Abstract
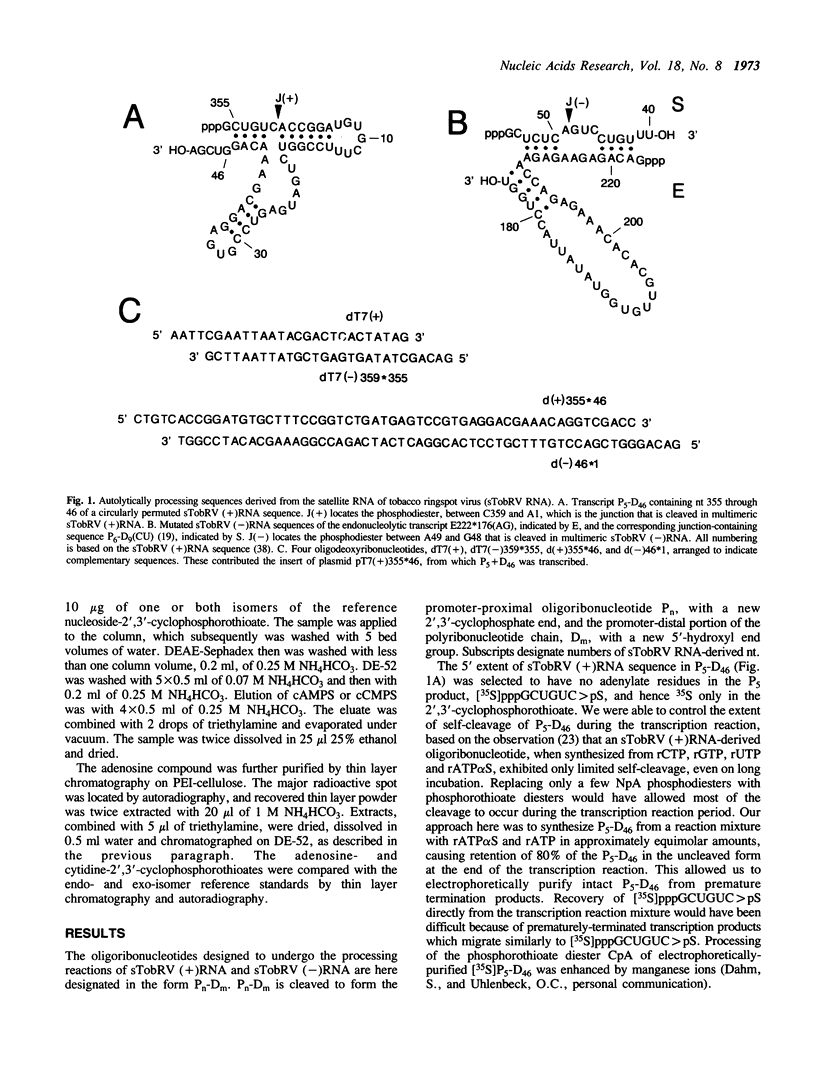
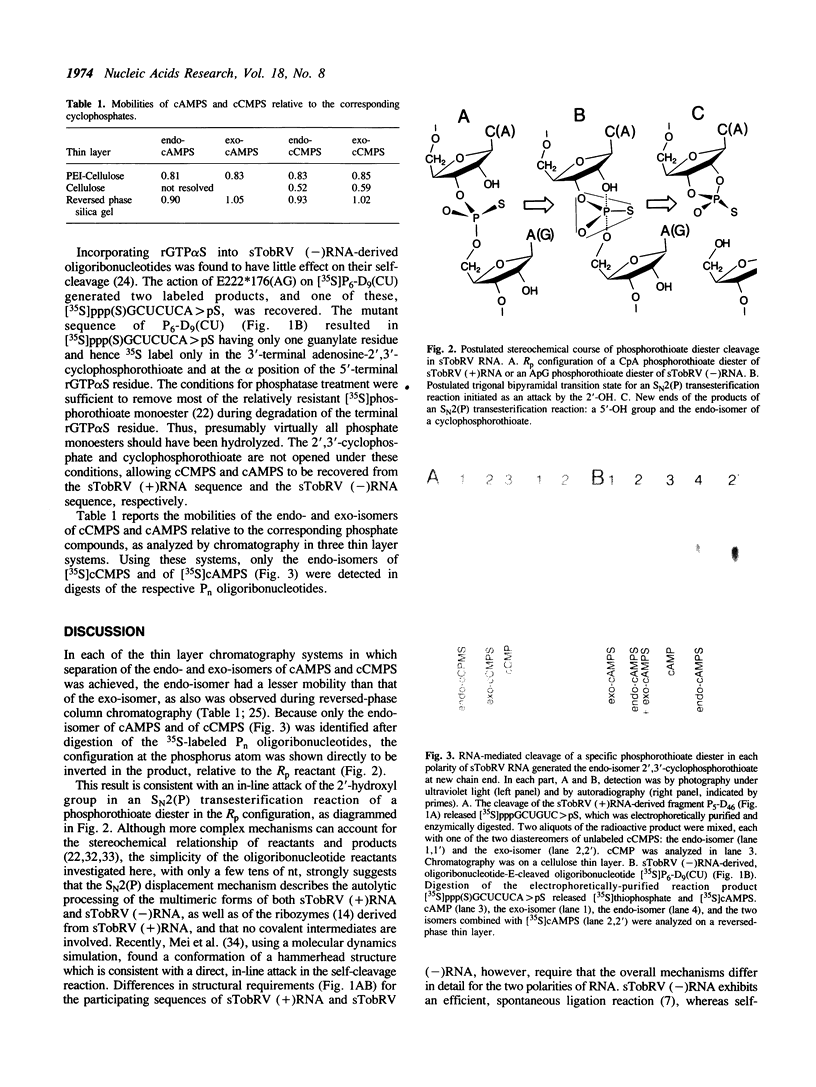
Both polarities of the satellite RNA of tobacco ringspot virus occur in infected cells in multimeric forms which are capable of autolytic processing, using different sequences and structures [Feldstein, P.A., et al., Proc. Nat. Acad. Sci. USA (1990) 87 (in press)]. These transesterification reactions generate a 2',3'-cyclophosphate and a 5'-hydroxyl as the two new end groups. Cleavage is at a CpA for the (+) polarity RNA and at an ApG for the (-) polarity RNA. We enzymically synthesized oligoribonucleotides with processing capability and with specific 35S-labeled phosphorothioate diesters in the Rp configuration. After processing had occurred, the terminal nucleoside-2',3'-cyclophosphorothioate diester residues were recovered from the appropriate product by digestion with nuclease and phosphatase. Comparisons with specially prepared endo- and exoisomer reference compounds by thin layer chromatography and autoradiography revealed that the [35S]cytidine- and [35S]adenosine-2',3'-cyclophosphorothioate both were endo-isomers. The results are consistent with transesterification occurring by an inline SN2(P) attack of the 2'-hydroxyl group in the autolytic processing reactions of both polarities of the satellite RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buzayan J. M., Feldstein P. A., Bruening G., Eckstein F. RNA mediated formation of a phosphorothioate diester bond. Biochem Biophys Res Commun. 1988 Oct 14;156(1):340–347. doi: 10.1016/s0006-291x(88)80846-5. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Feldstein P. A., Segrelles C., Bruening G. Autolytic processing of a phosphorothioate diester bond. Nucleic Acids Res. 1988 May 11;16(9):4009–4023. doi: 10.1093/nar/16.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzayan J. M., Hampel A., Bruening G. Nucleotide sequence and newly formed phosphodiester bond of spontaneously ligated satellite tobacco ringspot virus RNA. Nucleic Acids Res. 1986 Dec 22;14(24):9729–9743. doi: 10.1093/nar/14.24.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. J Am Chem Soc. 1970 Jul 29;92(15):4718–4723. doi: 10.1021/ja00718a039. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Feldstein P. A., Buzayan J. M., Bruening G. Two sequences participating in the autolytic processing of satellite tobacco ringspot virus complementary RNA. Gene. 1989 Oct 15;82(1):53–61. doi: 10.1016/0378-1119(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Potter B. V., Eperon I. C. Stereospecificity of nucleases towards phosphorothioate-substituted RNA: stereochemistry of transcription by T7 RNA polymerase. Nucleic Acids Res. 1987 May 26;15(10):4145–4162. doi: 10.1093/nar/15.10.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Sequences required for self-catalysed cleavage of the satellite RNA of tobacco ringspot virus. Gene. 1989 Oct 15;82(1):43–52. doi: 10.1016/0378-1119(89)90028-0. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Steger G. Nucleotide sequence predicts circularity and self-cleavage of 300-ribonucleotide satellite of arabis mosaic virus. Biochem Biophys Res Commun. 1988 Jul 15;154(1):318–325. doi: 10.1016/0006-291x(88)90687-0. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Bruening G., Russell M. L. RNA and capsid accumulation in cowpea protoplasts that are resistant to cowpea mosaic virus strain SB. Virology. 1984 Sep;137(2):371–381. doi: 10.1016/0042-6822(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Hayase Y., Iwai S., Kamiya H., Inoue H., Ohtsuka E. Design of RNA enzymes distinguishing a single base mutation in RNA. Nucleic Acids Res. 1989 Sep 12;17(17):7059–7071. doi: 10.1093/nar/17.17.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M., Iwai S., Ohtsuka E. Cleavage of specific sites of RNA by designed ribozymes. FEBS Lett. 1988 Nov 7;239(2):285–288. doi: 10.1016/0014-5793(88)80935-9. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Iwai S., Ohtsuka E. Construction of a series of several self-cleaving RNA duplexes using synthetic 21-mers. FEBS Lett. 1988 Feb 15;228(2):228–230. doi: 10.1016/0014-5793(88)80004-8. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- McSwiggen J. A., Cech T. R. Stereochemistry of RNA cleavage by the Tetrahymena ribozyme and evidence that the chemical step is not rate-limiting. Science. 1989 May 12;244(4905):679–683. doi: 10.1126/science.2470150. [DOI] [PubMed] [Google Scholar]
- Mei H. Y., Kaaret T. W., Bruice T. C. A computational approach to the mechanism of self-cleavage of hammerhead RNA. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9727–9731. doi: 10.1073/pnas.86.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Rajagopal J., Doudna J. A., Szostak J. W. Stereochemical course of catalysis by the Tetrahymena ribozyme. Science. 1989 May 12;244(4905):692–694. doi: 10.1126/science.2470151. [DOI] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. Mutagenesis analysis of a self-cleaving RNA. Nucleic Acids Res. 1989 Jul 25;17(14):5679–5685. doi: 10.1093/nar/17.14.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. RNA stem stability in the formation of a self-cleaving hammerhead structure. Nucleic Acids Res. 1989 Jul 25;17(14):5665–5677. doi: 10.1093/nar/17.14.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]



