Abstract
Galanin-like peptide (GALP) is a neuropeptide that has complex actions on energy balance, producing orexigenic effects in the short-term in rats, but anorexigenic and febrile effects over the longer-term in rats and mice. GALP is thought to promote feeding via neuropeptide Y and orexin neurons, but the mediators of the anorexia are unknown. However, the anorexic and febrile actions of GALP are similar in magnitude and profile to those seen after central injections of the cytokine IL-1. Thus, the aim of this study was to test the hypothesis that IL-1 mediates the effects of GALP on energy balance. Intracerebroventricular (icv) injection of GALP (1.5 nmol) in male Sprague-Dawley rats stimulated production of IL-1α and IL-1β protein in macrophages and/or microglia in selected brain areas, including the meninges, and peri-ventricular brain regions. Icv injection of GALP in rats stimulated food intake over 1 h, but decreased feeding and body weight at 24 h, and caused a rise in core body temperature over 8 h. Co-infusion of the IL-1 receptor antagonist had no effect on the GALP-induced orexigenic response, but significantly reduced the longer-term actions of GALP observed at 24 h, and its effect on body temperature. Furthermore, the actions of GALP on feeding, body weight and body temperature were significantly reduced in IL-1α/β-, IL-1β-, or IL-1 type I receptor (IL-1RI)-deficient mice. These data suggest that GALP induces expression of IL-1 in the brain, and its anorexic and febrile actions are mediated by this cytokine acting via IL-1RI.
Keywords: galanin-like peptide (GALP), interleukin-1 (IL-1), metabolism, neuropeptides, inflammation
Galanin-like peptide (GALP) is a 60-amino-acid neuropeptide that is expressed in the arcuate nucleus of the hypothalamus, the median eminence and pituicytes of the posterior pituitary. Following its discovery in 1999 (1), GALP was believed to be an orexigenic neuropeptide that stimulated feeding over 1 h after central nervous system administration (CNS) in rats (2). However, GALP expression is positively regulated by the adipose tissue hormone leptin, a mechanism that is more akin to the modulation of peptides that are anorexigenic (3-5). It is now clear that GALP has dichotomous actions on energy balance after intracerebroventricular (icv) injection in rats, stimulating feeding over the first hour, but reducing food intake and body weight at 24 h (6). These dual effects of GALP are in contrast to galanin, which stimulates feeding acutely (over 1 h), but has no effect on energy balance measured 24 h after injection in rats. In contrast to the effects in the rat, GALP does not stimulate food intake over 1 h in mice, but produces a suppression in food intake and body weight over 24 h (7-10). Central administration of GALP (but not galanin) in rats or mice also causes an increase in core body temperature that is mediated by prostaglandins (6,10). However, in mice, GALP initially causes a transient period of hypothermia that precedes the GALP-induced hyperthermic phase (10). Taken together, these data suggest that GALP has complex actions on energy balance, with the predominant effect being catabolic.
Recent data suggest that GALP promotes feeding in the rat via activation of known orexigenic neurons that contain orexin and neuropeptide Y (11,12), but the mediators of the anorexic effect are unknown. Central administration of GALP in rats or mice induces a specific pattern of Fos activation in the brain (10,13,14), with robust c-Fos protein expression observed in the ependymal cells of the ventricles and peri-ventricular regions, a response not seen with galanin. The cells that are activated in the peri-ventricular hypothalamic region are astrocytes, rather than neurons or microglia (13). Activated astrocytes are involved in inflammatory responses to infection and injury in the brain, and release key mediators of inflammation, such as cytokines (15). Analogous to actions of GALP, infection and inflammation are associated with negative energy balance, characterised by weight loss, reduced food intake and fever (rise in core body temperature). The prostaglandin-dependent rise in temperature and anorexia induced by GALP are similar in intensity and pattern to those produced by the cytokine IL-1 (16). IL-1 is a classical mediator of inflammation that is found in two forms, IL-1α and IL-1β, and acts via the IL-1 type I receptor (IL-1RI), although recent data also suggests that IL-1 may also act through a novel receptor (17,18). The similarities between the cytokine-mediated response to infection and the actions of GALP on energy balance raise the possibility that GALP may be inducing anorexia, body weight loss and fever non-physiologically.
The overall aim of the present study, therefore, was to test the hypothesis that IL-1 mediates the actions of GALP on energy balance in the rodent. Firstly, the effect of GALP on IL-1α and IL-1β expression in the brain of the rat was investigated. Following this, the effect of inhibiting endogenous IL-1 was assessed in the rat by co-administering GALP with the IL-1 receptor antagonist, IL-1RA. Finally, the IL-1 ligand and receptor responsible for the actions of GALP on energy balance were assessed by testing the effects of GALP in transgenic mice deficient in IL-1α/β, IL-1β or IL-1RI.
Materials and Methods
Animals
At the time of injections, male Sprague-Dawley rats (Charles River Laboratories Inc., Sandwich, UK) were used between 280-320 g. IL-1α/β- and IL-1β-deficient male mice (IL-1α/β −/− and IL-1β −/−) were kindly provided by Dr Yoichiro Iwakura, and had been backcrossed for eight generations on a C57BL/6 background. IL-1RI-deficient male mice (IL-1RI −/−) were obtained from Charles River, and had previously undergone nine backcrosses on a C57BL/6 background by Professor Martin Nicklin (University of Sheffield, UK). Toll-like receptor 4-deficient mice (TLR4 −/−) were also on a C57BL/6 background, and were provided by Dr Richard Grencis (University of Manchester, UK). IL-1α/β −/−, IL-1β −/−, IL-1RI −/− and TLR4 −/− mice, and their wild-type control (C57BL/6) mice were kept and bred at the Faculty of Life Sciences animal facility (University of Manchester, UK), and used at a weight range of 26-32 g. All animals were housed at a constant ambient temperature of 21 ± 2 C on a 12-h light, 12-h dark cycle (lights on 0800 h). Rodent chow (Beekay International, Hull, UK) and tap water were provided ad libitum. All procedures conformed to the requirements of the United Kingdom Animals (Scientific Procedures) Act, 1986.
Materials
Rat GALP (Bachem, Merseyside, UK) and rat galanin (Bachem) were used at a equimolar concentration of 1.5 nmol (in 1 μl). Human IL-1 receptor antagonist (IL-1RA; R & D Systems, Abingdon, UK) was administered in three separate injections at a concentration of 200 μg/injection (in 2 μl). Lipopolysaccharide (LPS; 0127:B8; Sigma-Aldrich Co Ltd., Poole, UK) was administered peripherally at 100 μg/kg or 100 ng icv. All compounds were dissolved in sterile saline and stored at −20 C until required.
Intracerebroventricular cannulation and injections
Rats or mice were anaesthetised with isoflurane (1.5-3 % in O2) and stereotaxically implanted with guide cannulae into the lateral cerebral ventricle. Injection co-ordinates relative to bregma for rats were: posterior 0.8 mm, lateral 1.5 mm, and ventral 4 mm, according to the atlas of Paxinos and Watson (19), and for mice: posterior 0.2 mm, lateral 1 mm and ventral 2.5 mm, according to the atlas of Franklin and Watson (20). In experiments involving measurements of core body temperature, radiotransmitters (TA10TA-F40 for rats and TA10TA-F20 for mice; Data Sciences, Minneapolis, MN, USA) were implanted abdominally into the peritoneum at the same time as cannulation. After surgery, mice were housed individually and allowed to recover for at least 7 d. Rats were also left to recover for the same period and housed individually 24-48 h before experimental injections. Animals were assigned randomly to treatment groups, and each animal was used only once. All icv injections were performed in conscious unrestrained rats, or lightly restrained mice commencing 2 h after lights on (1000 h), and were given in a total volume of 1-3 μl for rats, or 1 μl for mice. After injections, animals were given a pre-weighed amount of food that was re-weighed at several time points later.
ELISA
Rat plasma IL-6 concentrations were analysed by ELISA (Duoset; R & D Systems), according to the manufacturer’s instructions.
Experiment 1: effect of GALP on IL-1α or IL-1β expression
Rats (n = 3/group) were given a single icv injection of either rat GALP (1.5 nmol in 1 μl), rat galanin (1.5 nmol in 1 μl) or vehicle (1 μl sterile saline). After injections, animals were given a pre-weighed amount of food that was re-weighed 2 h later. Animals were then culled at 2, 4, 8 or 24 h post-injection with an overdose of sodium pentobarbitone (90 mg/kg ip; Sagatal, Rhône-Mérieux, Harlow, UK) and transcardially-perfused with heparinised saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Plasma was obtained from anti-coagulated (sodium citrate) blood samples taken by cardiac puncture, immediately before perfusions.
After transcardial perfusion, brains were removed, post-fixed in the same fixative overnight, immersed in 30% sucrose until the tissue sank, and then frozen. Coronal brain sections were cut at 30 μm on a freezing sledge microtome and stored in cryoprotectant at −20 C until required.
Immunohistochemistry for IL-1α or IL-1β was performed on free-floating sections at room temperature, unless otherwise stated. To remove endogenous peroxidise activity, sections were treated with 1.5% H2O2 (in 20% methanol, 0.2% triton X-100 in 0.1 M PB). After pre-incubation in 2% normal horse serum (in 0.1% saponin) or 10% normal rabbit serum (in 0.3% triton) for 1 h for IL-1α or IL-1β respectively, the sections were incubated at 4 C in either goat anti-rat IL-1α (1μg/ml in 2% normal horse serum/0.1% saponin; R & D systems), or sheep anti-rat IL-1β antibody diluted 1:1000 (in 2% normal rabbit serum/0.3% triton; National Institute for Biological Standards and Control (NIBSC), Potters Bar, UK). After 24 h, sections were rinsed in PB/0.1% saponin (for IL-1α), or PB/0.3% triton (for IL-1β), and incubated in the appropriate secondary biotinylated antibody (anti-goat IgG 1:500 in 2% normal horse serum/0.1% saponin for IL-1α, or anti-sheep IgG 1:500 in 2% normal rabbit serum/0.3% triton for IL-1β; Vector Laboratories Ltd, Peterborough, UK) for 2 h. All sections were then washed in 0.1 M PB, immersed in avidin-biotin-peroxidase complex (ABC, Vector Laboratories Ltd) for 30 min, rinsed in 0.1 M PB and colour-developed using a 0.05% diaminobenzidine solution (in 0.01% H2O2). Sections were mounted onto gelatine-coated slides, dried, and coverslips were applied before viewing under a light microscope.
In a separate experiment rats (n = 2-3/group) were given an icv injection of LPS (100 ng in 1μl) or vehicle (1 μl saline). Food intake and body weight were assessed 24 h later, or animals were culled at 2, 8 or 24 h, and immunohistochemistry for IL-1α or IL-1β was performed as above.
Experiment 2: phenotype of IL-1α and IL-1β-expressing cells
For dual fluorescence immunohistochemistry, rat brain sections (taken from experiment 1) were treated with 2% normal horse serum (for IL-1α) or 10% normal donkey serum (for IL-1β) before incubation for 24 h at 4 C in the goat anti-rat IL-1α antibody (1μg/ml; in 2% normal horse serum/0.1% saponin) or the sheep anti-rat IL-1β antibody (1:1000; in 2% normal rabbit serum/0.3% triton), along with either a mouse monoclonal antibody against glial fibrillary acidic protein (GFAP) (1:64,000; Sigma-Aldrich Co. Ltd.) to detect astrocytes, rabbit anti-Iba1 (1:5000; Wako Pure Chemicals Ltd, Japan) for microglia and macrophages, or mouse monoclonal anti-NeuN (1:1000; Chemicon International, UK) for neurons. After washes in 0.1M PB/0.1% saponin (for IL-1α) or 0.1 M PB/0.3% triton (for IL-1β), sections were incubated in an anti-goat Alexa 594 (for IL-1α; Molecular Probes, UK) or anti-sheep Alexa 594 secondary antibody (for IL-1β; Molecular Probes), along with the appropriate Alexa 488 IgG antibody (anti-rabbit or anti-mouse; Molecular Probes). After further washes (in 0.1 M PB), sections were mounted and coverslipped with ProLong Gold mounting medium (Invitrogen, Paisley, UK), before examination under an Olympus widefield fluorescence microscope.
Experiment 3: effect of IL-1RA on GALP-induced changes in food intake, body weight, and core body temperature in rats
Rats (n = 8-13/group) were given an icv injection of either vehicle (1 μl saline) or GALP (1.5 nmol in 1 μl). IL-1RA (200 μg in 2 μl) or vehicle (2 μl saline) were administered icv at −1 h, 0 h and 1 h relative to GALP injections. Food consumption was measured at 1 h and 24 h, and body weight at 24 h after injections. Core body temperature was monitored continuously for 24 h by remote radiotelemetry.
Experiments 4-6: effect of GALP on food intake, body weight, and core body temperature in IL-1α/β −/−, IL-1β −/− and IL-1RI −/− mice
In separate experiments IL-1α/β −/−, IL-1β −/− or IL-1RI −/− mice and their respective wild-type C57BL/6 controls (n = 5/group) were injected icv with either vehicle (1 μl saline) or GALP (1.5 nmol in 1 μl). As GALP does not stimulate food intake at 1 h after injection in mice (7,10), food consumption was measured only at 24 h. Body weight was also assessed 24 h after injections, and core body temperature was monitored continuously for 24 h by remote radiotelemetry.
Experiments 7-8: effect of GALP or LPS on food intake, body weight and core body temperature in toll-like receptor 4 (TLR4) −/− mice
The effects of GALP on the IL-1 system are similar to those seen after injection of LPS, the bacterial endotoxin that mediates its actions via the receptor TLR4. Thus, in order to test whether the actions of GALP on energy homeostasis were due to endotoxin sample contamination, its effects were tested in TLR4 −/− mice. Icv injections of GALP (1.5 nmol) or vehicle (1 μl saline) were administered to TLR4 −/− or wild-type mice (n = 4-5/group). In order to confirm the lack of responsiveness of TLR4 −/− to LPS, a separate experiment was performed whereby TLR4 −/− or wild-type mice were given an ip injection of LPS (100 μg/kg) or vehicle (5 ml/kg saline; n = 4-5/group). For both experiments, food intake and body weight were assessed at 24 h, and core body temperature was monitored continuously for 24 h by remote radiotelemetry.
Statistical Analyses
All data are presented as mean ± SEM. Body temperatures are plotted as the mean change from the point of injection (time zero) over 10 h. The integrated temperature response between 0 and 10 h for rats [area under the curve (AUC), C/h] was calculated for each animal by the trapezoidal method. Average AUC values were then determined for each treatment group. For mice, AUC was calculated between 2 and 10 h to avoid the conflicting hypothermia that was observed between 0 and 2 h after GALP treatment. For both rats and mice only data up to 10 h was analysed, as GALP-induced changes in temperature are no longer apparent after this time (6,10). In experiments involving LPS, the dose administered did not cause hypothermia in control wild-type mice, but an increase in core body temperature that lasted approximately 8 h. Thus, in experiments using LPS, core body temperature was analysed by calculating AUC between 0-8 h.
Statistical comparisons for food intake, body weight and AUC were performed using a one-way ANOVA followed by a Tukey’s multiple comparisons test for the experiment using IL-1RA in rats, or for experiments employing transgenic mice, a parametric two-way ANOVA, followed by Scheffé multiple comparisons test was used. For data involving transgenic mice, statistical significances are plotted on figures for GALP treatment versus their respective controls only. Statistical significance was taken when P < 0.05.
Results
Experiment 1: effect of GALP on IL-1α and IL-1β expression
Icv injection of equimolar doses (1.5 nmol) of either GALP or galanin significantly increased food intake at 2 h compared to vehicle-injected rats (vehicle, 0.1 ± 0.1 g; GALP, 3.4 ± 1.1 g; galanin, 2.7 ± 0.2 g; P < 0.05 vs vehicle for both GALP and galanin, n = 12/treatment group, which includes all time point groups).
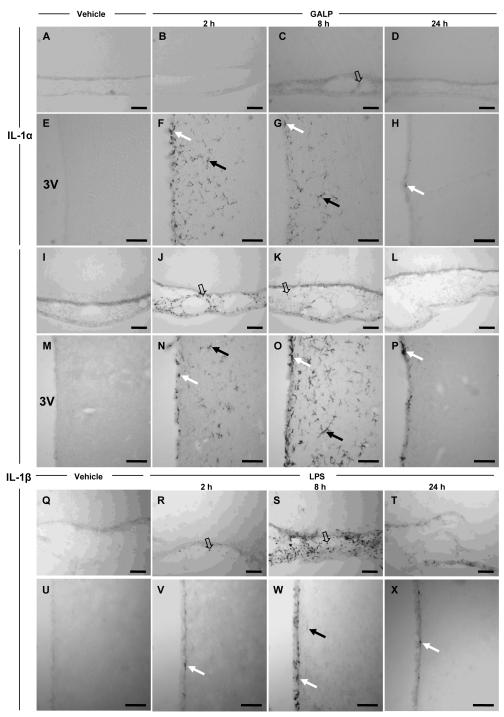
No cells expressing IL-1α or IL-1β were found in the brain after icv injection of vehicle (Fig. 1A, E, I and M) or galanin (1.5 nmol; data not shown), at any time point studied, and in any area of the brain. However, IL-1α and IL-1β immunoreactive cells were observed in the brain after icv injection of GALP (Fig. 1). At 2, 4 (data not shown) and 8 h after GALP treatment, IL-1α- and IL-1β-positive cells were found within the ependymal cells lining the ventricles and in the brain parenchyma surrounding the ventricles (the lateral, dorsal third, third and fourth ventricles). The staining in the parenchyma appeared to be almost limited exclusively to the areas surrounding the ventricles, and was strongest in the peri-third hypothalamic region (Fig. 1F-G and N-O). IL-1β-positive cells were also seen within the meninges (Fig. 1J-K) and the choroid plexus (data not shown). The cells expressing IL-1β in the meninges were pre-dominantly in the ventral region of the brain. However, in contrast to IL-1β, only a few cells expressing IL-1α were identified in the meninges (Fig. 1C).
FIG. 1.
Effect of galanin-like peptide (GALP) or lipopolysaccharide (LPS) on IL-1α and IL-1β expression in the rat brain. The pictures are representative photomicrographs of IL-1α- (A-H) and IL-1β- (I-P) expressing cells after intracerebroventricular (icv) injection of either 1 μl saline (n = 3, A, E, I and M at 8 h) or 1.5 nmol GALP (in 1 μl, n = 3, B-D, F-H, J-L and N-P). Expression of IL-1α and IL-1β was observed at 2 and 8 h in the ependymal cells of the ventricles and the parenchyma surrounding the ventricles, e.g. the peri-third ventricular hypothalamic region (F-H and N-P), following GALP injection. Expression of IL-1β was also seen at 2 and 8 h in the meninges (J-K). Expression of IL-1α and IL-1β at 24 h was observed only in a few ependymal cells of the ventricles (H and P). IL-1α- and IL-1β- positive cells were also observed at 4 h but as expression was virtually identical to that observed at 2 h, data are not shown. Photomicrographs for vehicle-treated animals are only shown for 8 h, but no IL-1α- or IL-1β-expressing cells were observed at any of the time points studied (2, 4, 8, and 24 h). The morphology of IL-1α- and IL-1β-expressing cells appears to be microglia in parenchyma (solid arrows) and ependyma (white arrows), and macrophages (open arrows) in the meninges. For each time point, the pictures represent IL-1α or IL-1β expression in sections taken from the same animal. Q-X represent the effect of icv injection of 100 ng LPS or vehicle on IL-1β expression in the meninges (Q-T) and the peri-third ventricular hypothalamic region at 2 (R and V), 8 (S and W) and 24 h (T and X). Scale bars, 100μm
The number of IL-1α-positive cells in the parenchyma and ependymal lining appeared to be maximal at 2 h, when compared to 8 h. In contrast, IL-1β-expressing cells in the parenchyma and ependymal cells increased between 2-8 h after GALP injection, although the number of cells expressing IL-1β in the meninges appeared to be maximal at 2 h. There were no apparent cells immunoreactive for either IL-1α or IL-1β, at 24 h after injection of GALP, in any brain region apart from a few scattered cells in the ependymal lining of the ventricles (Fig. 1H and P).
Morphologically, the IL-1β-positive cells in the meninges and the choroid plexus were spherical, and are likely to represent macrophages. In contrast, IL-1α- and IL-1β-expressing cells within the brain parenchyma and the ependymal lining had a distinct morphology that resembled microglia.
IL-1α- and IL-1β-expressing cells were also observed in the brains of mice treated with 1.5 nmol GALP (data not shown). The effect of GALP on IL-1α and IL-1β protein was studied only at 2 h post-injection in mice, but was found to be expressed in an identical pattern to that observed in rats at the equivalent time point. In particular, strong expression was seen in the ependymal cells of the ventricles and the peri-ventricular regions.
In order to assess if icv injection of GALP caused a general inflammatory response in the periphery, plasma IL-6 concentration was analysed as a marker of systemic inflammation in the rat. There was no change in plasma IL-6 concentration at all time points (2, 4, 8 and 24 h) analysed, and in all samples, IL-6 concentrations were below the limit of detection (data not shown).
In order to compare the effect of GALP on IL-1 expression in the brain, a separate group of animals were injected icv with LPS (100 ng), a known stimulator of IL-1 expression. LPS caused a significant reduction in food intake (vehicle, 27.8 ± 0.7 g; LPS, 17.9 ± 3.5 g; P < 0.05) and body weight (vehicle, 10 ± 1 g; LPS, −1 ± 3 g; P < 0.01) at 24 h in rats. This anorexic effect of LPS was similar in magnitude to that observed after injection of GALP into rats (see experiment 3 and (6,13)). This dose of LPS (100 ng) was then used to assess the effect of LPS on expression of IL-1 in the same brain regions that were positive for IL-1 after icv injection of GALP. Icv injection of LPS caused an induction of IL-1β in the meninges at 2 h, which peaked at 8 h (Fig. 1R and S), and was almost absent at 24 h (Fig. 1T). In general, there were more cells expressing IL-1β at 8 h after injection of LPS compared to GALP (Fig. 1K). However, in contrast to GALP, LPS did not induce a robust expression of IL-1β in the parenchyma of the brain surrounding the ventricles at any time point studied (Fig. 1V-X), and only the occasional cell was detected for IL-1β immunoreactivity, albeit at a much lower intensity (Fig. 1W), although a few IL-1β-positive cells were detected in the ependymal cells lining the ventricles. No cells expressing IL-1β were observed in the brain after icv injection of vehicle at any time point. Icv injection of LPS also induced the expression of IL-1α in the same areas as where IL-1β was found. However, the effect of LPS on IL-1α was minimal, and only a few weakly-stained IL-1α positive cells were observed (data not shown).
Experiment 2: phenotype of IL-1α and IL-1β-expressing cells
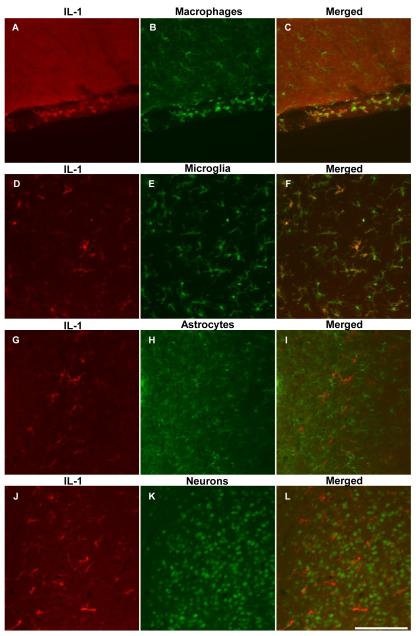
To determine the phenotype of the GALP-induced IL-1α and IL-1β-expressing cells in the rat, double fluorescence immunohistochemistry was performed to co-localise IL-1α or IL-1β with either astrocytes, microglia, or neurons. Figure 2 illustrates that the IL-1β-positive cells in the parenchyma surrounding the ventricles were microglia (Fig. 2F), and not astrocytes (Fig. 2I) or neurons (Fig. 2L). The IL-1β-expressing cells in the meninges (Fig. 2C) and choroid plexus (not shown) were also positive for Iba1, a marker for microglia and macrophages, and are therefore likely to be macrophages due to their spherical morphology.
FIG. 2.
Representative photomicrographs showing dual fluorescence immunohistochemistry for IL-1β, with markers for microglia and macrophages, astrocytes and neurons in the rat brain after icv injection of 1.5 nmol galanin-like peptide (GALP). IL-1β-positive cells in the meninges (A-C) and the parenchyma of the peri-third ventricular region (D-F) and were found to be macrophages (Iba1-positive), and microglia (Iba1-positive) respectively. However, cells expressing IL-1β did not co-localize with markers for astrocytes (GFAP-positive; G-I) or neurons (NeuN-positive; J-L). Cells expressing IL-1α in the parenchyma were also found to be microglia (data not shown for clarity). Scale bar, 100μm
Like IL-1β, IL-1α-expressing cells in the parenchyma were microglia, and not astrocytes or neurons (data not shown). As there were only a few cells expressing IL-1α in the meninges, the phenotype of these cells could not be determined.
Microglia and macrophages, astrocytes and neurons were detected in the brains of vehicle-injected rats, but they did not express IL-1α or IL-1β (data not shown).
Experiment 3: effect of IL-1RA on actions of GALP in the rat
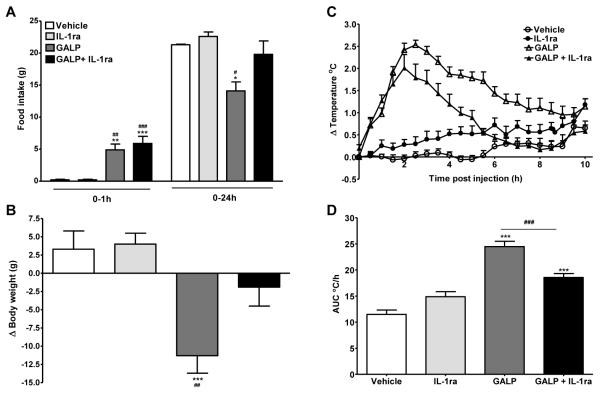
In rats, central administration of IL-1RA alone had no effect on food intake at 1 and 24 h, or body weight at 24 h, compared to vehicle-treated animals. Icv injection of GALP stimulated food intake at 1 h, but suppressed feeding at 24 h after treatment (1 h: vehicle, 0.2 ± 0.1 g vs GALP, 4.9 ± 0.9 g, P < 0.01; 24 h: vehicle, 21.3 ± 0.1 g vs GALP, 14.1 ± 1.4 g, P < 0.05; Fig. 3A). IL-1RA had no affect on GALP-induced feeding at 1 h, but significantly inhibited the reduction in feeding at 24 h in response to GALP, and the values for GALP plus IL-1RA-treated rats were no different from vehicle-injected rats (1 h: vehicle, 0.2 ± 0.1 g vs GALP+IL-1RA, 5.9 ± 1.1 g, P < 0.001; 24 h: vehicle, 21.3 ± 0.1 g vs GALP+IL-1RA, 19.8 ± 2.1 g; Fig. 3A). The reduction in the change in body weight 24 h after central administration of GALP was partially reversed by IL-1RA treatment (vehicle, 3 ± 3 g vs GALP, −11 ± 2 g vs GALP+IL-1RA, −2 ± 3 g; P < 0.001 GALP vs vehicle; Fig. 3B), and there was no significant difference between vehicle-, and GALP plus IL-1RA-treated rats.
FIG. 3.
Effect of interleukin-1 receptor antagonist (IL-1RA) on galanin-like peptide (GALP)-induced changes in food intake (A), body weight (B) and the change (Δ) in core body temperature (C and D) in rats. GALP (1.5 nmol in 1 μl) or vehicle (1 μl saline) was administered intracerebroventricularly (icv) in rats. Three separate icv injections of 200 μg (in 2 μl) of IL-1RA or vehicle (2 μl saline) were given at one hour before, at the time of, and one hour post injection of GALP (or vehicle). Food intake was measured at 1 and 24 h, and body weight at 24 h after injections. Body temperature was monitored continuously by remote radiotelemetry for 24 h but for clarity, data are shown only for 0-10 h. Analysis of the change in core body temperature over 0-10 h after injections is illustrated in D) as the area under the curve (AUC C/h). Data are mean ± SEM for n = 8-13 rats per group. *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs vehicle-treated animals and ##, P < 0.01, ###, P < 0.001 vs IL-1RA-treated animals.
Icv injection of GALP in rats caused a significant rise in body temperature that commenced immediately, peaked at 2-3 h, and lasted until approximately 10 h after injection (Fig. 3 C and D). Although IL-1RA administration alone had no effect on core body temperature compared to vehicle-treated rats, it significantly reduced the rise in core body temperature in rats treated with GALP (Fig. 3C and D). IL-1RA did not completely inhibit the GALP-induced rise in body temperature, as an immediate rise in temperature was observed in GALP plus IL-1RA-treated rats, which was temporally identical to rats injected with GALP alone. However, IL-1RA appeared to affect the maintenance of the temperature response to GALP. The duration of the febrile response in animals treated with IL-1RA plus GALP was shorter than in animals treated with GALP alone, and body temperature of rats injected with GALP plus IL-1RA was similar to vehicle-treated animals by 6 h after injections.
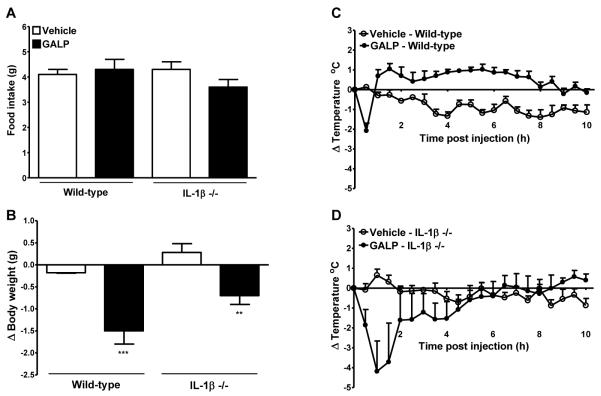
Experiment 4: effect of GALP on food intake, body weight, and core body temperature in IL-1α/β −/− mice
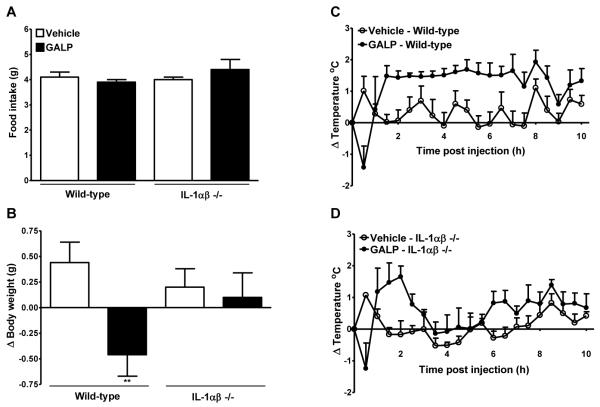
Icv injection of GALP in wild-type mice had no effect on food intake, but caused a significant reduction in the change in body weight at 24 h after injection. However, 24 h after icv injection of GALP in IL-1α/β −/− mice, there was no significant difference in food intake and body weight compared to vehicle-treated IL-1α/β mice (Fig. 4A and B).
FIG. 4.
Effect of galanin-like peptide (GALP) in mice lacking interleukin-1α and β (IL-1αβ −/−). GALP or vehicle was injected intracerebroventricularly (icv) into wild-type or IL-1αβ −/− mice. Food intake (A) and the change in body weight (B) were assessed at 24 h. Core body temperature was monitored continuously for 24 h but for clarity, data are plotted as change (Δ) in body temperature for 0-10 h only (C and D). Analysis of the change in core body temperature over 0-10 h after injections is illustrated in Table 1. Data are mean ± SEM for n = 5 mice per group. **, P < 0.01 vs the respective vehicle-treated animals.
Immediately after icv injection of GALP in wild-type and IL-1α/β −/− mice, a drop in core body temperature was observed that reached a nadir at 30 min (Table 1A, and Fig. 4C and D). The extent of hypothermia after GALP injection was not significantly different between wild-type and IL-1α/β −/− mice. In wild-type mice, this period of hypothermia was followed by an immediate rise in core body temperature that lasted until approximately 10 h after injection (Table 1B and Fig. 4C). However, there was no significant difference in the change in core body temperature after central injection of GALP in IL-1α/β −/− mice, when compared to vehicle-treated IL-1α/β −/− mice between 2-10 h after injection (Fig. 4D).
TABLE 1.
The temperature response after icv injection of galanin-like peptide (GALP) in IL-1α/β, IL-1β or IL-1RI −/− mice
A) The change in temperature at 30 min
| Δ °C at 30 min | ||
|---|---|---|
|
| ||
| Vehicle | GALP | |
| Wild-type | 1.0 ± 0.5 | −1.4 ± 0.7 a |
| IL-1α/β −/− | 1.1 ± 0.1 | −1.2 ± 0.8 a |
|
| ||
| Wild-type | 0.1 ± 0.1 | −2.1 ± 0.4 a |
| IL-1β −/− | 0.4 ± 0.2 | −1.9 ± 0.8 a |
|
| ||
| Wild-type | 1.3 ± 0.4 | −2.9 ± 0.4 b |
| IL-1R1 −/− | 0.7 ± 0.3 | −3.2 ± 0.2 b |
The change (Δ) in temperature was measured at 30 min after icv injection of 1.5 nmol GALP or vehicle (1 μl saline)
Data are mean ± SEM for n = 5 per group.
P < 0.05
P < 0.001 vs respective vehicle
B) The integrated temperature response (AUC, °C h) for 2-10 h
| AUC (°C h) | ||
|---|---|---|
|
| ||
| Vehicle | GALP | |
| Wild Type | 10.7 ± 2.4 | 19.7 ± 1.9a |
| IL-1α/β −/− | 8.2 ± 1.7 | 12.8 ± 1.9 |
|
| ||
| Wild Type | 16.1 ± 1.1 | 29.1 ± 1.6b |
| IL-1β −/− | 21.0 ± 1.6 | 21.1 ± 4.1 |
|
| ||
| Wild Type | 17.7 ± 1.7 | 24.8 ± 1.6c |
| IL-1R −/− | 13.3 ± 1.3 | 16.3 ± 1.5 |
The AUC for the temperature response was measured between 2-10 h after icv injection of 1.5 nmol GALP or vehicle (1 μl saline)
Data are mean ± SEM for n = 5 per group.
P < 0.05
P < 0.01
P < 0.001 vs respective vehicle
Experiment 5: effect of GALP on food intake, body weight, and core body temperature in IL-1β −/− mice
To identify if IL-1β was the ligand responsible for the actions of IL-1, the effect of GALP was tested in IL-1β −/− mice. Icv injection of GALP had no effect on food intake, but caused a significant reduction in the change in body weight at 24 h after injection in wild-type and IL-1β −/− mice (Fig. 5A and B). However, there was a non-significant trend in the extent of body weight change being less in the IL-1β −/− compared to wild-type mice (difference in change in body weight compared to respective vehicle-treated mice: wild-type, −1.32 ± 0.32 g vs IL-1β −/−, −1.01 ± 0.22 g; P > 0.05).
FIG. 5.
Effect of galanin-like peptide (GALP) in mice lacking interleukin-1β (IL-1β −/−). GALP or vehicle was injected intracerebroventricularly (icv) into wild-type or IL-1β −/− mice. Food intake (A) and the change in body weight (B) were assessed at 24 h. Core body temperature was monitored continuously for 24 h but for clarity, data are plotted as change (Δ) in body temperature for 0-10 h only (C and D). Analysis of the change in core body temperature over 0-10 h after injections is illustrated in Table 1. Data are mean ± SEM for n = 5 mice per group. **, P < 0.01, ***, P < 0.001 vs the respective vehicle-treated animals.
Icv injection of GALP caused an immediate drop in core body temperature in wild-type and IL-1β −/− mice that reached a nadir at 30 min in wild-type mice (Fig. 5C and D, and Table 1A). The hypothermic effect of GALP in IL-1β −/− mice was more prolonged than in wild-type mice, and significant differences were observed up to 90 min after injection of GALP in IL-1β −/− mice compared to vehicle-treated mice (Δ in temperature at 60 min: vehicle, 0.7 ± 0.3 g vs GALP, −4.2 ± 1.5 g, P < 0.05; 90 min: vehicle, 0.3 ± 0.3 g vs GALP; −3.7 ± 2.0 g, P < 0.05). However, this greater effect of GALP on hypothermia in IL-1β −/− mice was likely to be due to one animal that had an extreme and prolonged response. In wild-type mice, the transient period of hypothermia was followed by a prolonged rise in core body temperature that was significantly different to vehicle-treated wild-type mice over 2-10 h (Table 1B). However, following the hypothermic phase in GALP-treated IL-1β −/− mice, body temperature was the same as in IL-1β −/− mice injected with vehicle (Table 1B). Furthermore, there was no significant difference in body temperature over 2-10 h in GALP-versus vehicle-treated IL-1β −/−, even after the animal with the prolonged hypothermic response had been omitted (data not shown).
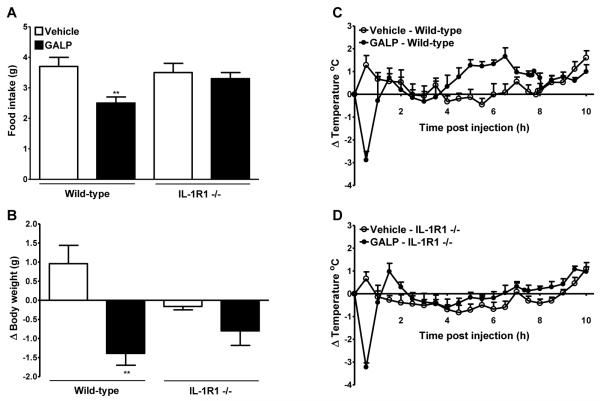
Experiment 6: effect of GALP on food intake, body weight, and core body temperature in IL-1RI −/− mice
It has been suggested that in addition to the known IL-1 receptor, IL-1RI, IL-1 may also signal via an unidentified novel receptor (17,18). Thus, to identify if IL-1RI mediates the actions of IL-1, the effect of GALP was tested in IL-1RI −/− mice. Icv injection of 1.5 nmol GALP in wild-type mice led to a significant reduction in food intake and change in body weight at 24 h. However, GALP administration had no significant effect on food intake and body weight in IL-1RI −/− mice at 24h, compared to vehicle treated IL-1RI −/− mice (Fig. 6A and B).
FIG. 6.
Effect of galanin-like peptide (GALP) in mice lacking the interleukin-1 type I receptor (IL-1RI −/−). GALP or vehicle was injected intracerebroventricularly (icv) into wild-type or IL-1R1 −/− mice. Food intake (A) and the change in body weight (B) were assessed at 24 h. Core body temperature was monitored continuously for 24 h but for clarity, data are plotted as change (Δ) in body temperature for 0-10 h only (C and D). Analysis of the change in core body temperature over 0-10 h after injections is illustrated in Table 1. Data are mean ± SEM for n = 5 mice per group. **, P < 0.01 vs respective vehicle-treated animals.
Icv injection of GALP in wild-type and IL-1RI −/− mice caused an immediate drop in core body temperature that reached a nadir at 30 min (Table 1A, and Fig. 6C and D). The extent of hypothermia was not significantly different in both wild-type and IL-1RI −/− mice, following GALP injection. In wild-type mice, this period of hypothermia was followed by a significant rise in core body temperature that lasted until approximately 8 h after injection, an effect that was not observed in IL-1RI −/− mice (Fig. 6C and D, and Table 1B).
Experiments 7-8: effect of GALP or LPS on food intake, body weight and core body temperature in TLR4 −/− mice
Icv injection of GALP had no effect on food intake at 24 h in wild-type or TLR4 −/− mice. However, GALP administration caused a significant reduction in the change in body weight in both wild-type and TL4R −/− mice. In addition, GALP caused an increase in core body temperature in wild-type and TLR4 −/−, and temperature was significantly elevated between 2-10 h (Table 2).
TABLE 2.
The effect of galanin-like peptide (GALP) and lipopolysaccharide (LPS) on food intake, body weight and core body temperature in toll-like receptor 4-deficient mice (TLR4 −/−)
| Food Intake (g) | Body weight change (Δ g) | AUC (°C h) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Wild-type | TLR4 −/− | Wild-type | TLR4 −/− | Wild-type | TLR4 −/− | |
| Vehicle | 3.8 ± 0.4 | 3.5 ± 0.4 | 0.4 ± 0.2 | 0.1 ± 0.2 | 11.7 ± 1.8 | 13.5 ± 1.9 |
| GALP | 3.5 ± 0.2 | 3.2 ± 0.3 | −0.1 ± 0.1a | −0.8 ± 0.3a | 24.5 ± 1.4c | 21.1 ± 0.4a |
|
| ||||||
| Vehicle | 3.7 ± 0.2 | 3.8 ± 0.1 | 0.4 ± 0.3 | −0.3 ± 0.1 | 14.1 ± 2.4 | 15.5 ± 3.9 |
| LPS | 2.6 ± 0.2b | 3.7 ± 0.2 | −0.7 ± 0.2a | −0.3 ± 0.2 | 23.9 ± 2.1a | 20.5 ± 1.6 |
Food intake and body weight were measured 24 h after icv injection of GALP (1.5 nmol) or vehicle (1 μl saline), or i.p. injection of LPS (100 μg/kg) or vehicle (5 ml/kg saline). For core body temperature, AUC was calculated between 2-10 h or 0-8 h for experiments with GALP or LPS respectively.
Data are mean ± SEM for n = 4-5 per group.
P < 0.05
P < 0.01
P < 0.001 vs respective vehicle.
Peripheral administration of LPS reduced food intake and caused a decrease in body weight at 24 h in wild-type mice, but had no effect in TLR4 −/− mice (Table 2). Core body temperature was elevated after peripheral injection of LPS in wild-type mice, but there was no significant difference in temperature over 8 h in TLR4 −/− mice treated with LPS, compared to vehicle-injected TLR4 −/− mice.
Discussion
Central administration of GALP has previously been shown to have dichotomous actions on energy balance in the rat; stimulating food intake over 1 h, but reducing food intake and body weight at 24 h (2,6). GALP also causes a prostaglandin-dependent rise in core body temperature in the rat (6). In contrast to effects in the rat, icv injection of GALP in mice induces only an anorexic response, and a transient hypothermic response that is followed by a period of hyperthermia (7,10). Data from the present study also confirm these findings and further support the differing actions of GALP in rats and mice. However, central administration of GALP reliably reduces body weight at 24 h in rats and mice (6-8,13), although the ability of GALP to reduce food intake at this time point has been reported to be variable in rats (6,13). Experiments in the present study also indicate that although GALP causes a reduction in body weight in mice at 24 h, an effect on food intake is not always observed at this time point. These data are in contrast to other reports demonstrating GALP reduces food intake in mice 24 h after icv injection, although in these studies higher doses of GALP (3-5 nmol) were used compared to present study (1.5 nmol) (7,8,10). The ability of GALP to affect body weight in the absence of an affect on food intake supports the suggestion that GALP is also affecting energy expenditure (21) and a recent report demonstrates that central administration of GALP increases metabolic rate (22).
The anorexic and febrile responses observed after GALP injection in rats and mice are similar to how rodents respond to an immune challenge, and in particular to injection of the pro-inflammatory cytokine IL-1. Peripheral or central injection of IL-1α or IL-1β in rodents reduces food intake, body weight and causes fever (23-26), effects that are similar in magnitude and profile to GALP. These similarities between IL-1 and GALP led us to test our main hypothesis that IL-1 mediates the effects of GALP on energy balance, and questions the physiological relevance of the effects of GALP on energy balance. The present study demonstrates that central administration of GALP (but not galanin) in rodents induced IL-1α and IL-1β expression in the brain, and the effects of GALP on feeding, body weight and core body temperature are mediated by IL-1 acting via IL-1RI. These data suggest that GALP may induce an inflammatory response in the brain. However, these effects of GALP on inflammation are likely to be localised to the brain, as GALP did not affect plasma IL-6 levels (a marker of peripheral infection).
In rats, inhibition of endogenous IL-1, using the antagonist IL-1RA, significantly reduced the GALP-induced reduction in food intake and body weight at 24 h, but had no effect on the acute orexigenic action. The latter result is not surprising since studies in the rat have demonstrated that the acute stimulatory effect of GALP on feeding is mediated by the orexigenic neuropeptides orexin and NPY (11,12). IL-1RA also partially reduced the rise in core body temperature observed in rats after GALP, with a predominant effect on the maintenance of the febrile response. The failure of IL-1RA to completely reverse the GALP-induced rise in body temperature is not unusual as several studies have demonstrated the inability of central administration of IL-1RA to fully inhibit the febrile response to LPS (27,28). Thus, in order to avoid any possible uncertainties about the dose of IL-1RA, time of administration and/or its short half life, we studied the effects of GALP in mice that were defective in the IL-1 system. Our data show that the acute hypothermia induced by GALP is not mediated by IL-1α or IL-1β. However, the GALP-induced hyperthermia is due to the action of IL-1β on the IL-1RI, as mice deficient in IL-1β or IL-1RI were resistant to these effects of GALP temperature. As IL-1β −/− mice also fail to show GALP-induced hyperthermia, the contribution of IL-1α in the febrile effects of GALP cannot be fully disregarded, but is unlikely, as IL-1β is the predominant ligand involved in the febrile response in rodents (29,30). It is also difficult to conclude whether IL-1α or IL-1β are involved in the anorexic effects of GALP, as this neuropeptide did not significantly reduce food intake in wild-type mice in experiments involving IL-1α/β- and IL-1β-deficient mice. However, one or both of these IL-1 ligands are mediating the effects of GALP on food intake through IL-1RI, as IL-1RI −/− mice did not respond to the anorexic actions of GALP, which is in contrast to the wild-type control mice. In addition, as both IL-1α/β −/− and IL-1RI −/− mice were resistant to the actions of GALP on body weight, and IL-1β −/− mice still lost body weight in response to GALP (albeit less than in wild-type mice), IL-1α must be playing a role in the effect of GALP on body weight. As in our hands, IL-1α is equally or more potent at reducing food intake and body weight when compared to IL-1β in mice (unpublished observation), it can be hypothesised that the effects of GALP on food intake and body weight are through the actions of both IL-1α and IL-1β, but the febrile response is mediated by IL-1β. However, to fully understand the contribution of IL-1α in the actions of GALP, studies would need to be performed in IL-1α deficient mice.
Central administration of GALP (but not galanin) in rats stimulated the expression of IL-1α and IL-1β in the parenchyma surrounding the ventricles, predominantly in the region surrounding the third ventricle of the hypothalamus, the ependymal cells of the ventricles. GALP also stimulated expression of IL-1β in the meninges and choroid plexus, whereas only a few cells expressing IL-1α were found in these regions. The IL-1α- and IL-1β-positive cells found in the parenchyma were microglia, while the cells expressing IL-1β in the meninges and choroid plexus were macrophages. The time course of expression of IL-1α versus IL-1β appeared to be slightly different, with maximal expression of IL-1α was earlier compared with IL-1β. The relevance of the temporal difference between IL-1α and IL-1β expression, and the lack of IL-1α expression in the meninges is unclear. However, in order to verify these qualitative findings, the expression of the two IL-1 ligands needs to be assessed quantitatively (e.g. by ELISA). The effect of central administration of LPS on IL-1 expression in the rat was also assessed. Although IL-1β-positive cells were found within the meninges and the ependymal cells of the ventricles after icv LPS, there were very few cells expressing IL-1β found within the parenchyma surrounding the ventricles. The lack of IL-1β-positive cells in the parenchyma is in contrast to the effect seen here with GALP, and suggests that this neuropeptide is more potent at inducing IL-1β in the brain after central administration compared to the inflammatory stimulus LPS.
The mechanism by which GALP leads to this production of IL-1 in the brain also remains to be determined. Galanin and GALP are reported to act via the three known galanin receptors GALR1-3. In vitro studies show that galanin exhibits equal affinity for all three receptors, while GALP has higher affinity for GALR2 and GALR3 (1,31). However, it is likely that GALP is initiating IL-1 expression via a novel receptor as galanin did not induce expression of this cytokine in the brain. These findings are in agreement with data from other groups, suggesting that GALP acts via a novel receptor. For example, GALP produces several different physiological responses compared to galanin (e.g. (6,13,32)), and the anorexic actions of GALP are still present in mice deficient in GALR1 or GALR2 (8).
Central administration of GALP induces a specific pattern of Fos activation in the brain when compared to galanin (13,14,33). In particular, GALP activates cells within the peri-ventricular hypothalamic area (13), the region where microglial IL-1 expression is observed here. It is unlikely that GALP directly activates microglia to express IL-1, as the cells that express Fos in the peri-ventricular hypothalamic area are astrocytes and not microglia or neurons (13). The actions of GALP on astrocytes may be direct as galanin receptors are expressed on astrocytes in culture (34), however, this effect may also be due to a novel GALP receptor as galanin does not induce peri-ventricular hypothalamic Fos expression (13). The relevance of the GALP-induced astrocytic activation is unclear and remains to be tested, but these cells have been shown to release prostaglandins that may be involved in the febrile response to GALP. It is also possible that IL-1 production within microglia is mediated via key inflammatory mediators released by astrocytes.
Peripheral infections activate the immune system leading to cytokine production, which results in changes in brain-mediated responses including sickness behaviour, fever and anorexia. The effects of GALP in the present study on feeding, body weight, body temperature and IL-1 expression are analogous to those observed during infection and inflammation. The endotoxin LPS is a component of gram-negative bacteria cell walls and is a potent inducer of the inflammatory response. Like GALP, peripheral or central administration of LPS to rodents causes a reduction in food intake and body weight, and a rise in core body temperature (35). LPS stimulates the expression of IL-1 in the brain (36-38), and its effects on feeding, body weight and temperature are mediated by this cytokine (39-41), actions similar to GALP in the present study. GALP mRNA in the arcuate nucleus and pituitary are increased in response to LPS administration in rats (42,43). It is possible, therefore, that LPS may mediate its affects on IL-1, and hence fever and anorexia, via actions of GALP. In order to test this hypothesis, the effects of LPS in a GALP-deficient mouse, or in the presence of a GALP specific antagonist, needs to be determined. However, as the actions of GALP are similar to those of LPS it could be argued that the preparations of GALP used in the present study were contaminated with endotoxin. In order to test this, we injected GALP into mice deficient in the receptor that is responsible for the actions of LPS, TLR4 (44). In contrast to LPS that had no effect in TLR4 −/− mice, GALP caused a reduction in body weight and a rise in body temperature in these mice. These data confirm that the GALP samples do not contain endotoxin, or that GALP is a ligand for TLR4.
Taken together, the findings in the present study question the relevance of GALP in the physiological regulation of food intake. It is possible that endogenous GALP has an acute physiological effect in rats to increase feeding via the actions of orexin and/or NPY (11,12). However, in contrast, the anorexia, body weight loss and fever induced by central administration of GALP may represent a non-GALR-mediated non-specific sickness response that is dependent on IL-1. Caution is therefore needed in the interpretation of studies involving the central administration of GALP.
In summary, we have demonstrated that the anorexic and febrile effects of GALP in the rodent are mediated by IL-1 in the brain. The relevance of the localised IL-1 production in response to central administration of GALP, and how this relates to energy balance, remain to be determined. However, as GALP neurons are located in the arcuate nucleus, they are ideally positioned to receive and respond to peripheral signals, and it is therefore interesting to speculate that GALP may be an intermediary in how peripheral infections lead to anorexic and febrile effects.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council and Research Councils UK (RCUK).
Footnotes
Disclosure summary: The authors have nothing to disclose
References
- 1.Ohtaki T, Kumano S, Ishibashi Y, Ogi K, Matsui H, Harada M, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J Biol Chem. 1999;274:37041–37045. doi: 10.1074/jbc.274.52.37041. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto Y, Watanabe T, Adachi Y, Itoh T, Ohtaki T, Onda H, Kurokawa T, Nishimura O, Fujino M. Galanin-like peptide stimulates food intake in the rat. Neurosci Lett. 2002;322:67–69. doi: 10.1016/s0304-3940(01)02515-0. [DOI] [PubMed] [Google Scholar]
- 3.Jureus A, Cunningham MJ, McClain ME, Clifton DK, Steiner RA. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology. 2000;141:2703–2706. doi: 10.1210/endo.141.7.7669. [DOI] [PubMed] [Google Scholar]
- 4.Jureus A, Cunningham MJ, Li D, Johnson LL, Krasnow SM, Teklemichael DN, Clifton DK, Steiner RA. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology. 2001;142:5140–5144. doi: 10.1210/endo.142.12.8542. [DOI] [PubMed] [Google Scholar]
- 5.Kumano S, Matsumoto H, Takatsu Y, Noguchi J, Kitada C, Ohtaki T. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology. 2003;144:2634–2643. doi: 10.1210/en.2002-221113. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence CB, Baudoin FM, Luckman SM. Centrally administered galanin-like peptide modifies food intake in the rat: a comparison with galanin. J Neuroendocrinol. 2002;14:853–860. doi: 10.1046/j.1365-2826.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 7.Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144:813–822. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- 8.Krasnow SM, Hohmann JG, Gragerov A, Clifton DK, Steiner RA. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology. 2004;79:268–277. doi: 10.1159/000079632. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman AS, Buenzle J, Fraley GS, Rissman EF. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav. 2005;48:141–151. doi: 10.1016/j.yhbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Man PS, Lawrence CB. The Effects of Galanin-Like Peptide on Energy Balance, Body Temperature and Brain Activity in the Mouse and Rat Are Independent of the GALR2/3 Receptor. J Neuroendocrinol. 2008;20:128–137. doi: 10.1111/j.1365-2826.2007.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kageyama H, Kita T, Toshinai K, Guan JL, Date Y, Takenoya F, Kato S, Matsumoto H, Ohtaki T, Nakazato M, Shioda S. Galanin-like peptide promotes feeding behaviour via activation of orexinergic neurones in the rat lateral hypothalamus. J Neuroendocrinol. 2006;18:33–41. doi: 10.1111/j.1365-2826.2005.01382.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuramochi M, Onaka T, Kohno D, Kato S, Yada T. Galanin-like peptide stimulates food intake via activation of neuropeptide Y neurons in the hypothalamic dorsomedial nucleus of the rat. Endocrinology. 2006;147:1744–1752. doi: 10.1210/en.2005-0907. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence CB, Williams T, Luckman SM. Intracerebroventricular galanin-like peptide induces different brain activation compared with galanin. Endocrinology. 2003;144:3977–3984. doi: 10.1210/en.2003-0391. [DOI] [PubMed] [Google Scholar]
- 14.Fraley GS, Shimada I, Baumgartner JW, Clifton DK, Steiner RA. Differential patterns of Fos induction in the hypothalamus of the rat following central injections of galanin-like peptide and galanin. Endocrinology. 2003;144:1143–1146. doi: 10.1210/en.2002-0114. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence CB, Rothwell NJ. Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. J Neuroendocrinol. 2001;13:490–495. doi: 10.1046/j.1365-2826.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 17.Andre R, Moggs JG, Kimber I, Rothwell NJ, Pinteaux E. Gene regulation by IL-1beta independent of IL-1R1 in the mouse brain. Glia. 2006;53:477–483. doi: 10.1002/glia.20302. [DOI] [PubMed] [Google Scholar]
- 18.Touzani O, Boutin H, LeFeuvre R, Parker L, Miller A, Luheshi G, Rothwell N. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type I receptor. J Neurosci. 2002;22:38–43. doi: 10.1523/JNEUROSCI.22-01-00038.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 20.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: 1997. [Google Scholar]
- 21.Hansen KR, Krasnow SM, Nolan MA, Fraley GS, Baumgartner JW, Clifton DK, Steiner RA. Activation of the sympathetic nervous system by galanin-like peptide--a possible link between leptin and metabolism. Endocrinology. 2003;144:4709–4717. doi: 10.1210/en.2003-0748. [DOI] [PubMed] [Google Scholar]
- 22.Rich N, Reyes P, Reap L, Goswami R, Fraley GS. Sex differences in the effect of prepubertal GALP infusion on growth, metabolism and LH secretion. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moldawer LL, Andersson C, Gelin J, Lundholm KG. Regulation of food intake and hepatic protein synthesis by recombinant-derived cytokines. Am J Physiol. 1988;254:G450–G456. doi: 10.1152/ajpgi.1988.254.3.G450. [DOI] [PubMed] [Google Scholar]
- 24.Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello CA. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Invest. 1989;84:228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence CB, Rothwell NJ. Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. J Neuroendocrinol. 2001;13:490–495. doi: 10.1046/j.1365-2826.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 26.Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, Lundkvist J, Michaud B, Mistry Y, Van Dam AM, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJ, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw. 1998;9:279–288. [PubMed] [Google Scholar]
- 27.Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- 28.Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busbridge NJ, Dascombe MJ, Tilders FJ, van Oers JW, Linton EA, Rothwell NJ. Central activation of thermogenesis and fever by interleukin-1 beta and interleukin-1 alpha involves different mechanisms. Biochem Biophys Res Commun. 1989;162:591–596. doi: 10.1016/0006-291x(89)92351-6. [DOI] [PubMed] [Google Scholar]
- 30.Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, Lundkvist J, Michaud B, Mistry Y, Van Dam AM, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJ, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw. 1998;9:279–288. [PubMed] [Google Scholar]
- 31.Lang R, Berger A, Santic R, Geisberger R, Hermann A, Herzog H, Kofler B. Pharmacological and functional characterization of galanin-like peptide fragments as potent galanin receptor agonists. Neuropeptides. 2005;39:179–184. doi: 10.1016/j.npep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Fraley GS, Thomas-Smith SE, Acohido BV, Steiner RA, Clifton DK. Stimulation of sexual behavior in the male rat by galanin-like peptide. Horm Behav. 2004;46:551–557. doi: 10.1016/j.yhbeh.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto H, Noguchi J, Takatsu Y, Horikoshi Y, Kumano S, Ohtaki T, Kitada C, Itoh T, Onda H, Nishimura O, Fujino M. Stimulation effect of galanin-like peptide (GALP) on luteinizing hormone-releasing hormone-mediated luteinizing hormone (LH) secretion in male rats. Endocrinology. 2001;142:3693–3696. doi: 10.1210/endo.142.8.8432. [DOI] [PubMed] [Google Scholar]
- 34.Hosli E, Ledergerber M, Kofler A, Hosli L. Evidence for the existence of galanin receptors on cultured astrocytes of rat CNS: colocalization with cholinergic receptors. J Chem Neuroanat. 1997;13:95–103. doi: 10.1016/s0891-0618(97)00024-0. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy DO, Kluger MJ, Vander AJ. The role of fever in appetite suppression after endotoxin administration. Am J Clin Nutr. 1984;40:310–316. doi: 10.1093/ajcn/40.2.310. [DOI] [PubMed] [Google Scholar]
- 36.Van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 37.Van Dam AM, Poole S, Schultzberg M, Zavala F, Tilders FJ. Effects of peripheral administration of LPS on the expression of immunoreactive interleukin-1 alpha, beta, and receptor antagonist in rat brain. Ann N Y Acad Sci. 1998;840:128–138. doi: 10.1111/j.1749-6632.1998.tb09557.x. [DOI] [PubMed] [Google Scholar]
- 38.Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- 39.Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–R98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- 40.Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- 42.Saito J, Ozaki Y, Ohnishi H, Nakamura T, Ueta Y. Induction of galanin-like peptide gene expression in the rat posterior pituitary gland during endotoxin shock and adjuvant arthritis. Brain Res Mol Brain Res. 2003;113:124–132. doi: 10.1016/s0169-328x(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 43.Saito J, Ozaki Y, Kawasaki M, Ohnishi H, Okimoto N, Nakamura T, Ueta Y. Induction of galanin-like peptide gene expression in the arcuate nucleus of the rat after acute but not chronic inflammatory stress. Brain Res Mol Brain Res. 2005;133:233–241. doi: 10.1016/j.molbrainres.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–120. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]