imagery rehearsal.84
2. POSSIBLE ROLE OF SLEEP ON THE HOMEOSTATIC CONTROL OF SODIUM IONS AND OSMOLARITY IN THE INTERNAL ENVIRONMENT
The fundamental theme in Homer Smith's book36 is that evolution in general, and the evolution of renal functions in particular, have contributed to the maintenance of a constant internal environment. It is true that maintenance of homeostasis requires a number of organs and systems, the kidney being the main effector organ in mammals because it takes part in many fluid-regulation and hormonal processes: for example, maintenance of extracellular fluid volume, the concentration of osmotically active substances, the regulation of plasma pH, the excretion of unwanted products of metabolism, and the catabolism of peptide hormones, as well as substances that modulate the effects of hormones.85 In addition, the kidneys metabolise proteins, carbohydrates and lipids, and produce physiologically active substances regulating blood pressure, blood coagulation, and calcium balance. The high dependence of the CNS upon ion composition and osmolarity of the internal environment can be surmised from not only the small range of physiological osmolarity values (280–296 mOsm/L) but also from pathological observations, in particular the inappropriate ADH secretion syndrome. The clinical features of this syndrome are principally neuro-muscular, such as confusion, lethargy, anorexia and cramps, the severity of which is related to both absolute serum sodium concentration and osmolarity.86,87
Thus, as stated above, and in agreement with Homer Smith’s view, it is suggested that, in terrestrial mammals, one of the most important functional meanings of the integrated actions of the various apparatuses is the maintenance of ion composition and especially the primacy of osmolarity of the internal environment; circadian rhythms of the organism have these features also as one of their main goals.
This assumption can be investigated through an analysis of the connections between sleep-wakefulness cycles, HPA hormones, RAAS and ANS, all of which have been briefly analyzed in Section 1 in connection with the homeostasis of the internal environment as well as being the means whereby the subject copes with stress.
In this Section 2, new data of the highest relevance will be reported, which support our proposal for a role of sleep in the osmolarity control of the internal environment, namely the demonstration that ADH affects sleep, and that this action is related to osmoregulation.88 Actually, it has been demonstrated that osmoregulated vasopressin release is facilitated during the late sleep period (LSP) and that this action is dependent upon the circadian activity of clock neurons in the suprachiasmatic nucleus (SCN) which have low firing rates during the LSP.
It is well demonstrated that renal excretion of water and major electrolytes exhibit a significant circadian rhythm that is dependent upon both a central and a peripheral mechanism:
• Central mechanism: As mentioned above, vasopressin release increases during the sleep of mammals, in particular a facilitated release of ADH late in sleep has also been observed.88 These phenomena are important because they blunt the rise in plasma osmolality caused by evaporative and respiratory water loss at a time when water intake is suppressed.
• Peripheral mechanism: Renal transport mechanisms show an intrinsic periodicity caused by a circadian rhythm in the excretion of water and major electrolytes. This phenomenon is the result, at least in part, of temporally related changes in the secretion/reabsorption capacities of the distal nephron and collecting ducts.89
The central mechanism is based on the closely controlled ADH secretion by the posterior pituitary gland. Plasma osmolarity is monitored by osmosensory neurons located mainly in the organum vasculosum lamina terminalis (OVLT), which is not isolated by the blood-brain barrier. Osmosensory neurons of the OVLT project toward vasopressin magnocellular neurosecretory cells (MNCs) in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus and thereby facilitate increased osmoregulated vasopressin release. During dehydration, the electrical activity of MNCs is enhanced such that a proportional relationship exists between plasma osmolality and vasopressin concentration and, hence, water is reabsorbed by the kidney in proportion to the increased extracellular fluid osmolality.
The SCN, where clock neurons are located, have a peak of action potential firing rates during the night period. The neurons project toward the PVN and SON, thereby modulating the sensitivity of signals directed toward these nuclei from the osmosensor neurons. In fact, SCN projections inhibit pre-synaptically this sensory input to the MNCs during part of the daily cycle and this inhibition is removed during the late sleep phase, resulting in an increase release of vasopressin and water retention in the kidneys. Thus, as Trudel and Bourque88 suggest, clock neurons of the SCN mediate a pre-synaptic ‘silencing’ of those OVLT osmosensory afferent synapses which contact MNC vasopressin neurons. This means that during the late sleep phase the silencing action is removed, and ADH is secreted and water retained. Basically, by changing the relationship between osmolarity and vasopressin release, the SCN is effectively modulating the ‘gain’ of this homeostatic circuit, promoting the release of vasopressin.
As far as the second mechanism is concerned it has been shown that both the distal convoluted tubule and the connecting tubule, as well as the cortical collecting duct, possess an intrinsic circadian timing system characterized by robust oscillations in the expression of circadian core clock genes. Knockout mice exhibit alterations in water or sodium balance and sodium excretion rhythm. Hence, these mice display a complex phenotype characterized by partial diabetes insipidus and a significant decrease in blood pressure.
The discovery of such a molecular clock provides the basis for one of the possible ‘‘predictive’’ homeostatic mechanisms namely, a circadian timing system of the kidney that has control over renal tubular function at both a transcriptional and a functional level. The existence of such a ‘‘predictive’’ component provides the kidney with a significant functional advantage through anticipation of changes in the organism’s requirement for water and for solute reabsorption/secretion.
Furthermore, it can be surmised that similar predictive components may be in operation at other another, lower organ level and, hence, the sleep-wakefulness cycle may also have a peripheral origin. Thus, the mechanisms regulating sleep-wakefulness at the brain level affect the peripheral organs via the pituitary hormones and the ANS. However, it should also be considered that these organs, in turn, and as a consequence of their activities (which are modulated by their intrinsic circadian rhythms) can affect the brain. It should, therefore, be considered important that there are functional regulatory mechanisms around and within each of the peripheral organs (see Figure 2 the box “activity in the peripheral organs”).
In agreement with the view that there are reciprocal interactions between the circadian rhythm in the brain and circadian rhythms in peripheral organs, it has been observed that the transition from wakefulness to sleep is associated with a pronounced decline in diuresis, a necessary physiological process that allows uninterrupted sleep.90 Furthermore, the relations between sleep and renal physiology have also been investigated in healthy young volunteers by evaluating the effects of acute sleep deprivation (SD) on their urine output and renal water, sodium, and solute handling. SD markedly increased diuresis and led to excess renal sodium excretion; the effect was more pronounced in men than in women.90
Summing up, acute SD acts on several systems that govern sodium homeostasis because nocturnal levels of plasma renin, ANG II, and ALDO are suppressed during SD, leading directly to reduced sodium reabsorption in the renal tubuli.
3. POSSIBLE ROLE OF SLEEP IN MEMORY PROCESSING
3A. Sleep and epigenetic memory processes
Sensory experiences, coded in the form of electrochemical signals, are transmitted to the CNS where they are translated into a biochemical code and thence into changes in molecular and cellular networks. As underlined by our group and others,2,91,92 learning, sensory inputs, as well as continuing development and aging, bring about the reorganization of neuronal networks and are not “purely neuronal” events. On the contrary, it is accompanied by changes in glial, immune and vascular arrangements, as well as by changes in the molecular make up of the neural extracellular matrix. Thus, basic features of WT (e.g., dendritic spine density) and VT (e.g., features of the extra-cellular diffusion pathways) can be altered, as well as causing the alteration of the integrative actions of complex cellular networks.2 Furthermore, modulatory changes can take place in the structure and composition of the Global Molecular Network. This was surmised more than a century ago by Apathy93,94 and it is still a not well explored world. On this basis, our group has applied the Jacob’s concept of the “Russian Doll”95 to the organization of the CNS.96,97 According to this view, the computational devices of the CNS are structurally organized by analogy to a “Russian doll,” as a nested hierarchy of items.95 Molecular networks are nested within cells, multimeric macromolecular complexes are nested within molecular networks, and molecules are nested within multimeric macromolecular complexes. It should be noted that the hierarchical organization, as well as the elements at each level which are interconnected to carry out a computational task (i.e., the “building of the Russian doll”), is not done once for all time, but it is often a moment-to-moment changing complex of computational systems, arranged according to need and frequency of usage. Thus, not only can information handling take place at several miniaturization levels, but memories can also be stored similarly.98-101 Recent investigations have demonstrated how, in some instances, information handling can propagate inside the Russian Doll analog of the CNS to result in long-term storage of memories. Central for comprehension has been the finding that signal-transduction cascades can modify gene expression by remodelling the chromatin through epigenetic mechanisms. In other words, chromatin remodelling is a further process by which experiences can be “imprinted”;102-105 and this process takes place especially during sleep.106,107
A memorisation, or memory process, encompasses the stages of acquisition, consolidation, retrieval, and updating. Acquisition refers to the uptake of new information during learning and its encoding within a vulnerable, or labile, memory trace. Subsequent consolidation stabilizes the newly encoded memory, and includes also the processes of enhancement and integration with other pre-existing, long-term memories. Memory updating is both a conscious and an unconscious process, causing a continuous rearrangement of the memory traces. Sleep, by being a period during which complex cellular networks work off-line, may represent a privileged period during which these processes — and in particular the epigenetic-based ones — may occur. Several findings support this view. For example, there is now compelling evidence that sleep promotes the long-term consolidation of declarative and procedural memories. And, moreover, behavioral studies suggest that sleep preferentially consolidates explicit aspects of memories which, during encoding, are possibly associated with activation of the prefrontal–hippocampal circuitry.108 It has been proposed that the two major phases of sleep play distinct and complementary roles in memory consolidation: pre-transcriptional recall occurs during SW sleep, and transcriptional storage occurs during REM sleep.109 Hippocampus-dependent, declarative memory benefits particularly from SWS, whereas procedural aspects of memory seem to benefit from REM sleep. Furthermore, it has been hypothesized that tonic REM sleep supports off-line mnemonic processing, whereas pulsed or phasic bursts of activity during REM may promote memory consolidation.110 Consolidation of hippocampus-dependent memories is likely to rely on a dialog between the neocortex and hippocampus. In this regard, Buzsaki111,112 has suggested that sharp-wave bursts initiated in the hippocampus during SWS, and associated with theta and gamma oscillations, are likely to provide the mechanism(s) by which ‘‘quanta’’ of information are relayed to the neocortex during memory consolidation. Strong support of such a view has been provided by a recent study demonstrating a correlation between neocortical and hippocampal activities during SWS. This finding suggests that these hippocampal sharp-wave bursts are coupled selectively to those neocortical cell groups which have recently participated in the triggering of earlier bursts in the hippocampus.
According to this model, neocortical signaling to the hippocampus predominates during both waking and REM sleep periods. At the same time, feedback from the hippocampus to the neocortex is suppressed by acetylcholine. Memory traces encoded and temporarily stored with the hippocampal circuitry may then be relayed back to the neocortex and associated with relevant, already-stored traces during SWS. At this time, the cholinergic suppression of hippocampal feedback to the neocortex is released.113
Recently, an important paper has shed light upon a possible biochemical mechanism that links epigenetic mechanisms with memory processing and sleep.107 The amount of exploratory behavior was correlated with the mRNA levels for BDNF protein synthesis measured within the entire cerebral cortex. That is, the higher the induction of BDNF in the cerebral cortex during waking, the stronger the Slow-Wave Activity (SWA; 0.5–4.0Hz) response during subsequent sleep. In view of compelling evidence linking BDNF with adult cortical potentiation, these results strongly suggest that synaptic potentiation during waking, via BDNF signaling, can affect sleep homeostasis.
On the basis of this and other evidence, it has been suggested that the homeostatic SWA response is a direct consequence of the amount of synaptic plasticity, in particular that of BDNF-induced synaptic potentiation, which occurs prior to awakening. According to this hypothesis, sleep SWA should depend not only on the duration of the period prior to awakening, but also on the quality of awakening. Thus, there is evidence supporting a causal link between sleep, BDNF signaling and epigenetic coding of long-term memories.
Summing up, it seems that, because BDNF is a major mediator of synaptic plasticity in adult animals and facilitates the acquisition of cortically mediated memory tasks, it can be surmised that there is a direct link between the synaptic plasticity triggered by waking activities, the homeostatic sleep response, and BDNF. The latter should be considered as a major mediator of this link at the molecular level.107 Thus, in addition to its trophic function during development, BDNF is critical for learning-related synaptic plasticity and the maintenance of long-term memory, possibly via epigenetic mechanisms similar to those mechanisms which are BDNF-dependent during early life.106 The importance of these findings is further underlined by recent evidence showing that changes in BDNF expression which affect its release and neuromodulatory activity, and mediated by epigenetic and post-translational mechanisms, are also associated with many physiological conditions such as environmental enrichment and pathological conditions (depression) as well as with developmental experiences, such as maternal deprivation. These investigations might also shed some light on the proposed role of the amygdala in emotional memory formation.114 In this respect, functional neuroimaging studies support the critical involvement of the amygdala in facilitating emotional memory encoding. Moreover, the role of BDNF in fear conditioning is well defined. Thus, within the amygdala of the rat, both fear conditioning and its extinction lead to an increase in BDNF gene transcripts and the synthesis of this protein.115-117 Since interactions between amygdala and medial prefrontal cortex (mPFC) play an important role in fear learning,118-120 it has been proposed that regulation of BDNF in the prefrontal cortex plays an important role in the tuning of the emotional tone of mental activity that occurs during sleep, particularly with respect to fear learning and fear extinction. Also, data from humans allow important deductions on the critical role of amygdala and PFC in the “cleaning” of memorised experiences. Thus, in healthy individuals, the PFC and amygdala have been shown to be critical for processing fearful and other emotional stimuli, and also for the ability to extinguish fear in situations which are no longer threatening. In contrast, patients suffering from PTSD or anxiety disorders describe persistent anxiety-provoking memories. These are severely debilitating and cannot be extinguished.121 Furthermore, a REM-sleep hypothesis of emotional-memory processing has been proposed, the implications of which may provide brain-based insights into the association between sleep abnormalities and the initiation and maintenance of mood disturbances.22 All these findings can be viewed in the frame of our hypothesis on the special role of sleep and dreams on psychic homeostasis.
3B. Modulatory actions of sleep and dream mentation on the emotional tone
The strong emotional tone of mental activity occurring during sleep (often referred to as ‘dream mentation122) has long encouraged speculation about sleep-dependent affective processing (for recent review see ref. 123). As has been discussed above, a remarkable overlap exists between the known neurochemical anatomy of sleep, especially that of REM sleep, and the neurochemical anatomy that modulates emotions. Furthermore, abnormal sleep (including REM sleep) coincides with almost all affective psychiatric and mood disorders, in particular, PTSDs.22
It is still unclear whether changes in sleep composition and dream characteristics in PTSD reflect the brain’s attempt at functional compensation, or whether they are symptoms of dysfunctional processes. The two possibilities would represent, respectively, either a feature, or a failure, of the proposed psychic homeostasis (see below Section 4.). The link between sleep, dream content and psychic homeostasis is also underlined by the observation that emotional episodic memory events pervade the mental experience of dreaming in patients with PTSD, and that these are potentially related to both aberrant consolidation mechanisms and to the etiology of the disorder itself.22 In agreement with such a view, it should be noted that several studies in the last few decades have shown that nightmares can be treated with several cognitive behavioral techniques, e.g., by direct psycho-therapeutic interventions upon psychic homeostasis. Thus, Imagery Rehearsal Therapy (IRT) is a promising method of cognitive–behavioral therapy for PTSD nightmares. Although several forms of IRT have been proposed, the patient is encouraged to select one of his repetitive nightmares, and then to change its story line so making it less distressing and/or bringing it to a safe conclusion. The patient is then encouraged to rehearse mentally (and thus consolidate) the changed dream imagery.84
This therapeutic approach postulates that different memory representations acquired in relation to the same event may compete for retrieval. Therefore, rehearsal of an alternative, positive or neutral, image should tend to make this new memory more accessible to retrieval – because of its more recent consolidation – than the imagery contained in the original nightmare. Hence the nightmare scenario becomes less likely to re-occur. The cognitive-restructuring technique of IRT has been proposed as the treatment of choice for nightmares,124 and its results can be related to a reconstruction of the memory stores that are consolidated during sleep (see below Section 4.).
There is now considerable evidence, in both animals and humans that SD prior to encoding can significantly, but also selectively, alter and impair the canonical profile of emotional memory enhancement.22 SD studies in humans have demonstrated, in the amygdala, an amplified reaction in response to negative emotional stimuli. It is interesting to note that this enhanced response is associated with a loss of functional connectivity with the mPFC, which may imply a failure of top-down inhibition by the prefrontal lobe. These data support the proposal that sleep may “reset” the correct affective brain reactivity to subsequent next-day emotional challenges by maintaining the functional integrity of this mPFC-amygdala circuit. This, in turn, governs the subsequent behavioral repertoires of, for example, more appropriate social judgments and rational decisions. In view of the role of both amygdala and mPFC in memory processing, it may also be surmised that, during sleep, an updating of the memory stores takes place, especially of those stores that have a highly disturbing emotional content (see next Section).
4. THE SPECIAL ROLE OF SLEEP IN HUMANS: THE ‘PREDICTIVE PSYCHIC HOMEOSTASIS’
As mentioned above, it has been proposed that important affective processing occurs during dreaming: sleep, and especially dreaming, are viewed as playing a fundamental role in psychic homeostasis (see Figure 4). In agreement with this view, neurobiological findings in humans demonstrate that, during sleep, there occurs an overnight modulation of affective neural systems as well as the (re)processing of recent emotional experiencesb. Both phenomena appear to re-address the question of what should be the appropriate next-day reactivity of limbic and associated autonomic networks. They also point to the special role played by sleep and dreams in psychic homeostasis, a phenomenon which should be considered not simply as an updating of the neuro-endocrine controls for that particular type of homeostasis, but rather as contributing to a PPH.
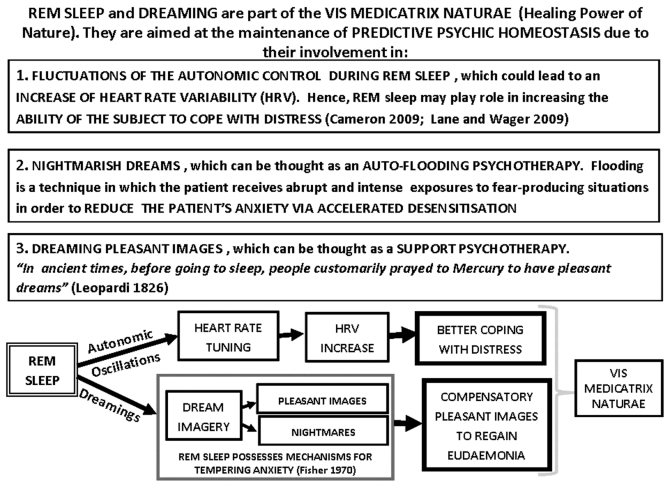
Figure 4.
Schematic representation of some aspects of the present hypothesis of ‘Psychic Predictive Homeostasis’ (PPH). The main assumption is that, together, sleep and dreaming play a role in the maintenance of psychic homeostasis in a predictive mode. Thus, sleep and dreaming are aimed not only at correcting the psychic allostatic loads suffered by the subject during the daytime, but also at preparing him for the likely psychic loads of the next day. For further details, see text.
In order to clarify further the present proposal on the functional meaning of sleep and dreams, two classical concepts will be used:
• The homeostasis concept embodies the maintenance of a dynamic balance of physico-chemical parameters of the internal environment (see above and Figure 1). A new type of homeostasis, a so-called “psychic homeostasis” (eudemonia, which is an auto-regulated state of well-being) is the analog of the physiological homeostasis and is one which maintains a dynamic balance within the psychic sphere. This psychic homeostasis has obviously unique features in humans.95
• Jacob’s concept of evolutionary tinkering, in which it is held that evolution tinkers together contraptions, allows natural selection to give direction to changes, orient chance, and slowly and progressively produce more complex structures, new organs, and new species. Hence, novelties come from unexpected associations of old material.40 It is surmised that evolution also tinkers old processes to produce novel functions, which may be of paramount importance for the adaptation of a species to novel surroundings. We could propose that the ancient processes of sleep and dreaming have been tinkered to produce a novel function in humans – the PPH – which is of vital and, adaptive importance.
Let us briefly discuss these two aspects:
The classical term ‘homeostasis’ is due to Claude Bernard (1813–1878)125 and Walter Cannon (1871–1945),126 both of whom proposed that the functions of certain physiological systems of a multi-cellular organism have as their final goal the maintenance of the constancy of composition of the internal environment, this being mainly comprised of the liquid which bathes the cells of the organism and by means of which they exchange solutes and metabolites within the nested levels of the organism within its environment. This condition of dynamic constancy is called homeostasis.
The maintenance of homeostasis implies an energy cost. Thus, McEwen, in his consideration of homeostasis, introduced three important additional concepts:127
- allostasis: the process by which the organism actively adjusts the activity of its systems in order to maintain the stability of the internal environment, notwithstanding the challenges due to changes of the external environment;
- allostatic load: which represents the cumulative energy cost to the body for the allostatic process;
- allostatic overload: a state in which the compensatory actions of the organism are overwhelmed, in consequence of which serious pathologies can occur.
The concept of PPH has been proposed by analogy with the concept of physiological predictive homeostasis of the internal environment. Thus, it has been proposed that the human organism in toto has as its main goal not simply the homeostasis of the internal environment but also a ‘psychic homeostasis’. The homeostasis of the psyche predicates a state of emotional balance that can be markedly altered during psychic disturbances, such as depression, paranoia and post-traumatic anxiety95 or even in conditions of excessive stressful inputs which bring about psychic allostatic overload.
Thus, as mentioned above for the homeostasis of the internal milieu, there should also be allostatic overloads for the psychic homeostasis which affect cognitive and emotional controls and, hence, lead to psycho-somatic diseases.
Taken all together, we suggest that evolution has tinkered old functions, such as the resetting of the complex cellular networks of ANS and CNS, as well as the epigenetic processes concerned with memory storage. In addition, there is an evolutionary input, via tinkering of the psychic elements of dreaming during sleep, and of fear and its processing during dreaming. This novel fear-processing function is also involved in the maintenance of the ‘psychic homeostasis’. By means of its circadian, clock-type of regulation, a ‘predictive’ processing is also introduced (see also ref. 128). Thus, although the tuning of the ANS and associated neuroendocrine responses prepares the subject for the challenges of wakefulness, as well as consolidating memories, both of which are functions of sleep in humans and in other animals, dreams also have a special and unique function in humans: the PPH. Not only do dreams possess a psycho-therapeutic action as the Balm of hurt minds (Shakespeare Macbeth Act II Sc II) but their recollection can also bring into daily life a predictive element due to the topic recalled (a fear, say, or an ever-present anxiety). These could be in the form of anxiety dreams rather than of the more crippling nightmares. Dreaming may have led to solutions to anxious problems via a ludic processing129 (see below).
Moreover, it may be surmised that while consolatory dreams work as an auto-psychotherapy of supportc, nightmares can play a role as an auto-flooding therapy. As a matter of fact, flooding is a form of behavior therapyd based on the principles of respondent conditioning. It is a psychotherapeutic technique used to treat phobia and anxiety disorders, including PTSDs. It works by exposing the patient to their painful memories, with the goal of reintegrating their repressed emotions with their current awareness.
As mentioned above, one of the main symptoms of the mental disorders of nightmares and PTSD is the re-experiencing of the original trauma. It is this which is commonly expressed in the form of chronic trauma-related nightmares. In these patients, nightmares can fragment sleep, decrease sleep quality, and even cause fear about going to sleep. One promising psychological treatment for chronic nightmares is IRT. Imagery rehearsal therapy presumes that nightmares are a learned behavior, and that activating the visual imagery system may facilitate emotional processing of the trauma. This treatment involves deliberately rewriting a nightmare while awake, and then mentally rehearsing images from the newly scripted scenario in preparation for sleep and memory reprocessing. Accordingly, IRT has been found to reduce nightmares and associated distress.131
FINAL COMMENTS
Three important theories have recently been put forward as functions for sleep and dreaming, namely:
• The threat simulation theory.132 According to this theory, dream consciousness (of non-aggressive, mild anxiety states) is a mechanism for simulating threat perception and rehearsing threat-avoidance responses and behaviors.
• Costly signaling theory.133 In relation to the former theory, dreams can facilitate production of signals when they produce some daytime effects on memory, mood or behavior that communicate a message to an observer.
• Simulation theory in a ludic context.129 Dreaming can be best understood as an expression of an innate capacity for playful creativity.
The present proposal of PPH maintains that sleep in humans is not simply involved in metabolic restoration; nor is it a careful orchestration of the circadian rhythms of the peripheral organs, with primacy being given to the precise control of natraemia, osmolarity and thermoregulation.134-136 Rather, and in agreement with the above-mentioned three theories, sleep and dreams in humans are involved in psychological processes of a fundamental importance for the maintenance of psychic homeostasis, and that this process can take upon itself a predictive form. To this end, the epigenetic processes occurring during sleep allow a profound resetting of the ANS and CNS complex cellular networks. In conclusion, dreams in humans can be thought as an example of the “vis medicatrix naturae,” allowing a type of auto-psychotherapy which can be of the highest importance for the eudemonia.
Acknowledgments
Economical support from IRCCS San Camillo Via Alberoni 70 Lido VE, Italy
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- ADH
antidiuretic hormone
- ALDO
aldosterone
- ANG II
angiotensin II
- ANS
autonomic nervous system
- BDNF
brain-derived neurotrophic factor
- CAN
central autonomic network
- CNS
central nervous system
- CRH
corticotrophin-releasing hormone
- CRH-COE-Cam
mouse mutants over-expressing CRH in the forebrain
- CRH-COE-Nes
mouse mutants over-expressing CRH in entire central nervous system
- ECG
electrocardiography
- EEG
electroencephalogram
- GH
growth hormone
- HF
high frequency
- HPA
hypothalamic–pituitary–adrenocortical
- HRV
heart-rate variability
- IRT
imagery rehearsal therapy
- LSP
late sleep period
- LF
low frequency
- MNCs
magnocellular neurosecretory cells
- mPFC
medial prefrontal cortex
- NREM
non-rapid eye movement
- OVLT
organum vasculosum lamina terminalis
- PET
positron emission tomography
- PFC
prefrontal cortex
- PGO waves
electrical waves in the pons, lateral geniculate nuclei of the thalamus and occipital cortex
- PPH
predictive psychic homeostasis
- PRA
plasma-rennin activity
- PTSD
post-traumatic-stress-disorder
- PVN
paraventricular nucleus
- RAAS
renin-angiotensin-aldosterone system
- rCBF
regional cerebral blood flow
- REM
rapid eye movement
- SCN
suprachiasmatic nucleus
- SD
sleep deprivation
- SON
supraoptic nucleus
- SWS
slow wave sleep
- VT
volume transmission
- WT
wiring transmission
Note
aSpectral analysis of heart rate variability provides a noninvasive technique for indirectly measuring sympathetic and parasympathetic modulation during sleep.56
bThe sleep that knits up the ravelled sleave of care,/ The death of each day’s life, sore labor’s bath,/ Balm of hurt minds, great nature’s second course,/ Chief nourisher in life’s feast. (Shakespeare Macbeth Act II Sc II).
cGli antichi avanti di coricarsi solevano orare e fare libazioni a Mercurio, conduttore dei sogni, acciò ne menasse loro di quei lieti [In the ancient times, before going to sleep, people were used to pray Mercury to give them pleasant dreams] (Leopardi Operette Morali 1826). Several examples of dreams as ‘auto-psychotherapy of support’ are reported in the Bible. One for all is the Jacob’s dream of a ladder connecting the earth with the sky. The dream offered to Jacob a strong psychological support in a critical situation.
dFlooding was invented by psychologist Thomas Stampfl in 1967 and it still is used in behavior therapy today.130
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17602
References
- 1.Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnati LF, Fuxe K. Volume transmission as a key feature of information handling in the central nervous system possible new interpretative value of the Turing's B-type machine. Prog Brain Res. 2000;125:3–19. doi: 10.1016/S0079-6123(00)25003-6. [DOI] [PubMed] [Google Scholar]
- 3.Fuxe K, Dahlström AB, Jonsson G, Marcellino D, Guescini M, Dam M, et al. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog Neurobiol. 2010;90:82–100. doi: 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Bass J, Takahashi JS. Circadian Integration of Metabolism and Energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaway CW, Lydic R, Baghdoyan HA, Hobson JA. Pontogeniculooccipital waves: spontaneous visual system activity during rapid eye movement sleep. Cell Mol Neurobiol. 1987;7:105–49. doi: 10.1007/BF00711551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects editors. In: Rechtschaffen A, Kales ed, Los Angeles: Brain Information Service/Brain Research Institute, University of California, 1968. [Google Scholar]
- 7.Steriade M, Amzica F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res Online. 1998;1:1–10. [PubMed] [Google Scholar]
- 8.Llinás R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90:2078–81. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steriade M, Amzica F, Contreras D. Synchronization of fast (30-40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–8. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 11.Lydic R, Baghdoyan HA. Handbook of behavioural state control: Cellular and molecular mechanisms. Buca Raton, FL: CRC Press, 1988. [Google Scholar]
- 12.Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci. 1990;47:421–6. doi: 10.1016/0024-3205(90)90300-G. [DOI] [PubMed] [Google Scholar]
- 13.Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 14.Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–7. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 16.Kiianmaa K, Fuxe K. The effects of 5,7-dihydroxytryptamine-induced lesions of the ascending 5-hydroxytryptamine pathways on the sleep wakefulness cycle. Brain Res. 1977;131:287–301. doi: 10.1016/0006-8993(77)90521-2. [DOI] [PubMed] [Google Scholar]
- 17.Fuxe K, Kiianmaa K. 5-Hydroxytryptamine neurons and the sleep-wakefulness cycle. Effects of methergoline and zimelidine. Neurosci Lett. 1978;8:55–8. doi: 10.1016/0304-3940(78)90097-6. [DOI] [PubMed] [Google Scholar]
- 18.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shima K, Nakahama H, Yamamoto M. Firing properties of two types of nucleus raphe dorsalis neurons during the sleep-waking cycle and their responses to sensory stimuli. Brain Res. 1986;399:317–26. doi: 10.1016/0006-8993(86)91522-2. [DOI] [PubMed] [Google Scholar]
- 20.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–93. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 21.Nofzinger EA. Neuroimaging and sleep medicine. Sleep Med Rev. 2005;9:157–72. doi: 10.1016/j.smrv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara P, Johnson P, McLaren D, Harris E, Beauharnais C, Auerbach S. REM and NREM sleep mentation. Int Rev Neurobiol. 2010;92:69–86. doi: 10.1016/S0074-7742(10)92004-7. [DOI] [PubMed] [Google Scholar]
- 24.Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–76. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10:747–53. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol. 1986;250:R737–52. doi: 10.1152/ajpregu.1986.250.5.R737. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P. Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep. 2010;33:113–22. doi: 10.1093/sleep/33.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24:1–15. doi: 10.1016/j.janxdis.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12:169–84. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–53. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 31.Cohen H, Benjamin J. Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Auton Neurosci. 2006;128:1–8. doi: 10.1016/j.autneu.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Payne JD, Nadel L. Sleep, dreams, and memory consolidation: The role of the stress hormone cortisol. Learn Mem. 2004;11:671–8. doi: 10.1101/lm.77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neylan TC, Otte C, Yehuda R, Marmar CR. Neuroendocrine regulation of sleep disturbances in PTSD. Ann N Y Acad Sci. 2006;1071:203–15. doi: 10.1196/annals.1364.015. [DOI] [PubMed] [Google Scholar]
- 34.Wittmann L, Schredl M, Kramer M. Dreaming in posttraumatic stress disorder: A critical review of phenomenology, psychophysiology and treatment. Psychother Psychosom. 2007;76:25–39. doi: 10.1159/000096362. [DOI] [PubMed] [Google Scholar]
- 35.Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407. doi: 10.1016/S0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 36.Smith H. From the Fish to the Philosopher. Little Brown, Boston, 1953. [Google Scholar]
- 37.Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–71. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- 38.Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117:134–74. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–4. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 40.Jacob F. Evolution and tinkering. Science. 1977;196:1161–6. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 41.Agnati LF, Guidolin D, Carone C, Dam M, Genedani S, Fuxe K. Understanding neuronal molecular networks builds on neuronal cellular network architecture. Brain Res Brain Res Rev. 2008;58:379–99. doi: 10.1016/j.brainresrev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Gould SJ, Vrba ES. Exaptation–a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- 43.Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, et al. Organization of circadian functions: interaction with the body. Prog Brain Res. 2006;153:341–60. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 44.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008;456:1005–24. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muccioli G, Broglio F, Valetto MR, Ghè C, Catapano F, Graziani A, et al. Growth hormone-releasing peptides and the cardiovascular system. Ann Endocrinol (Paris) 2000;61:27–31. [PubMed] [Google Scholar]
- 47.McCall GE, Gosselink KL, Bigbee AJ, Roy RR, Grindeland RE, Edgerton VR. Muscle afferent-pituitary axis: a novel pathway for modulating the secretion of a pituitary growth factor. Exerc Sport Sci Rev. 2001;29:164–9. doi: 10.1097/00003677-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34:271–92. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Fehm HL, Späth-Schwalbe E, Pietrowsky R, Kern W, Born J. Entrainment of nocturnal pituitary-adrenocortical activity to sleep processes in man–a hypothesis. Exp Clin Endocrinol. 1993;101:267–76. doi: 10.1055/s-0029-1211243. [DOI] [PubMed] [Google Scholar]
- 50.Steiger A. Sleep and the hypothalamo–pituitary–adrenocortical system. Sleep Med Rev. 2002;6:125–38. doi: 10.1053/smrv.2001.0159. [DOI] [PubMed] [Google Scholar]
- 51.Murck H, Held K, Ziegenbein M, Künzel H, Koch K, Steiger A. The renin-angiotensin-aldosterone system in patients with depression compared to controls–a sleep endocrine study. BMC Psychiatry. 2003;3:15. doi: 10.1186/1471-244X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schüssler P, Yassouridis A, Uhr M, Kluge M, Bleninger P, Holsboer F, et al. Sleep and active renin levels–interaction with age, gender, growth hormone and cortisol. Neuropsychobiology. 2010;61:113–21. doi: 10.1159/000279301. [DOI] [PubMed] [Google Scholar]
- 53.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol. 1999;276:E43–9. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 54.Kimura M, Müller-Preuss P, Lu A, Wiesner E, Flachskamm C, Wurst W, et al. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry. 2010;15:154–65. doi: 10.1038/mp.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog Brain Res. 2000;126:117–32. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- 56.Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.PSY.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 57.Ako M, Kawara T, Uchida S, Miyazaki S, Nishihara K, Mukai J, et al. Correlation between electroencephalography and heart rate variability during sleep. Psychiatry Clin Neurosci. 2003;57:59–65. doi: 10.1046/j.1440-1819.2003.01080.x. [DOI] [PubMed] [Google Scholar]
- 58.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 59.Vanoli E, Adamson PB. Ba-Lin, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–22. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 60.Mezzacappa E, Kindlon D, Earls F. The utility of spectral analytic techniques in the study of the autonomic regulation of beat-to-beat heart rate variability. Int J Met Psych Res. 1994;4:29–44. [Google Scholar]
- 61.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 62.Elsenbruch S, Harnish MJ, Orr WC. Heart rate variability during waking and sleep in healthy males and females. Sleep. 1999;22:1067–71. doi: 10.1093/sleep/22.8.1067. [DOI] [PubMed] [Google Scholar]
- 63.Lechin F, van der Dijs B, Lechin AE. Autonomic nervous system assessment throughout the wake-sleep cycle and stress. Psychosom Med. 2004;66:974–6. doi: 10.1097/01.psy.0000146793.90058.c3. [DOI] [PubMed] [Google Scholar]
- 64.Appelhans BM, Luecken LJ. Heart Rate Variability as an Index of Regulated Emotional Responding. Rev Gen Psychol. 2006;10:229–40. doi: 10.1037/1089-2680.10.3.229. [DOI] [Google Scholar]
- 65.Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 66.George MS, Nahas Z, Borckardt JJ, Anderson B, Burns C, Kose S, et al. Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders. Expert Rev Neurother. 2007;7:63–74. doi: 10.1586/14737175.7.1.63. [DOI] [PubMed] [Google Scholar]
- 67.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–55. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 68.Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213–22. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 69.Ehrenthal JC, Herrmann-Lingen C, Fey M, Schauenburg H. Altered cardiovascular adaptability in depressed patients without heart disease. World J Biol Psychiatry. 2010;11:586–93. doi: 10.3109/15622970903397714. [DOI] [PubMed] [Google Scholar]
- 70.Nahshoni E, Aravot D, Aizenberg D, Sigler M, Zalsman G, Strasberg B, et al. Heart rate variability in patients with major depression. Psychosomatics. 2004;45:129–34. doi: 10.1176/appi.psy.45.2.129. [DOI] [PubMed] [Google Scholar]
- 71.Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79:503–13. doi: 10.1016/S0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 72.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cameron OG. Visceral brain–body information transfer. Neuroimage. 2009;47:787–94. doi: 10.1016/j.neuroimage.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Thayer JF, Siegle GJ. Neurovisceral integration in cardiac and emotional regulation. IEEE Eng Med Biol Mag. 2002;21:24–9. doi: 10.1109/MEMB.2002.1032635. [DOI] [PubMed] [Google Scholar]
- 75.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 76.Spoormaker VI, Schredl M, Van den Bout J. Nightmares: from anxiety symptom to sleep disorder. Sleep Med Rev. 2006;10:19–31. doi: 10.1016/j.smrv.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 78.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 79.Mellman TA, Hipolito MM. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 2006;11:611–5. doi: 10.1017/s1092852900013663. [DOI] [PubMed] [Google Scholar]
- 80.Bryant RA, Harvey AG, Guthrie RM, Moulds ML. Acute psychophysiological arousal and posttraumatic stress disorder: a two-year prospective study. J Trauma Stress. 2003;16:439–43. doi: 10.1023/A:1025750209553. [DOI] [PubMed] [Google Scholar]
- 81.Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.PSY.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 82.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–67. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 83.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–92. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 84.Gehrman PR, Harb GC. Treatment of nightmares in the context of posttraumatic stress disorder. J Clin Psychol. 2010;66:1185–94. doi: 10.1002/jclp.20730. [DOI] [PubMed] [Google Scholar]
- 85.Natochin YV. Evolutionary aspects of renal function. Kidney Int. 1996;49:1539–42. doi: 10.1038/ki.1996.220. [DOI] [PubMed] [Google Scholar]
- 86.Felig P, Baxter JD, Brodaus AB, Frohman LA. Endocrinology and Metabolism, in McGraw ed, Hill NY, 1987. [Google Scholar]
- 87.Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35:1495–9. doi: 10.1016/S1357-2725(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 88.Trudel E, Bourque CW. Central clock excites vasopressin neurons by waking osmosensory afferents during late sleep. Nat Neurosci. 2010;13:467–74. doi: 10.1038/nn.2503. [DOI] [PubMed] [Google Scholar]
- 89.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA. 2009;106:16523–8. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–11. doi: 10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- 91.Agnati LF, Zunarelli E, Genedani S, Fuxe K. On the existence of a global molecular network enmeshing the whole central nervous system: physiological and pathological implications. Curr Protein Pept Sci. 2006;7:3–15. doi: 10.2174/138920306775474086. [DOI] [PubMed] [Google Scholar]
- 92.Báez-Saldana A, Fetter-Pruneda I, Fuentes-Farías A, Granados-Rojas L, Gutierrez-Ospina G, Matinez-Mendez R, et al. Brain Plasticity, Signal Transduction and Epigenesis: a Missing Link Revealed. Ann Rev Biomed Scie. 2009;11:T114–22. [Google Scholar]
- 93.Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K, et al. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol (Oxf) 2006;187:329–44. doi: 10.1111/j.1748-1716.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 94.Agnati LF, Genedani S, Leo G, Rivera A, Guidolin D, Fuxe K. One century of progress in neuroscience founded on Golgi and Cajal's outstanding experimental and theoretical contributions. Brain Res Brain Res Rev. 2007;55:167–89. doi: 10.1016/j.brainresrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 95.Jacob F. La logique du vivant. Une histoire de l'hérédité. In: Gallimard Ed, Paris, 1970. [Google Scholar]
- 96.Agnati LF, Fuxe K. New concepts on the structure of the neuronal networks: the miniaturization and hierarchical organization of the central nervous system. (Hypothesis) Biosci Rep. 1984;4:93–8. doi: 10.1007/BF01120304. [DOI] [PubMed] [Google Scholar]
- 97.Agnati LF. Neuroscienze e filosofia: antiche e nuove “mitologie”. Hermeneutica 2008; 269-299. [Google Scholar]
- 98.Agnati LF, Fuxe K, Zoli M, Rondanini C, Ögren SO. New vistas on synaptic plasticity: mosaic hypothesis on the engram. Med Biol. 1982;60:183–90. [PubMed] [Google Scholar]
- 99.Agnati LF, Benfenati F, Ferri M, Morpurgo A, Apolloni B, Fuxe K. Molecular basis of learning and memory: modeling based on receptor mosaics. In: Apolloni B, Kurfess F eds. From synapses to rules: discovering symbolic rules from neural processed data. Kluwer Academic/Plenum Press Publisher, New York, 1988: 165-196. [Google Scholar]
- 100.Agnati LF, Franzen O, Ferré S, Franco R, Fuxe K. Possible role of intramembrane receptor-receptor interactions in memory and learning via formation of long-lived heteromeric complexes: focus on motor learning in the basal ganglia. J Neural Transm. 2003;65(Suppl):195–222. doi: 10.1007/978-3-7091-0643-3_1. [DOI] [PubMed] [Google Scholar]
- 101.Guidolin D, Fuxe K, Neri G, Nussdorfer GG, Agnati LF. On the role of receptor-receptor interactions and volume transmission in learning and memory. Brain Res Brain Res Rev. 2007;55:119–33. doi: 10.1016/j.brainresrev.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 102.Báez-Saldana A, Fetter-Pruneda I, Fuentes-Farías A, Granados-Rojas L, Gutierrez-Ospina G, Matinez-Mendez R, et al. Brain Plasticity, Signal Transduction and Epigenesis: a Missing Link Revealed. Ann Rev Biomed Sci. 2009;11:T114–22. [Google Scholar]
- 103.Sapolsky RM. Mothering style and methylation. Nat Neurosci. 2004;7:791–2. doi: 10.1038/nn0804-791. [DOI] [PubMed] [Google Scholar]
- 104.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 105.Champagne FA, Curley JA. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huber R, Tononi G, Cirelli C. Exploratory behaviour, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–39. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 108.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–50. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja P, et al. Long lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2:126–37. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Montgomery SM, Sirota A, Buzsáki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–41. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buzsáki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 112.Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 113.Power AE. Slow-wave sleep, acetylcholine, and memory consolidation. Proc Natl Acad Sci USA. 2004;101:1795–6. doi: 10.1073/pnas.0400237101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 115.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–2. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–96. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- 118.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 119.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–94. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 120.Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–10. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci USA. 2010;107:2675–80. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23:793–842. doi: 10.1017/S0140525X00003976. [DOI] [PubMed] [Google Scholar]
- 123.Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11:295–310. doi: 10.1016/j.smrv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Spoormaker VI, Schredl M, Van den Bout J. Nightmares: from anxiety symptom to sleep disorder. Sleep Med Rev. 2006;10:19–31. doi: 10.1016/j.smrv.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 125.Bernard C. Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux. In: Albert Dastre, eds. Baillière et fils, Paris, 1878-1879. [Google Scholar]
- 126.Cannon WE. A discussion of the regulation of body fluids, hunger, thirst, temperature, oxygen supply, water, sugar, and proteins of the body, and the role of the asympathetic-adrenal mechanism. In: Cannon eds. The wisdom of the body. Norton & Co, New York, 1932. [Google Scholar]
- 127.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52(Suppl 2):10–6. doi: 10.1053/S0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 128.Horowski R, Benes H, Fuxe K. Striatal plasticity and motor learning-importance of circadian rhythms, sleep stages and dreaming. Parkinsonism Relat Disord. 2004;10:315–7. doi: 10.1016/j.parkreldis.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 129.Bulkeley K. Dreaming as inspiration evidence from religion, philosophy, literature, and film. Int Rev Neurobiol. 2010;92:31–46. doi: 10.1016/S0074-7742(10)92002-3. [DOI] [PubMed] [Google Scholar]
- 130.Stampfl TG, Lewis DJ. Essentials of implosive therapy: A learning-theory-based psychodynamic behavioral therapy. J Abnorm Psychol. 1967;72:496–503. doi: 10.1037/h0025238. [DOI] [PubMed] [Google Scholar]
- 131.Berlin KL, Means MK, Edinger JD. Nightmare reduction in a Vietnam veteran using imagery rehearsal therapy. J Clin Sleep Med. 2010;6:487–8. [PMC free article] [PubMed] [Google Scholar]
- 132.Revonsuo A. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav Brain Sci. 2000;23:877–901. doi: 10.1017/S0140525X00004015. [DOI] [PubMed] [Google Scholar]
- 133.McNamara P, McLaren D, Durso K. Representation of the Self in REM and NREM Dreams. Dreaming. 2007;17:113–26. doi: 10.1037/1053-0797.17.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci. 2010;15:604–25. doi: 10.2741/3636. [DOI] [PubMed] [Google Scholar]
- 135.Parmeggiani PL. Interaction between sleep and thermoregulation: an aspect of the control of behavioral states. Sleep. 1987;10:426–35. doi: 10.1093/sleep/10.5.426. [DOI] [PubMed] [Google Scholar]
- 136.Menet JS, Rosbash M. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.06.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neckelmann D, Amzica F, Steriade M. Spike-wave complexes and fast components of cortically generated seizures. III. Synchronizing mechanisms. J Neurophysiol. 1998;80:1480–94. doi: 10.1152/jn.1998.80.3.1480. [DOI] [PubMed] [Google Scholar]