Summary
The Bacillus subtilis extracytoplasmic function (ECF) σ factor σM is inducible by, and confers resistance to, several cell envelope acting antibiotics. Here, we demonstrate that σM is responsible for intrinsic β-lactam resistance, with σX playing a secondary role. Activation of σM upregulates several cell wall biosynthetic enzymes including one, PBP1, shown here to be a target for the beta-lactam cefuroxime. However, σM still plays a major role in cefuroxime resistance even in cells lacking PBP1. To better define the role of σM in β-lactam resistance we characterized suppressor mutations that restore cefuroxime resistance to a sigM null mutant. The most frequent suppressors inactivated gdpP (yybT) which encodes a cyclic-di-AMP phosphodiesterase (PDE). Intriguingly, σM is a known activator of disA encoding one of three paralogous c-di-AMP cyclases (DAC). Overproduction of the GdpP PDE greatly sensitized cells to β-lactam antibiotics. Conversely, genetic studies indicate that at least one DAC is required for growth with depletion leading to cell lysis. These findings support a model in which c-di-AMP is an essential signal molecule required for cell wall homeostasis. Other suppressors highlight the roles of ECF σ factors in counteracting the deleterious effects of autolysins and reactive oxygen species in β-lactam treated cells.
Keywords: ECF σ factor, β-lactam, cefuroxime, c-di-AMP, Bacillus subtilis
Introduction
The bacterial cell envelope is crucial for maintaining cell shape and counteracting turgor pressure and is an important target for many antimicrobial compounds (Walsh, 2003). The cell envelope of Bacillus subtilis contains a cytoplasmic membrane surrounded by layers of cross-linked peptidoglycan (PG), membrane-associated lipoteichoic acids (LTA), and wall-associated teichoic acids (WTA) (Foster & Popham, 2002, Scheffers & Pinho, 2005). PG is a polymer of alternating N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) glycan chains cross-linked by peptide sidechains. The newly synthesized lipid-linked NAG-NAM units are polymerized to glycan strands by the action of transglycosylase (TG). Concurrent with, or soon after this polymerization, the peptide side chains on the NAM residue are cross-linked by transpeptidase (TP). Both TG and TP are activities of high molecular weight penicillin binding proteins (HMW PBPs), and they are the targets of moenomycin and the β-lactam antibiotics, respectively (Waxman & Strominger, 1983, Macheboeuf et al., 2006, Foster & Popham, 2002).
β-lactam antibiotics are characterized by the presence of a β-lactam ring which mimics the D-Ala-D-Ala dipeptide substrate of HMW PBP and inhibits the transpeptidation reaction by covalent modification of the TG active site (Macheboeuf et al., 2006). This inhibition disrupts cell wall biosynthesis, triggers the formation of reactive oxygen species (ROS), and results in cell lysis and death (Kohanski et al., 2007, Kohanski et al., 2010, Gusarov et al., 2009). Synthesis and incorporation of new PG glycan strands into the existing cell wall requires close coordination between the biosynthetic machinery (including HMW-PBPs) and autolytic enzymes that allow the separation of already crosslinked glycan strands. When properly coordinated, the cell grows normally and maintains proper cell shape. Conversely, agents that prevent this coordination by inhibiting TG or TP activities of PBPs, or by activating autolysins, lead to lysis and cell death (Fig. 1). Several models have been advanced to explain how this coordination occurs, but the existence and precise architecture of the proposed biosynthetic holoenzyme is still unclear (Vollmer & Bertsche, 2008, Carballido-Lopez & Formstone, 2007).
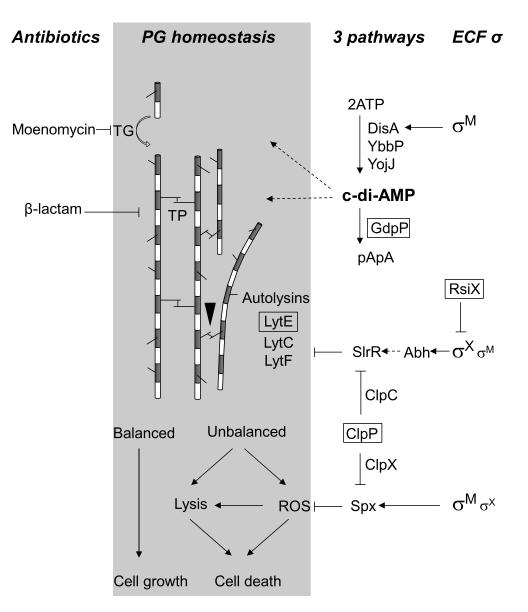
Fig. 1.
Model of peptidoglycan (PG) homeostasis and the contributions of σM and σX to cell wall antibiotic resistance. The alternating grey and white bars represent N-acetylmuramic acid and N-acetylglucosamine, respectively, which comprise the glycan chains. Peptide crosslinks between strands are introduced by transpeptidation (TP) and are broken by autolytic endopeptidases (black triangle). Moenomycin targets the transglycosylation (TG) step in glycan chain elongation while ß-lactams inhibit TP-mediated crosslinking. The results reported herein, combined with previous results (see text), indicate that σM and σX contribute to antibiotic resistance by three distinct pathways as shown on the right. Genes identified by Tn7 mutagenesis are boxed. ROS, reactive oxygen species; straight arrow, direct positive regulation; dashed arrow, indirect positive regulation; ---| negative regulation.
There are three major mechanisms that confer high level β-lactam resistance as described for the Gram positive genera Staphylococcus and Streptococcus and the Gram negative species Escherichia coli and Pseudomonas spp. These are: (i) expression of β-lactamase(s) that inactivate the antibiotics; (ii) expression of mutated or mosaic PBP alleles that have low affinity for β-lactams; and (iii) the expression of a β-lactam specific efflux pump (Poole, 2004, Wilke et al., 2005). B. subtilis displays a significant level of intrinsic resistance against a variety of β-lactam antibiotics, but the underlying mechanisms are poorly understood. Although there are three putative β-lactamase genes (penP, ybbE, and yblX) in the genome, no β-lactamase activity is detected in the growing cells or supernatant (Colombo et al., 2004). No penicillin-insensitive PBP alleles have been identified nor does an efflux pump-based mechanism appear to be applicable to B. subtilis and other Gram positive bacteria. Therefore, the molecular basis of this intrinsic, moderate level β-lactamase resistance is unclear. Recent results suggest that extracytoplasmic function (ECF) σ factors play a role in resistance to β-lactam antibiotics: a triple mutant (strain sigMWX) as well as a mutant lacking all 7 ECF σ factors (strain Δ7ECF) is sensitive to β-lactam antibiotics including ampicillin, penicillin G, aztreonam, and cefuroxime (Mascher et al., 2007, Luo et al., 2010).
B. subtilis harbors 7 ECF σ factors, σM, σX, σW, σV, σY, σZ and σYlaC. Of these, the physiological roles of σM, σW, σX, and more recently σV, have been well characterized, and their target regulons have been defined (Helmann, 2002, Jervis et al., 2007, Eiamphungporn & Helmann, 2008, Guariglia-Oropeza & Helmann, 2011). Both expression and activity of these ECF σ factors are often stimulated by cell wall-active antibiotics. σM is strongly induced by vancomycin and moenomycin, and confers resistance to moenomycin (Thackray & Moir, 2003, Mascher et al., 2007, Eiamphungporn & Helmann, 2008). Activation of the σW regulon contributes to resistance to fosfomycin, sublancin, and a toxic peptide SdpC (Cao et al., 2002, Butcher & Helmann, 2006). The σX regulon is involved in the resistance to nisin and other cationic antimicrobial peptides (Cao & Helmann, 2002, Cao & Helmann, 2004). Finally, σV is induced by and provides resistance to lysozyme (Ho et al., 2011, Guariglia-Oropeza & Helmann, 2011).
In this study, we investigated the roles of ECF σ factors in providing intrinsic resistance to β-lactam antibiotics and, in particular, to cefuroxime (CEF). We found that σM plays a primary role in β-lactam resistance, with σX as a secondary determinant. We identified Tn7 insertions mutations that restored CEF resistance to a sigM mutant. Genetic analysis reveals a central role for the recently identified signal molecule cyclic-di-AMP (c-di-AMP), synthesized in part by a σM-activated diadenylate cyclase (DAC), in cell wall homeostasis. In addition, our results highlight the key role of previously defined pathways by which ECF σ factors regulate autolysin activity and resistance to reactive oxygen species.
Results and Discussion
σM is the major ECF σ factor involved in the intrinsic resistance to cefuroxime
Previously, we showed that a null mutant lacking all 7 ECF σ factors (strain Δ7ECF) has higher sensitivity to numerous antibiotics (including several β-lactams) compared to the wild type (WT) strain (Luo et al., 2010). To clarify the role of ECF σ factors in mediating the intrinsic resistance to β-lactam antibiotics, we here sought to identify both the ECF σ factor(s) and the relevant pathways responsible for resistance using cefuroxime (CEF) as a model ß-lactam.
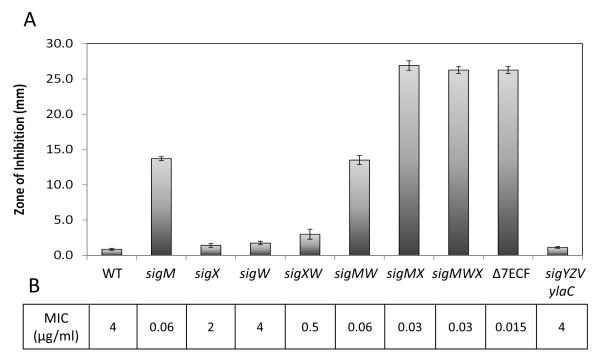
Isogenic strains carrying single or multiple mutations in genes encoding ECF σ factors were tested for CEF susceptibility using disk diffusion and minimal inhibition concentration (MIC) assays. A sigM null mutant showed elevated sensitivity to CEF, whereas other single mutants showed little or no change (Fig. 2). The double sigM sigX mutant displayed high sensitivity equivalent to the Δ7ECF strain. A sigW mutant showed no effect, although effects on ß-lactam resistance have been seen in other B. subtilis strain backgrounds (Lee et al., 2012). None of the other four ECF σ factors played a role in CEF resistance, even when a multiple mutant strain was tested (Fig. 2). We conclude that σM is the major ECF σ involved in the intrinsic resistance to CEF, with σX playing a secondary role apparent in strains lacking σM. These results suggest that the major resistance pathway(s) depend exclusively on σM for their expression, with one or more additional pathways that can be activated by either σM or σX (as revealed in the double sigM sigX mutant). As described previously, several ECF σ factor promoters can be recognized by more than one ECF σ factor (Huang et al., 1998, Qiu & Helmann, 2001, Mascher et al., 2007). As we will show later in this study, genes encoding the transcription factors Abh and Spx are recognized by both σM and σX and are involved in CEF resistance.
Fig. 2.
σM is the major ECF σ involved in the intrinsic resistance to CEF and σX plays a secondary role. A. The susceptibility of each strain was tested using disk diffusion assay with 6 μg CEF. The zone of inhibition is expressed as the total diameter of the clearance zone minus the diameter of filter paper disk (7mm). The means and SE from at least 3 biological replicates are reported. B. MIC values are shown under the bar graph.
Antibiotic resistance pathways are often transcriptionally activated in the presence of the cognate antibiotic. ECF σ factors typically autoregulate their own expressions and we and others have previously characterized the relevant autoregulatory promoters (Helmann, 2002, Asai et al., 2003, Thackray & Moir, 2003). We therefore monitored the effect of CEF on expression from the autoregulatory promoters for sigM, sigW, and sigX. In each case, a 2~3 fold induction was observed (Table 1). In contrast, low (basal) activity and no induction were detected for the other four ECF σ factors (sigY, sigV, sigZ, ylaC) (data not shown). This induction profile is consistent with prior results demonstrating that σM, σX and σW are responsive to cell envelope stress and are activated by an overlapping set of inducers (Mascher et al., 2007, Eiamphungporn & Helmann, 2008, Hachmann et al., 2009, Minnig et al., 2003).
Table 1. ECF σ promoter activities induced after treatment with 8 μg/ml CEF for 30 min.
Activities (Miller Units) were measured using β-galactosidase assays and the means and SE are reported.
| Reporter fusion | Untreated | CEF treated |
|---|---|---|
| PsigM-lacZ | 3.7±0.5 | 10.1±0.5 |
| PsigX-lacZ | 38.6±1.3 | 99.7±3.5 |
| PsigW-lacZ | 35.6±1.9 | 71.3±2.3 |
CEF targets PBP1, 2a, 2b and 4
The σM regulon is known to include several enzymes involved in various aspects of cell wall synthesis including one HMW PBP (PBP1, encoded by ponA) (Eiamphungporn & Helmann, 2008). In most cases, σM-dependent promoters serve to up-regulate gene expression in response to stress, but are not solely responsible for expression due to the presence of other promoters. In the case of ponA, this gene can be transcribed from two promoters: one is σM dependent, and the other is σA-dependent. We here hypothesized that one mechanism of resistance might be the σM-dependent upregulation of PBP1 or other factors involved in assembly or function of cell wall biosynthetic complexes.
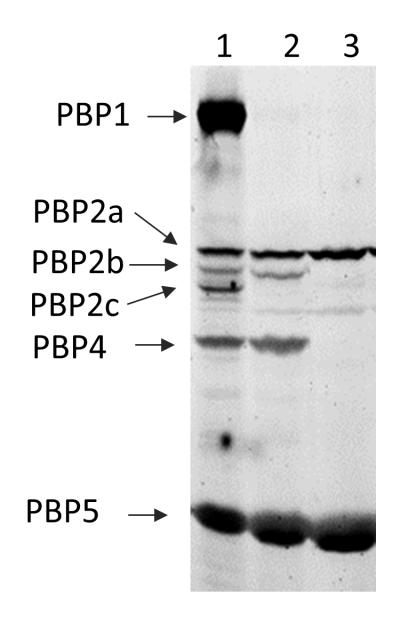
To identify the targets of CEF, we performed bocillin-FL competitive labeling assays (Zhao et al., 1999, Kawai et al., 2009). Five HMW PBPs (PBP1, 2a, 2b, 2c, 4) and one low molecular weight penicillin-binding protein (LMW PBP) (PBP5) were detected by bocillin-FL labeling and CEF competed with bocillin-FL for binding to PBP1, 2b, 2c and 4 (Fig. 3). Since only six PBPs can be detected in this assay, it is possible that other PBPs are also targets for CEF. No differences in either PBP profile or relative affinity for CEF binding were apparent in a comparison of the CEF sensitive sigMWX mutant and the WT strain using the bocillin-FL labeling assay (data not shown). This suggests that mutants lacking ECF σ factors are not altered in their CEF susceptibility due to a gross change in the levels of PBPs.
Fig. 3.
CEF binds to PBP 1, 2b, 2c, and 4. PBPs in vegetatively growing cells were labeled with bocillin-FL (lane 1). The binding of bocillin-FL to PBPs was subjected to competitive inhibition by the addition of aztreonam (Lane 2) or CEF (Lane 3). Proteins were separated by 4~12% gradient SDS-PAGE, and visualized by using a Typhoon Fluorimager.
Since PBP1 is a target for CEF, we hypothesized that σM-mediated upregulation of PBP1 might contribute to β-lactam resistance. However, deletion of ponA did not alter CEF susceptibility (a ponA null mutant and WT have an identical zone of inhibition). Thus, upregulation of PBP1 by σM does not appear to be a major mechanism of CEF resistance. We next tested whether B. subtilis expresses β-lactamase using the chromogenic substrate nitrocefin (Ross et al., 2009). No β-lactamase activity could be detected (either prior to or after CEF treatment) in the WT, sigMWX or Δ7ECF strains (data not shown). Thus, the role of ECF σ factors in CEF resistance does not appear to be due to alterations in CEF targets or due to degradation by β-lactamases.
Tn7 mutagenesis reveals multiple pathways involved in CEF resistance
To gain insights into the pathways contributing to CEF resistance, we performed Tn7 transposon mutagenesis and selected for mutations that restored CEF resistance to a sigM mutant. The Tn7 transposon derivative we used harbors an outward-facing, xylose-inducible promoter which thereby allows recovery of both loss of function (gene disruption) and gain of function (xylose-dependent up-regulation) mutations (Bordi et al., 2008). Insertion libraries were generated in vitro using WT genomic DNA as a target and then transformed into competent B. subtilis cells with selection for both the transposon (spcR) and CEF resistance. In an initial study, we recovered numerous insertions linked to sigM. In these strains, a functional copy of sigM had been co-transformed into the recipient cells. Although this result confirms the importance of σM in CEF resistance, it was otherwise uninformative. Therefore, all subsequent experiments used a Tn7 mutant library generated in a sigM mutant (HB10216) background.
A total of 520 CEF resistant colonies were obtained in 10 separate experiments. DNA sequence analysis identified 25 unique insertions localized to 10 different genes (Table 2). All of the insertions increased CEF resistance in a sigM mutant, although none restored resistance to WT levels (Table 2). The most frequently observed insertion occurred in yybT, an ortholog of a gene recently renamed gdpP (see below). We therefore performed an additional round of selection, transforming the sigM Tn7 library into a sigM gdpP double mutant strain (HB10257). This selection led to the recovery of insertions in two genes (lytE and clpP). Both triple mutants (sigM gdpP lytE::Tn7 and sigM gdpP clpP::Tn7) were at least as CEF resistant as WT (Table 2). These results indicate that gdpP likely affects a different resistance pathway than lytE and clpP. Although our selection plates contained xylose, in no case was CEF resistance dependent on xylose suggesting that in each case we have recovered gene disruption mutations that lead to increased CEF resistance.
Table 2.
Tn7 insertions that can restore CEF resistance in a sigM or a sigM gdpP mutant.
| Tn7 Mutants | Unique insertions |
Gene annotation | Resistance to CEFa |
Growth rate relative to WT (%)b. |
|---|---|---|---|---|
| Insertions in a sigM background | ||||
| gdpP::Tn7 | 11 | phosphodiesterase | ++ | 98 |
| rsiX::Tn7 | 1 | anti-sigma X | ++ | 100 |
| lytE::Tn7 | 1 | autolysin | ++ | 98 |
| pbpX::Tn7 | 1 | penicillin-binding endopeptidase X | ++ | 95 |
| tagA::Tn7 | 1 | wall teichoic acid biosynthesis | ++ | 81 * |
| ymdB::Tn7 | 1 | Regulate expression of SlrR | + | 68 * |
| kinD::Tn7 | 1 | negative regulator of Spo0A~P | + | 95 |
| spo0A::Tn7 | 2 | initiation of sporulation | + | 102 |
| qoxAB:Tn7 | 3 | cytochrome aa3-600 quinol oxidase | + | 52 * |
| ssrA::Tn7 | 1 | transfer-messenger RNA (tmRNA) | + | 79 * |
| Insertions in a sigM gdpP background | ||||
| lytE::Tn7 | 2 | autolysin | +++ | 96 |
| clpP::Tn7 | 2 | ATP-dependent Clp protease proteolytic subunit |
++++ | 81 * |
The resistance to CEF was tested using disk diffusion assay with biological triplicates, and repeated twice. The zone of inhibition (mean ± SE) was used for the score. The resistance level of wt is defined as “+++”, and ΔsigM is “-”.
The sigM strain has the same growth rate as WT (100%). Strains with noticeably reduced growth rates are labeled with *.
Genes identified in this suppressor mutation are involved in a variety of pathways and functions (Table 2). We categorized them into three groups using two criteria: (i) direct or indirect involvement in cell wall metabolism, and (ii) mild or strong effect on CEF resistance. The first group included several insertions that inactivated genes directly involved in cell wall metabolism including lytE, pbpX, tagA, and ymdB. LytE is a major autolytic endopeptidase in vegetative cells (Margot et al., 1998, Smith et al., 2000). LytE interacts with the actin-like protein MreBH along the cylindrical part of cell wall and with FtsZ and PBP2b at the division septum. It is, therefore, closely related to cell wall synthesis (Carballido-Lopez et al., 2006). The inactivation of lytE presumably increases ß-lactam resistance by delaying cell lysis. PbpX is a LMW PBP that is located at the septum during vegetative growth (Scheffers et al., 2004). Its function is unknown, although it was shown previously to be activated by σX (Cao & Helmann, 2004). YmdB was recently reported to regulate the expression and/or activity of a transcriptional regulator SlrR, which in turn affects the activity of both σD and the regulator of biofilm formation, SinR, and likely indirectly modulates autolysin activity (Diethmaier et al., 2011). Finally, TagA is a key enzyme in the synthesis of teichoic acids, a major component of the cell wall (Mauel et al., 1991, D’Elia et al., 2009). The second and third groups of insertions are not directly linked to cell wall homeostasis. The second group, including kinD, spo0A, qoxAB and ssrA insertions, had relatively mild effects on CEF resistance. Further studies are needed to define the mechanisms of these effects, but in several cases the mutant strains grew more slowly than WT strain under our experimental conditions and this may contribute to their increased ß-lactam resistance (Table 2).
Here, we focus on the third group of mutations (gdpP, rsiX, and clpP) for further analysis since they resulted in strong CEF resistance and have been linked to σM and its regulon members. We recovered 11 independent insertions within the 1980 bp coding sequence of gdpP (formerly yybT). GdpP is a transmembrane protein containing three functional domains: a heme-binding PAS domain, a degenerate GGDEF domain, and a DHH/DHHA1 phosphodiesterase (PDE) domain (Rao et al., 2010, Rao et al., 2011). The S. aureus ortholog has recently been renamed GdpP to indicate that it is a GGDEF domain protein containing phosphodiesterase (Corrigan et al., 2011) and we therefore adopt this same designation for B. subtilis. RsiX is the anti-σ factor cognate for σX. We hypothesized that the rsiX::Tn7 insertion increased ß-lactam resistance by upregulation of σX. Tn7 insertions in clpP led to the highest level of CEF resistance observed in this study (Table 2). ClpP is a component of the Clp protease. In B. subtilis, the ClpP proteolytic core can pair with any of the three Clp ATPases (ClpX, ClpC and ClpE) and form a large hetero-oligomeric Clp protease. Clp protease recognizes and degrades a wide range of proteins, including non-native proteins and stress response regulators, and it is therefore involved in multiple cell development and stress response pathways (Frees et al., 2007). Here, we present evidence that these three insertion mutations affect three inter-related pathways for CEF resistance (Fig. 1).
The role of σX in CEF resistance is in part through regulation of abh and spx
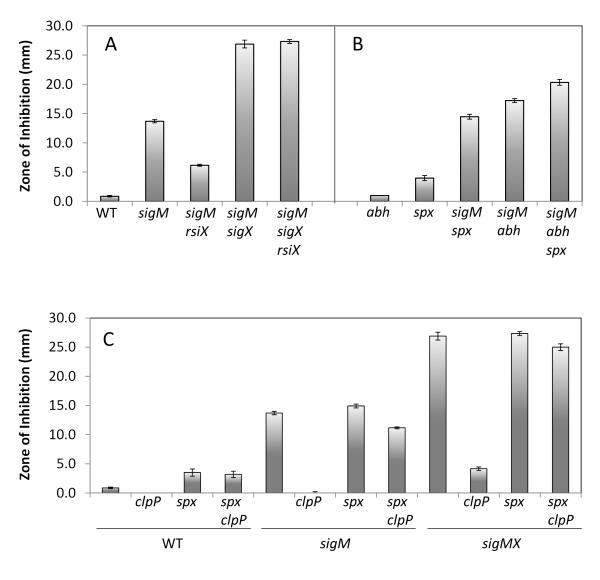
We hypothesized that the Tn7 insertion in rsiX restored CEF resistance by up-regulation of σX which, as noted above, plays a secondary role in CEF resistance that becomes important in the absence of σM (Fig. 1). As predicted, epistasis experiments indicated that σX is downstream of RsiX: a sigM sigX rsiX strain was as sensitive to CEF as the sigM sigX strain (Fig. 4A).
Fig. 4.
The role of rsiX and clpP mutations in CEF resistance. A. Increased CEF resistance due to an rsiX null mutation depends on σX. B. abh and spx mutations are additive to sigM with respect to CEF sensitivity. C. Increased CEF resistance due to a clpP null mutation depends on Spx in all three strain backgrounds. For ease of comparison, some strains are shown in multiple panels. The susceptibility of each strain was tested using disk diffusion assays with 6 μg CEF. The means and SE at least from 3 biological replicates and 2 independent experiments are reported.
Since the effect of sigX on CEF resistance is greatly enhanced in a sigM mutant background (Figs. 2 and 4A), we hypothesized that the relevant genes involved in CEF resistance can be activated by either σM or σX. The regulons of σM and σX have been characterized, and several target promoters have been defined that are activated by both ECF σ factors (Eiamphungporn & Helmann, 2008, Cao & Helmann, 2004). We chose six such target operons (abh, spx, dltABCDE, lytR, yceCDEF, and bcrC) for further analysis. In a WT background, only the spx null mutant showed increased CEF susceptibility. When introduced into the sigM null mutant, the abh and spx mutations both increased CEF sensitivity (Fig. 4B). The abh and spx CEF sensitive phenotypes in both sigM and sigMX background can be complemented using IPTG-inducible abh or spx alleles, respectively (Figs. S1 and S2). These results suggest that spx and abh can account for at least part of the role of σX in CEF resistance. We also defined the MIC of single and multiple mutant strains of sigM, abh, and spx. Although their differences in CEF susceptibility are readily detected in the disk diffusion assay (Fig. 4A and B), mutant strains of sigMX, sigM abh, sigM abh spx have the same MIC of 0.03 μg/ml when measured in liquid medium (Table S3). We therefore focus here on the differences observed on solid medium.
Abh is a paralog of AbrB and together these two transition state regulators regulate biofilm formation, autolysin activity, and antibiotic production and resistance (Strauch et al., 2007, Murray et al., 2009, Luo & Helmann, 2009, Murray & Stanley-Wall, 2010). The transcription of abh is dependent on σX and σM, with σX being the major regulator (Huang & Helmann, 1998, Luo & Helmann, 2009). Recently, an abh mutant was shown to be sensitive to β-lactam antibiotics ampicillin, carbenicillin, and cephalexin (Murray & Stanley-Wall, 2010). Resistance to ampicillin was restored by inducing the expression of the transcriptional regulator slrR, or by inactivating genesencoding major autolysins (lytC and lytF encoding amidase and DL-endopeptidase, respectively) (Murray & Stanley-Wall, 2010). These results support a model (Fig. 1) in which Abh indirectly activates the expression of SlrR (Murray et al., 2009). SlrR forms a heteromeric complex with SinR which represses both the lytABC and lytF operons (Chai et al., 2010b). Thus, σX and σM play partially redundant roles in ß-lactam resistance by activating Abh, which in turn activates SlrR to enable repression of autolytic enzymes.
Accumulation of Spx can increase CEF resistance
Next, we investigated the genetic basis for increased CEF resistance in the clpP mutant strains. Several of the reported phenotypes of clpP mutants have been linked to increased accumulation of Spx (Nakano et al., 2001, Nakano et al., 2003), a global regulator of oxidative stress responses (Zuber, 2009). There are at least four promoters that control expression of Spx, including one activated by σM and σX (Eiamphungporn & Helmann, 2008). Previously, we determined that spx was transcriptionally activated ~3-fold by vancomycin in a σM-dependent manner (Eiamphungporn & Helmann, 2008) and a similar induction was also reported by Jervis et al. (2007) using lacZ-fusions. Other cell wall antibiotics also induce the Spx regulon including amoxicillin (Hutter et al., 2004, Eiamphungporn & Helmann, 2008) and enduracidin (Rukmana et al., 2009).
β-lactam antibiotics trigger the production of ROS (Kohanski et al., 2007, Gusarov et al., 2009), and Spx is known to protect against oxidative stress (Nakano et al., 2003, Choi et al., 2006, Pamp et al., 2006, You et al., 2008). We therefore hypothesized that the upregulation of Spx by σM might provide a pathway by which ECF σ factors contribute to antibiotic resistance (Fig. 1). Indeed, in S. aureus mutation of the adaptor protein YjbH was recently found to lead to a modest increase in β-lactam resistance which may be due to stabilization of Spx (Gohring et al., 2011).
We used a genetic approach to explore the role of ClpP and Spx in ß-lactam resistance. As noted above (Table 2), a clpP::Tn7 mutation greatly increased CEF resistance in the sigM gdpP mutant strain (HB10264). The clpP null mutation also increased CEF resistance in WT and null mutant strains of sigM and both sigM and sigX (Fig. 4C). Spx is a ClpXP substrate (Nakano et al., 2002). The spx mutation masked the effect of clpP in the WT, sigM, and sigM sigX strain backgrounds (Fig. 4C). These epistasis results imply that spx is downstream of clpP in the CEF resistance pathway and is the major ClpP substrate that plays a role in ß-lactam resistance. Thus, we predict that the major impact of the clpP mutation is to enhance accumulation of Spx in the cell. To test this idea, an IPTG inducible copy of spx or spxDD (a Clp protease insensitive variant; Nakano et al., 2003) was introduced in the sigM and sigM sigX mutant strains. An increase in CEF resistance was observed when either spx or spxDD was induced (although the effect was much more dramatic with the protease-insensitive allele), suggesting that the accumulation of Spx can increase resistance to CEF in B. subtilis (Fig. S2). In addition, we performed disk diffusion assays with strains lacking either clpX or clpC (Fig. S3). Deletion of clpX can strongly increase CEF resistance in both strain backgrounds of WT and sigM mutant, while deletion of clpC only showed minor effect. This result is consistent with the major role of ClpP in CEF resistance being the ClpXP-dependent degradation of Spx.
We also note that the effect of the clpP mutation may not be limited to enhancing accumulation of Spx, since mutation of clpP also led to a small increase in CEF resistance in an spx mutant background. This effect was most notable in strains mutant for sigM or sigM and sigX (Fig. 4C). A small increased in CEF resistance was also found with a clpC mutant (Fig. S3). Therefore, we suggest that there are other ClpP protease substrates that also contribute, albeit modestly, to CEF resistance. One candidate is SlrR which, as noted above, has been implicated in the down-regulation of autolysins and is subjected to degradation by ClpCP (Chai et al., 2010a) (Fig. 1). A second candidate and a ClpCP-degraded substrate is MurAA. MurAA is a UDP-N-acetylglucosamine 1-carboxyvinyltransferase, which catalyzes the first committed step in PG biosynthesis (Kock et al., 2004).
c-di-AMP as an emerging second messenger found in Bacteria
The most frequent insertions recovered in our selection (Table 2) were in gdpP and inactivate a PDE known to degrade c-di-AMP, an emerging second messenger found in Bacteria and likely in Archaea (Romling, 2008). c-di-AMP was discovered as a metabolite bound in the crystal structure of DisA which catalyzes its synthesis from ATP (Witte et al., 2008). DisA was initially characterized as a DNA integrity scanning protein that signals the integrity of the DNA and thereby enables sporulation to proceed (Bejerano-Sagie et al., 2006). This led to a model in which the DisA diadenylate cyclase (DAC; DUF147 domain) signals chromosome integrity: DAC activity can be strongly inhibited by binding of DisA to branched chain nucleic acid structures that might form as recombination intermediates.
DisA is the only confirmed c-di-AMP cyclase (DAC) in B. subtilis (Witte et al., 2008, Oppenheimer-Shaanan et al., 2011). However, B. subtilis encodes two additional candidate DAC proteins (containing DUF147 domains): YbbP and YojJ (Romling, 2008). The DisA DAC domain is linked to a helix-hairpin-helix non-specific DNA-binding domain which allows DAC activity to be regulated by DNA integrity. In contrast, YbbP is predicted to be membrane-localized and YojJ cytosolic, but little is known of how their activities might be regulated. Of relevance to the present study, transcription of disA is regulated by both σA and σM (Eiamphungporn & Helmann, 2008).
The level of c-di-AMP in the cell is controlled by both its rate of synthesis by DAC and its degradation by a c-di-AMP specific phosphodiesterase (PDE) (Fig. 1). B. subtilis GdpP (formerly YybT) is a c-di-AMP PDE in vitro (Rao et al., 2010, Rao et al., 2011) and in vivo (Oppenheimer-Shaanan et al., 2011). In vegetatively growing B. subtilis, 1.7 μM c-di-AMP was measured which increased, in a DisA-dependent manner, to near 5 μM early during sporulation. A gdpP deletion strain of B. subtilis was shown to have a >4-fold increase in c-di-AMP levels in early sporulating cells (Oppenheimer-Shaanan et al., 2011). Similarly, a ~15 fold increase was observed with a gdpP mutation in S. aureus (from 2.8 μM to 42.9 μM). In S. aureus, elevated levels of c-di-AMP suppress the growth defects associated with an inability to synthesize LTA and alter both autolysin expression and the level of PG crosslinking (Corrigan et al., 2011).
In B. subtilis, the synthesis and degradation of c-di-AMP is correlated with ß-lactam resistance
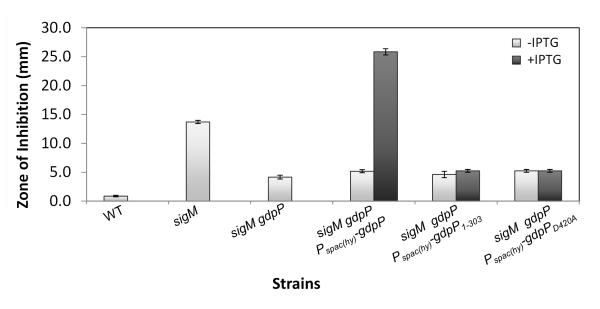
GdpP is a transmembrane protein with three functional domains: a heme-binding PAS domain, a degenerate GGDEF domain, and a DHH/DHHA1 PDE domain (Rao et al., 2010, Rao et al., 2011). In accordance with the emerging model of c-di-AMP as a signal molecule, we hypothesized that it was the loss of GdpP PDE activity that conferred CEF resistance. We therefore complemented the sigM gdpP strain with an IPTG-inducible GdpP, a truncated GdpP lacking the DHH/DHHA1 domain (GdpP1-303), or a mutated GdpP (GdpPD420A) carrying a single amino acid substitution which abolishes PDE activity (Rao et al., 2010). Induction of WT GdpP conferred an extreme CEF sensitivity (Fig. 5). In contrast, neither of the mutant GdpP proteins increased sensitivity to CEF (Fig. 5), suggesting that it is the PDE activity that affects CEF sensitivity.
Fig.5.
The DHH/DHHA1 domain of GdpP is required to restore CEF sensitivity to the resistant strain sigM gdpP. Disk diffusion tests were performed with 6 μg CEF. The means and SE based on 3 biological replicates and 2 independent experiments are shown. 1 mM IPTG was added where indicated.
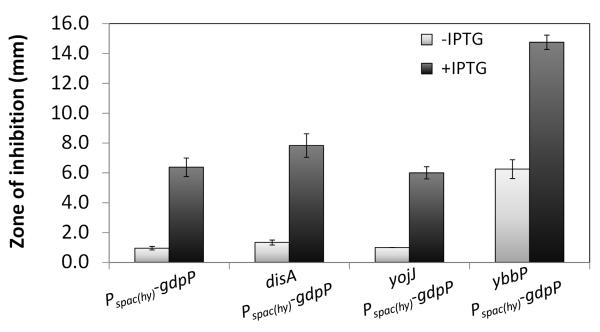
One consequence of antibiotic stress is the activation of σM which leads to elevated expression of the DisA DAC. We therefore hypothesized that a sigM null mutant might have decreased c-di-AMP levels that could be compensated by mutation of GdpP, the c-di-AMP degrading PDE. Indeed, a disA deletion mutant displayed a small but reproducible increase in sensitivity to CEF, with an MIC of 3 μg/ml compared to 4 μg/ml for WT (Table S3). This is consistent with the recent report that DisA accounts for perhaps 50% of the c-di-AMP present in cells as monitored early in sporulation (Oppenheimer-Shaanan et al., 2011). As expected, the induction of GdpP in the disA mutant led to a large increase in CEF susceptibility (Fig. 6), consistent with the notion that even disA cells contain substantial c-di-AMP that contributes to CEF resistance. This suggests that B. subtilis contains at least one additional DAC, presumably encoded by either or both the DAC-domain containing proteins YbbP and YojJ.
Fig.6.
Induction of the GdpP PDE increases CEF sensitivity in WT and cells individual DAC enzymes. Disk diffusion tests were performed with 6 μg CEF. The means and SE based on 3 biological replicates and 2 independent experiments are reported. 1 mM IPTG was added where indicated.
c-di-AMP is essential for cell growth
To gain insights into the relative contributions of disA, ybbP, and yojJ to c-di-AMP synthesis we mutated each of these loci individually and in combination. Deletion of ybbP resulted in the highest CEF sensitivity (as seen in the uninduced sample in Fig. 6, and MIC of 1 μg/ml, Table S3). Deletion of yojJ, however, had no effect. Induction of GdpP increased CEF sensitivity in all three DAC mutant backgrounds (Fig. 6). We conclude that YbbP is the major DAC contributing to intrinsic β-lactam resistance in growing cells, and that both synthesis and degradation of c-di-AMP affects CEF resistance. This result is consistent with the recent suggestion that DisA functions primarily in early sporulation, with a comparatively minor contribution in (unstressed) vegetative phase cells (Oppenheimer-Shaanan et al., 2011). It is interesting to note that YbbP and GdpP are both membrane-localized, although the signals that might control their synthesis and activity are unknown.
The expression of YbbP is poorly characterized, but it is noteworthy that it is encoded immediately downstream of the sigW-rsiW operon and it may be, in part, activated by σW. However, σW has no effect in CEF resistance in our B. subtilis WT strain background (Fig. 2). We therefore asked whether σM or σX have a role in regulating ybbP. Multiple null mutants of sigM, sigX, and ybbP were constructed and tested for their susceptibilities to CEF. The mutation in ybbP is clearly additive to both sigM and sigX mutations (Fig. S4). In addition, the transcriptional start site of ybbP was mapped to 72 bp upstream of its start codon using 5′RACE. A σA promoter is present upstream of the assigned start site (TTCACTtgctaaatcgaaatgtggTATAATgggctcG; upper case letters indicate the −35, −10, +1 regions, respectively). Together, these results suggest that ybbP is not part of the σM or σX regulatory pathways.
We next sought to construct double and triple null mutants of disA, ybbP, and yojJ. A disA ybbP double mutant strain could not be obtained, suggesting that this combination of mutations is lethal, whereas double mutants of disA yojJ and ybbP yojJ were viable. We conclude that c-di-AMP is essential for viability and that the basal level of expression of either DisA or YbbP is sufficient to support growth. An essential role for DAC proteins has also been suggested in Listeria monocytogenes since it was impossible to disrupt the single DAC encoding gene in this organism (Woodward et al., 2010). Similarly, DAC genes were identified in screens for essential genes in Mycoplasma spp., Streptococcus pneumoniae, and S. aureus (Chaudhuri et al., 2009, Glass et al., 2006, French et al., 2008, Song et al., 2005).
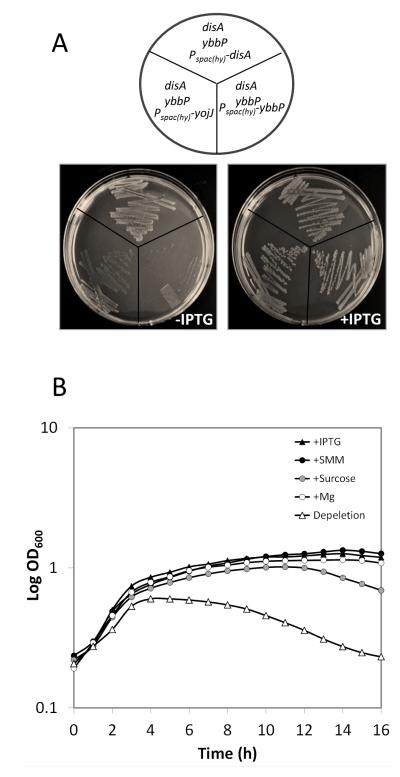
To determine whether all three DAC proteins (DisA,YbbP, YojJ) are active and could support growth, we integrated an IPTG-inducible copy of each gene into a ybbP null mutant and then attempted to introduce a disA null mutation by chromosomal transformation. Indeed, a disA ybbP double mutant could be obtained when any one of the three genes (disA, ybbP, or yoj) was induced (Fig. 7A). This strategy also allowed construction of IPTG-dependent disA ybbP yojJ triple mutant strains in which growth could be supported by any one of three DAC-encoding genes. We note that the Pspac(hy) promoter used in this work is slightly leaky and, as a result, the disA ybbP Pspac(hy)-disA strain was able to grow even in the absence of IPTG. However, the disA ybbP Pspac(hy)-yojJ strain grew slowly and the disA ybbP Pspac(hy)-ybbP was unable to grow unless at least 50 μM IPTG was present (data not shown). These results suggest that all three of these putative DAC proteins are biologically active and able to support growth when expressed.
Fig.7.
disA and ybbP are synthetically lethal. A. Strains of disA ybbP harboring IPTG inducible disA, ybbP or yojJ were grown on MH agar plates supplemented with or without 1 mM IPTG. B. Depletion of ybbP in strain disA ybbP Pspac(hy)-ybbP results in cell lysis. Cells were grown in presence of 1 mM IPTG to mid-log phase, washed, resuspended in fresh MH medium alone or with additional 1 mM IPTG, SMM, 10% sucrose or 10 mM Mg, and returned to 37°C incubation with vigorous shaking. Growth was measured by OD600 using a Bioscreen incubator. Ten biological replicates were tested, and showed similar growth pattern. Growth curves from one representative experiment are shown.
The essential role of c-di-AMP is linked to PG homeostasis
Since a reduced level of c-di-AMP is linked to high CEF sensitivity, we tested whether c-di-AMP is involved in cell wall homeostasis. Depletion of c-di-AMP in strain disA ybbP Pspac(hy)-ybbP by growth in the absence of inducer IPTG led to cell lysis as monitored both by following optical density (Fig. 7B) and by light microscopy (Fig. S5). The lysis phenotype can be suppressed either by the presence of IPTG (inducing the expression of ybbP), or by supplementation of the growth medium with SMM (sucrose, MgSO4 and maleic acid), sucrose, or MgSO4. SMM has been used previously to stabilize protoplasts and support the growth of cell wall-free L-form cells (Chang & Cohen, 1979, Leaver et al., 2009). Similarly, sucrose likely functions as an osmotic protectant, and Mg2+ has been shown to restore growth and WT morphology of many PG defective mutants including single mutants of ponA, rodA, mreB, mreC, mreD, mbl and a double mutant of pbpAH (Murray et al., 1998, Formstone & Errington, 2005, Leaver & Errington, 2005, Kawai et al., 2009, Schirner & Errington, 2009, Kawai et al., 2011). This is reminiscent of recent results from Corrigan et al. (2011) who showed that osmotic protectants support the growth of a LTA deficient mutant of S. aureus and that this requirement can be bypassed by a gdpP mutation. The S. aureus gdpP mutant displayed an increase in both c-di-AMP and PG cross-linking. Collectively, these results suggest that c-di-AMP plays an essential role in PG homeostasis (Fig. 1).
σM and c-di-AMP are involved in resistance to other cell wall antibiotics
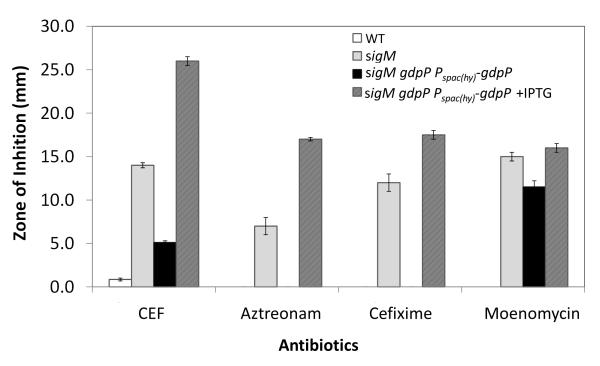
We next tested whether c-di-AMP is involved in resistance to other antibiotics. Induction of GdpP in strain sigM gdpP Pspac(hy)-gdpP leads to high sensitivity to aztreonam, cefixime, and moenomycin in addition to CEF as monitored using disk diffusion assays (Fig. 8). Cefixime is a third generation cephalosporin, aztreonam is a monobactam, and moenomycin is a glycolipid. As β-lactams, cefixime and aztreonam target PBP transpeptidases. Moenomycin, on the other hand, targets the TG activity of HMW-PBPs (Lovering et al., 2007). Although aztreonam is generally found to have poor activity against Gram positive bacteria (Georgopapadakou et al., 1982, Guay & Koskoletos, 1985) we observed using bocillin-FL labeling that aztreonam can derivatize PBP 1, 2c, and 4 in B. subtilis (Fig. 3). As also noted for CEF, mutation of sigM converts B. subtilis from an aztreonam non-susceptible to a susceptible strain, and this susceptibility is modulated by gdpP (Fig. 8). Thus, the function of c-di-AMP is not limited to CEF resistance, as would be expected if it functions to support balanced cell wall synthesis.
Fig. 8.
c-di-AMP is involved in intrinsic resistance to other cell wall antibiotics. Disk diffusion tests were performed with CEF (6 μg), aztreonam (30 μg), cefixime (5 μg) and moenomycin (50 μg). The means and SE from biological triplicates are shown. Note that no zone of inhibition could be detected with aztreonam or cefixime in WT and the uninduced sigM gdpP Pspac(hy)-gdpP strain.
A model for the role of ECF σ factors in ß-lactam resistance
The genetic analyses presented herein lead to an integrated model in which the ECF σ factors σM and σX contribute to ß-lactam resistance by the antibiotic-inducible activation of regulatory proteins that affect three distinct pathways (Fig. 1). B. subtilis PG is a dynamic structure, which is continuously synthesized, modified, and hydrolyzed. It is notable that σM-activated promoters have been previously mapped preceding several genes involved in PG synthesis (including mreB, bcrC, divIB, divIC, ddl, murB, murF, rodA, pbpX, and ponA), one of the four paralogous LTA synthases (yfnI), and cell wall modification enzymes (dltABCDE) (Eiamphungporn & Helmann, 2008). Thus, σM appears to function to positively regulate cell wall assembly and structure in response to antibiotic stress. β-lactam antibiotics inhibit the TP activity of PBPs and thereby inhibit glycan strand cross-linking. This inhibition disrupts the balance between PG synthesis and hydrolysis and endogenous autolysins trigger cell lysis. In addition, ß-lactams trigger ROS formation and cell death. Both autolysin-dependent and independent mechanisms contribute to the bactericidal effect (Dubee et al., 2011, Kohanski et al., 2007).
ECF σ factors counteract the effects of ß-lactams by activating at least three distinct pathways (Fig.1). First, σM contributes to the expression of one of three c-di-AMP synthases (DisA). The cellular level of c-di-AMP is regulated by both DAC synthases (DisA, YbbP and YojJ) and the cognate PDE (GdpP). At least one DAC is required for cell growth, indicating an essential role of c-di-AMP. The cell lysis phenotype of our DAC depletion strain together with the recent report from Corrigan et al. (2011) suggest a positive link between c-di-AMP and PG cross-linking. However, the role of c-di-AMP may be not limited to cross-linking, since c-di-AMP also modulates susceptibility to moenomycin, which targets the TG domain of PBP and thereby inhibits the polymerization of the PG glycan strands.
Second, ECF σ factors affect the expression and regulation of autolysins. Both σM and σX activate the transcription of abh, whose product indirectly activates the expression of SlrR, which directly represses expression of LytC and LytF (Luo & Helmann, 2009, Murray & Stanley-Wall, 2010, Chai et al., 2010b). Another autolytic endopeptidase (LytE) was identified by Tn7 mutagenesis as a contributor to ß-lactam susceptibility. These findings support the notion that preventing autolysis can increase ß-lactam resistance.
Third, our analysis of the ß-lactam resistance phenotype of a clpP null mutant identified Spx, a regulator of pathways that protect the cell against ROS (Zuber, 2009), as a contributor to ß-lactam resistance. The clpP mutant strain may also have elevated levels of SlrR, a known inhibitor of autolysin expression (Chai et al., 2010a). Although the model we have developed here (Fig. 1) is already quite complex, it certainly underestimates the true complexity of the adaptive responses mediated by ECF σ factors and other regulators that conspire to protect cells against antibiotics and other chemical insults.
Experimental Procedures
Bacterial strains and growth conditions
B. subtilis strains used are derivatives of strain168 (trpC2) and are shown in Table 3. Escherichia coli strain DH5α was used for standard cloning procedures. Bacteria were grown in Luria-Bertani (LB) (10 g tryptone, 5 g yeast extract and 5 g NaCl per liter) broth at 37°C with vigorous shaking. Antibiotics were added to the growth medium when appropriate: 100 μg/ml ampicillin for E. coli, and 1 μg/ml erythromycin plus 25 μg/ml of lincomycin (MLS, macrolide-lincomycin-streptogramin B resistance), 10 μg/ml chloramphenicol, 100 μg/ml spectinomycin (Spc), 5 μg/ml tetracycline and 10 μg/ml kanamycin for B. subtilis. OD600 readings were taken on a Spectronic 21 spectrophotometer.
Table 3.
Strains used in this study
| Strain1 | Genotype | Reference / construction2 |
|---|---|---|
| 168 | trpC2 | lab strain |
| CU1065 | trpC2 attSPβ | lab strain |
| PS832 | Prototrophic revertant of strain 168 | lab strain |
| BSU2007 | 168 sigMWXYZV ylaC (Δ7ECF) | (Asai et al., 2008) |
| HB0031 | CU1065 sigM::kan | (Cao et al., 2002) |
| HB10216 | 168 sigM::kan | chrDNA of HB0031 -->168 |
| HB10016 | 168 sigM::tet | (Luo & Helmann, 2009) |
| HB10103 | 168 sigX::kan | (Luo & Helmann, 2009) |
| HB10102 | 168 sigW::mls | (Luo & Helmann, 2009) |
| HB10114 | 168 sigX::kan, sigW::mls | (Luo & Helmann, 2009) |
| HB10117 | 168 sigM::tet, sigW::mls | (Luo & Helmann, 2009) |
| HB10113 | 168 sigM::tet sigX::kan | (Luo & Helmann, 2009) |
| HB7007 | CU1065 sigX::spc | (Huang et al., 1997) |
| HB15815 | 168 sigM::kan sigX::spc | chrDNA of HB7007 --> HB10216 |
| HB10107 | 168 sigM::tet, sigX::kan sigW::mls | (Luo & Helmann, 2009) |
| HB10236 | 168 sigZ::kan sigV::cat sigY::mls ylaC::spc | (Luo et al., 2010) |
| HB5421 | CU1065 amyE::PsigX-lacZ cat | Lab strain |
| HB5422 | CU1065 amyE::PsigW-lacZ cat | Lab strain |
| HB5423 | CU1065 amyE::PsigM-lacZ cat | Lab strain |
| HB10183 | 168 amyE::PsigM-lacZ cat | chrDNA of HB5423 --> 168 |
| HB10184 | 168 amyE::PsigX-lacZ cat | chrDNA of HB5421 --> 168 |
| HB10185 | 168 amyE::PsigW-lacZ cat | chrDNA of HB5422 --> 168 |
| PS2062 | PS832 ponA::spc | (Popham & Setlow, 1995) |
| HB10386 | 168 ponA::spc | chrDNA of PS2062 --> 168 |
| HB0047 | CU1065 rsiX::spc | lab strain |
| HB10118 | 168 rsiX::spc | chrDNA of HB0047 --> 168 |
| HB10379 | 168 sigM::tet rsiX::spc | chrDNA of HB10118 --> |
| HB10016 | HB10536 CU1065 sigX rsiX::kan | LFH -->CU1065 |
| HB10378 | 168 sigM::tet sigX rsiX::kan | chrDNA of HB10536 -->HB10016 |
| HB10131 | 168 abh::spc | (Luo & Helmann, 2009) |
| HB4728 | CU1065 spx::spc | lab strain |
| HB10328 | 168 spx::spc | chrDNA of HB4728 --> 168 |
| HB10348 | 168 spx::mls | LFH -->168 |
| HB10329 | 168 sigM::kan spx::spc | chrDNA of HB4728 --> HB10216 |
| HB15808 | 168 sigM::kan abh::spc | chrDNA of HB10131 --> HB10216 |
| HB15811 | 168 sigM::kan abh::spc spx::mls | chrDNA of HB10348--> HB15808 |
| HB10316 | 168 clpP::tet | LFH-->168 |
| HB10332 | 168 spx::spc clpP::tet | chrDNA of HB10316 --> HB10328 |
| HB10320 | 168 sigM::kan clpP::tet | chrDNA of HB10316 --> HB10216 |
| HB15814 | 168 sigM::kan spx::spc clpP::tet | chrDNA of HB10316 --> HB10329 |
| HB15816 | 168 sigM::kan sigX::spc clpP::tet | chrDNA of HB10316 --> HB15815 |
| HB15823 | 168 sigM::kan sigX::spc spx::mls | chrDNA of HB10348 --> HB15815 |
| HB15824 | 168 sigM::kan sigX::spc spx::mls clpP::tet | chrDNA of HB10316 --> HB15823 |
| HB10278 | 168 amyE:: Pspac(hy)- gdpP cat | pPL82-gdpP -->168 |
| HB10287 | 168 amyE::Pspac(hy)- gdp1-303 cat | pPL82-gdpP1-303 -->168 |
| HB10309 | 168 amyE::Pspac(hy)- gdpPD420A cat | pPL82- gdpPD420A -->168 |
| HB10352 | 168 gdpP::mls | LFH -->168 |
| HB10257 | 168 sigM::kan gdpP::mls | chrDNA of HB10352 --> HB10216 |
| HB10295 | 168 sigM::kan gdpP::mls amyE::Pspac(hy)- gdpP cat |
chrDNA HB10278--> HB10257 |
| HB10298 | 168 sigM::kan gdpP::mls amyE::Pspac(hy)- gdpP1- 303cat |
chrDNA HB10287 --> HB10257 |
| HB10310 | 168 sigM::kan gdpP::mls amyE::Pspac(hy)- gdpPD420A cat |
chrDNA HB10309 --> HB10257 |
| HB10353 | 168 disA::spc | LFH -->168 |
| HB10334 | 168 ybbP::tet | LFH -->168 |
| HB10335 | 168 yojJ::kan | LFH -->168 |
| HB10365 | 168 disA::spc amyE:: Pspac(hy)- gdpP cat | chrDNA of HB10278 --> HB10353 |
| HB10366 | 168 ybbP::tet amyE:: Pspac(hy)- gdpP cat | chrDNA of HB10278 --> HB10334 |
| HB10367 | 168 yojJ::kan amyE:: Pspac(hy)- gdpP cat | chrDNA of HB10278 --> HB10335 |
| HB10354 | 168 disA::spc yojJ::kan | chrDNA of HB10353 -->HB10335 |
| HB10356 | 168 ybbP::tet yojJ::kan | chrDNA of HB10334 -->HB10335 |
| HB10281 | 168 amyE::Pspac(hy)-disA cat | pPL82-disA -->168 |
| HB10283 | 168 amyE::Pspac(hy)-ybbP cat | pPL82-ybbP -->168 |
| HB10285 | 168 amyE::Pspac(hy)-yojJ cat | pPL82-yojJ -->168 |
| HB10357 | 168 disA::spc amyE:: Pspac(hy)-disA cat | chrDNA of HB10353 --> HB10281 |
| HB10358 | 168 ybbP::tet amyE:: Pspac(hy)-ybbP cat | chrDNA of HB10334 --> HB10283 |
| HB10374 | 168 ybbP::tet amyE:: Pspac(hy)-yojJ cat | chrDNA of HB10334 --> HB10285 |
| HB10359 | 168 disA::spc ybbP::tet amyE:: Pspac(hy)-ybbP cat | chrDNA of HB10353 --> HB10358 |
| HB10360 | 168 disA::spc ybbP::tet amyE:: Pspac(hy)-disA cat | chrDNA of HB10334 --> HB10357 |
| HB10375 | 168 disA::spc ybbP::tet amyE:: Pspac(hy)-yojJ cat | chrDNA of HB10353 --> HB10374 |
| HB15802 | 168 ybbP::tet yojJ::kan amyE:: Pspac(hy)-ybbP cat | chrDNA of HB10358 --> HB10356 |
| HB15803 | 168 ybbP::tet yojJ::kan amyE:: Pspac(hy)-yojJ cat | chrDNA of HB10374 --> HB10356 |
| HB15801 | 168 disA::spc ybbP::tet yojJ::kan amyE:: Pspac(hy)--disA cat |
chrDNA of HB10354 --> HB10360 |
| HB15806 | 168 disA::spc ybbP::tet yojJ::kan amyE:: Pspac(hy)-ybbP cat |
chrDNA of HB10353 --> HB15802 |
| HB15807 | 168 disA::spc ybbP::tet yojJ::kan amyE:: Pspac(hy)-yojJ cat |
chrDNA of HB10353 --> HB15803 |
| HB10209 | 168 sigM::tet spo0A::Tn7 | WT Tn7 library --> HB10016 |
| HB10210 | 168 sigM::tet tagA::Tn7 | WT Tn7 library --> HB10016 |
| HB10253 | 168 sigM::kan gdpP::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10247 | 168 sigM::kanrsiX::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10248 | 168 sigM::kan lytE::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10246 | 168 sigM::kan pbpX::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10273 | 168 sigM::kan ymdB::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10249 | 168 sigM::kan kinD::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10245 | 168 sigM::kan qoxAB:Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10274 | 168 sigM::kan ssrA::Tn7 sigM::kan | Tn7 library --> HB10216 |
| HB10263 | 168 sigM::kan gdpP::mls lytE::Tn7 sigM::kan | Tn7 library --> HB10257 |
| HB10264 | 168 sigM::kan gdpP::mls clpP::Tn7 sigM::kan | Tn7 library --> HB10257 |
Some genes have multiple Tn7 insertion positions. Only one representative strain number for each gene is listed here.
The donor DNA and recipient strain of transformation are indicated before and after the arrows, respectively.
Strain Constructions
Gene deletions were generated by replacing the coding region with an antibiotic resistance cassette using long flanking homology PCR (LFH-PCR) followed by DNA transformation as previously described (Mascher et al., 2003). Chromosomal DNA transformations were performed as described previously (Harwood & Cutting, 1990).
The IPTG inducible constructs were generated using vector pPL82 (Quisel et al., 2001). PCR products were amplified from B. subtilis 168 chromosomal DNA, digested with endonucleases, and cloned into pPL82. pPL82 contains a chloramphenicol resistance cassette, a multiple cloning site downstream of the Pspac(hy) promoter, and the lacI gene between the two arms of the amyE gene. Primer pairs used for PCR amplification are 5249/5250 for disA, 5252/5253 for ybbP, 5255/5256 for yojJ, 5244/5245 for gdpP, and 5244/5258 for gdpP1-303. All oligonucleotide sequences are listed in SI Table S1. The sequences of the inserts were verified by DNA sequencing (Cornell DNA sequencing facility). pPL82-gdpPD420A was generated using overlap joining PCR with pPL82-gdpP as DNA template. Primer pairs 5244/5293, and 5294/5245 were first used to amplify the up and down fragments of gdp, respectively. The gdpPD420A mutation was generated using primers 5293 and 5294. A joining PCR was then performed with the up and down fragments as template and primer pairs 5244/5245. The PCR product was cloned into pPL82 as above, and the insert was verified by DNA sequencing. Plasmids were linearized by ScaI and used to transform B. subtilis, where they integrated into the amyE locus.
Antibiotic susceptibility tests
Susceptibility tests for antibiotics were conducted using disk diffusion assay and minimal inhibitory concentration (MIC) test. Mueller Hinton (MH, Sigma-Aldrich) medium was used for both assays. Disk diffusion assays were performed as previously described (Luo et al., 2010). The bottom agar is 15 ml MH broth supplemented with1.5% agar, and the top agar is 4 ml MH broth supplemented with 0.75% agar. We used BBL™ Sensi-Disc™ Susceptibility Test Discs (BD; cefixime 5 μg, cefoxitin 30 μg, ceftriaxone 30 μg, ceftazidime 30 μg, cefoperazone 75 μg, amoxicillin 30 μg, ampicillin 10 μg, piperacillin 100 μg, oxacillin 1 μg, piperacillin 100 μg, imipenem 10 μg, meropenem 10 μg, and Isoniazid 1μg) and also prepared disks using Whatman filter paper disks (7 mm in diameter) and freshly made stocks of antibiotics (aztreonam 30 μg, cefuroxime 6 μg , penicillin G 10 U, nalidixic acid 30 μg, novobiocin 250 μg, vancomycin 30 μg, polymycin B 250 μg, and moenomycin 50 μg). The zone of growth inhibition was measured after overnight growth at 37°C. For MIC test, fresh single colonies were first grown in MH broth to an OD600 of 0.4, and diluted 1:100 in MH broth, and 200 μl of the diluted culture was dispensed in Bioscreen 100-well microtiter plate. Growth was measured spectrophotometrically (OD600) using a Bioscreen incubator (Growth Curves USA, Piscataway, NJ) at 37°C with vigorous shaking. The absorbance was recorded every 30 minutes for 24 hours. Inhibition was defined as a final OD600<0.2 at the 12 hour time point (after 12 h, suppressor mutants started to grow up). All antibiotics susceptibility tests were performed with biological triplicates and repeated at least twice.
Bocillin-FL competitive labeling assay
The bocillin-FL labeling assay was performed as previously described (Zhao et al., 1999, Kawai et al., 2009) with modifications. Overnight cultures of B. subtilis cells in LB were diluted 1:100 into 5 ml fresh LB broth, and incubated at 37°C with vigorous shaking. When cell cultures reached mid-log phase (OD600 0.4), the cultures were treated with either 0.05 μg/ml (final conc.) of bocillin-FL, or with additional challenge of 0.00625μg/ml (final conc.) of CEF, or an additional 5 μg/ml aztreonam (final conc.) for 10 min. The cells were pelleted by centrifugation and kept at −20°C overnight. The pellet was thawed on ice and resuspended in 50 μl 0.85% NaCl. The cell resuspension was boiled for 5 min with SDS loading buffer, and proteins were separated by 4~12% SDS-PAGE. To visualize the labeled PBPs, the gels were scanned with a Molecular Dynamics Typhoon PhosporImager (excitation at 488 nm and emission at 530 nm), and the images were analyzed using ImageQuant TL (Amersham Biosciences).
Tn7 mutagenesis
The Tn7 mutagenesis libraries were generated with chromosomal DNA using in vitro transposition as described (Bordi et al., 2008). The library DNA was transformed into WT B. subtilis or a sigM mutant strain (HB10216), and the resulting transposants were grown in the presence of 100 μg/ml spectinomycin (Spc) with and without xylose (final concentration of 1%). Chromosomal DNA was prepared from these cultures using phenol-chloroform extraction (Sambrook & Russell, 2001) and considered an amplified Tn7 library. The amplified Tn7 library DNA was transformed into the sigM mutant strains (HB10016 or HB10216), and cells were plated on LB agar supplemented with 100 μg/ml Spc, 1% xylose and 2 μg/ml CEF (32 x MIC of the sigM strain). Resulting transformants were streaked onto the same selection plate twice. In order to confirm that the increased CEF resistance was due to the presence of the transposon, we performed linkage tests by transforming the chromosomal DNA of the Tn7 mutants into the sigM mutant again and selecting with 100 μg/ml Spc. The resulting transformants (20 colonies for each strain) were then streaked on LB agar supplemented either with 100 μg/ml Spc or with 100 μg/ml Spc plus 2 μg/ml CEF. The transformants that can grow on both plates were counted as linked mutants, and strains with 100% linkage were subjected to Tn7 insertion position mapping using arbitrary PCR as previously described (Bordi et al., 2008). The dependence on xylose was tested by streaking cells on LB agar supplemented with 2 μg/ml CEF or with 2 μg/ml CEF plus 1% xylose. Tn7 mutagenesis with strain sigM gdpP (HB10257) was performed as described above, except that 4 μg/ml of CEF (MIC of the WT strain, and 64 x MIC of the sigM strain) was used for selection.
β-galactosidase activity test
Strains harboring ECF σ promoter-lacZ fusions were grown overnight in LB broth containing appropriate antibiotics and diluted 1:100 into 5 ml LB medium. The culture was grown at 37°C with vigorous shaking to OD600~0.4 (mid-log growth phase), and then split into two aliquots. One was challenged with 8 μg/ml of CEF and the other was untreated. The cultures were returned to 37°C, and samples were collected after 30 min. β-galactosidase assays were performed as described by Miller (26), and each strain was tested in biological triplicates and repeated three times. Data were reported as the mean and SE.
5′-RACE
The transcriptional start site of ybbP was determined using 5′ rapid amplification of cDNA ends (5′-RACE). Five micrograms of total RNA from a mid-log-phase LB culture was reversed transcribed to cDNA using TaqMan reverse transcription reagents (Roche) and oligo ybbP-rev-GSP3 (5584) as primer. The 3′ end of cDNA was tailed with poly-dCTP using terminal deoxynucleotidyl transferase (New England Biolabs). The tailed cDNAs were then amplified by PCR with primers AAP (3314) and ybbP-rev-GSP4 (5585). The PCR products were subjected to DNA sequencing (Cornell DNA sequencing facility).
Growth rate test
Fresh single colonies were first grown in MH broth to OD600 of 0.4, and diluted 1:100 in MH broth, and inoculated in Bioscreen microtiter plates with a total inoculum of 200 μl. Growth was measured spectrophotometrically (OD600) using a Bioscreen incubator (Growth Curves USA, Piscataway, NJ) at 37°C with vigorous shaking. The specific growth rate of each strain was calculated from the exponential growth phase. Each test was performed with biological triplicates and repeated twice.
Depeletion of c-di-AMP and microscopic imaging
Strain HB10359 was grown in MH broth supplemented with 1mM IPTG to mid-exponential phase, and collected by centrifugation. The cells were washed twice with MH medium, and resuspended to OD600 of 0.2 in fresh MH broth, or MH broth supplemented with 1mM IPTG, SMM (20 mM MgCl2, 10% sucrose, 20 mM maleic acid, pH 7.0), 10% sucrose, or 10mM MgSO4. 200ul of each cell resuspension was added a Bioscreen microtiter plate, and incubated at 37°C with vigorous shaking. For phase contrast and fluorescence microscopy, 1μg/ml (final concentration) of cell membrane stain FM 4-64 (Invitrogen) was added to the cell culture, and incubated at 37 °C for 30min with shaking. 5 μl of cells were then mounted on microscope slide coated with a thin film of 1% agarose as previously described in (Glaser et al., 1997). Microscopy was performed using an Olympus BX61 epifluorescence microscope. Images were acquired using Cooke SensiCam and Slidebook software (Intelligent Imaging Inc.).
Supplementary Material
Acknowledgments
We thank Dr. Peter Zuber (Oregon Health & Science University) for strains ORB4342 and ORB4342, and Dr. David Popham (Virginia Tech) for stain PS2062, Dr. Win Chai (Harvard University) for strains RL2173 and RL2774, Dr. Esther Angert (Cornell University) for assistance with microscopy, and Marilyn Wang (Cornell University) for performing antibiotic screening. This work was supported by grant GM-047446.
Reference
- Asai K, Ishiwata K, Matsuzaki K, Sadaie Y. A viable Bacillus subtilis strain without functional extracytoplasmic function σ genes. J Bacteriol. 2008;190:2633–2636. doi: 10.1128/JB.01859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai K, Yamaguchi H, Kang CM, Yoshida K, Fujita Y, Sadaie Y. DNA microarray analysis of Bacillus subtilis σ factors of extracytoplasmic function family. FEMS Microbiol Lett. 2003;220:155–160. doi: 10.1016/S0378-1097(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Bejerano-Sagie M, Oppenheimer-Shaanan Y, Berlatzky I, Rouvinski A, Meyerovich M, Ben-Yehuda S. A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell. 2006;125:679–690. doi: 10.1016/j.cell.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Bordi C, Butcher BG, Shi Q, Hachmann AB, Peters JE, Helmann JD. In vitro mutagenesis of Bacillus subtilis by using a modified Tn7 transposon with an outward-facing inducible promoter. Appl Environ Microbiol. 2008;74:3419–3425. doi: 10.1128/AEM.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher BG, Helmann JD. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol Microbiol. 2006;60:765–782. doi: 10.1111/j.1365-2958.2006.05131.x. [DOI] [PubMed] [Google Scholar]
- Cao M, Helmann JD. Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function σ factors. J Bacteriol. 2002;184:6123–6129. doi: 10.1128/JB.184.22.6123-6129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Helmann JD. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J Bacteriol. 2004;186:1136–1146. doi: 10.1128/JB.186.4.1136-1146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang T, Ye R, Helmann JD. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol Microbiol. 2002;45:1267–1276. doi: 10.1046/j.1365-2958.2002.03050.x. [DOI] [PubMed] [Google Scholar]
- Carballido-Lopez R, Formstone A. Shape determination in Bacillus subtilis. Curr Opin Microbiol. 2007;10:611–616. doi: 10.1016/j.mib.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Carballido-Lopez R, Formstone A, Li Y, Ehrlich SD, Noirot P, Errington J. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev Cell. 2006;11:399–409. doi: 10.1016/j.devcel.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Chai Y, Kolter R, Losick R. Reversal of an epigenetic switch governing cell chaining in Bacillus subtilis by protein instability. Mol Microbiol. 2010a;78:218–229. doi: 10.1111/j.1365-2958.2010.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Norman T, Kolter R, Losick R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 2010b;24:754–765. doi: 10.1101/gad.1915010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Cohen SN. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979;168:111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Chaudhuri RR, Allen AG, Owen PJ, Shalom G, Stone K, Harrison M, Burgis TA, Lockyer M, Garcia-Lara J, Foster SJ, Pleasance SJ, Peters SE, Maskell DJ, Charles IG. Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH) BMC Genomics. 2009;10:291. doi: 10.1186/1471-2164-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Reyes D, Leelakriangsak M, Zuber P. The global regulator Spx functions in the control of organosulfur metabolism in Bacillus subtilis. J Bacteriol. 2006;188:5741–5751. doi: 10.1128/JB.00443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo ML, Hanique S, Baurin SL, Bauvois C, De Vriendt K, Van Beeumen JJ, Frere JM, Joris B. The ybxI gene of Bacillus subtilis 168 encodes a class D β-lactamase of low activity. Antimicrob Agents Chemother. 2004;48:484–490. doi: 10.1128/AAC.48.2.484-490.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. c-di-AMP is a new second messenger in staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elia MA, Henderson JA, Beveridge TJ, Heinrichs DE, Brown ED. The N-acetylmannosamine transferase catalyzes the first committed step of teichoic acid assembly in Bacillus subtilis and Staphylococcus aureus. J Bacteriol. 2009;191:4030–4034. doi: 10.1128/JB.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, Hubner S, Stulke J. A novel factor controlling bistability in bacillus subtilis: the ymdb protein affects flagellin expression and biofilm formation. J Bacteriol. 2011;193:5997–6007. doi: 10.1128/JB.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubee V, Chau F, Arthur M, Garry L, Benadda S, Mesnage S, Lefort A, Fantin B. The in vitro contribution of autolysins to bacterial killing elicited by amoxicillin increases with inoculum size in Enterococcus faecalis. Antimicrob Agents Chemother. 2011;55:910–912. doi: 10.1128/AAC.01230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiamphungporn W, Helmann JD. The Bacillus subtilis σM regulon and its contribution to cell envelope stress responses. Mol Microbiol. 2008;67:830–848. doi: 10.1111/j.1365-2958.2007.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstone A, Errington J. A magnesium-dependent mreB null mutant: implications for the role of MreB in Bacillus subtilis. Mol Microbiol. 2005;55:1646–1657. doi: 10.1111/j.1365-2958.2005.04506.x. [DOI] [PubMed] [Google Scholar]
- Foster SJ, Popham DL. Structure and synthesis of cell wall, spore cortex, teichoic acids, S-layers, and capsules. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and its closest relatives: From genes to cells. Amer Society for Microbiology; Washington, DC: 2002. pp. 21–41. [Google Scholar]
- Frees D, Savijoki K, Varmanen P, Ingmer H. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol Microbiol. 2007;63:1285–1295. doi: 10.1111/j.1365-2958.2007.05598.x. [DOI] [PubMed] [Google Scholar]
- French CT, Lao P, Loraine AE, Matthews BT, Yu H, Dybvig K. Large-scale transposon mutagenesis of Mycoplasma pulmonis. Mol Microbiol. 2008;69:67–76. doi: 10.1111/j.1365-2958.2008.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou NH, Smith SA, Sykes RB. Mode of action of azthreonam. Antimicrob Agents Chemother. 1982;21:950–956. doi: 10.1128/aac.21.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P, Sharpe ME, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA, 3rd, Smith HO, Venter JC. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohring N, Fedtke I, Xia G, Jorge AM, Pinho MG, Bertsche U, Peschel A. New role of the disulfide stress effector YjbH in Staphylococcus aureus β-lactam susceptibility. Antimicrob Agents Chemother. 2011;55:5452–5458. doi: 10.1128/AAC.00286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariglia-Oropeza V, Helmann JD. Bacillus subtilis σV confers lysozyme resistance by activation of two cell wall modification pathways: peptidoglycan O-acetylation and D-alanylation of teichoic acids. J Bacteriol. 2011;193:6223–6232. doi: 10.1128/JB.06023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay DR, Koskoletos C. Aztreonam, a new monobactam antimicrobial. Clin Pharm. 1985;4:516–526. [PubMed] [Google Scholar]
- Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachmann AB, Angert ER, Helmann JD. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob Agents Chemother. 2009;53:1598–1609. doi: 10.1128/AAC.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CR, Cutting SM. Molecular biological methods for Bacillus. Wiley; 1990. [Google Scholar]
- Helmann JD. The extracytoplasmic function (ECF) σ factors. Adv Microb Physiol. 2002;46:47–110. doi: 10.1016/s0065-2911(02)46002-x. [DOI] [PubMed] [Google Scholar]
- Ho TD, Hastie JL, Intile PJ, Ellermeier CD. The Bacillus subtilis Extra-Cytoplasmic Function σ factor, σV, is induced by lysozyme and provides resistance to lysozyme. J Bacteriol. 2011;193:6215–6222. doi: 10.1128/JB.05467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Decatur A, Sorokin A, Helmann JD. The Bacillus subtilis σX protein is an extracytoplasmic function σ factor contributing to survival at high temperature. J Bacteriol. 1997;179:2915–2921. doi: 10.1128/jb.179.9.2915-2921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Fredrick KL, Helmann JD. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J Bacteriol. 1998;180:3765–3770. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Helmann JD. Identification of target promoters for the Bacillus subtilis σX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- Hutter B, Fischer C, Jacobi A, Schaab C, Loferer H. Panel of Bacillus subtilis reporter strains indicative of various modes of action. Antimicrobial agents and chemotherapy. 2004;48:2588. doi: 10.1128/AAC.48.7.2588-2594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis AJ, Thackray PD, Houston CW, Horsburgh MJ, Moir A. σM-responsive genes of Bacillus subtilis and their promoters. J Bacteriol. 2007;189:4534–4538. doi: 10.1128/JB.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Daniel RA, Errington J. Regulation of cell wall morphogenesis in Bacillus subtilis by recruitment of PBP1 to the MreB helix. Mol Microbiol. 2009;71:1131–1144. doi: 10.1111/j.1365-2958.2009.06601.x. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Marles-Wright J, Cleverley RM, Emmins R, Ishikawa S, Kuwano M, Heinz N, Bui NK, Hoyland CN, Ogasawara N, Lewis RJ, Vollmer W, Daniel RA, Errington J. A widespread family of bacterial cell wall assembly proteins. Embo J. 2011 doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock H, Gerth U, Hecker M. MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol. 2004;51:1087–1102. doi: 10.1046/j.1365-2958.2003.03875.x. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Leaver M, Dominguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. Life without a wall or division machine in Bacillus subtilis. Nature. 2009;457:849–853. doi: 10.1038/nature07742. [DOI] [PubMed] [Google Scholar]
- Leaver M, Errington J. Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis. Mol Microbiol. 2005;57:1196–1209. doi: 10.1111/j.1365-2958.2005.04736.x. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kingston AW, Helmann JD. Glutamate dehydrogenase affects resistance to cell wall antibiotics in Bacillus subtilis. J Bacteriol. 2012 doi: 10.1128/JB.06547-11. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AL, de Castro LH, Lim D, Strynadka NC. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science. 2007;315:1402–1405. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- Luo Y, Asai K, Sadaie Y, Helmann JD. Transcriptomic and phenotypic characterization of a Bacillus subtilis strain without extracytoplasmic function σ factors. J Bacteriol. 2010;192:5736–5745. doi: 10.1128/JB.00826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Helmann JD. Extracytoplasmic function σ factors with overlapping promoter specificity regulate sublancin production in Bacillus subtilis. J Bacteriol. 2009;191:4951–4958. doi: 10.1128/JB.00549-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- Margot P, Wahlen M, Gholamhoseinian A, Piggot P, Karamata D. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J Bacteriol. 1998;180:749–752. doi: 10.1128/jb.180.3.749-752.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Hachmann AB, Helmann JD. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function σ factors. J Bacteriol. 2007;189:6919–6927. doi: 10.1128/JB.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- Mauel C, Young M, Karamata D. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J Gen Microbiol. 1991;137:929–941. doi: 10.1099/00221287-137-4-929. [DOI] [PubMed] [Google Scholar]
- Minnig K, Barblan J, Kehl S, Moeller S, Maueel C. In Bacillus subtilis W23, the duet σX σM, two σ factors of the extracytoplasmic function subfamily, are required for septum and wall synthesis under batch culture conditions. Molecular Microbiology. 2003;49:1435–1447. doi: 10.1046/j.1365-2958.2003.03652.x. [DOI] [PubMed] [Google Scholar]
- Murray EJ, Stanley-Wall NR. The sensitivity of Bacillus subtilis to diverse antimicrobial compounds is influenced by Abh. Arch Microbiol. 2010;192:1059–1067. doi: 10.1007/s00203-010-0630-4. [DOI] [PubMed] [Google Scholar]
- Murray EJ, Strauch MA, Stanley-Wall NR. σX is involved in controlling Bacillus subtilis biofilm architecture through the AbrB homologue Abh. J Bacteriol. 2009;191:6822–6832. doi: 10.1128/JB.00618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray T, Popham DL, Setlow P. Bacillus subtilis cells lacking penicillin-binding protein 1 require increased levels of divalent cations for growth. J Bacteriol. 1998;180:4555–4563. doi: 10.1128/jb.180.17.4555-4563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Hajarizadeh F, Zhu Y, Zuber P. Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol Microbiol. 2001;42:383–394. doi: 10.1046/j.1365-2958.2001.02639.x. [DOI] [PubMed] [Google Scholar]
- Nakano S, Kuster-Schock E, Grossman AD, Zuber P. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc Natl Acad Sci U S A. 2003;100:13603–13608. doi: 10.1073/pnas.2235180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Zheng G, Nakano MM, Zuber P. Multiple pathways of Spx (YjbD) proteolysis in Bacillus subtilis. J Bacteriol. 2002;184:3664–3670. doi: 10.1128/JB.184.13.3664-3670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 2011;12:594–601. doi: 10.1038/embor.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamp SJ, Frees D, Engelmann S, Hecker M, Ingmer H. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. Journal of Bacteriology. 2006;188:4861. doi: 10.1128/JB.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K. Resistance to beta-lactam antibiotics. Cell Mol Life Sci. 2004;61:2200–2223. doi: 10.1007/s00018-004-4060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham DL, Setlow P. Cloning, nucleotide sequence, and mutagenesis of the Bacillus subtilis ponA operon, which codes for penicillin-binding protein (PBP) 1 and a PBP-related factor. Journal of Bacteriology. 1995;177:326. doi: 10.1128/jb.177.2.326-335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Helmann JD. The −10 region is a key promoter specificity determinant for the Bacillus subtilis extracytoplasmic-function σ factors σX and σW. J Bacteriol. 2001;183:1921–1927. doi: 10.1128/JB.183.6.1921-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisel JD, Burkholder WF, Grossman AD. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J Bacteriol. 2001;183:6573–6578. doi: 10.1128/JB.183.22.6573-6578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao F, Ji Q, Soehano I, Liang ZX. Unusual heme-binding PAS domain from YybT family proteins. J Bacteriol. 2011;193:1543–1551. doi: 10.1128/JB.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J Biol Chem. 2010;285:473–482. doi: 10.1074/jbc.M109.040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal. 2008;1:pe39. doi: 10.1126/scisignal.133pe39. [DOI] [PubMed] [Google Scholar]
- Ross CL, Thomason KS, Koehler TM. An extracytoplasmic function σ factor controls β-lactamase gene expression in Bacillus anthracis and other Bacillus cereus group species. J Bacteriol. 2009;191:6683–6693. doi: 10.1128/JB.00691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukmana A, Morimoto T, Takahashi H, Giyanto, Ogasawara N. Assessment of transcriptional responses of Bacillus subtilis cells to the antibiotic enduracidin, which interferes with cell wall synthesis, using a high-density tiling chip. Genes Genet Syst. 2009;84:253–267. doi: 10.1266/ggs.84.253. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. CSHL press; 2001. [Google Scholar]
- Scheffers DJ, Jones LJ, Errington J. Several distinct localization patterns for penicillin-binding proteins in Bacillus subtilis. Mol Microbiol. 2004;51:749–764. doi: 10.1046/j.1365-2958.2003.03854.x. [DOI] [PubMed] [Google Scholar]
- Scheffers DJ, Pinho MG. Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev. 2005;69:585–607. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirner K, Errington J. The cell wall regulator σI specifically suppresses the lethal phenotype of mbl mutants in Bacillus subtilis. J Bacteriol. 2009;191:1404–1413. doi: 10.1128/JB.01497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Blackman SA, Foster SJ. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146(Pt 2):249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- Song JH, Ko KS, Lee JY, Baek JY, Oh WS, Yoon HS, Jeong JY, Chun J. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol Cells. 2005;19:365–374. [PubMed] [Google Scholar]
- Strauch MA, Bobay BG, Cavanagh J, Yao F, Wilson A, Le Breton Y. Abh and AbrB control of Bacillus subtilis antimicrobial gene expression. J Bacteriol. 2007;189:7720–7732. doi: 10.1128/JB.01081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray PD, Moir A. σM, an extracytoplasmic function σ factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J Bacteriol. 2003;185:3491–3498. doi: 10.1128/JB.185.12.3491-3498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W, Bertsche U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim Biophys Acta. 2008;1778:1714–1734. doi: 10.1016/j.bbamem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Walsh C. Where will new antibiotics come from? Nat Rev Microbiol. 2003;1:65–70. doi: 10.1038/nrmicro727. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Strominger JL. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Wilke MS, Lovering AL, Strynadka NC. β-lactam antibiotic resistance: a current structural perspective. Curr Opin Microbiol. 2005;8:525–533. doi: 10.1016/j.mib.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Witte G, Hartung S, Buttner K, Hopfner KP. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell. 2008;30:167–178. doi: 10.1016/j.molcel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Sekowska A, Francetic O, Martin-Verstraete I, Wang Y, Danchin A. Spx mediates oxidative stress regulation of the methionine sulfoxide reductases operon in Bacillus subtilis. BMC Microbiol. 2008;8:128. doi: 10.1186/1471-2180-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother. 1999;43:1124–1128. doi: 10.1128/aac.43.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P. Management of oxidative stress in Bacillus. Annu Rev Microbiol. 2009;63:575–597. doi: 10.1146/annurev.micro.091208.073241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.