Abstract
Dysregulation of cholesterol balance contributes significantly to atherosclerotic cardiovascular disease (ASCVD), the leading cause of death in the United States. The intestine has the unique capability to act as a gatekeeper for entry of cholesterol into the body, and inhibition of intestinal cholesterol absorption is now widely regarded as an attractive non-statin therapeutic strategy for ASCVD prevention. In this chapter we discuss the current state of knowledge regarding sterol transport across the intestinal brush border membrane. The purpose of this work is to summarize substantial progress made in the last decade in regards to protein-mediated sterol trafficking, and to discuss this in the context of human disease.
Keywords: Intestinal cholesterol absorption, Niemann-Pick C1-Like 1, sterol-sensing domain, ATP-binding cassette transporters G5 and G8, scavenger receptor class B type I, animal model, cell model, ezetimibe
1. INTRODUCTION
As discussed in detail in previous chapters, cholesterol is essential for the growth and function of all mammalian cells. However, elevated low-density lipoprotein (LDL) cholesterol (LDL-C) represents a major risk factor for the development of atherosclerotic cardiovascular disease (ASCVD). For many years now, statin-mediated inhibition of endogenous cholesterol biosynthesis has been the major therapeutic means to lower LDL-C, yet ASCVD still persists in most of the world (Rosamond et al., 2007, 2002). Therefore, additional LDL-C lowering is now recommended, and the search for therapeutic strategies that work in synergy with statins has now begun. As a result of this search, drugs that inhibit intestinal cholesterol absorption have become an attractive therapeutic strategy to use in combination with statins. However, only within the last decade have we begun to understand how intestinal sterol absorption occurs at the molecular level. Recently we have learned that intestinal sterol absorption is tightly regulated by key proteins located at the brush border membrane. One of these proteins, Niemann-Pick C1-Like 1 (NPC1L1), was recently identified to be essential for intestinal cholesterol absorption, and will be discussed in detail. In direct opposition of NPC1L1, the heterodimer of ATP-binding cassette transporters G5 and G8 (ABCG5/G8) has been shown to be critical for intestinal disposal of sterols. In addition, the scavenger receptor class B type I (SR-BI) has been implicated in modulating intestinal cholesterol absorption. The purpose of this chapter is to summarize the current state of knowledge regarding the structure and function of these apically-localized cholesterol transporters, and to provide discussion about how these proteins and others interact to regulate the complex process of intestinal sterol absorption.
2. INTESTINAL CHOLESTEROL ABSORPTION AND EZETIMIBE
Cholesterol in the intestinal lumen is mainly derived from bile and diet. A physiological process by which cholesterol enters intestinal or thoracic duct lymph across the small intestine is called intestinal cholesterol absorption (Wang, 2007) (Figure 1). Cholesterol absorption involves at least the following three phases: 1) intralumenal solubilization; 2) movement across the apical membrane of absorptive enterocytes; and 3) intracellular metabolism for incorporation into chylomicrons destined for lymph. Intestinal cholesterol absorption rates range widely from 29% to 80% in normal men and women consuming a moderately low cholesterol diet (Bosner et al., 1999). Molecular mechanisms underlying this large inter-individual variation remain to be elucidated.
Figure 1.
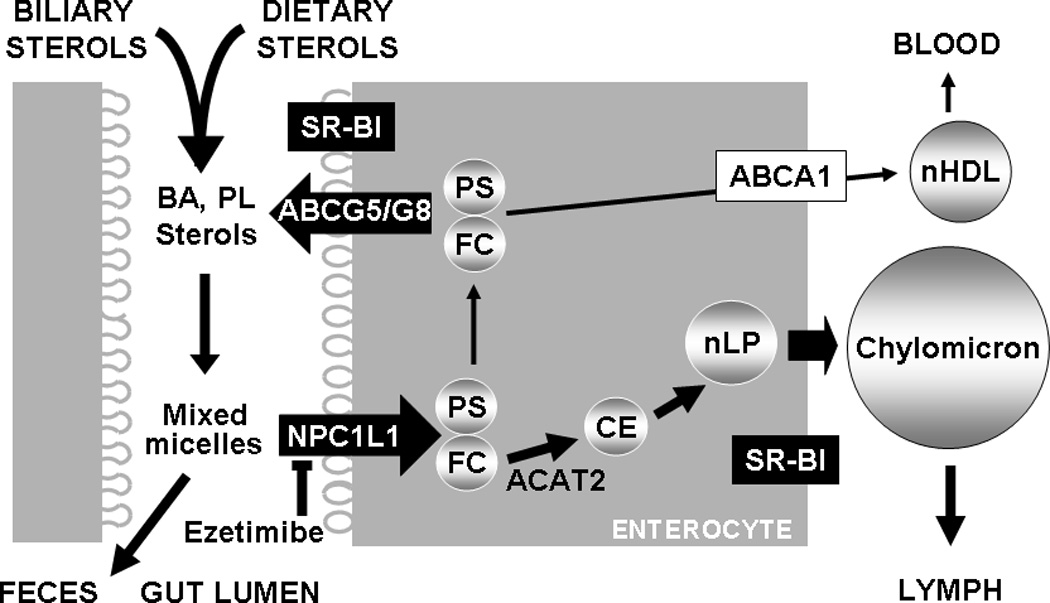
Intestinal sterol absorption and secretion. Sterols including free cholesterol (FC) and free plant sterols (PS) from diet and bile are mixed with phospholipids (PL) and bile acids (BA) to form micelles. FC and PS solubilized in mixed micelles are transported into absorptive enterocytes via an NPC1L1-dependent and ezetimibe-inhibitable mechanism. FC is delivered to the ER for esterification by acyl-CoA:cholesterol acyltransferase-2 (ACAT2) to form cholesterol esters (CE) that is then packaged into nascent lipoprotein particles (nLP) and secreted as a constituent of chylomicron. PS and FC that escapes ACAT2 esterification may be directly transported to nascent HDL (nHDL) through basolateral ABCA1, or back to the gut lumen via ABCG5/G8.
Since a detailed review of intestinal cholesterol absorption and its potential protein mediators is beyond the focus of this chapter, readers interested in this topic are referred to many excellent reviews available (Wilson and Rudel, 1994, Dawson and Rudel, 1999, Davis and Veltri, 2007, Levy et al., 2007, Wang, 2007, Turley and Dietschy, 2003, Iqbal and Hussain, 2009, Hui and Howles, 2005).
Intestinal cholesterol absorption represents an attractive target for developing cholesterol-lowering drugs because it is a major pathway governing whole-body cholesterol homeostasis. Schering-Plough Research Institute successfully identified ezetimibe as a potent and specific inhibitor of intestinal cholesterol absorption using in vivo models of cholesterol absorption (Clader, 2004). The drug is now widely used in monotherapy or in combination with statins (inhibitors of cholesterol biosynthesis) to efficiently treat hypercholesterolemia in the general population (Davis and Veltri, 2007).
Intestinal cholesterol absorption was once thought to be a passive process. The fact that ezetimibe potently inhibits intestinal cholesterol absorption at very low doses (Van Heek et al., 1997, van Heek et al., 2001, Sudhop et al., 2002) suggests that specific protein(s) must be involved in cholesterol absorption (Turley and Dietschy, 2003). In a search for the ezetimibe-inhibitory protein(s), NPC1L1, a previously-identified protein of unknown function (Davies et al., 2000), was discovered in the ezetimibe-sensitive pathway because disruption of NPC1L1 in mice reduces intestinal cholesterol absorption to the level seen in ezetimibe-treated animals (Altmann et al., 2004, Davis et al., 2004). Whether NPC1L1 is the molecular target of ezetimibe has been under considerable debate (Smart et al., 2004, Kramer et al., 2005, Labonte et al., 2007, Knopfel et al., 2007). Ezetimibe can bind to intestinal brush border membrane vesicles from wild-type mice but not mice lacking NPC1L1 (Garcia-Calvo et al., 2005). Recently, Weinglass and associates from Merck Research Laboratories purified NPC1L1-ezetimibe complex from NPC1L1-expressing and ezetimibe-treated cells and found that NPC1L1 is the only protein to account for ezetimibe binding (Weinglass et al., 2008). These pieces of biochemical evidence, together with findings from animal, genetic and cell biology studies (Yu, 2008) (see Sections below), strongly supports that NPC1L1 is the molecular target of ezetimibe.
3. NPC1L1
3.1 Structure: gene, mRNA, and protein domains
In human genome, NPC1L1 gene spans about 29 kb in chromosome 7p13 and contains 20 exons. It produces a predominant mRNA transcript that skips exon 15. This transcript, like that from rodents, encodes a 1332-amino acid protein, and has been used in most, if not all, studies (Davies et al., 2000, Yu et al., 2006, Altmann et al., 2004). Human NPC1L1 gene also produces two alternatively spliced transcripts (Davies et al., 2000). One contains a 27-amino acid insertion transcribed from the in-frame exon 15. The other skips exon 7 and terminates within intron 8, encoding a truncated protein of 724 amino acids. The physiological significance of these two alternatively spliced variants has yet to be defined.
The human NPC1L1 protein is a homolog of Niemann-Pick C1 (NPC1), having ~50% amino acid homology to NPC1 protein (Davies et al., 2000, Davies and Ioannou, 2006). Deficiency of human NPC1 causes an autosomal recessive lipid storage disorder, Niemann-Pick disease type C1 that is characterized by defective trafficking of intracellular cholesterol and lysosomal accumulation of free cholesterol, gangliosides and other lipids (Carstea et al., 1997, Loftus et al., 1997). NPC1L1, like its homolog NPC1, also shares similarity with the resistance-nodulation-division family of prokaryotic permeases that can pump out lipophilic drugs, detergents, fatty acids, bile acids, metal ions, and dyes from the cytosol of bacteria (Davies et al., 2000, Davies and Ioannou, 2006).
Based on the amino acid sequences, human NPC1L1 is predicted to have a typical signal peptide of 21 amino acids, 13 putative transmembrane domains (Figure 2A), and extensive potential N-linked glycosylation sites located within the extracellular loops of the protein facing intestinal lumen (Altmann et al., 2004, Davies et al., 2000) or within the luminal loops of the protein if endocytosed from plasma membrane into intracellular vesicles (Yu et al., 2006, Ge et al., 2008, Wang et al., 2009). The membrane topology and N-glycosylation of NPC1L1 have been examined experimentally and the findings are consistent with sequence-based prediction (Iyer et al., 2005, Temel et al., 2007, Altmann et al., 2004, Davies et al., 2000, Wang et al., 2009).
Figure 2.
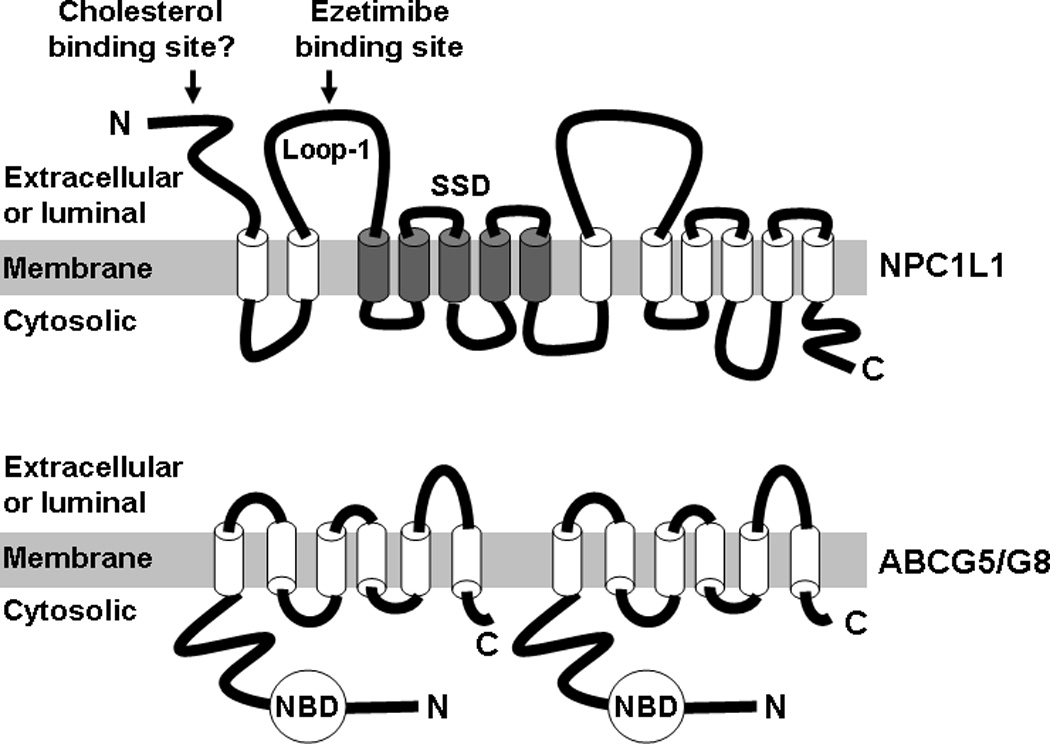
Proposed membrane topologies and domains of NPC1L1 (A) and ABCG5/G8 (B) proteins. SSD, sterol-sensing domain; N, N-terminus; C, C-terminus; NBD, nucleotide-binding domain.
A sterol-sensing domain (SSD) is another signature of NPC1L1 protein (Davies et al., 2000, Altmann et al., 2004) (Figure 2A). This domain consists of ~180 amino acids that form five predicted membrane-spanning helices with short intervening loops (Radhakrishnan et al., 2004). The SSD is conserved in at least 7 other membrane proteins, all of which have relations to cholesterol (Kuwabara and Labouesse, 2002), including the aforementioned NPC1 (Davies et al., 2000, Carstea et al., 1997, Loftus et al., 1997); 3-hydroxy-3-methylglutaryl (HMG) CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis (Brown and Goldstein, 1999, Goldstein and Brown, 1990); sterol regulatory element-binding protein (SREBP)-cleavage activating protein (SCAP), a protein that controls the endoplasmic reticulum (ER)-to-Golgi transport and proteolytic activation of membrane-bound transcription factors SREBPs (Brown and Goldstein, 1999, Horton et al., 2002, Goldstein and Brown, 1990); and Patched, a membrane receptor for the cholesterol-linked signaling peptide Hedgehog (Cooper et al., 2003). The function of NPC1L1 SSD remains unknown and may be implicated in cholesterol regulation of NPC1L1 protein trafficking (Yu et al., 2006).
3.2. Function: Lessons learned from animal models and human genetics
In mammals, NPC1L1 mRNA and protein are highly expressed in small intestine (Altmann et al., 2004, Davies et al., 2005). In the small intestine, NPC1L1 protein localizes at the apical surface of absorptive enterocyte (Altmann et al., 2004, Davis et al., 2004). Gene knockout studies in mice have unambiguously established an essential role of NPC1L1 in intestinal cholesterol absorption (Altmann et al., 2004, Davis et al., 2004, Tang et al., 2008, Tang et al., 2008, Temel et al., 2009). The cholesterol absorption inhibitor ezetimibe cannot further reduce intestinal cholesterol absorption in NPC1L1-deficient mice, demonstrating that NPC1L1 is in the ezetimibe-sensitive pathway (Altmann et al., 2004, Davis et al., 2004).
Interestingly, genetic ablation of NPC1L1 in mice also protects against obesity, insulin resistance and fatty liver induced by a nutrient-rich diet (Labonte et al., 2008, Davies et al., 2005) (Yu, L., unpublished observation). Ezetimibe treatment also improves dyslipidemia, hepatic steatosis, non-alcoholic fatty liver disease, and insulin resistance in several animal models (van Heek et al., 2001, Assy et al., 2006, Deushi et al., 2007, Zheng et al., 2008). Another interesting observation is that NPC1L1 ablation in mice greatly attenuates dyslipidemia, lipogenic gene overexpression, and hepatic steatosis induced by activation of nuclear receptor liver X receptors (LXRs) (Tang et al., 2008). LXR forms a heterodimer with retinoid X receptor to regulate expression of their target genes in response to fluctuations of cellular cholesterol content (Repa and Mangelsdorf, 2002). Given that rodent NPC1L1 is almost exclusively expressed in the small intestine and the primary defect of NPC1L1-null mice is the blockade of intestinal cholesterol absorption (Altmann et al., 2004), the observed phenotypes are likely attributable to reduced intestinal cholesterol absorption. Efficient intestinal cholesterol absorption may be essential for maximizing LXR activities in the liver and small intestine, and perhaps other tissues where cholesterol and its derivatives are likely used as endogenous LXR ligands. LXR target genes involve the regulation of many metabolic pathways, including metabolism of cholesterol, fatty acids, and carbohydrates (Kalaany and Mangelsdorf, 2006). Inhibition of NPC1L1-dependent intestinal cholesterol absorption may improve some metabolic disorders by down-regulating tissue LXR activities. Consistent with this notion, LXR knockout mice are protected against obesity induced by a high fat diet (Kalaany et al., 2005).
Although NPC1L1 mRNA and protein are very abundant in the small intestine of mammals examined, their levels in the liver differ remarkably among species. Rodents express only a negligible amount of NPC1L1 in the liver (Altmann et al., 2004, Tang et al., 2006). In contrast, human livers have readily detectable levels of NPC1L1 mRNA and proteins (Altmann et al., 2004, Davies et al., 2005, Temel et al., 2007). The reason for different expression pattern of NPC1L1 among species is unknown, but may result from differences in cholesterol metabolism among species (Dietschy and Turley, 2002, Yu, 2007). In the liver of nonhuman primates and humans, NPC1L1 localizes to the canalicular membrane of hepatocyte (Yu et al., 2006, Temel et al., 2007). When overexpressed in the mouse liver by transgenic technology, human NPC1L1 also concentrates to the canalicular membrane of hepatocyte (Temel et al., 2007). Whereas the function of NPC1L1 in human livers remains to be elucidated, transgenic overexpression of human NPC1L1 in the mouse liver dramatically reduces biliary cholesterol concentrations without altering hepatic expression levels of the cholesterol efflux transporter ABCG5/G8 (Temel et al., 2007). This finding implies that hepatic NPC1L1 may inhibit biliary cholesterol excretion by transporting cholesterol from the canalicular bile back into hepatoctyes (Yu, 2008). The inhibition of NPC1L1 overexpression on biliary cholesterol excretion can be rescued by ezetimibe treatment, suggesting that hepatic NPC1L1 is another target for ezetimibe, at least in mice (Temel et al., 2007).
Increased cholesterol concentrations in the bile can result in gallstone formation. If ezetimibe treatment results in an increase in biliary cholesterol concentration in humans by inhibiting hepatic NPC1L1, it may have a potential to promote gallstone formation particularly in subjects in whom NPC1L1 is more abundantly expressed in the liver than in the intestine. Currently, there is no evidence that ezetimibe increases the incidence of gallstone disease and it is unknown whether hepatic NPC1L1 regulates biliary cholesterol excretion in humans. In a small human study, inhibition of NPC1L1 for 30 days by ezetimibe treatment at 20 mg/day did not alter biliary cholesterol molar percentage, cholesterol to phospholipid ratio, and cholesterol saturation index in 5 overweight subjects without gallstones, but did reduce these parameters significantly in 7 patients with gallstones (Wang et al., 2008). Ezetimibe may have its predominant effect at the intestinal level, thereby reducing cholesterol that is transported from the gut lumen to the liver for biliary secretion. In animals that express NPC1L1 predominantly in the small intestine, inhibition of NPC1L1 by ezetimibe may reduce biliary cholesterol levels and prevent gallstone disease. This notion is consistent with the following observations: 1) NPC1L1 knockout mice have lower biliary cholesterol concentrations, even after being challenged with a high cholesterol diet (Davis et al., 2004); 2) In Golden Syrian hamsters, ezetimibe prevents high cholesterol diet-induced increase in biliary cholesterol (Valasek et al., 2008); and 3) In wild-type mice, ezetimibe treatment protects against lithogenic diet-induced increase in biliary cholesterol concentration and gallstone formation (Zuniga et al., 2008).
Human genetic studies have shown that sequence variations in NPC1L1 are associated with sterol absorption efficiency, LDL-C levels, and LDL-C response to ezetimibe therapy (Cohen et al., 2006, Wang et al., 2005, Hegele et al., 2005, Simon et al., 2005, Fahmi et al., 2008). These findings strongly support a key role of NPC1L1 and NPC1L1-depedent cholesterol transport in whole-body cholesterol homeostasis in humans.
3.3 Function: Lessons learned from cell model systems
In whole animals, NPC1L1 protein is asymmetrically enriched in the intestinal brush border membrane (Altmann et al., 2004, Garcia-Calvo et al., 2005, Iyer et al., 2005, Labonte et al., 2007, Sane et al., 2006) or hepatic canalicular membrane (Yu et al., 2006, Temel et al., 2007). In cultured cells, NPC1L1 proteins can localize to both the plasma membrane and intracellular compartments (Yu et al., 2006, Davies et al., 2005, Yamanashi et al., 2007, Iyer et al., 2005). Human NPC1L1 with a C-terminal green fluorescent protein tag predominantly localizes at the endocytic recycling compartment in actively growing McArdle RH7777 rat hepatoma cells (Yu et al., 2006). Intriguingly, the intracellular itinerary of NPC1L1 protein in these cells and in HepG2 hepatic carcinoma cells is under control of cellular cholesterol availability (Yu et al., 2006). Cholesterol depletion results in a redistribution of NPC1L1 from the intracellular endocytic recycling compartment to the cell surface likely via a mechanism involved in microfilament-associated myosin Vb/Rab11a/Rab11-FIP2 complex, and conversely, cholesterol reloading causes the proteins to transit from the cell surface back into the cell interior likely through clathrin-mediated enocytosis, which is coupled to NPC1L1-facilitated and ezetimibe-inhibitable cholesterol uptake (Yu et al., 2006, Ge et al., 2008, Brown et al., 2007, Chu et al., 2009, Petersen et al., 2008). This cholesterol regulated trafficking may explain why both intracellular and cell surface locations have been observed for NPC1L1 protein (Altmann et al., 2004, Garcia-Calvo et al., 2005, Iyer et al., 2005, Labonte et al., 2007, Sane et al., 2006, Knopfel et al., 2007, Yamanashi et al., 2007, Temel et al., 2007, Yu et al., 2006).
The establishment of NPC1L1-dependent and ezetimibe-sensitive cholesterol uptake assay in cell models allows an opportunity to examine if NPC1L1 differentiates plant sterols from cholesterol (Figure 3). Each day, a large amount of plant-derived sterols (mainly sitosterol and campesterol) are consumed. Although these phytosterols are structurally similar to cholesterol, in normal individuals phytosterols are poorly absorbed. The rank order of fractional intestinal sterol absorption is cholesterol (~45%) > campesterol (~20%) > sitosterol (~5%) (Lutjohann et al., 1995). In mammals, this discrimination for phytosterols may be protective because phytosterols can displace cholesterol in the cell membrane and interfere with cell functions (Wang et al., 1981, Su et al., 2006, Kruit et al., 2008, Kim et al., 2008). Mechanisms underlying the defense against phytosterols remain elusive. Although mutations in ABCG5 and/or ABCG8 cause accumulation of phytosterols in the body (Berge et al., 2000, Lee et al., 2001), the rank order of intestinal sterol absorption rates is maintained in mice lacking ABCG5 and ABCG8 (Yu et al., 2002), implying that ABCG5/G8 is a gatekeeper rather than a discriminator for phytosterols. Is NPC1L1 a discriminator of phytosterols? Studies in mice and humans suggest that NPC1L1 is a common transporter for all sterols because NPC1L1 and ezetimibe-sensitive pathway are essential for phytosterol absorption (Davis et al., 2004, Salen et al., 2004, Salen et al., 2006, Yu et al., 2005, Tang et al., 2008). However, cell culture studies suggest that NPC1L1 may not mediate cellular uptake of all sterols equally. NPC1L1-mediated uptake is 60% lower for sitosterol than cholesterol in intestine-derived CaCo2 cells overexpressing NPC1L1 (Yamanashi et al., 2007). Overexpression of NPC1L1 in McArdle RH7777 rat hepatoma cells facilitates cholesterol, but not sitosterol uptake (Yu et al., 2006, Brown et al., 2007, Yamanashi et al., 2007, Ge et al., 2008). These cell-based assays imply that NPC1L1 has lower affinity to sitosterol than cholesterol. The lower affinity of NPC1L1 to phytosterols has the potential to determine the rank order of intestinal sterol absorption rates. NPC1L1 might be the first genetic defense against phytosterol absorption. But this defense is not complete; otherwise, mutations in ABCG5/G8 would not cause sitosterolemia in the presence of NPC1L1.
Figure 3.
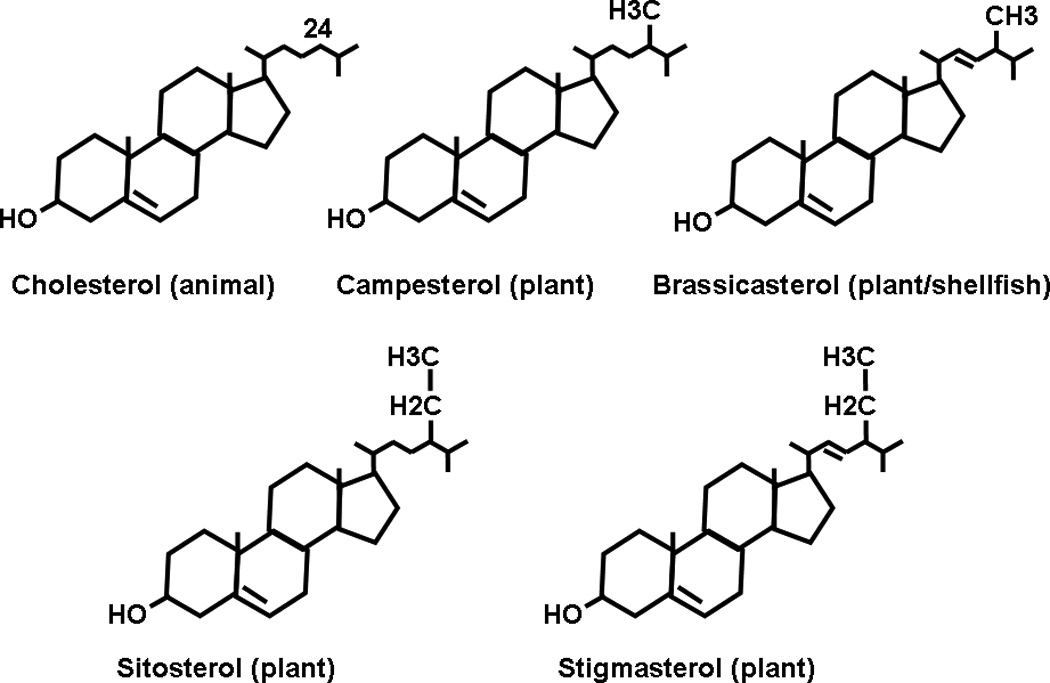
Structures of sterols derived from animals and plants.
3.4 Regulation of expression
Regulation of NPC1L1 gene expression is largely unknown and inconsistent. Since activation of many nuclear receptors, including peroxisome proliferators-activated receptor alpha (PPARα), PPARδ, liver X receptor (LXR), and retinoid X receptor (RXR), reduces intestinal cholesterol absorption (Knight et al., 2003, Repa et al., 2000, McNamara et al., 1980, Umeda et al., 2001, Vanhanen and Miettinen, 1995, Yu et al., 2003, Oliver et al., 2001, van der Veen et al., 2005), effects of these nuclear receptors on intestinal NPC1L1 expression have been examined in CaCo-2 cells and in mice. In 2005, van der Veen et al showed that PPARδ activation reduces intestinal cholesterol absorption by 43% in DBA mice after 8 days of treatment, coinciding with a significant reduction in intestinal NPC1L1 mRNA levels by 40% (van der Veen et al., 2005). PPARδ activation may explain why fish oil and docosahexaenoic acid treatments reduces NPC1L1 expression in CaCo-2 cells and in the proximal small intestine of hamsters (Mathur et al., 2007). In 2007, Valasek et al made an interesting observation that fenofibrate significantly reduces intestinal NPC1L1 mRNA and protein levels via a PPARα-dependent mechanism in mice, but NPC1L1 gene does not seem to be the direct target of PPARα because short-term treatment with fenofibrate does not reduce NPC1L1 mRNA expression and yet nuclear receptors generally enhance expression of their direct targets within several hours (Valasek et al., 2007). In the same study, Valasek et al also found that the effect of PPARα on intestinal NPC1L1 expression is LXR-independent because the similar effect was observed in the LXRα/LXRβ double knockout mice (Valasek et al., 2007).
Whether LXR directly modulates NPC1L1 expression is also uncertain. In a cell culture study, LXR activation by 1 µM of a synthetic agonist T0901317 or GW3965 for 24 h reduces NPC1L1 mRNA levels by ~30% or ~60%, respectively, in CaCo-2/TC7 cells (Duval et al., 2006). In studies with whole animals, LXR activation reduces NPC1L1 mRNA levels by ~35% in the duodena of apolipoprotein (apo) E2-KI mice (lacking endogenous apoE but expressing human apoE2 (Sullivan et al., 1998) fed a Western diet (Duval et al., 2006), and ~50% in the small intestine of ABCB4 knockout mice (Kruit et al., 2005). However, LXR activation by T0901317 or GW3965 does not alter intestinal mRNA levels significantly in the chow-fed wild-type mice (Yu L, unpublished data) (Kruit et al., 2005). NPC1L1 mRNA levels remains unaffected in the proximal small intestine of chow-fed LXRα/LXRβ double knockout mice (Valasek et al., 2007). Thus, it is unlikely that intestinal NPC1L1 is a direct target of LXR.
The human NPC1L1 promoter has a putative sterol regulatory element (Davies et al., 2000). In 2007, Alrefai et al reported that SREBP-2 (Horton et al., 2002) can bind to the two putative sterol regulatory elements in the human NPC1L1 promoter (Alrefai et al., 2007) that differ from the one predicted previously (Davies et al., 2000). They also showed that the NPC1L1 mRNA levels and promoter activities in CaCo2 cells are decreased by 25-hydroxycholesterol that suppresses SREBP activation, and are increased by mevinolin that induces SREBP activation (Alrefai et al., 2007). This study is consistent with that NPC1L1 mRNA levels are ~3-fold and ~2.4-fold higher in cholesterol-depleted CaCo2 cells induced by cyclodextrin and by taurocholate/phosphatidylcholine for 24h, respectively, and are significantly lower in CaCo-2 cells treated with cholesterol and 25-hydroxycholesterol (Field et al., 2007). Alrefai et al further showed that co-expression of the human NPC1L1 promoter-luciferase reporter and the active form of SREBP-2 dramatically increases human NPC1L1 promoter activity (Alrefai et al., 2007). Interestingly, SREBP-2 and the hepatocyte nuclear factor 4α (HNF4α) can synergistically increase human NPC1L1 promoter activity despite HNF4α alone having no effect, and the presence of HNF4α appears to be essential for cholesterol-dependent regulation of NPC1L1 expression (Iwayanagi et al., 2008).
Taken together, these studies suggest that NPC1L1 expression may be regulated by cellular cholesterol availability in a SREBP-dependent manner. Consistent with this regulation, we found that the hepatic NPC1L1 mRNA level is drastically increased in ABCG5/G8 transgenic mice (Yu et al., 2002) treated with lovastatin, a condition that causes a compensatory increase in hepatic mRNA levels of all cholesterol biosynthetic genes (Tang et al., 2006). Additionally, the intestinal NPC1L1 mRNA level is reduced by ~35% in mice lacking acyl-CoA:cholesterol acyltransferase-2 versus wild-type mice fed a synthetic diet containing 20% energy from palm oil and 0.17% cholesterol (Temel et al., 2005), and by ~45% in phospholipid transport protein-deficient versus wild-type mice fed a chow diet (Liu et al., 2007), which are two conditions under which free cholesterol is accumulated in the intestine. Further, in ezetimibe-treated miniature pigs fed a Western-type diet, intestinal and hepatic NPC1L1 mRNA levels are significantly increased (Telford et al., 2007).
Despite several pieces of evidence supporting regulation of NPC1L1 expression by cellular cholesterol availability, discrepancies exist in animal and cell culture studies. For example, high cholesterol diet feeding does not suppress intestinal NPC1L1 expression in mice (Valasek et al., 2007, Plosch et al., 2006), unless 0.5% cholate is added to the diet (Davis et al., 2004). Ezetimibe treatment that reduces cholesterol entry into enterocytes does not increase intestinal NPC1L1 expression in the chow-fed mice (Valasek et al., 2007). In one cell culture study, ezetimibe treatment for 16 h reduces rather than increases NPC1L1 mRNA by 65% in CaCo-2 cells (During et al., 2005). In another study, 24h treatment of CaCo-2 cells with ezetimibe causes no alterations in NPC1L1 mRNA levels or protein mass (Field et al., 2007). Currently, it is unclear if these obvious discrepancies in cholesterol regulation of NPC1L1 expression are related to differences in animal species, diet compositions, duration of drug treatment, or experimental systems used or other factors.
Other factors influencing NPC1L1 expression include estrogen receptors and diabetic state. Administration of high-doses of 17β-estradiol (6 µg/day) to ovariectomized AKR or C57L mice increases NPC1L1 mRNA expression in duodena and jejuna, but not ilea (Duan et al., 2006). Intestinal and hepatic NPC1L1 mRNA levels are significantly higher in streptozotosin-induced diabetic versus nondiabetic rats (Lally et al., 2007), and in Zucker diabetic fatty versus lean rats (Lally et al., 2007). NPC1L1 mRNA levels are also ~2-fold higher in the intestinal biopsy samples from type 2 diabetic patients than nondiabetic patients (Lally et al., 2006, Lally et al., 2007). Given the diabetes epidemic and clinical use of ezetimibe (NPC1L1 inhibitor), exploring mechanisms underlying the relationship between diabetes and NPC1L1 expression levels represents an important future direction and has enormous translation potential.
3.5 Cholesterol and ezetimibe binding studies
It is currently unknown if NPC1L1 protein binds cholesterol and other sterols. The purified SSD-containing membrane region of SCAP can directly bind cholesterol through receptor-ligand interaction and SCAP is thus considered as an ER receptor for cholesterol (Radhakrishnan et al., 2004). The binding between NPC1 (a homolog of NPC1L1) and photoactivatable cholesterol analog appears to be SSD-dependent (Ohgami et al., 2004). Unexpectedly, detailed cholesterol binding assays using purified NPC1 protein and its truncated versions localize the binding site of cholesterol and oxysterols to the luminal loop-1 (a 240-amino acid domain with 18 cysteines), instead of SSD (Infante et al., 2008, Infante et al., 2008). The N-terminal extracellular domain of NPC1L1 protein consists of 263-amino acids (the signal peptide of 21 amino acids excluded), 18 of which are also cysteines. NPC1L1, like its homolog NPC1, may also bind cholesterol via this N-terminal cysteine-rich domain (Figure 2A).
NPC1L1 protein has two large extracellular loops facing intestinal lumen (Figure 2A). A 61-amino acid region in the extracellular loop-1 has been recently shown to be critical for ezetimibe binding by Weinglass and associates (Weinglass et al., 2008). In this study, Weinglass et al purified an NPC1L1-ezetimibe complex from cultured cells and analyzed its constituents by mass spectrometry. They found that NPC1L1 is the only protein to account for ezetimibe binding. Taking advantage of the large difference in affinity between dog and mouse NPC1L1 for ezetimibe, they further identified two residues in this loop of NPC1L1 that are mostly responsible for the large differences in affinity between the two species. These residues reside adjacent to a hotspot of human NPC1L1 polymorphisms that are associated with reduced intestinal cholesterol absorption (Cohen et al., 2006). These findings indicate that the extracellular loop-1 of NPC1L1 plays an important role in mediating ezetimibe action and intestinal cholesterol absorption.
3.6 Potential mechanisms for NPC1L1 to mediate sterol uptake
Several lines of evidence suggest that NPC1L1 may mediate cholesterol uptake via the clathrin-mediated endocytic pathway (Yu, 2008). These include: 1) NPC1L1 cycles in a cholesterol-regulated manner to and from the cell surface (Yu et al., 2006); 2) NPC1L1 physically resides at both plasma membrane and intracellular compartments in cultured cells and in absorptive enterocytes of small intestine (Altmann et al., 2004, Garcia-Calvo et al., 2005, Iyer et al., 2005, Labonte et al., 2007, Sane et al., 2006, Knopfel et al., 2007, Yamanashi et al., 2007, Temel et al., 2007, Yu et al., 2006); 3) After extracted from cultured McArdle rat hepatoma cells, NPC1L1 co-immunoprecipitates with the μ2 (mu2) subunit of an adaptor protein complex AP2 and the clathrin heavy chain (Ge et al., 2008), two proteins that are involved in the clathrin endocytic pathway; 4) Potassium depletion, a condition known to arrest clathrin-mediated endocytosis (Larkin et al., 1983), inhibits NPC1L1-dependent cholesterol uptake (Brown et al., 2007); and 4) Mice lacking caveolin-1, a structural molecule of caveolae, display normal intestinal cholesterol absorption (Valasek et al., 2005), demonstrating that caveolin-mediated endocytosis (another important endocytic pathway) is not the cellular basis for NPC1L1-dependent cholesterol uptake. Clathrin-mediated endocytosis appears to be the cellular basis for intestinal fat absorption (Hansen et al., 2007, Hansen et al., 2003). Similar mechanism may operate for intestinal cholesterol absorption and perhaps hepatic cholesterol retrieval from canalicular bile (Temel et al., 2007). Ezetimibe appears to inhibit the sterol-induced internalization of NPC1L1 via clathrin-mediated endocytosis in cultured hepatoma cells (Ge et al., 2008, Chang and Chang, 2008, Petersen et al., 2008).
To definitively identify molecular mechanisms underlying NPC1L1-mediated cholesterol transport, many important questions have to be answered. For example, how does cholesterol regulate NPC1L1 trafficking? What sorting signals in the protein and sorting platforms in cells are involved in this regulation? Does NPC1L1 bind cholesterol? If it does, what is the physiological significance for cholesterol binding to NPC1L1? Do NPC1L1-cholesterol interactions function as a signal for inducing clathrin-mediated endocytosis of NPC1L1 or as a process to recruit and handle free cholesterol to NPC1L1-containing membrane microdomains? Does NPC1L1 bind cholesterol that resides in the plasma membrane or cholesterol present in extracellular spaces such as the gut lumen and hepatic bile canaliculus (Temel et al., 2007, Yu, 2008)? How does ezetimibe inhibit NPC1L1 internalization from the plasma membrane? Does ezetimibe interfere with NPC1L1-cholesterol binding? In addition, due to the dense and rigid microvillar cytoskeleton (Yamamoto, 1982), the base of the microvilli of small intestine is likely the only site for endocytic membrane traffic to occur (Hansen et al., 2003). Given that NPC1L1 abundantly localizes at the microvilli, a dilemma is how the microvillus-localized NPC1L1 and its cargos are endocytosed. If the microvillus-localized NPC1L1 has to move to the base of microvilli to mediate cholesterol uptake, what are signals triggering this translocation or movement? Elucidation of all these questions will greatly enhance our understanding of how cells such as enterocytes and hepatocytes handle extracellular unesterfied cholesterol.
3.7 Therapeutic perspectives
NPC1L1 is undoubtedly a gatekeeper of intestinal cholesterol absorption, thus positioning itself as an attractive drug target for prevention of cholesterol-driven diseases such as ASCVD and gallstone disease. Additionally, several studies have shown that NPC1L1 inhibition may provide benefits for important metabolic diseases such as hepatic steatosis, dyslipidemia, high fat diet-induced obesity and insulin resistance (Labonte et al., 2008, Davies et al., 2005, van Heek et al., 2001, Assy et al., 2006, Deushi et al., 2007, Zheng et al., 2008) (Yu, L., unpublished data). Further studies are needed to define molecular mechanisms underlying these intriguing observations. Intestinal cholesterol has been reported to regulate fat storage (Kalaany et al., 2005). Perhaps, simply by controlling how much cholesterol enters the body via small intestine, NPC1L1 can regulate responses of other metabolic pathways and physiological processes to overnutrition, thereby improving metabolic diseases induced by metabolic overload.
Although the NPC1L1 inhibitor ezetimibe can efficiently lower blood cholesterol, it is important to point out that inter-individual variations exist in response to ezetimibe treatment (Hegele et al., 2005). Comparison of multiple species NPC1L1 orthologs have shown that the in vivo responsiveness to ezetimibe correlates with NPC1L1 binding affinity (Hawes et al., 2007). Ezetimibe non-responders may have distinct NPC1L1 sequence variations, and these individuals may be responsive to other NPC1L1 inhibitors that work via distinct mechanisms. In addition, small molecule NPC1L1 inhibitors, after entering the body, have the potential to cause adverse effects. NPC1L1 localizes at the intestinal brush border membrane and its extracellular domains are exposed to contents in the gut lumen. This unique location and membrane topology makes NPC1L1 an ideal target for large molecule nonabsorbable NPC1L1 inhibitors (Davidson, 2009). Thus, NPC1L1 will remain an attractive drug target in the future.
4. ATP-BINDING CASSETTE TRANSPORTERS G5 AND G8 (ABCG5/G8)
4.1 Discovery of ABCG5/G8: The power of human genetics
The discovery of the heterodimeric transporters ABCG5 and ABCG8 represents a powerful example of human genetics leading to mechanistic understanding of the underlying disease process. In this case, it has long been appreciated that cholesterol is structurally very similar to plant sterols, with only minor differences in side chain configurations (Schoenheimer, 1929) (Figure 3). However, it has been known for nearly a century that mammals consume large amounts of dietary plant sterols, yet these phytosterols are excluded from the body, primarily at the level of the intestine (Sudhop et al., 2005, Schoenheimer and Breusch, 1933, Borgstrom, 1968, Gould et al., 1969, Hernandez et al., 1954, Huang and Kuksis, 1965). A major breakthrough regarding this issue came when Bhattacharayya and Connor described a novel disease in which two sisters presented to the clinic with tendon xanthomas, and were surprisingly shown to have extremely elevated levels of plasma plant sterols (primarily β-sitosterol) (Bhattacharyya and Connor, 1974). Hence, the disease was named β-sitosterolemia, and the authors proposed that this disease was caused by a single genetic defect, which prevented the intestine from excluding plant sterols from being absorbed. It was later discovered that sitosterolemic patients have elevated fractional absorption of dietary sterols and diminished ability to secrete multiple sterols into bile (Miettinen, 1980, Lutjohann et al., 1995, Gregg et al., 1986). As a result, sitosterolemia manifests as the accumulation of both plant and animal sterols in the plasma, skin, tendons, coronary arteries and other tissues, and most affected individuals suffer from premature coronary heart disease (Salen et al., 1985, Kolovou et al., 1996, Mymin et al., 2003, Bjorkhem et al., 2001). Sitosterolemia locus was subsequently mapped to a single sitosterolemia locus on human chromosome 2p21 that contains two genes, ABCG5 and ABCG8 (Patel et al., 1998), and mutations in either of these genes is causative of sitosterolemia (Berge et al., 2000, Lee et al., 2001).
4.2 Structure: gene, mRNA, and protein domains
ABCG5 and ABCG8 genes are arranged in a head-to-head orientation with less than 400 base pairs between their respective start codons, and each has 13 exons and twelve introns (Berge et al., 2000). The two genes encode two distinct proteins known as sterolin-1 (ABCG5) and sterolin-2 (ABCG8), which are mammalian homologues of the drosophila gene White. In addition to being in the same genetic neighborhood, ABCG5 and ABCG8 are also in close proximity at the protein level. Both of these proteins contain an ATP-binding cassette near the N-terminus followed by six putative transmembrane domains (Figure 2B), and serve only as non-functional half-transporters when expressed alone (Graf et al., 2002, Graf et al., 2003, Graf et al., 2004). ABCG5 and ABCG8 must heterodimerize to transport sterols across membranes (Graf et al., 2002, Graf et al., 2003, Graf et al., 2004). This idea is supported by data demonstrating that, when expressed together, the proteins colocalize in the ER and the plasma membrane, they can be co-immunoprecipitated, and the exit of these proteins from the ER to the plasma membrane requires coexpression of both proteins (Graf et al., 2002, Graf et al., 2003, Graf et al., 2004). Even stronger evidence for obligate heterodimerization comes from the fact single mutations in either of these genes alone causes sitosterolemia (Berge et al., 2000, Lee et al., 2001). The current data support a model where ABCG5 and ABCG8 heterodimerize in the ER, traffic together through the Golgi apparatus, and subsequently target to apical subdomains in the plasma membrane. Both ABCG5 and ABCG8 undergo N-linked glycosylation, and glycosylation at Asn-619 in ABCG8 is critical for efficient trafficking of the heterodimer (Graf et al., 2004). The ABCG5/G8 heterodimer requires the molecular chaperones, calnexin and calreticulin for proper folding and trafficking out of the ER (Graf et al., 2004, Okiyoneda et al., 2006). Subsequent site-directed mutagenesis experiments demonstrated that the majority of mutants causative of sitosterolemia exhibit impaired transport of the heterodimer from the ER to the plasma membrane (Graf et al., 2004).
4.3 Function: Lessons learned from animal models
Since the discovery of the genetic basis for sitosterolemia, there has been intensive effort put forth to understand the function of the ABCG5/G8 heterodimer. Like NPC1L1, ABCG5 and ABCG8 are expressed almost exclusively on the apical membrane of enterocytes in the intestine and hepatocytes in the liver (Patel et al., 1998, Berge et al., 2000, Lee et al., 2001, Graf et al., 2003, Klett et al., 2004). It is generally accepted that the ABCG5/G8 heterodimeric complex serves as an efflux pump to remove sterols (cholesterol and phytosterols) from hepatocytes and enterocytes. In the intestinal brush border membrane, this function would allow for transport of intracellular sterols back into the lumen of small intestine for fecal excretion. If this were true, ABCG5/G8 would likely play an important role in intestinal sterol absorption by opposing the action of NPC1L1.
A potential role for ABCG5/G8 in intestinal cholesterol absorption was first discovered in sitosterolemic patients, who have elevated intestinal absorption of both plant sterols and cholesterol (Miettinen, 1980, Lutjohann et al., 1995, Gregg et al., 1986, Salen et al., 1992, Bhattacharyya et al., 1991, Salen et al., 1989). In fact, sitosterolemic patients absorb roughly 20–30% of dietary sitosterol, compared to <5% absorption in unaffected individuals (Bhattacharyya and Connor, 1974, Miettinen, 1980, Salen et al., 1992, Salen et al., 1989). Importantly, mice lacking either ABCG5 alone (Plosch et al., 2004), ABCG8 alone (Klett et al., 2004), or both transporters (Yu et al., 2002) exhibit sitosterolemia, which can likely in part be explained by increased intestinal absorption of phytosterols. In support of this concept, in mice lacking both ABCG5 and ABCG8 fed a chow diet, intestinal absorption of cholesterol is not significantly altered, yet the absorption of sitosterol, cholestanol, campesterol is significantly increased (Yu et al., 2002). In a recent study, Wang and colleagues demonstrated that the lymphatic transport rate of cholesterol and sitostanol is increased by ~40% and 500%, respectively in mice lacking ABCG8 (Wang et al., 2007). Furthermore, mice transgenically overexpressing ABCG5 and ABCG8 in the intestine and liver exhibit a 50% reduction in fraction cholesterol absorption, and fecal neutral sterol levels increase 3–6 fold (Yu et al., 2002). Collectively, studies in both sitosterolemic humans and animal models support an important role for ABCG5/G8 in intestinal sterol absorption. Although ABCG5 and ABCG8 heterodimer is thought to influence intestinal sterol absorption by serving as an efflux pump to deliver sterols from enterocytes back into the gut lumen for fecal disposal, direct experiment evidence of the concept is still lacking.
4.4 Function: Lessons learned from cell model systems
There is a strong body of work examining the subcellular trafficking and dimerization of ABCG5 and ABCG8, but characterization of the cellular sterol transport properties of the heterodimer is still incomplete. For unknown reasons, although ABCG5 and ABCG8 can be readily expressed and properly localized in mammalian cells, a standard reproducible functional assay for ABCG5/G8-dependent sterol transport has not been established. Recently, one group was able to show that overexpression of ABCG5 and ABCG8 in human kidney and gallbladder epithelial cells promotes the efflux of cholesterol and plant sterols (Vrins et al., 2007, Tachibana et al., 2007). In these studies it was shown that ABCG5/G8-dependent efflux is dependent on the presence of mixed bile salt micelles as an acceptor, whereas other cholesterol acceptors such as apoAI, high-density lipoprotein (HDL), or methyl-β-cyclodextrin do not efficiently promote ABCG5/G8-dependent efflux.
Given that mutations in either ABCG5 or ABCG8 cause abnormal accumulation of plant sterols in the body (Berge et al., 2000, Lee et al., 2001), most have assumed that the ABCG5/G8 heterodimer is the primary, if not the sole protein complex, responsible for sterol discrimination in the intestine. However, the current knowledge base does not firmly support this conclusion. For example, genetically sitosterolemic patients (Lutjohann et al., 1995), rats (Hamada et al., 2007), and mice (Yu et al., 2002) still possess the ability to discriminate between cholesterol, campesterol, and sitosterol at the level of intestinal absorption, indicating that ABCG5/G8 is a gatekeeper rather than the intestinal sterol discriminator. Given this information, caution should be taken when assuming that the ABCG5/G8 heterodimer is the sole mediator of sterol selectivity in the intestine. Much more work is needed in this area, and with recent progress using cell-based systems for ABCG5/G8-dependent sterol transport, we may gain further mechanistic insights into the critical question of substrate specificity.
4.5 Regulation of expression
Both ABCG5 and ABCG8 seem to be primarily controlled at the transcriptional level. The sterol-sensing transcription factors LXRα and LXRβ appear to be the primary regulator of ABCG5 and ABCG8 mRNA expression. In support of this, both dietary cholesterol and synthetic LXR agonists upregulate ABCG5 and ABCG8 mRNA expression in the small intestine and liver of wild type mice, but not LXR knockout mice (Berge et al., 2000, Repa et al., 2002, Plosch et al., 2002, Kaneko et al., 2003). This is indicative of a direct effect, but the presence of a bona fide LXR response element in the ABCG5/G8 intergenic promoter or surrounding areas has not been identified. The physiological relevance of LXR-driven upregulation of ABCG5 and ABCG8 mRNA has recently been highlighted in two separate studies. It was first shown that LXR-driven increases in hepatobiliary and fecal cholesterol excretion rely on functional ABCG5 and ABCG8, since ABCG5/G8 knockout mice could not elevate biliary and fecal sterol secretion in response to a synthetic LXR agonist (Yu et al., 2003). In agreement, using ABCG5/G8 knockout mice, Calpe-Berdiel et al. demonstrated that LXR-mediated induction of macrophage to feces reverse cholesterol transport requires functional ABCG5/G8 (Calpe-Berdiel et al., 2008).
Another transcriptional activator of the sitosterolemia locus is the liver receptor homolog-1 (LRH-1) (Freeman et al., 2004). However, it is important to point out that a functional LRH-1 binding site is only present in the human gene, and rodent orthologs do not possess LRH-1-sensitive transcriptional activation (Freeman et al., 2004). More recently, it was shown that three additional transcription factors known as HNF-4α, GATA binding protein 4, and GATA binding protein 6 act in a cooperative fashion to transactivate the human intergenic promoter (Sumi et al., 2007). There is also evidence that bacterial endotoxin can downregulate ABCG5 and ABCG8 mRNA levels (Khovidhunkit et al., 2003), yet the transcription factors involved in this response have yet to be clearly elucidated. Collectively, it is quite clear that ABCG5 and ABCG8 are coordinately regulated at the transcriptional level. More work in this area may prove to be critical for future ABCG5/G8-centered therapies.
Most recently, there has been support for hormonal control of ABCG5/G8 expression at both transcriptional and post-transcriptional levels. In support of this, hepatic insulin resistance promotes cholesterol gallstone formation, which is driven in part by transcriptional regulation of ABCG5/G8 (Biddinger et al., 2008). In this study it was shown that hepatocyte-specific deletion of the insulin receptor results in reduced insulin-driven inhibition of the transcription factor forkhead box O1 (FOXO1), and that FOXO1 is a direct transcriptional activator of ABCG5/G8. In addition to insulin-mediated regulation, ABCG5/G8 expression has also been linked to the leptin axis in the liver (Sabeva et al., 2007). In this case, the hepatic protein (not mRNA) abundance of ABCG5/G8 is reduced in mice lacking leptin signaling. Furthermore, hypophesectomized rats have dramatic reductions in hepatic ABCG5/G8 mRNA levels that are coupled to decreased fecal sterol loss, and these alterations can be normalized by thyroid hormone replacement (Galman et al., 2008). The mechanism by which thyroid hormone and leptin regulates ABCG5/G8 expression requires further exploration.
4.5 Biochemical studies on ABCG5/G8-dependent sterol transport
Although cellular functional assays have been somewhat elusive, ABCG5/G8-dependent sterol transport has been successfully reconstituted in vitro with either a recombinant or purified native ABCG5/G8, and the heterodimer can directly transport sterols, sterol esters, and phospholipids from donor vesicles to proteoliposomes in an ATP-dependent fashion and vanadate-sensitive manner (Wang et al., 2006, Wang et al., 2008). With these established in vitro assays and complimentary cell-based models (Vrins et al., 2007, Tachibana et al., 2007) for ABCG5/G8-dependent sterol transport we now have the tool to address important unanswered questions. For instance, does the ABCG5/G8-transported pool of sterols originate from a cytosolic pool, or does the heterodimer act primarily on a membrane-associated pool as a flippase? Are other proteins required for ABCG5/G8-dependent sterol transport? Additionally, what is the substrate specificity for ABCG5/G8?
4.6 Therapeutic perspectives for ABCG5/G8
ABCG5/G8 plays a crucial role in cholesterol excretion from the body and therefore it seems logical to assume that mice lacking the heterodimer should have increased atherosclerotic burden, while mice overexpressing ABCG5/G8 should be protected against atherosclerosis. Consistent with these hypotheses, transgenic overexpression of ABCG5/G8 in both the intestine and liver of LDL receptor knockout mice results in reduced LDL-C and significantly less atherosclerosis (Wilund et al., 2004). In contrast, hepatocyte-specific ABCG5/G8 overexpression, which elevates biliary sterol secretion by ~2-fold, does not protect against atherosclerosis in the LDL receptor or apoE knockout backgrounds (Wu et al., 2004). This result is very confusing, since it was anticipated that a 2-fold increase in biliary cholesterol output would result in atheroprotection. In a subsequent study, these same authors clarified this confusing outcome, and showed that ezetimibe treatment in mice overexpressing ABCG5/G8 specifically in the liver produces profound protection against atherosclerosis (Basso et al., 2007). These data strongly support the opposing roles of ABCG5/G8 and NPC1L1 (target of ezetimibe), and demonstrate that therapies that simply increase biliary sterol output may not be effective at altering sterol balance and atherosclerosis, since these sterols can re-enter the body via the action of intestinal NPC1L1 (Altmann et al., 2004). However, dual therapies that increase biliary sterol output and block re-uptake of biliary sterols by the intestine continue to hold promise.
It is well known that many sitosterolemic patients suffer from premature ASCVD (Salen et al., 1985, Kolovou et al., 1996, Mymin et al., 2003, Bjorkhem et al., 2001). It is however not known whether this ASCVD is caused by the massive accumulation of plant sterols in the blood or the associated hypercholesterolemia. Without a doubt hypercholesterolemia is a major driving force in ASCVD progression, but little is known about the consequences of elevated blood plant sterols because in organisms with intact ABCG5/G8 plant sterols are efficiently excluded from the body. Sitosterolemic patients and ABCG5/G8 knockout mice provide a unique research opportunity to ask the age-old question: How are phytosterols metabolized, and do phytosterols like their animal counterparts contribute to atherogenesis? Importantly, the plant sterol stigmasterol has been shown to regulate cholesterol metabolism by directly inhibiting SREBP-2 processing and activating LXR-driven gene expression (Yang et al., 2004). In addition sitosterol has recently been shown to drive macrophage cell death (Bao et al., 2006), an important feature of the late stages of ASCVD. These and many other findings have promoted a potential role for plant sterols in the progression of ASCVD in sitosterolemic patients. Recently, a study examined the relationship between blood sitosterol levels and atherosclerosis in both ABCG5/G8 knockout mice and in sitosterolemic patients (Wilund et al., 2004). It was found that plasma levels of plant sterols do not correlate with atherosclerosis extent in either of these sitosterolemic models, leading the authors to conclude that perhaps elevated plasma cholesterol levels are responsible for the development of premature ASCVD in sitosterolemic patients.
Inter-individual variations exist in responses to statins (Kajinami et al., 2004), and it was thought to be attributable to differences in the baseline of intestinal cholesterol absorption and/or endogenous cholesterol synthesis among individuals (Miettinen et al., 2000, Gylling and Miettinen, 2002). Stimulation of ABCG5/G8-mediated cholesterol excretion not only promotes fecal disposal of cholesterol, but also increases endogenous cholesterol synthesis (Yu et al., 2002, Yu et al., 2004). Coadministration of a statin and a yet-to-be developed ABCG5/G8 activator is expected to have a synergistic cholesterol-lowering effect. Consistent with this notion is that transgenic overexpression of ABCG5/G8 in the liver and intestine confers mice hypersensitivity to lovastatin in reducing plasma cholesterol (Tang et al., 2006).
Unlike the successful story for NPC1L1 and its inhibitor ezetimibe, a specific pharmacological activator for ABCG5/G8-dependent sterol transport has yet to be developed. Most current attempts at promoting ABCG5/G8-dependent sterol transport involve the use of synthetic LXR agonists, which robustly and transcriptionally upregulates heterodimer expression in the liver and intestine (Berge et al., 2000, Repa et al., 2002, Plosch et al., 2002, Kaneko et al., 2003). LXR activation, however, elicits the unwanted side effect of increased de novo lipogenesis, resulting in pronounced hepatic steatosis (Rader, 2007, Cao et al., 2004), which rules out synthetic LXR agonists as a safe way to promote ABCG5/G8 function. Alternative strategies for ABCG5/G8 modulation need to be pursued. ABCG5/G8 heterodimer may be directly targeted by small molecule activators. With in vitro and cell-based functional assays for ABCG5/G8-dependent sterol transport in place, it is now possible to screen for such compounds. Based on the limited data generated in mice transgenically overexpressing ABCG5 and ABCG8 (Yu et al., 2002, Tang et al., 2006), pharmacologic activators of this pathway could serve as powerful promoters of cholesterol removal from the body, and thus providing additional protection against atherosclerosis when given in combination with a statin.
5 SCAVENGER RECEPTOR CLASS B TYPE I (SR-BI)
5.1 Discovery of SR-BI
SR-BI was originally discovered based on its sequence homology to another scavenger receptor known as cluster determinant 36 (CD36) (Calvo and Vega, 1993, Acton et al., 1994). SR-BI is expressed in a wide variety of tissues, with the highest level of expression in tissues regulating cholesterol metabolism such as the liver, intestine, adrenal gland, testes, and ovary (Acton et al., 1994, Acton et al., 1996, Landschulz et al., 1996). SR-BI is widely accepted as an HDL receptor, and can bi-directionally transport sterols across biological membranes (Landschulz et al., 1996, Acton et al., 1996). SR-BI-driven selective uptake into the liver promotes biliary and fecal excretion of cholesterol (Kozarsky et al., 1997, Ji et al., 1999, Zhang et al., 2005). In addition, SR-BI-driven selective uptake into the adrenal gland is linked to steroid hormone production (Rigotti et al., 1996, Rigotti et al., 1997, Temel et al., 1997). Since the role of SR-BI in these processes has been the focus of previous reviews (Trigatti, 2005, Connelly, 2009), these concepts will not be discussed in detail here. The purpose of this chapter is to specifically discuss the structure and function of SR-BI in regards to intestinal sterol transport.
5.2 Structure: gene, mRNA, and protein domains
SR-BI belongs to the class B scavenger receptor superfamily, which includes other proteins such as lysosomal integral membrane protein II (LIMP II), Drosophila melanogaster croquemort membrane protein, and Snmp-1 (Franc et al., 1996, Franc et al., 1999, Hart and Wilcox, 1993, Karakesisoglou et al., 1999, Vega et al., 1991). The human SR-BI gene contains 13 exons, and resides on chromosome 12q24.2-qter (Cao et al., 1997). The pattern of SR-BI full-length mRNA expression is similar in humans and rodents, with the highest level of mRNA in the adrenal gland, ovary, liver, intestine, and placenta (Acton et al., 1996, Landschulz et al., 1996, Cao et al., 1997). The full-length SR-BI mRNA encodes a 509 amino acid protein containing a short N-terminal cytoplasmic domain of 9 amino acids, a transmembrane spanning domain of 22 amino acids, a predominant extracellular domain of 408 amino acids, a second transmembrane domain of 23 amino acids, and a cytoplasmic C-terminus of 44 amino acids (Krieger, 1999, Williams et al., 1999). The predicted molecular weight of SR-BI is ~57 kDa, yet extensive N-linked glycosylation yields a protein that runs ~82–84 kDa on a Western blot (Krieger, 1999, Williams et al., 1999). SR-BI is also myristoylated and palmitoylated (Babitt et al., 1997), yet these post-translational modifications do not seem to impact SR-BI function. The extracellular domain of SR-BI is likely to form disulfide bridges since it contains six cysteine residues (Krieger, 1999). An alternatively spliced variant for of SR-BI mRNA was identified, and this protein is known as SR-BII (Webb et al., 1998, Eckhardt et al., 2004, Eckhardt et al., 2006). This alternatively spliced form has a slightly modified C-terminal tail, yet can also function to transport sterols across biological membranes (Webb et al., 1998, Eckhardt et al., 2004, Eckhardt et al., 2006). Most recently, the PDZ domain-containing adaptor protein PDZK1 has been shown to bind to the C-terminus of SR-BI and this protein-protein interaction controls SR-BI activity via a tissue-specific posttranscriptional mechanism (Silver, 2002, Yesilaltay et al., 2006, Kocher et al., 2003). In fact, mice deficient in PDZK1 have elevated plasma cholesterol levels due to liver-specific downregulation of SR-BI, implicating PDZK1 as a critical regulator of hepatic SR-BI function (Kocher et al., 2003).
5.3 Function: Lessons learned from animal models
Animal models of SR-BI deficiency or overexpression have shed light on the critical role of this protein in whole body sterol balance. Transgenic overexpression of SR-BI results in diminished very low density lipoprotein cholesterol, LDL-C, and HDL-cholesterol levels, increased biliary cholesterol secretion, and marked protection against atherosclerosis (Wang et al., 1998, Ueda et al., 1999, Ueda et al., 2000, Arai et al., 1999, Kozarsky et al., 2000, Ji et al., 1999). Conversely, mice lacking SR-BI accumulate cholesterol in the plasma, have decreased biliary sterol excretion and steroid hormone insufficiency, and develop severe atherosclerosis (Rigotti et al., 1997, Varban et al., 1998, Trigatti et al., 1999). Mice lacking SR-BI in the apoE-deficient background develop occlusive coronary artery atherosclerosis, myocardial infarction, and die at a very early age (Braun et al., 2002, Zhang et al., 2005). The hyperlipidemic and proatherogenic effects seen with SR-BI deficiency are thought to be primarily due to impairment of SR-BI’s critical role in selective uptake of HDL cholesteryl esters into the liver. However, SR-BI is also abundantly expressed in the intestine, and has been proposed to play a role in intestinal cholesterol absorption. The primary focus of this chapter is to discuss the current state of knowledge regarding SR-BI’s role in the intestine, which is still a matter of debate.
Using immunohistochemical and biochemical approaches SR-BI protein expression has been documented to be expressed in enterocytes, and can be found on both apical and basolateral membranes (Cai et al., 2001, Voshol et al., 2001, Hauser et al., 1998, Hatzopoulos et al., 1998). The intestinal gradient of SR-BI expression is found most prominently along the proximal regions of the gastrocolic axis, including the duodenum and jejunum where cholesterol absorption is thought to occur (Cai et al., 2001, Voshol et al., 2001, Hauser et al., 1998, Hatzopoulos et al., 1998),. The concept of SR-BI being involved in intestinal cholesterol absorption was first supported by in vitro studies demonstrating that cholesterol uptake in intestinal brush border membranes is reduced by anti-SR-BI blocking antibodies (Hauser et al., 1998). Further, early evidence showed that intestinal cholesterol absorption inhibitor ezetimibe could physically interact with SR-BI (Hatzopoulos et al., 1998). Also, it was shown that SR-BI is a high-affinity cholesterol binding protein in the intestinal brush border membrane (Altmann et al., 2002). However, mice genetically lacking SR-BI have either slightly elevated or normal intestinal cholesterol absorption (Altmann et al., 2002, Mardones et al., 2001, Nguyen et al., 2009). SR-BI-null mice still exhibit reduced fractional cholesterol absorption when treated with ezetimibe, ruling out SR-BI as the ezetimibe-sensitive transport protein (Altmann et al., 2002). In contrast to the results from SR-BI-deficient mice, intestine-specific transgenic overexpression of SR-BI promotes the appearance of radiolabeled cholesterol into the plasma following an oil-based gavage administration (Bietrix et al., 2006). This was interpreted as an increase in intestinal cholesterol absorption, yet this method may not provide an accurate quantitative measure of fractional cholesterol absorption. Collectively, there is evidence to support the ability of SR-BI to facilitate the binding of cholesterol from bile salt micelles donors into brush border membrane vesicles (Nguyen et al., 2009, Labonte et al., 2007, Knopfel et al., 2007), but data from knockout mice confirm that this phenomenon is not rate limiting in net cholesterol absorption (Altmann et al., 2002, Mardones et al., 2001, Nguyen et al., 2009).
5.4 Function: Lessons learned from cell model systems
Although SR-BI may have been implicated in the apical to basolateral transport process of intestinal cholesterol absorption, most cell models of SR-BI-dependent sterol transport suggest a quite different trafficking pattern of SR-BI-delivered sterols. Despite a matter of debate, in the steady state SR-BI seems to localize to both apical and basolateral membranes in polarized cells (Cai et al., 2001, Voshol et al., 2001, Hauser et al., 1998, Hatzopoulos et al., 1998, Silver et al., 2001, Burgos et al., 2004, Harder et al., 2007, Wustner et al., 2004, Sehayek et al., 2003). In polarized cells SR-BI undergoes sterol-dependent directional basolateral to apical transcytosis (Silver et al., 2001, Burgos et al., 2004, Harder et al., 2007, Wustner et al., 2004, Sehayek et al., 2003). This directional pattern of sterol transport is consistent with SR-BI’s role as an HDL receptor promoting selective uptake of cholesteryl esters from circulation to liver for biliary disposal (Silver et al., 2001, Burgos et al., 2004, Harder et al., 2007, Wustner et al., 2004, Sehayek et al., 2003). This selective uptake involves the internalization of HDL-associated cholesteryl esters into the cell, without the net internalization and degradation of the lipoprotein itself. Additionally, SR-BI can also promote selective uptake of free sterols, phospholipids, triglycerides, and cholesteryl ethers (Stangl et al., 1999, Urban et al., 2000, Greene et al., 2001). Selective uptake may involve the “retro-endocytosis” pathway, which involves holoparticle uptake and the subsequent re-secretion of the cholesteryl-ester poor HDL (Silver et al., 2001, Rhainds et al., 2004, de la Llera-Moya et al., 1999). The precise molecular mechanism by which SR-BI facilitates selective sterol uptake remains incompletely understood, but purified SR-BI reconstituted into liposomes can recapitulate high-affinity HDL binding and selective uptake, indicating that these processes are intrinsic to the receptor itself (Liu and Krieger, 2002).
Although SR-BI is generally thought to be an HDL receptor, it can bind a wide variety of ligands. In fact, the first SR-BI ligands described were modified apoB-containing lipoproteins including acetylated-LDL and oxidized-LDL (Acton et al., 1994, Gillotte-Taylor et al., 2001). In addition to binding native and modified lipoproteins, SR-BI binds maleylated BSA (Acton et al., 1994), advanced glycated proteins (Ohgami et al., 2001), anionic phospholipids (Rigotti et al., 1995, Fukasawa et al., 1996), and β-amyloid (Paresce et al., 1996, Husemann et al., 2001, Husemann and Silverstein, 2001). The majority of SR-BI ligand binding studies have been carried out using native lipoproteins. Lipoprotein binding to SR-BI exhibits intrinsic nonreciprocal cross competition (Ashkenas et al., 1993, Gu et al., 2000). In this case, HDL binding efficiently blocks subsequent LDL binding, yet the ability of LDL to block HDL binding is relatively weak (Ashkenas et al., 1993, Gu et al., 2000). Therefore, it is generally accepted that LDL does not typically interfere with HDL binding in vivo, further supporting the claim that SR-BI is the HDL receptor (Acton et al., 1996, Landschulz et al., 1996). Importantly, not all HDL particles bind to SR-BI with the same affinity. Lipid-free apoA-I and pre-β HDL particles are poor substrates for SR-BI, whereas spherical large α-HDL particles bind with much higher affinity (Xu et al., 1997, Liadaki et al., 2000, de Beer et al., 2001, Williams et al., 2000). The interaction of HDL particles with SR-BI relies on interactions with intrinsic HDL apolipoproteins including apoA-I, apoA-II, and apoE (Li et al., 2002, Pilon et al., 2000, Bultel-Brienne et al., 2002, Liu et al., 2002).
In addition to facilitating cholesterol uptake from lipoproteins, SR-BI can serve as a bi-directional sterol transporter, facilitating cholesterol efflux from cells (Kozarsky et al., 1997, Ji et al., 1997, Jian et al., 1998, Yancey et al., 2000, Gu et al., 2000). This activity was originally established when SR-BI expression levels in a variety of different cell lines are highly correlated to HDL-mediated sterol efflux (Ji et al., 1997). Later it was shown in SR-BI gain of function experiments in cells that SR-BI promotes cholesterol efflux to HDL and phosphatidylcholine-containing liposomes (Jian et al., 1998). Studies done with mutated forms of apoA-I suggest that a direct interaction between apoA-I and SR-BI must occur for efficient cholesterol efflux (Liu et al., 2002), yet other studies have challenged the concept that physical binding of the HDL particle to SR-BI is required for sterol efflux (Kellner-Weibel et al., 2000, de la Llera-Moya et al., 1999). In the context of atherosclerosis, the process of cholesterol efflux is thought to be most important in immune cells present in the artery wall such as macrophages, but macrophage SR-BI does not seem to play a major role in macrophage cholesterol efflux or in vivo reverse cholesterol transport (Yu et al., 2004, Yvan-Charvet et al., 2008, Wang et al., 2007). The physiological implication of SR-BI-mediated sterol efflux has yet to be understood.
5.5 Regulation of expression
Since SR-BI is so critical to sterol balance and steroid hormone production, its expression is highly controlled at the transcriptional level. In fact SR-BI expression is regulated by trophic hormones, cholesterol, and fatty acids in a tissue specific manner (Rigotti et al., 1996, Wang et al., 1996, Cao et al., 1999, Towns et al., 2005, Fluiter et al., 1998, Spady et al., 1999, Li et al., 1998, McLean and Sandhoff, 1998, Mizutani et al., 1997, Mizutani et al., 2000, Reaven et al., 1998, Reaven et al., 1999, Azhar et al., 1998, Li et al., 2001, Svensson et al., 1999). In the case of hormone regulation, SR-BI expression is upregulated by adrenocorticotrophic hormone (Kozarsky et al., 1997, Rigotti et al., 1996, Wang et al., 1996, Cao et al., 1999), luteinizing hormone (Landschulz et al., 1996), human chorionic gonadotropin (Kozarsky et al., 1997, Li et al., 1998, McLean and Sandhoff, 1998), pregnant mare serum gonadotropin (Li et al., 1998, Mizutani et al., 1997, McLean and Sandhoff, 1998), cyclic AMP analogs (Azhar et al., 1998), and insulin (Li et al., 2001). It is generally accepted that trophic hormone-driven stimulation of SR-BI expression in steroidogenic cells relies on cAMP-dependent activation of protein kinase A, and subsequent promoter transactivation that depends on both steroidogenic factor-1 (SF-1) (Cao et al., 1997, Cao et al., 1999, Parker, 1998, Lopez and McLean, 1999) and CCAAT/enhancer binding proteins (Lekstrom-Himes and Xanthopoulos, 1998). SF-1 is critical for both basal and trophic hormone-stimulated transactivation of the SR-BI promoter, and directly promotes the expression of SR-BI during the development of several steroidogenic tissues (Cao et al., 1997, Cao et al., 1999, Parker, 1998, Lopez and McLean, 1999).
In addition to hormonal regulation, SR-BI expression is also sensitive to alteration in cellular cholesterol levels (Wang et al., 1996, Cao et al., 1999, Sun et al., 1999). In this case, a bona fide sterol response element has been identified in the SR-BI promoter (Lopez and McLean, 1999). This element confers SREBP-1α-dependent transactivation of the gene. Interestingly, in the ovary, gonadotropin treatment induces the expression of SREBP-1α and subsequent enhancement of SR-BI expression (McConihay et al., 2001). In line with this, depletion of plasma cholesterol levels results in induction of SR-BI expression in the adrenal gland, an effect that does not depend on plasma levels of adrenocorticotrophic hormone (Sun et al., 1999). In further support of sterol-dependent transcriptional regulation, SR-BI expression levels are dramatically altered in mouse models of altered cholesterol metabolism (Wang et al., 1996, Ng et al., 1997). For example, mice genetically lacking the steroidogenic acute regulatory (StAR) protein have elevated levels of adrenal SR-BI (Cao et al., 1999). Mice lacking either apoA-I, lecithin:cholesterol acyltransferase, or hepatic lipase all exhibit increased levels of adrenal SR-BI (Cao et al., 1999, Wang et al., 1996, Ng et al., 1997). Further, the human SR-BI promoter can be regulated by LXR in hepatocytes and adipocyte (Malerod et al., 2002).
Two transcriptional pathways are known to repress SR-BI expression, including YY1 (Yin Yang 1) zinc transcription factor and DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia congenital, critical region on the X chromosome, gene 1). YY1-mediated repression occurs in the basal state due to direct binding of the SR-BI promoter, and can alternatively bind directly to SREBP-1 functionally preventing its transactivation of the SR-BI promoter (Shea-Eaton et al., 2001). In contrast, DAX-1 represses SR-BI expression by directly interacting with the known transcriptional activators such as SF-1 and SREBP-1a (Lopez et al., 2001). In addition to these defined pathways, numerous additional modes of transcriptional regulation of the SR-BI promoter are being discovered including PPARγ, HNF4α, and the prolactin regulatory element-binding (PREB) transcription factor (Malerod et al., 2003, Murao et al., 2008). Collectively, the SR-BI promoter is under complex transcriptional control, and additional work is needed to more completely define physiological implications of these regulatory pathways.
5.6 Cholesterol binding studies
Although it is well accepted that SR-BI can bi-directionally transfer cholesterol across biological membranes, the precise molecular mechanism by which this is carried out is a matter of debate. The vast majority of work has focused on the direct interactions between SR-BI and apolipoproteins resident on HDL. However, there has been some recent evidence that SR-BI can directly bind cholesterol (Assanasen et al., 2005). In this work the authors tested whether SR-BI could bind a photoactivatable cholesterol analog, and indeed found direct binding between the C-terminal transmembrane domain of SR-BI and this cholesterol analog. The molecular base for SR-BI to bind cholesterol has yet to be determined. The C-terminal transmembrane region of SR-BI does not contain a cholesterol recognition/interaction amino acid consensus (CRAC) or steroidogenic acute regulatory protein-related lipid transfer (START) domain found in other cholesterol-binding proteins (Li and Papadopoulos, 1998, Ponting and Aravind, 1999, Epand, 2006). However, SR-BI is not alone in its lacking of a canonical cholesterol-binding motif. Proteins in the tetraspanin and synaptophysin families also bind cholesterol through a CRAC-, or START-domain independent fashion (Thiele et al., 2000, Charrin et al., 2003). Recent evidence suggests that SR-BI may dimerize or form higher order oligomers, providing the potential for multiple C-termini to serve as a functional cholesterol binding pocket (Reaven et al., 2004, Sahoo et al., 2007, Sahoo et al., 2007). Additional work is needed to define this possibility.
5.7 Therapeutic perspectives for SR-BI
The hyperlipidemic and proatherogenic effects seen with SR-BI deficiency are thought to be primarily due to loss of SR-BI’s critical role in selective uptake of HDL cholesteryl esters into the liver, thereby reducing biliary and fecal sterol excretion. However, effects of SR-BI deficiency in the intestine and other tissues on whole-body cholesterol homeostasis should be considered as well. Several investigators have proposed that SR-BI facilitates intestinal cholesterol absorption (Altmann et al., 2002, Mardones et al., 2001, Nguyen et al., 2009, Bietrix et al., 2006, Labonte et al., 2007, Knopfel et al., 2007). The main argument for this is that SR-BI is present in intestinal brush border membrane vesicles, and an SR-BI antibody reduces cholesterol binding to brush border membrane vesicles. However these in vitro findings have not been substantiated in mice with targeted disruption of SR-BI. In fact, mice lacking SR-BI have normal or enhanced intestinal cholesterol absorption (Altmann et al., 2002, Mardones et al., 2001, Nguyen et al., 2009). The most thorough of these studies revealed that SR-BI deficiency significantly increases fractional cholesterol absorption, under several conditions of dietary cholesterol challenge (Mardones et al., 2001). Collectively, these studies have revealed that SR-BI is highly expressed in the intestine, but its role in intestinal cholesterol absorption has not been definitively established. In contrast to a role of SR-BI in the apical to basolateral transport process of cholesterol absorption, we propose that intestinal SR-BI may play a role in basolateral to apical transport of cholesterol in enterocytes. This directional process of transintestinal cholesterol excretion or non-biliary fecal sterol loss is a critical pathway to rid the body of excess cholesterol directly through intestinal secretion (Kruit et al., 2006, Brown et al., 2008, van der Velde et al., 2008, van der Velde et al., 2007). Based on a number of cellular trafficking studies, SR-BI-mediated selective uptake results in directional basolateral to apical trafficking of sterol cargo (Silver et al., 2001, Burgos et al., 2004, Harder et al., 2007, Wustner et al., 2004, Sehayek et al., 2003). This directional trafficking pattern for SR-BI cargo has been described in vivo in the liver (Silver et al., 2001), and investigations are now underway to examine whether a similar pathway exists in the proximal small intestine. At this point, given our incomplete and opposite understanding of SR-BI’s role in intestinal sterol trafficking, it is difficult to know whether intestinal SR-BI remains a viable therapeutic target for ASCVD. More work is needed before this possibility can be realized.
6. OTHER PROTEINS INFLUENCING INTESTINAL CHOLESTEROL ABSORPTION
Other proteins thought to influence intestinal cholesterol absorption at the intestinal brush border membrane level include CD36 and aminopeptidase N (CD13). CD36 is a scavenger receptor. Deletion of CD36 in mice appear to reduce cholesterol absorption from the proximal small intestine, but increase cholesterol absorption from the distal small intestine, and therefore does not affect net cholesterol absorption (Nguyen et al., 2009, Nauli et al., 2006). CD13 localizes to the apical membrane of enterocyte and was reported to interact with ezetimibe (Kramer et al., 2005). Physiological evidence for a role of CD13 in intestinal cholesterol absorption is currently unavailable.
Cholesterol enters absorptive enterocytes as a free form. Cholesterol delivered to intestinal and thoracic duct lymph mainly exists as an esterified form. The enzyme that catalizes cholesterol esterification in absorptive enterocytes is acyl-CoA:cholesterol acyltransferase-2 (Anderson et al., 1998) (Figure 1). Thus, genetic inactivation of acyl-CoA:cholesterol acyltransferase-2 in mice significantly reduces intestinal cholesterol absorption (Buhman et al., 2000, Repa et al., 2004, Temel et al., 2005).
Unesterified cholesterol, if not transported out back to the gut lumen by the apically-localized heterodimer of ABCG5/G8, can enter the circulation via the basolaterally-localized ABCA1 (Temel et al., 2005) (Figure 1) and this ABCA1-dependent pathway plays a significant role in HDL biogenesis because mice lacking ABCA1 display a reduced plasma HDL-cholesterol concentration (Brunham et al., 2006).
In addition to proteins discussed in this article, many other cellular proteins may also regulate intestinal sterol absorption, such as serine palmitoytransferase (Li et al., 2009) and those listed in a classic review on regulation of intestinal cholesterol absorption (Wang, 2007). Readers interested are referred to this review (Wang, 2007).
7. CONCLUDING COMMENTS
Within the last decade, our understanding of protein-mediated intestinal cholesterol transport has seen immense progress. We have gained important insights into the structure, function, and regulation of a dedicated apical sterol transporter NPC1L1, and its opposing heterodimeric protein complex ABCG5/G8. Furthermore, the development of small molecule inhibitors of intestinal cholesterol absorption has turned out to be important tools to dissect the pathway. Such rapid progress in our understanding of this complex physiological process has been a powerful integration of many scientific disciplines including human genetics, pharmacology, cell biology, biochemistry, genetically altered mouse models, and physiological approaches. Although we have made substantial progress in identifying several protein-mediators of sterol transport across intestinal brush border membrane, it is unlikely this list is complete in its current form. Importantly, mechanistic understanding of protein-mediated sterol transport across plasma membrane and subsequent intracellular trafficking of sterols in the absorptive enterocyte is still in its infancy. By taking advantage of this same multidisciplinary approach, we now have the tools in place to further our understanding of intestinal cholesterol absorption and its protein mediators.
ACKNOWLEDGEMENTS
Dr. Mark Brown is supported by a career transition award (1K99HL096166-01) generously provided by the National Heart, Lung, and Blood Institute. Dr. Liqing Yu is supported by a Scientist Development Grant (#0635261N) from the American Heart Association, and by funds generously provided by the Department of Pathology of Wake Forest University Health Sciences.
ABBREVIATIONS
- ABC
ATP-binding cassette transporter
- Apo
Apolipoprotein
- ASCVD
Atherosclerotic cardiovascular disease
- CD36
Cluster determinant 36
- ER
Endoplasmic reticulum
- HDL
High density lipoprotein
- HNF4α
Hepatocyte nuclear factor 4 alpha
- LDL
Low density lipoprotein
- LDL-C
Low density lipoprotein-cholesterol
- LXR
Liver X receptor
- NPC1
Niemann-Pick C1
- NPC1L1
Niemann-Pick C1-Like 1
- PPAR
peroxisome proliferators-activated receptor
- SCAP
Sterol regulatory element-binding protein-cleavage activating protein
- SR-BI
Scavenger receptor class B type I
- SREBP
Sterol regulatory element-binding protein
REFERENCES
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: Role of sterol regulatory element binding protein 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G369–G376. doi: 10.1152/ajpgi.00306.2006. [DOI] [PubMed] [Google Scholar]
- Altmann SW, Davis HR, Jr, Yao X, Laverty M, Compton DS, Zhu LJ, Crona JH, Caplen MA, Hoos LM, Tetzloff G, Priestley T, Burnett DA, Strader CD, Graziano MP. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. Biochim. Biophys. Acta. 2002;1580:77–93. doi: 10.1016/s1388-1981(01)00190-1. [DOI] [PubMed] [Google Scholar]
- Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- Ashkenas J, Penman M, Vasile E, Acton S, Freeman M, Krieger M. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J. Lipid Res. 1993;34:983–1000. [PubMed] [Google Scholar]
- Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J. Clin. Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assy N, Grozovski M, Bersudsky I, Szvalb S, Hussein O. Effect of insulin-sensitizing agents in combination with ezetimibe, and valsartan in rats with non-alcoholic fatty liver disease. World. J. Gastroenterol. 2006;12:4369–4376. doi: 10.3748/wjg.v12.i27.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S, Nomoto A, Leers-Sucheta S, Reaven E. Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J. Lipid Res. 1998;39:1616–1628. [PubMed] [Google Scholar]
- Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J. Biol. Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- Bao L, Li Y, Deng SX, Landry D, Tabas I. Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J. Biol. Chem. 2006;281:33635–33649. doi: 10.1074/jbc.M606339200. [DOI] [PubMed] [Google Scholar]
- Basso F, Freeman LA, Ko C, Joyce C, Amar MJ, Shamburek RD, Tansey T, Thomas F, Wu J, Paigen B, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J. Lipid Res. 2007;48:114–126. doi: 10.1194/jlr.M600353-JLR200. [DOI] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J. Clin. Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya AK, Connor WE, Lin DS, McMurry MM, Shulman RS. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler. Thromb. 1991;11:1287–1294. doi: 10.1161/01.atv.11.5.1287. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 2008;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bietrix F, Yan D, Nauze M, Rolland C, Bertrand-Michel J, Comera C, Schaak S, Barbaras R, Groen AK, Perret B, Terce F, Collet X. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J. Biol. Chem. 2006;281:7214–7219. doi: 10.1074/jbc.M508868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I, Boberg K, Leitersdorf E. The Metabolic and Molecular Basis of Inherited Diseases. New York: McGraw-Hill; 2001. [Google Scholar]
- Borgstrom B. Quantitative aspects of the intestinal absorption and metabolism of cholesterol and beta-sitosterol in the rat. J. Lipid Res. 1968;9:473–481. [PubMed] [Google Scholar]
- Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 1999;40:302–308. [PubMed] [Google Scholar]
- Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- Brown JM, Bell TA, 3rd, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG, Graham MJ, Crooke RM, Rudel LL. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem. J. 2007:273–283. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- Bultel-Brienne S, Lestavel S, Pilon A, Laffont I, Tailleux A, Fruchart JC, Siest G, Clavey V. Lipid free apolipoprotein E binds to the class B Type I scavenger receptor I (SR-BI) and enhances cholesteryl ester uptake from lipoproteins. J. Biol. Chem. 2002;277:36092–36099. doi: 10.1074/jbc.M201943200. [DOI] [PubMed] [Google Scholar]
- Burgos PV, Klattenhoff C, de la Fuente E, Rigotti A, Gonzalez A. Cholesterol depletion induces PKA-mediated basolateral-to-apical transcytosis of the scavenger receptor class B type I in MDCK cells. Proc. Natl. Acad. Sci. USA. 2004;101:3845–3850. doi: 10.1073/pnas.0400295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SF, Kirby RJ, Howles PN, Hui DY. Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J. Lipid Res. 2001;42:902–909. [PubMed] [Google Scholar]
- Calpe-Berdiel L, Rotllan N, Fievet C, Roig R, Blanco-Vaca F, Escola-Gil JC. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J. Lipid Res. 2008;49:1904–1911. doi: 10.1194/jlr.M700470-JLR200. [DOI] [PubMed] [Google Scholar]
- Calvo D, Vega MA. Identification, primary structure, and distribution of CLA-1, a novel member of the CD36/LIMPII gene family. J. Biol. Chem. 1993;268:18929–18935. [PubMed] [Google Scholar]
- Cao G, Garcia CK, Wyne KL, Schultz RA, Parker KL, Hobbs HH. Structure and localization of the human gene encoding SR-BI/CLA-1. Evidence for transcriptional control by steroidogenic factor 1. J. Biol. Chem. 1997;272:33068–33076. doi: 10.1074/jbc.272.52.33068. [DOI] [PubMed] [Google Scholar]
- Cao G, Liang Y, Jiang XC, Eacho PI. Liver X receptors as potential therapeutic targets for multiple diseases. Drug News Perspect. 2004;17:35–41. doi: 10.1358/dnp.2004.17.1.829024. [DOI] [PubMed] [Google Scholar]
- Cao G, Zhao L, Stangl H, Hasegawa T, Richardson JA, Parker KL, Hobbs HH. Developmental and hormonal regulation of murine scavenger receptor, class, B. type 1. Mol. Endocrinol. 1999;13:1460–1473. doi: 10.1210/mend.13.9.0346. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Tagle DA, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Chang TY, Chang C. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell Metab. 2008;7:469–471. doi: 10.1016/j.cmet.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Charrin S, Manie S, Thiele C, Billard M, Gerlier D, Boucheix C, Rubinstein E. A physical and functional link between cholesterol and tetraspanins. Eur. J. Immunol. 2003;33:2479–2489. doi: 10.1002/eji.200323884. [DOI] [PubMed] [Google Scholar]
- Chu BB, Ge L, Xie C, Zhao Y, Miao HH, Wang J, Li BL, Song BL. Requirement of myosin Vb/Rab11a/Rab11-FIP2 complex in cholesterol-regulated translocation of Niemann-Pick C1 like 1 protein to the cell surface. J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.034355. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clader JW. The discovery of ezetimibe: a view from outside the receptor. J. Med. Chem. 2004;47:1–9. doi: 10.1021/jm030283g. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc. Natl. Acad. Sci. USA. 2006;103:1810–1815. doi: 10.1073/pnas.0508483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA. SR-BI-mediated HDL cholesteryl ester delivery in the adrenal gland. Mol. Cell Endocrinol. 2009;300:83–88. doi: 10.1016/j.mce.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Davidson MH. Novel nonstatin strategies to lower low-density lipoprotein cholesterol. Curr. Atheroscler. Rep. 2009;11:67–70. doi: 10.1007/s11883-009-0011-0. [DOI] [PubMed] [Google Scholar]
- Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- Davies JP, Ioannou YA. The role of the Niemann-Pick C1-like 1 protein in the subcellular transport of multiple lipids and their homeostasis. Curr. Opin. Lipidol. 2006;17:221–226. doi: 10.1097/01.mol.0000226112.12684.5e. [DOI] [PubMed] [Google Scholar]
- Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65:137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 2005;280:12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- Davis HR, Veltri EP. Zetia: Inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to Reduce Intestinal Cholesterol Absorption and Treat Hyperlipidemia. J. Atheroscler. Thromb. 2007;14:99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Rudel LL. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 1999;10:315–320. doi: 10.1097/00041433-199908000-00005. [DOI] [PubMed] [Google Scholar]
- de Beer MC, Durbin DM, Cai L, Jonas A, de Beer FC, van der Westhuyzen DR. Apolipoprotein A-I conformation markedly influences HDL interaction with scavenger receptor BI. J. Lipid Res. 2001;42:309–313. [PubMed] [Google Scholar]
- de la Llera-Moya M, Rothblat GH, Connelly MA, Kellner-Weibel G, Sakr SW, Phillips MC, Williams DL. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J. Lipid Res. 1999;40:575–580. [PubMed] [Google Scholar]
- Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, Yoshida M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581:5664–5670. doi: 10.1016/j.febslet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J. Biol. Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- Duan LP, Wang HH, Ohashi A, Wang DQ. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: gender and age effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G269–G276. doi: 10.1152/ajpgi.00172.2005. [DOI] [PubMed] [Google Scholar]
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 2006;340:1259–1263. doi: 10.1016/j.bbrc.2005.12.137. [DOI] [PubMed] [Google Scholar]
- Eckhardt ER, Cai L, Shetty S, Zhao Z, Szanto A, Webb NR, Van der Westhuyzen DR. High density lipoprotein endocytosis by scavenger receptor SR-BII is clathrin-dependent and requires a carboxyl-terminal dileucine motif. J. Biol. Chem. 2006;281:4348–4353. doi: 10.1074/jbc.M513154200. [DOI] [PubMed] [Google Scholar]
- Eckhardt ER, Cai L, Sun B, Webb NR, van der Westhuyzen DR. High density lipoprotein uptake by scavenger receptor SR-BII. J. Biol. Chem. 2004;279:14372–14381. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- Epand RM. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 2006;45:279–294. doi: 10.1016/j.plipres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Fahmi S, Yang C, Esmail S, Hobbs HH, Cohen JC. Functional characterization of genetic variants in NPC1L1 supports the sequencing extremes strategy to identify complex trait genes. Hum. Mol. Genet. 2008;17:2101–2107. doi: 10.1093/hmg/ddn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field FJ, Watt K, Mathur SN. Ezetimibe interferes with cholesterol trafficking from the plasma membrane to the endoplasmic reticulum in CaCo-2 cells. J. Lipid Res. 2007;48:1735–1745. doi: 10.1194/jlr.M700029-JLR200. [DOI] [PubMed] [Google Scholar]
- Fluiter K, van der Westhuijzen DR, van Berkel TJ. In vivo regulation of scavenger receptor BI and the selective uptake of high density lipoprotein cholesteryl esters in rat liver parenchymal and Kupffer cells. J. Biol. Chem. 1998;273:8434–8438. doi: 10.1074/jbc.273.14.8434. [DOI] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- Freeman LA, Kennedy A, Wu J, Bark S, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J. Lipid Res. 2004;45:1197–1206. doi: 10.1194/jlr.C400002-JLR200. [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Adachi H, Hirota K, Tsujimoto M, Arai H, Inoue K. SRB1, a class B scavenger receptor, recognizes both negatively charged liposomes and apoptotic cells. Exp. Cell Res. 1996;222:246–250. doi: 10.1006/excr.1996.0030. [DOI] [PubMed] [Google Scholar]
- Galman C, Bonde Y, Matasconi M, Angelin B, Rudling M. Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormone. Gastroenterology. 2008;134:1127–1136. doi: 10.1053/j.gastro.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill K A, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc. Natl. Acad. Sci. USA. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18:652–662. doi: 10.1016/0026-0495(69)90078-x. [DOI] [PubMed] [Google Scholar]
- Graf GA, Cohen JC, Hobbs HH. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J. Biol. Chem. 2004;279:24881–24888. doi: 10.1074/jbc.M402634200. [DOI] [PubMed] [Google Scholar]
- Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Skeggs JW, Morton RE. Elevated triglyceride content diminishes the capacity of high density lipoprotein to deliver cholesteryl esters via the scavenger receptor class B type I (SR-BI) J. Biol. Chem. 2001;276:4804–4811. doi: 10.1074/jbc.M008725200. [DOI] [PubMed] [Google Scholar]
- Gregg RE, Connor WE, Lin DS, Brewer HB., Jr Abnormal metabolism of shellfish sterols in a patient with sitosterolemia and xanthomatosis. J. Cli.n Invest. 1986;77:1864–1872. doi: 10.1172/JCI112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Kozarsky K, Krieger M. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J. Biol. Chem. 2000;275:29993–30001. doi: 10.1074/jbc.275.39.29993. [DOI] [PubMed] [Google Scholar]
- Gu X, Lawrence R, Krieger M. Dissociation of the high density lipoprotein and low density lipoprotein binding activities of murine scavenger receptor class B type I (mSR-BI) using retrovirus library-based activity dissection. J. Biol. Chem. 2000;275:9120–9130. doi: 10.1074/jbc.275.13.9120. [DOI] [PubMed] [Google Scholar]
- Gylling H, Miettinen TA. Baseline intestinal absorption and synthesis of cholesterol regulate its response to hypolipidaemic treatments in coronary patients. Atherosclerosis. 2002;160:477–481. doi: 10.1016/s0021-9150(01)00608-6. [DOI] [PubMed] [Google Scholar]
- Hamada T, Egashira N, Nishizono S, Tomoyori H, Nakagiri H, Imaizumi K, Ikeda I. Lymphatic absorption and deposition of various plant sterols in stroke-prone spontaneously hypertensive rats, a strain having a mutation in ATP binding cassette transporter G5. Lipids. 2007;42:241–248. doi: 10.1007/s11745-006-3015-3. [DOI] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Immerdal L, Danielsen EM. Scavenger receptor class B type I (SR-BI) in pig enterocytes: trafficking from the brush border to lipid droplets during fat absorption. Gut. 2003;52:1424–1431. doi: 10.1136/gut.52.10.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Immerdal L, Nystrom BT, Danielsen EM. Intestinal alkaline phosphatase: selective endocytosis from the enterocyte brush border during fat absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1325–G1332. doi: 10.1152/ajpgi.00379.2007. [DOI] [PubMed] [Google Scholar]
- Harder CJ, Meng A, Rippstein P, McBride HM, McPherson R. SR-BI undergoes cholesterol-stimulated transcytosis to the bile canaliculus in polarized WIF-B cells. J Biol Chem. 2007;282:1445–1455. doi: 10.1074/jbc.M604627200. [DOI] [PubMed] [Google Scholar]
- Hart K, Wilcox M. A Drosophila gene encoding an epithelial membrane protein with homology to CD36/LIMP II. J. Mol. Biol. 1993;234:249–253. doi: 10.1006/jmbi.1993.1580. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos AK, Rigotti A, Rosenberg RD, Krieger M. Temporal and spatial pattern of expression of the HDL receptor SR-BI during murine embryogenesis. J. Lipid Res. 1998;39:495–508. [PubMed] [Google Scholar]
- Hauser H, Dyer JH, Nandy A, Vega MA, Werder M, Bieliauskaite E, Weber FE, Compassi S, Gemperli A, Boffelli D, Wehrli E, Schulthess G, Phillips MC. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry. 1998;37:17843–17850. doi: 10.1021/bi982404y. [DOI] [PubMed] [Google Scholar]
- Hawes BE, O'Neill K A, Yao X, Crona JH, Davis HR, Jr, Graziano MP, Altmann SW. In vivo responsiveness to ezetimibe correlates with niemann-pick C1 like-1 (NPC1L1) binding affinity: Comparison of multiple species NPC1L1 orthologs. Mol. Pharmacol. 2007;71:19–29. doi: 10.1124/mol.106.027896. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Guy J, Ban MR, Wang J. NPC1L1 haplotype is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis. 2005;4:16. doi: 10.1186/1476-511X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez HH, Chaikoff IL, Dauben WG, Abraham S. The absorption of C14-labeled epicholesterol in the rat. J. Biol. Chem. 1954;206:757–765. [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb. Symp.Quant. Biol. 2002;67:491–498. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- Huang TC, Kuksis A. Lymphatic Absorption Of Cholesterol In The Dog Following Corn Oil And Butterfat Feeding. Can. J. Physiol. Pharmacol. 1965;43:299–311. doi: 10.1139/y65-029. [DOI] [PubMed] [Google Scholar]
- Hui DY, Howles PN. Molecular mechanisms of cholesterol absorption and transport in the intestine. Semin. Cell Dev. Biol. 2005;16:183–192. doi: 10.1016/j.semcdb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Kodama T, Silverstein SC. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar beta-amyloid. J. Neuroimmunol. 2001;114:142–150. doi: 10.1016/s0165-5728(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Husemann J, Silverstein SC. Expression of scavenger receptor class B, type, I, by astrocytes and vascular smooth muscle cells in normal adult mouse and human brain and in Alzheimer's disease brain. Am. J. Pathol. 2001;158:825–832. doi: 10.1016/S0002-9440(10)64030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Hussain MM. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwayanagi Y, Takada T, Suzuki H. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm. Res. 2008;25:1134–1141. doi: 10.1007/s11095-007-9496-9. [DOI] [PubMed] [Google Scholar]
- Iyer SP, Yao X, Crona JH, Hoos LM, Tetzloff G, Davis HR, Jr, Graziano MP, Altmann SW. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim. Biophys. Acta. 2005;1722:282–292. doi: 10.1016/j.bbagen.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J. Biol. Chem. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- Jian B, de la Llera-Moya M, Ji Y, Wang N, Phillips MC, Swaney JB, Tall AR, Rothblat GH. Scavenger receptor class B type I as a mediator of cellular cholesterol efflux to lipoproteins and phospholipid acceptors. J. Biol. Chem. 1998;273:5599–5606. doi: 10.1074/jbc.273.10.5599. [DOI] [PubMed] [Google Scholar]
- Kajinami K, Takekoshi N, Brousseau ME, Schaefer EJ. Pharmacogenetics of HMG-CoA reductase inhibitors: exploring the potential for genotype-based individualization of coronary heart disease management. Atherosclerosis. 2004;177:219–234. doi: 10.1016/j.atherosclerosis.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. LXRs regulate the balance between fat storage and oxidation. Cell Meta. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M. Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. J. Biol. Chem. 2003;278:36091–36098. doi: 10.1074/jbc.M304153200. [DOI] [PubMed] [Google Scholar]
- Karakesisoglou I, Janssen KP, Eichinger L, Noegel AA, Schleicher M. Identification of a suppressor of the Dictyostelium profilin-minus phenotype as a CD36/LIMP-II homologue. J. Cell Biol. 1999;145:167–181. doi: 10.1083/jcb.145.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner-Weibel G, de La Llera-Moya M, Connelly MA, Stoudt G, Christian AE, Haynes MP, Williams DL, Rothblat GH. Expression of scavenger receptor BI in COS-7 cells alters cholesterol content and distribution. Biochemistry. 2000;39:221–229. doi: 10.1021/bi991666c. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J. Lipid Res. 2003;44:1728–1736. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, Gustafsson JA. Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia. Proc. Natl. Acad. Sci. USA. 2008;105:2094–2099. doi: 10.1073/pnas.0711599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. doi: 10.1186/1471-230X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, Shefer S, Batta AK, Yu H, Chen J, Klein R, Looije N, Oude-Elferink R, Groen AK, Maeda N, Salen G, Patel SB. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;2:5. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight BL, Patel DD, Humphreys SM, Wiggins D, Gibbons GF. Inhibition of cholesterol absorption associated with a PPAR alpha-dependent increase in ABC binding cassette transporter A1 in mice. J. Lipid Res. 2003;44:2049–2058. doi: 10.1194/jlr.M300042-JLR200. [DOI] [PubMed] [Google Scholar]
- Knopfel M, Davies JP, Duong PT, Kvaerno L, Carreira EM, Phillips MC, Ioannou YA, Hauser H. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim. Biophys. Acta. 2007;1771:1140–1147. doi: 10.1016/j.bbalip.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Kocher O, Yesilaltay A, Cirovic C, Pal R, Rigotti A, Krieger M. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J. Biol. Chem. 2003;278:52820–52825. doi: 10.1074/jbc.M310482200. [DOI] [PubMed] [Google Scholar]
- Kolovou G, Voudris V, Drogari E, Palatianos G, Cokkinos DV. Coronary bypass grafts in a young girl with sitosterolemia. Eur. Heart. J. 1996;17:965–966. doi: 10.1093/oxfordjournals.eurheartj.a014983. [DOI] [PubMed] [Google Scholar]
- Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jahne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, Orso E, Schmitz G. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J. Biol. Chem. 2005;280:1306–1320. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- Krieger M. Charting the fate of the "good cholesterol": identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- Kruit JK, Drayer AL, Bloks VW, Blom N, Olthof SG, Sauer PJ, de Haan G, Kema IP, Vellenga E, Kuipers F. Plant sterols cause macrothrombocytopenia in a mouse model of sitosterolemia. J. Biol. Chem. 2008;283:6281–6287. doi: 10.1074/jbc.M706689200. [DOI] [PubMed] [Google Scholar]
- Kruit JK, Groen AK, van Berkel TJ, Kuipers F. Emerging roles of the intestine in control of cholesterol metabolism. World. J. Gastroenterol. 2006;12:6429–6439. doi: 10.3748/wjg.v12.i40.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruit JK, Plosch T, Havinga R, Boverhof R, Groot PH, Groen AK, Kuipers F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, Labouesse M. The sterol-sensing domain: multiple families, a unique role? Trends Genet. 2002;18:193–201. doi: 10.1016/s0168-9525(02)02640-9. [DOI] [PubMed] [Google Scholar]
- Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G776–G783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY. Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim. Biophys. Acta. 2007;1771:1132–1139. doi: 10.1016/j.bbalip.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally S, Owens D, Tomkin GH. The different effect of pioglitazone as compared to insulin on expression of hepatic and intestinal genes regulating post-prandial lipoproteins in diabetes. Atherosclerosis. 2007;193:343–351. doi: 10.1016/j.atherosclerosis.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Lally S, Owens D, Tomkin GH. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control and streptozotosin diabetic rats. Metabolism. 2007;56:430–438. doi: 10.1016/j.metabol.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Lally S, Tan CY, Owens D, Tomkin GH. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia. 2006;49:1008–1016. doi: 10.1007/s00125-006-0177-8. [DOI] [PubMed] [Google Scholar]
- Lally SE, Owens D, Tomkin GH. Sitosterol and cholesterol in chylomicrons of type 2 diabetic and non-diabetic subjects: the relationship with ATP binding cassette proteins G5 and G8 and Niemann-Pick C1-like 1 mRNA. Diabetologia. 2007;50:217–219. doi: 10.1007/s00125-006-0504-0. [DOI] [PubMed] [Google Scholar]
- Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J. Clin. Invest. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA. Intestinal cholesterol transport proteins: an update and beyond. Curr. Opin. Lipidol. 2007;18:310–318. doi: 10.1097/MOL.0b013e32813fa2e2. [DOI] [PubMed] [Google Scholar]
- Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- Li X, Kan HY, Lavrentiadou S, Krieger M, Zannis V. Reconstituted discoidal ApoE-phospholipid particles are ligands for the scavenger receptor BI. The amino-terminal 1–165 domain of ApoE suffices for receptor binding. J. Biol. Chem. 2002;277:21149–21157. doi: 10.1074/jbc.M200658200. [DOI] [PubMed] [Google Scholar]
- Li X, Peegel H, Menon KM. In situ hybridization of high density lipoprotein (scavenger, type 1) receptor messenger ribonucleic acid (mRNA) during folliculogenesis and luteinization: evidence for mRNA expression and induction by human chorionic gonadotropin specifically in cell types that use cholesterol for steroidogenesis. Endocrinology. 1998;139:3043–3049. doi: 10.1210/endo.139.7.6108. [DOI] [PubMed] [Google Scholar]
- Li X, Peegel H, Menon KM. Regulation of high density lipoprotein receptor messenger ribonucleic acid expression and cholesterol transport in theca-interstitial cells by insulin and human chorionic gonadotropin. Endocrinology. 2001;142:174–181. doi: 10.1210/endo.142.1.7865. [DOI] [PubMed] [Google Scholar]
- Li Z, Park TS, Li Y, Pan X, Iqbal J, Lu D, Tang W, Yu L, Goldberg IJ, Hussain MM, Jiang XC. Serine palmitoyltransferase (SPT) deficient mice absorb less cholesterol. Biochim. Biophys. Acta. 2009;1791:297–306. doi: 10.1016/j.bbalip.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liadaki KN, Liu T, Xu S, Ishida BY, Duchateaux PN, Krieger JP, Kane J, Krieger M, Zannis VI. Binding of high density lipoprotein (HDL) and discoidal reconstituted HDL to the HDL receptor scavenger receptor class B type I. Effect of lipid association and APOA-I mutations on receptor binding. J. Biol. Chem. 2000;275:21262–21271. doi: 10.1074/jbc.M002310200. [DOI] [PubMed] [Google Scholar]
- Liu B, Krieger M. Highly purified scavenger receptor class B, type I reconstituted into phosphatidylcholine/cholesterol liposomes mediates high affinity high density lipoprotein binding and selective lipid uptake. J. Biol. Chem. 2002;277:34125–34135. doi: 10.1074/jbc.M204265200. [DOI] [PubMed] [Google Scholar]
- Liu R, Iqbal J, Yeang C, Wang DQ, Hussain MM, Jiang XC. Phospholipid transfer protein-deficient mice absorb less cholesterol. Arterioscler. Thromb. Vasc. Biol. 2007;27:2014–2021. doi: 10.1161/ATVBAHA.107.149914. [DOI] [PubMed] [Google Scholar]
- Liu T, Krieger M, Kan HY, Zannis VI. The effects of mutations in helices 4 and 6 of ApoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and SR-BI is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. doi: 10.1074/jbc.M112103200. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- Lopez D, McLean MP. Sterol regulatory element-binding protein-1a binds to cis elements in the promoter of the rat high density lipoprotein receptor SR-BI gene. Endocrinology. 1999;140:5669–5681. doi: 10.1210/endo.140.12.7220. [DOI] [PubMed] [Google Scholar]
- Lopez D, Shea-Eaton W, Sanchez MD, McLean MP. DAX-1 represses the high-density lipoprotein receptor through interaction with positive regulators sterol regulatory element-binding protein-1a and steroidogenic factor-1. Endocrinology. 2001;142:5097–5106. doi: 10.1210/endo.142.12.8523. [DOI] [PubMed] [Google Scholar]
- Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J. Lipid Res. 1995;36:1763–1773. [PubMed] [Google Scholar]
- Malerod L, Juvet LK, Hanssen-Bauer A, Eskild W, Berg T. Oxysterol-activated LXRalpha/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem. Biophys. Res. Commun. 2002;299:916–923. doi: 10.1016/s0006-291x(02)02760-2. [DOI] [PubMed] [Google Scholar]
- Malerod L, Sporstol M, Juvet LK, Mousavi A, Gjoen T, Berg T. Hepatic scavenger receptor class B, type I is stimulated by peroxisome proliferator-activated receptor gamma and hepatocyte nuclear factor 4alpha. Biochem. Biophys. Res. Commun. 2003;305:557–565. doi: 10.1016/s0006-291x(03)00819-2. [DOI] [PubMed] [Google Scholar]
- Mardones P, Quinones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Trigatti B, Krieger M, VanPatten S, Cohen DE, Rigotti A. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 2001;42:170–180. [PubMed] [Google Scholar]
- Mathur SN, Watt KR, Field FJ. Regulation of intestinal NPC1L1 expression by dietary fish oil and docosahexaenoic acid. J. Lipid Res. 2007;48:395–404. doi: 10.1194/jlr.M600325-JLR200. [DOI] [PubMed] [Google Scholar]
- McConihay JA, Horn PS, Woollett LA. Effect of maternal hypercholesterolemia on fetal sterol metabolism in the Golden Syrian hamster. J. Lipid Res. 2001;42:1111–1119. [PubMed] [Google Scholar]
- McLean MP, Sandhoff TW. Expression and hormonal regulation of the high-density lipoprotein (HDL) receptor scavenger receptor class B type I messenger ribonucleic acid in the rat ovary. Endocrine. 1998;9:243–252. doi: 10.1385/ENDO:9:3:243. [DOI] [PubMed] [Google Scholar]
- McNamara DJ, Davidson NO, Samuel P, Ahrens EH., Jr Cholesterol absorption in man: effect of administration of clofibrate and/or cholestyramine. J. Lipid Res. 1980;21:1058–1064. [PubMed] [Google Scholar]
- Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Strandberg TE, Gylling H. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler. Thromb. Vasc. Biol. 2000;20:1340–1346. doi: 10.1161/01.atv.20.5.1340. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Sonoda Y, Minegishi T, Wakabayashi K, Miyamoto K. Cloning, characterization, and cellular distribution of rat scavenger receptor class B type I (SRBI) in the ovary. Biochem. Biophys. Res. Commun. 1997;234:499–505. doi: 10.1006/bbrc.1997.6646. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Yamada K, Minegishi T, Miyamoto K. Transcriptional regulation of rat scavenger receptor class B type I gene. J. Biol. Chem. 2000;275:22512–22519. doi: 10.1074/jbc.M001631200. [DOI] [PubMed] [Google Scholar]
- Murao K, Imachi H, Yu X, Cao WM, Muraoka T, Dobashi H, Hosomi N, Haba R, Iwama H, Ishida T. The transcriptional factor prolactin regulatory element-binding protein mediates the gene transcription of adrenal scavenger receptor class B type I via 3',5'-cyclic adenosine 5'-monophosphate. Endocrinology. 2008;149:6103–6112. doi: 10.1210/en.2008-0380. [DOI] [PubMed] [Google Scholar]
- Mymin D, Wang J, Frohlich J, Hegele RA. Image in cardiovascular medicine. Aortic xanthomatosis with coronary ostial occlusion in a child homozygous for a nonsense mutation in ABCG8. Circulation. 2003;107:791. doi: 10.1161/01.cir.0000050545.21826.ad. [DOI] [PubMed] [Google Scholar]
- Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DS, Francone OL, Forte TM, Zhang J, Haghpassand M, Rubin EM. Disruption of the murine lecithin:cholesterol acyltransferase gene causes impairment of adrenal lipid delivery and up-regulation of scavenger receptor class B type. I. J. Biol. Chem. 1997;272:15777–15781. doi: 10.1074/jbc.272.25.15777. [DOI] [PubMed] [Google Scholar]
- Nguyen DV, Drover VA, Knopfel M, Dhanasekaran P, Hauser H, Phillips MC. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J. Lipid Res. 2009 doi: 10.1194/jlr.M900036-JLR200. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc. Natl. Acad. Sci. USA. 2004;101:12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Miyazaki A, Ikemoto M, Arai H, Horiuchi S, Nakayama H. Scavenger receptor class B type I-mediated reverse cholesterol transport is inhibited by advanced glycation end products. J. Biol. Chem. 2001;276:13348–13355. doi: 10.1074/jbc.M011613200. [DOI] [PubMed] [Google Scholar]
- Okiyoneda T, Kono T, Niibori A, Harada K, Kusuhara H, Takada T, Shuto T, Suico MA, Sugiyama Y, Kai H. Calreticulin facilitates the cell surface expression of ABCG5/G8. Biochem. Biophys. Res. Commun. 2006;347:67–75. doi: 10.1016/j.bbrc.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Parker KL. The roles of steroidogenic factor 1 in endocrine development and function. Mol. Cell Endocrinol. 1998;140:59–63. doi: 10.1016/s0303-7207(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH, Brownstein MJ. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J. Clin. Invest. 1998;102:1041–1044. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NH, Faergeman NJ, Yu L, Wustner D. Kinetic imaging of NPC1L1 and sterol trafficking between plasma membrane and recycling endosomes in hepatoma cells. J. Lipid Res. 2008;49:2023–2037. doi: 10.1194/jlr.M800145-JLR200. [DOI] [PubMed] [Google Scholar]
- Pilon A, Briand O, Lestavel S, Copin C, Majd Z, Fruchart JC, Castro G, Clavey V. Apolipoprotein AII enrichment of HDL enhances their affinity for class B type I scavenger receptor but inhibits specific cholesteryl ester uptake. Arterioscler. Thromb. Vasc. Biol. 2000;20:1074–1081. doi: 10.1161/01.atv.20.4.1074. [DOI] [PubMed] [Google Scholar]
- Plosch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, Groen AK, Shan B, Kuipers F, Schwarz M. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126:290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- Plosch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- Plosch T, Kruit JK, Bloks VW, Huijkman NC, Havinga R, Duchateau GS, Lin Y, Kuipers F. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the Abcg5/8 transporter. J. Nutr. 2006;136:2135–2140. doi: 10.1093/jn/136.8.2135. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- Rader DJ. Liver X receptor and farnesoid X receptor as therapeutic targets. Am. J. Cardiol. 2007;100:n15–n19. doi: 10.1016/j.amjcard.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol. Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Reaven E, Cortez Y, Leers-Sucheta S, Nomoto A, Azhar S. Dimerization of the scavenger receptor class B type I: formation, function, and localization in diverse cells and tissues. J. Lipid Res. 2004;45:513–528. doi: 10.1194/jlr.M300370-JLR200. [DOI] [PubMed] [Google Scholar]
- Reaven E, Lua Y, Nomoto A, Temel R, Williams DL, van der Westhuyzen DR, Azhar S. The selective pathway and a high-density lipoprotein receptor (SR-BI) in ovarian granulosa cells of the mouse. Biochim. Biophys. Acta. 1999;1436:565–576. doi: 10.1016/s0005-2760(98)00169-6. [DOI] [PubMed] [Google Scholar]
- Reaven E, Nomoto A, Leers-Sucheta S, Temel R, Williams DL, Azhar S. Expression and microvillar localization of scavenger receptor, class B type I (a high density lipoprotein receptor) in luteinized and hormone-desensitized rat ovarian models. Endocrinology. 1998;139:2847–2856. doi: 10.1210/endo.139.6.6056. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Buhman KK, Farese RV, Jr, Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 2004;40:1088–1097. doi: 10.1002/hep.20439. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: potential new players in atherosclerosis. Nat. Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- Rhainds D, Bourgeois P, Bourret G, Huard K, Falstrault L, Brissette L. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J. Cell Sci. 2004;117:3095–3105. doi: 10.1242/jcs.01182. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Edelman ER, Seifert P, Iqbal SN, DeMattos RB, Temel RE, Krieger M, Williams DL. Regulation by adrenocorticotropic hormone of the in vivo expression of scavenger receptor class B type I (SR-BI), a high density lipoprotein receptor, in steroidogenic cells of the murine adrenal gland. J. Biol. Chem. 1996;271:33545–33549. doi: 10.1074/jbc.271.52.33545. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Sabeva NS, Rouse EJ, Graf GA. Defects in the leptin axis reduce abundance of the ABCG5-ABCG8 sterol transporter in liver. J. Biol. Chem. 2007;282:22397–22405. doi: 10.1074/jbc.M702236200. [DOI] [PubMed] [Google Scholar]
- Sahoo D, Darlington YF, Pop D, Williams DL, Connelly MA. Scavenger receptor class B Type I (SR-BI) assembles into detergent-sensitive dimers and tetramers. Biochim. Biophys. Acta. 2007;1771:807–817. doi: 10.1016/j.bbalip.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sahoo D, Peng Y, Smith JR, Darlington YF, Connelly MA. Scavenger receptor class B type I (SR-BI) homo-dimerizes via its C-terminal region: fluorescence resonance energy transfer analysis. Biochim. Biophys. Acta. 2007;1771:818–829. doi: 10.1016/j.bbalip.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G, Horak I, Rothkopf M, Cohen JL, Speck J, Tint GS, Shore V, Dayal B, Chen T, Shefer S. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J. Lipid Res. 1985;26:1126–1133. [PubMed] [Google Scholar]
- Salen G, Shore V, Tint GS, Forte T, Shefer S, Horak I, Horak E, Dayal B, Nguyen L, Batta AK, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J. Lipid Res. 1989;30:1319–1330. [PubMed] [Google Scholar]
- Salen G, Starc T, Sisk CM, Patel SB. Intestinal cholesterol absorption inhibitor ezetimibe added to cholestyramine for sitosterolemia and xanthomatosis. Gastroenterology. 2006;130:1853–1857. doi: 10.1053/j.gastro.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler. Thromb. 1992;12:563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane AT, Sinnett D, Delvin E, Bendayan M, Marcil V, Menard D, Beaulieu JF, Levy E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J. Lipid Res. 2006;47:2112–2120. doi: 10.1194/jlr.M600174-JLR200. [DOI] [PubMed] [Google Scholar]
- Schoenheimer R. Uber die bedeutung der pflanzensterine fur den tierschen organismus. Hoppe-Seyler's Fur Physiol. Chem. 1929;180:1–5. [Google Scholar]
- Schoenheimer R, Breusch F. Synthesis and destruction of cholesterol in the organism. J. Biol. Chem. 1933;103:439–448. [Google Scholar]
- Sehayek E, Wang R, Ono JG, Zinchuk VS, Duncan EM, Shefer S, Vance DE, Ananthanarayanan M, Chait BT, Breslow JL. Localization of the PE methylation pathway and SR-BI to the canalicular membrane: evidence for apical PC biosynthesis that may promote biliary excretion of phospholipid and cholesterol. J. Lipid Res. 2003;44:1605–1613. doi: 10.1194/jlr.M200488-JLR200. [DOI] [PubMed] [Google Scholar]
- Shea-Eaton W, Lopez D, McLean MP. Yin yang 1 protein negatively regulates high-density lipoprotein receptor gene transcription by disrupting binding of sterol regulatory element binding protein to the sterol regulatory element. Endocrinology. 2001;142:49–58. doi: 10.1210/endo.142.1.7868. [DOI] [PubMed] [Google Scholar]
- Silver DL. A carboxyl-terminal PDZ-interacting domain of scavenger receptor B, type I is essential for cell surface expression in liver. J. Biol. Chem. 2002;277:34042–34047. doi: 10.1074/jbc.M206584200. [DOI] [PubMed] [Google Scholar]
- Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J. Biol. Chem. 2001;276:25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- Simon JS, Karnoub MC, Devlin DJ, Arreaza MG, Qiu P, Monks SA, Severino ME, Deutsch P, Palmisano J, Sachs AB, Bayne ML, Plump AS, Schadt EE. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics. 2005;86:648–656. doi: 10.1016/j.ygeno.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Smart EJ, De Rose RA, Farber SA. Annexin 2-caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. Proc. Natl. Acad. Sci. USA. 2004;101:3450–3455. doi: 10.1073/pnas.0400441101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spady DK, Kearney DM, Hobbs HH. Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1 (SR-BI) expression and HDL cholesteryl ester uptake in the hamster. J. Lipid Res. 1999;40:1384–1394. [PubMed] [Google Scholar]
- Stangl H, Hyatt M, Hobbs HH. Transport of lipids from high and low density lipoproteins via scavenger receptor-BI. J. Biol. Chem. 1999;274:32692–32698. doi: 10.1074/jbc.274.46.32692. [DOI] [PubMed] [Google Scholar]
- Su Y, Wang Z, Yang H, Cao L, Liu F, Bai X, Ruan C. Clinical and molecular genetic analysis of a family with sitosterolemia and co-existing erythrocyte and platelet abnormalities. Haematologica. 2006;91:1392–1395. [PubMed] [Google Scholar]
- Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- Sudhop T, Lutjohann D, von Bergmann K. Sterol transporters: targets of natural sterols and new lipid lowering drugs. Pharmacol. Ther. 2005;105:333–341. doi: 10.1016/j.pharmthera.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J. Clin. Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi K, Tanaka T, Uchida A, Magoori K, Urashima Y, Ohashi R, Ohguchi H, Okamura M, Kudo H, Daigo K, Maejima T, Kojima N, Sakakibara I, Jiang S, Hasegawa G, Kim I, Osborne TF, Naito M, Gonzalez FJ, Hamakubo T, Kodama T, Sakai J. Cooperative interaction between hepatocyte nuclear factor 4 alpha and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol. Cell Biol. 2007;27:4248–4260. doi: 10.1128/MCB.01894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang N, Tall AR. Regulation of adrenal scavenger receptor-BI expression by ACTH and cellular cholesterol pools. J. Lipid Res. 1999;40:1799–1805. [PubMed] [Google Scholar]
- Svensson PA, Johnson MS, Ling C, Carlsson LM, Billig H, Carlsson B. Scavenger receptor class B type I in the rat ovary: possible role in high density lipoprotein cholesterol uptake and in the recognition of apoptotic granulosa cells. Endocrinology. 1999;140:2494–2500. doi: 10.1210/endo.140.6.6693. [DOI] [PubMed] [Google Scholar]
- Tachibana S, Hirano M, Hirata T, Matsuo M, Ikeda I, Ueda K, Sato R. Cholesterol and plant sterol efflux from cultured intestinal epithelial cells is mediated by ATP-binding cassette transporters. Biosci. Biotechnol. Biochem. 2007;71:1886–1895. doi: 10.1271/bbb.70109. [DOI] [PubMed] [Google Scholar]
- Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J. Lipid. Res. 2008;50:293–300. doi: 10.1194/jlr.M800439-JLR200. [DOI] [PubMed] [Google Scholar]
- Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Niemann-Pick C1-Like 1 Is Required for an LXR Agonist to Raise Plasma HDL Cholesterol in Mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:448–454. doi: 10.1161/ATVBAHA.107.160465. [DOI] [PubMed] [Google Scholar]
- Tang W, Ma Y, Yu L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 2006;44:1259–1266. doi: 10.1002/hep.21380. [DOI] [PubMed] [Google Scholar]
- Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PH, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J. Lipid Res. 2007;48:699–708. doi: 10.1194/jlr.M600439-JLR200. [DOI] [PubMed] [Google Scholar]
- Temel RE, Brown JM, Ma Y, Tang W, Rudel LL, Ioannou YA, Davies JP, Yu L. Diosgenin stimulation of fecal cholesterol excretion in mice is not NPC1L1-dependent. J. Lipid Res. 2009;50:915–923. doi: 10.1194/jlr.M800631-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ATP-binding cassette transporter A1 (ABCA1) and Acyl-CoA:Cholesterol O-acyltransferase 2 (ACAT2) J. Lipid Res. 2005;46:2423–2431. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel RE, Trigatti B, DeMattos RB, Azhar S, Krieger M, Williams DL. Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc. Natl. Acad. Sci. USA. 1997;94:13600–13605. doi: 10.1073/pnas.94.25.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat. Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- Towns R, Azhar S, Peegel H, Menon KM. LH/hCG-stimulated androgen production and selective HDL-cholesterol transport are inhibited by a dominant-negative CREB construct in primary cultures of rat theca-interstitial cells. Endocrine. 2005;27:269–277. doi: 10.1385/ENDO:27:3:269. [DOI] [PubMed] [Google Scholar]
- Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc. Natl. Acad. Sci. USA. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigatti BL. Hepatic high-density lipoprotein receptors: roles in lipoprotein metabolism and potential for therapeutic modulation. Curr. Atheroscler. Rep. 2005;7:344–350. doi: 10.1007/s11883-005-0045-x. [DOI] [PubMed] [Google Scholar]
- Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr. Opin. Lipidol. 2003;14:233–240. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Gong E, Royer L, Cooper PN, Francone OL, Rubin EM. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J. Biol. Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Royer L, Gong E, Zhang J, Cooper PN, Francone O, Rubin EM. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J. Biol. Chem. 1999;274:7165–7171. doi: 10.1074/jbc.274.11.7165. [DOI] [PubMed] [Google Scholar]
- Umeda Y, Kako Y, Mizutani K, Iikura Y, Kawamura M, Seishima M, Hayashi H. Inhibitory action of gemfibrozil on cholesterol absorption in rat intestine. J. Lipid Res. 2001;42:1214–1219. [PubMed] [Google Scholar]
- Urban S, Zieseniss S, Werder M, Hauser H, Budzinski R, Engelmann B. Scavenger receptor BI transfers major lipoprotein-associated phospholipids into the cells. J. Biol. Chem. 2000;275:33409–33415. doi: 10.1074/jbc.M004031200. [DOI] [PubMed] [Google Scholar]
- Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPAR{alpha}-dependent modulation of NPC1L1 expression in mouse. J. Lipid Res. 2007;48:2725–2735. doi: 10.1194/jlr.M700345-JLR200. [DOI] [PubMed] [Google Scholar]
- Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G813–G822. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasek MA, Weng J, Shaul PW, Anderson RG, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. J. Biol. Chem. 2005;280:28103–28109. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK, Kuipers F. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J. Lipid Res. 2005;46:526–534. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- van der Velde AE, Vrins CL, van den Oever K, Seemann I, Oude Elferink RP, van Eck M, Kuipers F, Groen AK. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G203–G208. doi: 10.1152/ajpgi.90231.2008. [DOI] [PubMed] [Google Scholar]
- van Heek M, Austin TM, Farley C, Cook JA, Tetzloff GG, Davis HR. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 2001;50:1330–1335. doi: 10.2337/diabetes.50.6.1330. [DOI] [PubMed] [Google Scholar]
- van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br. J. Pharmacol. 2001;134:409–417. doi: 10.1038/sj.bjp.0704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR., Jr In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J. Pharmacol. Exp. Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- Vanhanen HT, Miettinen TA. Cholesterol absorption and synthesis during pravastatin, gemfibrozil and their combination. Atherosclerosis. 1995;115:135–146. doi: 10.1016/0021-9150(94)05474-w. [DOI] [PubMed] [Google Scholar]
- Varban ML, Rinninger F, Wang N, Fairchild-Huntress V, Dunmore JH, Fang Q, Gosselin ML, Dixon KL, Deeds JD, Acton SL, Tall AR, Huszar D. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega MA, Rodriguez F, Segui B, Cales C, Alcalde J, Sandoval IV. Targeting of lysosomal integral membrane protein LIMP II. The tyrosine-lacking carboxyl cytoplasmic tail of LIMP II is sufficient for direct targeting to lysosomes. J. Biol. Chem. 1991;266:16269–16272. [PubMed] [Google Scholar]
- Voshol PJ, Schwarz M, Rigotti A, Krieger M, Groen AK, Kuipers F. Down-regulation of intestinal scavenger receptor class B, type I (SR-BI) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem J. 2001;356:317–325. doi: 10.1042/0264-6021:3560317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrins C, Vink E, Vandenberghe KE, Frijters R, Seppen J, Groen AK. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 2007;581:4616–4620. doi: 10.1016/j.febslet.2007.08.052. [DOI] [PubMed] [Google Scholar]
- Wang C, Lin HJ, Chan TK, Salen G, Chan WC, Tse TF. A unique patient with coexisting cerebrotendinous xanthomatosis and beta-sitosterolemia. Am. J. Med. 1981;71:313–319. doi: 10.1016/0002-9343(81)90134-0. [DOI] [PubMed] [Google Scholar]
- Wang DQ. Regulation of intestinal cholesterol absorption. Annu. Rev. Physio. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(−/−) mice. Hepatology. 2007;45:998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chu BB, Ge L, Li BL, Yan Y, Song BL. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J. Lipid Res. 2009 doi: 10.1194/jlr.M800669-JLR200. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun F, Zhang DW, Ma Y, Xu F, Belani JD, Cohen JC, Hobbs HH, Xie XS. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J. Biol. Chem. 2006;281:27894–27904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Williams C, Hegele R. Compound heterozygosity for two non-synonymous polymorphisms in NPC1L1 in a non-responder to ezetimibe. Clin. Genet. 2005;67:175–177. doi: 10.1111/j.1399-0004.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang DW, Lei Y, Xu F, Cohen JC, Hobbs HH, Xie XS. Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8. Biochemistry. 2008;47:5194–5204. doi: 10.1021/bi800292v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Arai T, Ji Y, Rinninger F, Tall AR. Liver-specific overexpression of scavenger receptor BI decreases levels of very low density lipoprotein ApoB, low density lipoprotein ApoB, and high density lipoprotein in transgenic mice. J. Biol. Chem. 1998;273:32920–32926. doi: 10.1074/jbc.273.49.32920. [DOI] [PubMed] [Google Scholar]
- Wang N, Weng W, Breslow JL, Tall AR. Scavenger receptor BI (SR-BI) is up-regulated in adrenal gland in apolipoprotein A-I and hepatic lipase knock-out mice as a response to depletion of cholesterol stores. In vivo evidence that SR-BI is a functional high density lipoprotein receptor under feedback control. J. Biol. Chem. 1996;271:21001–21004. doi: 10.1074/jbc.271.35.21001. [DOI] [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb NR, Connell PM, Graf GA, Smart EJ, de Villiers WJ, de Beer FC, van der Westhuyzen DR. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J. Biol. Chem. 1998;273:15241–15248. doi: 10.1074/jbc.273.24.15241. [DOI] [PubMed] [Google Scholar]
- Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, Thomas A, Schmalhofer W, Williams B, Bildl W, McMasters DR, Dai K, Beers L, McCann ME, Kaczorowski GJ, Garcia ML. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl. Acad. Sci. USA. 2008;105:11140–11145. doi: 10.1073/pnas.0800936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Connelly MA, Temel RE, Swarnakar S, Phillips MC, de la Llera-Moya M, Rothblat GH. Scavenger receptor BI and cholesterol trafficking. Curr. Opin. Lipidol. 1999;10:329–339. doi: 10.1097/00041433-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Williams DL, de La Llera-Moya M, Thuahnai ST, Lund-Katz S, Connelly MA, Azhar S, Anantharamaiah GM, Phillips MC. Binding and cross-linking studies show that scavenger receptor BI interacts with multiple sites in apolipoprotein A-I and identify the class A amphipathic alpha-helix as a recognition motif. J. Biol. Chem. 2000;275:18897–18904. doi: 10.1074/jbc.M002411200. [DOI] [PubMed] [Google Scholar]
- Wilson MD, Rudel LL. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 1994;35:943–955. [PubMed] [Google Scholar]
- Wilund KR, Yu L, Xu F, Hobbs HH, Cohen JC. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/− mice. J. Lipid Res. 2004;45:1429–1436. doi: 10.1194/jlr.M400167-JLR200. [DOI] [PubMed] [Google Scholar]
- Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler. Thromb. Vasc. Biol. 2004;24:2326–2332. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
- Wu JE, Basso F, Shamburek RD, Amar MJ, Vaisman B, Szakacs G, Joyce C, Tansey T, Freeman L, Paigen BJ, Thomas F, Brewer HB, Jr, Santamarina-Fojo S. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J. Biol. Chem. 2004;279:22913–22925. doi: 10.1074/jbc.M402838200. [DOI] [PubMed] [Google Scholar]
- Wustner D, Mondal M, Huang A, Maxfield FR. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J. Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- Xu S, Laccotripe M, Huang X, Rigotti A, Zannis VI, Krieger M. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J. Lipid Res. 1997;38:1289–1298. [PubMed] [Google Scholar]
- Yamamoto T. Ultrastructural basis of intestinal absorption. Arch. Histol. Jpn. 1982;45:1–22. doi: 10.1679/aohc.45.1. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y, Takada T, Suzuki H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in CaCo-2 cells. J. Pharmacol. Exp. Ther. 2007;320:559–564. doi: 10.1124/jpet.106.114181. [DOI] [PubMed] [Google Scholar]
- Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000;275:36596–36604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J. Clin. Invest. 2004;114:813–822. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilaltay A, Kocher O, Pal R, Leiva A, Quinones V, Rigotti A, Krieger M. PDZK1 is required for maintaining hepatic scavenger receptor, class B, type I (SR-BI) steady state levels but not its surface localization or function. J. Biol. Chem. 2006;281:28975–28980. doi: 10.1074/jbc.M603802200. [DOI] [PubMed] [Google Scholar]
- Yu L. Dual action of the ezetimibe target Niemann-Pick C1-Like 1. Future Lipidology. 2007;2:379–382. [Google Scholar]
- Yu L. The structure and function of Niemann-Pick C1-like 1 protein. Curr. Opin. Lipidol. 2008;19:263–269. doi: 10.1097/MOL.0b013e3282f9b563. [DOI] [PubMed] [Google Scholar]
- Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J. Biol. Chem. 2006;281:6616–6624. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- Yu L, Cao G, Repa J, Stangl H. Sterol regulation of scavenger receptor class B type I in macrophages. J. Lipid Res. 2004;45:889–899. doi: 10.1194/jlr.M300461-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J. Lipid Res. 2004;45:301–307. doi: 10.1194/jlr.M300377-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J. Lipid Res. 2005;46:1739–1744. doi: 10.1194/jlr.M500124-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol, Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, Rinninger F, Tall AR. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J. Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, Krieger M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation. 2005;111:3457–3464. doi: 10.1161/CIRCULATIONAHA.104.523563. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Hoos L, Cook J, Tetzloff G, Davis H, Jr, van Heek M, Hwa JJ. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur. J. Pharmacol. 2008;584:118–124. doi: 10.1016/j.ejphar.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Zuniga S, Molina H, Azocar L, Amigo L, Nervi F, Pimentel F, Jarufe N, Arrese M, Lammert F, Miquel JF. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28:935–947. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]


