Abstract
Since cloning of the ATP-binding cassette (ABC) family member breast cancer resistance protein (BCRP/ABCG2) and its characterization as a multidrug resistance efflux transporter in 1998, BCRP has been the subject of more than two thousand scholarly articles. In normal tissues, BCRP functions as a defense mechanism against toxins and xenobiotics, with expression in the gut, bile canaliculi, placenta, blood-testis and blood-brain barriers facilitating excretion and limiting absorption of potentially toxic substrate molecules, including many cancer chemotherapeutic drugs. BCRP also plays a key role in heme and folate homeostasis, which may help normal cells survive under conditions of hypoxia. BCRP expression appears to be a characteristic of certain normal tissue stem cells termed “side population cells,” which are identified on flow cytometric analysis by their ability to exclude Hoechst 33342, a BCRP substrate fluorescent dye. Hence, BCRP expression may contribute to the natural resistance and longevity of these normal stem cells. Malignant tissues can exploit the properties of BCRP to survive hypoxia and to evade exposure to chemotherapeutic drugs. Evidence is mounting that many cancers display subpopulations of stem cells that are responsible for tumor self-renewal. Such stem cells frequently manifest the “side population” phenotype characterized by expression of BCRP and other ABC transporters. Along with other factors, these transporters may contribute to the inherent resistance of these neoplasms and their failure to be cured.
Keywords: ATP binding cassette transporters; Breast Cancer Resistance Protein; ABCG2; drug absorption, distribution, metabolism and excretion; cancer; drug resistance
Section 1. Introduction
The influence of ATP-binding cassette (ABC) transporters – and breast cancer resistance protein (BCRP, ABCG2) in particular – on resistance of human cancers to antineoplastic pharmaceuticals should be viewed as the combined effects of the role that the transporter plays in normal tissues to affect drug absorption, distribution, metabolism and excretion (ADME), and the effects of the expression of the transporter in neoplastic tissues to produce active efflux of chemotherapeutic molecules. A review of the literature on this subject through the year 2008 was published by our group in 2010 [1]; the present paper will update findings on this topic since 2008, but does not include an extensive listing of the many drugs that are substrates or inhibitors of BCRP/ABCG2; such listings can be found in other recent reviews of BCRP/ABCG2 [2–9].
Section 2. Background: BCRP in cancer drug resistance
History and nomenclature
In the 1970’s multidrug resistance emerged as a significant cellular mechanism to explain/account for the clinical resistance of cancer cells to standard cancer chemotherapeutic agents. Initially P-glycoprotein (Pgp/ABCB1) and later multidrug resistance protein (MRP1/ABCC1) were identified as drug efflux proteins contributing to the multidrug resistance phenotype. However, in a subset of leukemia cells from patients, the transport activity of these two proteins could not account entirely for the efflux of chemotherapeutic agents [10]. Subsequently, generation of a drug-resistant cell line devoid of either Pgp or MRP1 expression [11] prompted investigations in our laboratory that identified a novel ATP-dependent efflux protein, named the breast cancer resistance protein (BCRP/ABCG2) [12].
BCRP derived its name as a result of its isolation from the drug-resistant breast cancer cell line MCF-7/AdrVp [12]. In addition to the identification of BCRP in MCF-7/AdrVp cells, sequences corresponding to BCRP cDNA were also identified in the human placenta and in a mitoxantrone-resistant cell line by two different groups, leading to the terms ABCP and MXR, respectively [13, 14]. Since BCRP belongs to the ABC transporter superfamily and is the second member of the G subfamily of proteins, the gene symbol ABCG2 was assigned to BCRP, using HUGO nomenclature. Because orthologs of human BCRP are present in several other species, human BCRP has been designated BCRP/ABCG2 in upper case letters, while rodent (mouse/rat) BCRP has been designated Bcrp1/Abcg2 with lower case letters. Additionally, in CD (clusters of differentiation) nomenclature, BCRP was assigned the term CD338 by the Human Cell Differentiation Molecules organization.
Physical properties
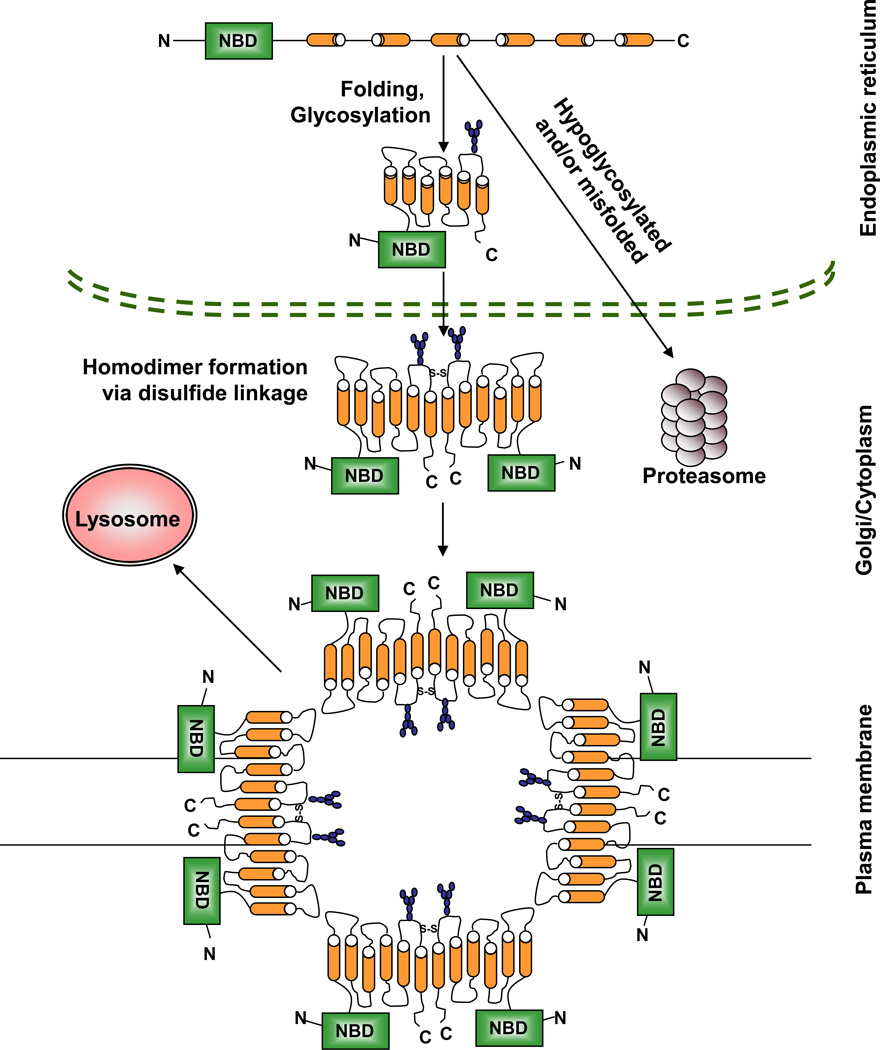
BCRP mRNA encodes a 655-amino acid, 72kDa protein with a single nucleotide binding domain (NBD) and six transmembrane domains (TMD). BCRP is a half-transporter, and thus requires at least two NBDs to function as a drug efflux pump. Hence, functional BCRP exists as either homodimers or homo-multimers [15–17]. Homodimer formation is believed to involve linkages by disulfide bridges at cysteine 603 [18, 19]. Additionally, recent crystallization studies suggest a functional tetrameric complex composed of four BCRP homodimers [20]. During its transit to the plasma membrane via the endoplasmic reticulum-Golgi pathway, BCRP undergoes N-linked glycosylation at asparagine 596 [21], and formation of an intramolecular disulfide bond between cysteines 592 and 608 which maintain its protein stability. Mature oligomeric fully glycosylated BCRP is degraded mainly via the lysosome, while underglycosylated, misfolded BCRP or BCRP without the intramolecular disulfide bond is targeted to the proteasome for degradation [19, 22, 23]. The physical properties and cellular trafficking of BCRP are diagrammed in Figure 1.
Figure 1.
Schematic of the physical properties of BCRP. BCRP is translated in the ER as a nonfunctional monomer consisting of one NBD and six TMDs. After proper protein folding and glycosylation of asparagine 596 in the ER, BCRP is transported through the Golgi to the plasma membrane where it localizes as a functional oligomer comprised of four homodimers joined by disulfide linkages involving cysteine 603. The exact site and sequence of homodimerization and oligomerization of BCRP is still unclear. Subsequent to its function, oligomeric BCRP is degraded in the lysosome. Any misfolded BCRP is recognized early on in the ER and is targeted to the proteasome for degradation.
BCRP Substrates / inhibitors
BCRP emerged as an important multidrug resistance protein because it confers cross-resistance to several structurally unrelated classes of cancer chemotherapeutic agents. Moreover, the known BCRP substrate spectrum has expanded to include physiological compounds, a growing list of cancer and noncancer chemotherapeutics, as well as common dietary xenobiotics. Additionally, the current BCRP substrate list also includes several novel molecularly-targeted therapeutics, such as the fatty acid amide hydrolase inhibitor URB937 [24] and the tyrosine kinase inhibitor danusertib [25]. Several identified BCRP substrates are substrates at lower concentrations and inhibitors at higher concentrations. In addition to the fumitremorgin C (FTC) analogues that were found to be specific inhibitors of BCRP, several novel classes of compounds such as poloxamines and acrylonitrile derivatives are emerging as potent BCRP inhibitors [26–29]. In addition, BCRP function is also inhibited by the tyrosine kinase inhibitor imatinib mesylate [30] but not by phosphodiesterase-5 inhibitors as is Pgp/ABCB1 [31]. Imatinib is also a substrate for BCRP [32]. Recently a Hedgehog (Hh) pathway inhibitor, HhAntag691, was identified as a modulator of BCRP[33]. BCRP modulatory activity of currently approved therapeutic agents opens up the possibility of overcoming BCRP-mediated drug resistance, but at the same time suggests possible drug-drug interactions.
Natural functions
BCRP is expressed in a subset of progenitor/stem cells in all of the major tissues of the body [34–37]. Efflux of Hoechst dye serves as a marker for the side population (SP) subset of cells that expresses BCRP as analyzed by flow cytometry [38]. SP cells generally possess stem cell properties such as resistance to chemotherapeutic drugs, self-renewal, enhanced engraftment capacity, quiescence, and activation of stem cell signaling pathways. A review of the role of BCRP in chemoresistance of stem cells is found in [39]. In one study, inhibition or silencing of BCRP suppressed cellular proliferation in cancer cell lines [40].
In addition to its expression in stem cells, BCRP is preferentially localized to the apical surface of enterocytes, the luminal surface of liver canaliculi, the luminal surface of the proximal convoluted tubule of the kidneys, and the blood-brain (BBB), blood-testis (BTB), blood-placental and blood-retinal barriers [41–45]. Because of its localization on the secretory surface of the major organs involved in drug transport, BCRP alters the ADME of substrate drugs [46]. BCRP exerts a protective function in human physiology by limiting the access of pharmaceutical agents to tissues. This protective function extends to endogenous substrates of BCRP as well.
Identification of the first endogenous BCRP substrate, porphyrin/heme, was based on studies of BCRP knockout mice [47]. BCRP regulation of heme homeostasis is exemplified under hypoxic conditions, wherein porphyrins accumulate within the cell and plasma membrane-localized BCRP effluxes them out of the cell [48]. Self-renewal of embryonic stem (ES) cells is dependent on BCRP-regulated heme homeostasis in SP cells. Increased porphyrin levels in ES cells are negatively correlated with expression of the pluripotent gene Nanog [49]. BCRP interacts with porphyrins via its large extracellular loop (ECL3) [50]. ABCB6, the only other major known porphyrin transporter, is localized on the outer mitochondrial cristae, thereby transporting porphyrins into them [51]. Recent localization of BCRP to inner mitochondrial cristae [52] suggests a hitherto unknown role for BCRP as the porphyrin transporter into the mitochondrial matrix for subsequent synthesis of heme.
In addition to heme/porphyrins, BCRP also transports endogenous folates, mainly the mono-, diand tri-glutamates of folic acid [53]. Decreased folic acid levels within the cell in turn upregulate BCRP expression and function, but restrict BCRP expression to the cytoplasm and endoplasmic reticulum [54, 55].
Most recently uric acid was identified as a BCRP substrate. The earliest BCRP localization and expression studies had revealed high levels of BCRP expression in the kidney [56]. Since BCRP plays a significant role in xenobiotic clearance, its high renal expression was considered to be related to the extensive number of its substrates cleared in the urine. Notably, in genome-wide screening of genetic determinants of gout, the Q141K BCRP single nucleotide polymorphism (SNP) was found to be associated with high uric acid levels, suggesting that uric acid is a substrate of BCRP [57, 58].
Regulation of BCRP expression and function
BCRP expression is regulated by alternative promoter usage in mice and in humans [59–62]. In humans these promoters are designated E1A and E1B/C; in mice they are termed E1a, E1b, and E1c. These alternative promoters regulate tissue-specific, disease stage-specific and tumor-specific expression of BCRP. A very good example is our laboratory’s finding that the E1b promoter controls Bcrp1 expression in the mouse gut, and that E1b, but no other mouse alternative promoter, is regulated by cAMP/CREB/CRE [61]. Use of a multitude of promoters which may be tissue-specific indicates very complex and intricate transcriptional machinery regulating BCRP expression.
The predominant BCRP promoter was characterized by Bailey-Dell et al in our laboratory [63]. Similar to other MDR protein gene promoters, this E1B/C BCRP promoter is TATA-less, contains several SP1 sites, and is downstream of a putative CpG island. To date, several transcription factors and their respective cis-regulatory elements in the BCRP promoter have been characterized.
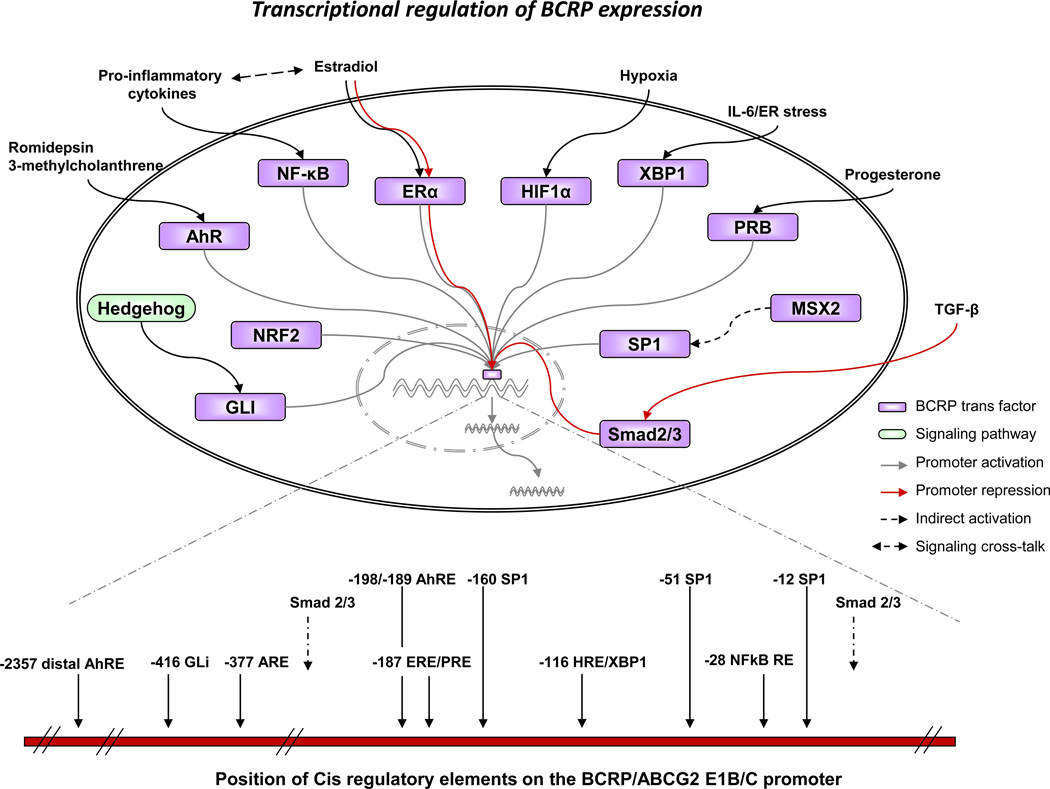
The cis and trans regulatory elements currently identified on the BCRP promoter are displayed in Figure 2. BCRP transcription is increased under hypoxic conditions by binding of hypoxia-inducible factor 1 (Hif-1α) to a hypoxia response element (HRE) in the BCRP promoter [64]. Estradiol (E2) activates or represses BCRP expression by binding of estrogen receptor α (ERα) to an estrogen response element (ERE) [65]. Interleukin-6 as well as endoplasmic reticulum stress stimulate BCRP expression in plasma cells by binding of XBP-1 and/or HIF-1α to the BCRP HRE [66]. Also, progesterone receptor isoform B (PRB) but not PRA binding to the progesterone response element (PRE), overlapping with the ERE site, enhances BCRP expression [67, 68]. In diffuse large B-cell lymphoma (DLBCL), as a response to activation of the Hh signaling pathway, Gli transcription factors bind to a Gli binding site on the BCRP promoter, enhancing BCRP expression [69]. Nrf2, an oxidative stress sensor, maintains the SP cell phenotype by upregulating BCRP expression through its direct interaction with an anti-oxidant response element (ARE) on the BCRP promoter [70]. In response to histone deacetylase inhibitor treatments, aryl hydrocarbon receptor (AhR) dissociates from its binding partner heat shock protein 90 (HSP90), binds to the aryl hydrocarbon response element (AhRE) and stimulates BCRP transcription [71]. However in the human colon adenocarcinoma cell line LS174T, AhR binds to a distal AhRE site, increasing BCRP transcription in response to 3-methylcholanthrene treatment [72]. TGF-β-induced interaction of smad2/3 with the BCRP promoter represses BCRP expression in SP cells in diffuse-type gastric carcinoma [73]. Besides binding of individual transcription factors, binding of ER facilitates co-operative binding and BCRP transcriptional regulation by p65 (NF-κB) [74]. Also, MSX2 by regulating binding of SP1 transcription factors to their cis elements on the BCRP promoter, regulates BCRP expression indirectly [75]. Upregulation of both ABCG2 and p50 (NF-κB) is observed when wild-type p53 is inhibited in MCF-7 cells, while mutant p53 does not alter the expression of BCRP [76]. ABCG2 is also upregulated during the development of gefitinib resistance and this is attributed to nuclear translocation of EGFR, which then interacts with the BCRP promoter, thereby increasing its expression [77]. Additionally, phospho-cAMP response element binding protein (p-CREB) and HIF2α binding sites have been identified in the mouse Bcrp1 promoter [61, 78]. Transcription factor overexpression or treatment with transcription factor-specific ligands further identified PPARγ, PXR, GR and c-Myc as trans-regulatory elements for BCRP expression [79–82]. Additionally, the stem cell gene SALL4 was recently shown to enhance BCRP expression indirectly [83].
Figure 2.
Schematic representation of transcriptional regulation of BCRP expression. Transcription factors that bind to cis elements upstream of the BCRP E1B/C promoter with subsequent activation or repression of the promoter are depicted diagrammatically. Signaling pathways and extracellular stimuli that stimulate binding of the transcription factors are also shown. The position of the various experimentally verified BCRP cis regulatory elements in relation to the transcription start site of the BCRP E1B/C first exon are also shown. The trans and cis regulatory elements were compiled from the literature cited in Section 2e, “Regulation of BCRP expression and function” of this review.
Binding of c-Myc to the Abcg2 promoter is restricted by the methylation status of the CpG islands [84]. The CpG islands in the BCRP promoter are hypomethylated or unmethylated in drug-resistant cell lines in comparison with drug-sensitive cell lines [85, 86]. In the quiescent state, the CpG islands associate with methylated lysine 9 on histone 3, the methyl CpG binding domain proteins MBD2 and MECP2, as well as histone deacetylase (HDAC) I and a co-repressor, mSin3A. In progression to a BCRP+ drug-resistant phenotype, histone hyperacetylation was frequently observed with CpG island demethylation [87]. Also, histone modifications in certain cell types subsequent to treatment with HDAC inhibitors were similar to the histone modifications observed in the development of a drug-resistant phenotype [88]. During the development of the drug-resistant phenotype after single-dose chemotherapy treatment, histone hyperacetylation of the BCRP promoter was observed [89]. Therefore the two epigenetic mechanisms appear to co-operatively regulate ABCG2 expression in the drug-resistant phenotype.
In addition to epigenetic and transcriptional regulation of BCRP in drug-resistant cells, a checkpoint in BCRP translation is also lost. An apparent loss of the BCRP 3’ UTR is observed in the drugresistant S1M1 colon carcinoma cell line [90]. The 3’ UTR of BCRP harbors numerous microRNA (miR) binding sites. miRs that bind to the BCRP 3’UTR and negatively regulate BCRP translation include miR-519c [91], miR-520h [92] and miR-328 [93]. The presence of a proximal response element (MRE) for sha-miR-519c in the truncated BCRP 3’UTR suggests that, even with 3’ UTR truncations, BCRP expression can still be regulated by miR binding to the shortened 3’ UTR [94].
Once translated, BCRP has to multimerize as well as translocate to the cell surface to function as a drug efflux pump. Pim-1 phosphorylation of BCRP at T362 promotes BCRP dimerization and plasma membrane trafficking [95]. PI3K/Akt pathway signaling is also essential for plasma membrane localization of BCRP [96, 97]. The PI3K/Akt pathway couples with the NF-κB pathway in the development of HER2-mediated drug resistance in MCF7/HER2 cells [98].
Section 3. Influence of BCRP/ABCG2 on cancer drug ADME and pharmacologic resistance
Summary of findings through 2008 [1]
BCRP expression in normal tissues can affect the ADME of antineoplastic drugs. For example BCRP expression in the intestine or liver (bile canaliculi) can affect absorption and elimination of BCRP substrate drugs; BCRP expression in the BBB or BTB can affect penetration of drugs transported by BCRP as well. The expression and function of endogenous BCRP can be affected by SNPs of the gene. The most frequently observed non-synonymous SNPs in the BCRP coding region occur in exon 2 (G34A, resulting in a V to M mutation in amino acid 12), and in exon 5 (C421A, resulting in a Q141K mutation). The altered BCRP primary structure resulting from these polymorphisms generally causes loss or diminution in transporter expression and/or function [1]. A review of the pharmacogenomics of ABC transporters was also published in 2008 [99].
Certain SNPs in the promoter region and in other non-coding regions (intron 1) were shown to alter transcription of BCRP, with both increases and decreases in mRNA observed [1]. Genetic polymorphisms of BCRP were found to alter the pharmacokinetics and bioavailability of some, but not all, BCRP substrates investigated. Increased toxicity of gefitinib (diarrhea) or of therapy for childhood acute lymphoblastic leukemia (ALL) (encephalopathy) were associated with presence of the C421A allele of BCRP; the same allele was associated with diarrhea in patients undergoing treatment for diffuse large B-cell lymphoma (DLBCL) with R-CHOP.
A decrease in BCRP function due to allelic variation may place an individual at greater risk of exposure to dietary/environmental carcinogens. Persons with the G34A or C421A alleles had an increased incidence of DLBCL; in contrast, the C421A allele was reported to decrease risk for developing renal cell carcinoma. No increased risk of developing prostate cancer was observed with carriers of the C421A allele; however, individuals with this allele were found to have higher intracellular concentrations of the dietary carcinogen PhIP, a known substrate for BCRP.
Disparate results concerning the influence of BCRP polymorphisms on treatment outcome were cited in the previous review [1]: one study reported better survival following docetaxel-based chemotherapy for men with hormone-resistant prostate cancer who carried the C512A allele; another showed worse overall survival for lung cancer patients with the C421A allele following treatment with platinum-based regimens.
Update of literature since 2008 [1]
BCRP Polymorphisms
Most of the observed BCRP SNPs are postulated to result in altered mRNA expression (usually diminished) or in a transporter protein with low or absent function. Recently, evidence was presented that polymorphisms resulting in the variants F208S and S441N cause diminished BCRP protein levels by virtue of ubiquitin-mediated proteasomal degradation [100].
The influence of BCRP polymorphisms on treatment outcome and toxicities has been investigated recently for acute myeloid leukemia (AML), chronic myelogenous leukemia (CML), DLBCL, and pancreatic cancer; these findings will be discussed subsequently in Sections 4 and 5, BCRP effects in hematological malignancies and solid tumors, respectively. A review of the importance of ABCG2 pharmacogenomics can be found in [101]. Another review of the pharmacogenomics of intestinal efflux transporters, including BCRP, is found in [102].
Effect of non-neoplastic expression of BCRP on cancer drug ADME
Considerable efforts have been made to determine which transporters affect ADME of antineoplastic drugs. Recent studies have shown that ABC transporters, including BCRP, profoundly affect the disposition of methotrexate (MTX). Using a knockout mouse model, Mrp2 (Abcc2), Mrp3 (Abcc3), and Bcrp1 (Abcg2) were found to be primarily responsible for the clearance of MTX and its primary toxic metabolite 7-hydroxyMTX [103, 104]. Loss of any one transporter is compensated for by increased activity of the others; however, loss of all 3 transporters results in a marked decrease in MTX/metabolite clearance. Caution was advised in patients with inactivating polymorphisms of one or more of these transporters if co-treatment with possible inhibitors of the remaining unaffected transporters is given [103, 104].
Work by Yang, et al. demonstrated that P-glycoprotein (Pgp, Abcb1a/1b) and Bcrp1 play an important role in oral absorption, systemic clearance and brain penetration of the investigational tyrosine kinase inhibitor (TKI) tandutinib in mice [105]. A study by Li, et al found that BCRP, Pgp and MRP2 expressed in gut are probably responsible for the low bioavailability of belotecan and topotecan, based on studies in Caco-2 and MDCKII cells [106]. Using mice bearing triple knockouts of Mdr1a, Mdr1b and Bcrp1, Pgp (Mdr1a/1b) and Bcrp1 were found to transport erlotinib (Tarceva®) efficiently and to affect its bioavailability in these animals [107]. Hence, erlotinib bioavailability may be influenced by drugs that inhibit Pgp and/or BCRP.
BCRP inhibitors as modulators of BCRP-mediated cancer drug ADME
In light of evidence that ABC transporters affect the clearance of MTX in animal models described above, it may be critical to consider limiting the concomitant use of drugs that inhibit BCRP in patients receiving MTX therapy. Benzimidazole proton pump inhibitors (PPIs) have been known for some time to be BCRP inhibitors [108]; however, studies of their effect on MTX clearance have shown mixed results [109, 110]. Investigators in Japan studied 74 patients receiving high dose MTX and found that, in addition to renal and hepatic dysfunction, co-administration of benzimidazole PPIs was a risk factor for delayed elimination of MTX [111]. Not every patient receiving PPIs displayed delayed elimination of MTX; BCRP polymorphisms were not investigated in this study [111]. In addition to PPIs, the BCR-ABL TKI nilotinib was found to be a potent inhibitor of both Pgp and BCRP. In membrane vesicles or cells overexpressing BCRP, nilotinib inhibited the transport of MTX and of mitoxantrone [112].
The list of drugs that inhibit BCRP is continually growing. Many common antipsychotic drugs inhibit BCRP [113]. Another commonly used drug, sildenafil, inhibits the transporter function of both Pgp and BCRP [114].
Many of the novel TKIs in use or under development are capable of inhibiting BCRP transport. Many of these are substrates of BCRP as well. For example, the TKI gefitinib (Iressa®) is a known inhibitor of BCRP and other ABC transporters [115, 116]. However, exposure of cells to gefitinib or another TKI, vandetanib, caused upregulation of BCRP and resistance to SN-38, suggesting that these TKIs may also be substrates of BCRP [117]; in contrast gefitinib or vandetanib were found only to be inhibitors of Pgp in this study [117]. In addition to being a BCRP substrate [107], erlotinib may also be an inhibitor of BCRP [118]. Apatinib, another small molecule TKI, was found to inhibit BCRP and Pgp transporter function [119]. Sunitinib was found to inhibit BCRP- and Pgp-mediated transport and may thus affect the bioavailability of other BCRP substrate drugs co-administered with sunitinib [120].
Inhibiting BCRP to allow anticancer drugs to penetrate the BBB
Drug penetration into the brain is limited by impermeable tight junctions between brain capillary endothelial cells, and by high expression of drug efflux ABC transporters in the brain capillary endothelial cells themselves. Although the ABC transporters classically associated with the BBB are Pgp and MRP (ABCC) family members, expression of BCRP in the BBB is now clearly and increasingly recognized. A recent review of the impact of BCRP expression on the BBB is available [121]. Similarly, Pgp, MRP1 and 2, and BCRP have been implicated in creating the BTB [7, 43]. The following discussion reports recent evidence that illuminates the extent to which BCRP and other barrier transporters limit anticancer drug penetration into the brain or testis. Such data may help to improve pharmacologic strategies to treat brain tumors.
ABC transporter knockout murine models have proven valuable for determining the contribution of individual ABC transporters to the BBB. A general theme emerging from these studies is that multiple transporters bestow protection from a given xenobiotic, providing redundancy to assure that loss of any one particular transporter (e.g., via inactivating SNPs or drug inhibition) would not result in the breakdown of the barrier. An early study with knockout mice found that Pgp and Bcrp1 cooperate to exclude topotecan from brain tissue [122]. Studies using polarized MDCKII cells transduced to express human Pgp and BCRP as a model of the BBB and also Mdr1a/Mdr1b/Bcrp1 triple knockout mice, found that both BCRP/Bcrp1 and Pgp/Mdr1a/1b are involved in the transport of topotecan, sorafenib, and sunitinib in both models [123]; the contribution of a given transporter in the barrier was impacted by drug concentration. Sorafenib brain accumulation in Mdr1a/1b or Bcrp1 knockout mice was studied at the Netherlands Cancer Institute. These investigators found that although Bcrp1 played a major role in restricting brain penetration, the highest levels of sorafenib achieved in the brain were in mice lacking both transporters or in mice treated with elacridar, a potent dual inhibitor of Pgp and BCRP [124]. Another study using in vitro and knockout models similarly found that Pgp and BCRP cooperate to restrict sorafenib brain penetration, with BCRP playing the dominant role [125].
Other work has generally but not always shown that Pgp and BCRP act cooperatively to exclude drugs from the brain in either an additive or a synergistic manner. For example, gefitinib brain penetration was found to be dependent on both Pgp and BCRP [77]. One study found BCRP/Abcg2 to be the primary transporter preventing erlotinib and its metabolite OSI-420 from penetrating mouse brain [126]; In contrast, another group, using knockout mouse models, found that although both Pgp and Bcrp1 appear to reduce brain penetration of erlotinib, Pgp is more effective than Bcrp1 in reducing the brain area under the curve (AUC) of erlotinib, compared to the AUC in mice that lack both transporters [127]. A study of the role of Pgp and Bcrp1 in the distribution of topotecan into the brain and CSF compartments of mice found that both Bcrp1 and Pgp transport topotecan into ventricular CSF and out of brain parenchyma via the BBB [128]. Bcrp1/Abcg2 and Pgp/Abcb1 were found to work in concert to exclude lapatinib from mouse brain [129]. For common substrates of Pgp and Bcrp such as flavopiridol, imatinib, and prazosin, these transporters were found to function synergistically to limit brain penetration [130]. Pgp/Abcb1 and Bcrp/Abcg2 were found to inhibit the penetration of the novel PI3 kinase inhibitor GDC-0941 into mouse brain [131], and Pgp and BCRP in the BBB were found to restrict entry of dasatinib into mouse brain [132]. In contrast to the combined role of transporters in limiting brain penetration, one study found that Pgp but not Bcrp1 is responsible for brain exclusion of the novel second-generation VEGF TKI axitinib [133]. Contrary to previous reports of synergism between Pgp and Bcrp1 in preventing drug penetration across the BBB or BTB, one group of investigators found a purely additive effect of the two transporters without any interaction between Pgp and Bcrp [134]. A 2009 study found a minimal role for Bcrp1 in BBB for a chemically diverse set of model compounds [135]. In view of the diverse findings presented, the factors determining the brain penetration of any given pharmaceutical clearly must be determined experimentally on a drug-by-drug basis.
The BBB is known to be reduced under inflammatory conditions. A group of investigators evaluated the effects of IL-1β, IL-6 and TNF-α on BCRP and Pgp expression in human hCMEC/D3 cells (a human adult brain endothelial cell line) in vitro. Profound suppression of BCRP mRNA expression by the pro-inflammatory cytokines was observed [136]. This finding is in contrast to findings in cultured human breast cancer cells where IL-1 and TNF-α increased BCRP expression [137], which will be discussed in Section 5. A recent review of redox regulation of MDR-associated proteins including BCRP is found in [138].
BCRP and Pgp expression is decreased in tumor capillary endothelial cells from primary CNS lymphoma, which may help to explain the chemosensitivity of CNS lymphomas [139].
Modulating the BBB by BCRP inhibition
In addition to the effects of elacridar in increasing brain penetration of sorafenib, elacridar was found to improve sunitinib brain penetration in mice [140]. Interestingly, E2 was found to induce downregulation of Bcrp1 protein and mRNA in rat brain capillaries [141]. We await future clinical trials to see if drug or hormonal modulation of BCRP and other transporters will improve treatment outcomes for patients with brain cancers.
Section 4. Recent findings in acute leukemia and other hematological malignancies
Hematologic and lymphoid malignancies
BCRP has the potential to play an important role in drug resistance in hematologic malignancies, as it is frequently expressed on malignant hematopoietic and lymphoid cells, and some of the drugs used to treat these cancers are BCRP substrates. Additionally, BCRP is expressed on stem cells in leukemias, potentially contributing to their resistance to eradication by chemotherapy or targeted therapies. Finally, an evolving literature associates BCRP SNPs not only with treatment response, but with leukemia incidence. Our group summarized the literature on the significance of BCRP in hematologic and lymphoid malignancies in 2010 [1]. Here we summarize the salient literature included in that review, and emphasize more recent work.
Acute myelogenous leukemia (AML)
Detection and measurement of BCRP in AML cells are associated with methodological issues. A study from the Baer and Ross laboratories found concordance of measurement of BCRP mRNA by realtime RT-PCR, BCRP protein by flow cytometry using three antibodies, including BXP21 and BXP34, which label intracellular BCRP epitopes and 5D3, which labels a cell surface BCRP epitope, and BCRP function by a flow cytometric functional assay in cell lines, but not in AML blasts, including a lack of expected correlation between BCRP surface expression and function [142]. Discordance of assays in AML blasts appears to reflect the complex biology of BCRP in AML. Of note, BCRP expression was found in small subpopulations of AML blasts [142].
BCRP is prognostic in AML, despite the fact that frontline therapy generally consists of non-BCRP substrate drugs. All AML patient samples studied to date have had the wild-type sequence, encoding arginine at codon 482 [142–144], and thus should be resistant to mitoxantrone, but not to anthracyclines [145]. Moreover, cytarabine, a highly effective drug for AML treatment, is not a substrate for BCRP [146]. Nevertheless, Steinbach et al. found a correlation between high BCRP levels and failure to achieve complete remission (CR) following induction therapy including cytosine arabinoside, an anthracycline and thioguanine or etoposide [147]. Benderra et al. [148, 149] also found a correlation between BCRP mRNA expression and lower CR rate and shorter survival, while Uggla et al. found a correlation with survival, but not CR rate [150]. Thus BCRP may be a marker for, rather than a mechanism of, resistance in AML.
Data from several groups support BCRP overexpression in subpopulations of AML cells likely to contain AML stem cells. In addition to Suvannasankha et al. [142], Abbott et al. [151] and van der Kolk et al. [152] also found BCRP to be restricted to small subpopulations of AML cells. van der Kolk et al. found a correlation between high BCRP expression and CD34 expression on AML blasts [152]. Likewise, Raaijmakers et al. [153] found highest BCRP expression and function in the CD34+/CD38− cells in AML marrows, and de Figueriedo-Pontes et al. [154] found that “leukemia stem cells,” defined as CD34+/CD38−/CD123+ cells, had higher expression of BCRP. Of note, SALL4, a zinc finger transcription factor constitutively expressed in AML cells [155] and important in maintaining the stem cell phenotype [156], was recently reported to be a marker for drug resistance in AML, to be expressed in the AML side population, and to upregulate expression of BCRP indirectly, possibly through its effects on Akt signaling [83].
BCRP is frequently co-expressed with Pgp, and their co-expression is associated with inferior outcomes [154, 157–160]. van den Heuvel-Eibrink et al. [160] found that BCRP and Pgp co-expression was the most significant poor prognostic indicator for attainment of CR, and Ho et al. [159] found that non-responders had significantly higher BCRP and/or MDR1 expression in CD34+/CD38− cells. Damiani et al. [157] found a high frequency of BCRP and Pgp co-expression (p=0.006) in cytogenetically normal de novo AML; Pgp expression was associated with lower CR rate (p=0.02), while BCRP was associated with shorter disease-free survival (DFS) (p=0.027).
Tiribelli et al. [161] found a significant positive correlation between BCRP overexpression and fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) (p=0.002), and while CR attainment correlated with neither, DFS was shorter in patients with blast BCRP expression (p=0.046), and shortest in those with both BCRP expression and FLT3-ITD (p=0.023). FLT3 signaling inhibitors are in clinical trials in AML for patients with a FLT3-ITD. Among these inhibitors, tandutinib has been found to be a substrate of BCRP as well as Pgp, affecting its absorption [105] and potentially its anti-tumor efficacy. Additionally, sorafenib is a Pgp and BCRP substrate [124, 125], while sunitinib is a Pgp and BCRP inhibitor [120].
Other chemotherapeutic agents have been incorporated into AML therapy to try to improve outcomes. Fludarabine is a BCRP substrate [162], but did not overcome the negative impact of BCRP overexpression in AML [163]. Flavopiridol is also a BCRP substrate [164]. In contrast, amonafide L-malate, a DNA intercalating agent and non-ATP-dependent topoisomerase 2 inhibitor, is not a Pgp or BCRP substrate [165], but unfortunately did not improve outcome in a randomized clinical trial in secondary AML [166].
The utility of BCRP modulation has been studied in vitro. Raaijmakers et al. [153] found that the BCRP inhibitor Ko143 increased mitoxantrone accumulation but not cytotoxicity in CD34+/CD38− leukemia cells, suggesting that selective modulation of BCRP is not sufficient to circumvent resistance of leukemic CD34+/CD38− cells, and that other factors contribute to resistance. Cyclosporine A, an inhibitor of BCRP as well as Pgp [167], has shown efficacy in some clinical trials [168], but not others, while the Pgp and BCRP inhibitors tariquidar [169, 170] and elacridar (GF120918, [171, 172]) have not been tested in AML.
Other agents being tested in AML may alter expression of BCRP and other resistance proteins. Notably, histone deacetylase inhibitors have been found to induce expression of multidrug resistanceassociated ATP-binding cassette proteins, including BCRP, in AML cells [173]. While the effects of hypomethylating agents have not been characterized, CpG islands in the BCRP promoter are hypomethylated or unmethylated in drug-resistant cell lines [85, 86], as discussed above.
Finally, BCRP SNPs have been studied. The 34GA/AA variant genotypes were associated with both improved survival and increased toxicity (OR=8.41, 95%CI= 1.10–64.28) in relation to the wild type (GG) genotype in one study [174]. Another study found BCRP mRNA expression to be significantly lower in liver tissue with the G34A variant genotype [175]. If, as in liver, individuals with the G34A allele have decreased BCRP expression in AML blast cells, the result may be better antitumor response along with increased toxicity because of lower BCRP expression in normal tissues. In contrast, Chinese AML patients with the 34GG genotype had longer DFS and overall survival than those with the 34GA/AA genotypes [176]. The reason for the discrepancy in results between the two studies is unclear.
Acute lymphoblastic leukemia (ALL)
BCRP is expressed in both B-lineage and T-lineage ALL in children and in adults [144, 177, 178]. As in AML, Suvannasankha et al. found poor correlation between BCRP mRNA expression, staining with three BCRP antibodies (BXP21, BXP34 and 5D3) and BCRP function in adult ALL [178]. Overall, expression was more common than in AML [178].
Chemotherapy drugs used in the treatment of ALL that are BCRP substrates include the mainstays of maintenance therapy, 6-mercaptopurine [162] and methotrexate [179, 180]. As with AML, all cases evaluated have had the wild-type sequence at codon 482 [144, 178], and thus should not efflux anthracyclines. Stam et al. found that infant ALL blasts with high BCRP mRNA expression had the highest in vitro resistance to cytarabine despite the fact that cytarabine is not a BCRP substrate; again, as in AML, this suggests that BCRP is a marker of, rather than a mechanism of, resistance [146].
Sauerbrey et al. [177] and Kourti et al. [181] found no correlation between BCRP mRNA expression and outcomes in children, while Suvannasankha et al. found a correlation between BXP21 antibody staining and short DFS in adults [178]. More recently, Cortez et al. [182] found that higher BCRP mRNA levels were associated with better, rather than worse, 5-year event-free survival in childhood ALL, with an association between lower BCRP mRNA levels and higher incidence of toxic deaths. Similarly, Fedasenka et al. [183] found a trend toward a correlation between lower BCRP expression and residual disease by flow cytometry. The discrepant findings between adult and childhood ALL likely reflect the markedly better clinical outcomes in childhood than in adult ALL, in turn likely reflecting greater chemosensitivity.
Considerations with regard to BCR-ABL inhibitors and BCRP are relevant to Philadelphia chromosome-positive (Ph+) ALL as well as chronic myelogenous leukemia (CML), and are discussed under CML below.
There is currently no evidence for a correlation between BCRP SNPs and the incidence of ALL [184].
Chronic myelogenous leukemia (CML)
BCR-ABL inhibitors have revolutionized treatment of CML and also significantly improved outcomes in Ph+ ALL, but CML stem cells in patient samples have been found to be insensitive to imatinib mesylate [185], which is likely the reason for persistent molecular disease in most patients treated with this drug. BCRP was found to be expressed on CD34+ CML cells [186], and in another study, lineage−/CD34+/CD38− cells from CML patients were found to express functional BCRP, as well as Pgp, and to also have low expression of human organic cation transporter 1 (OCT1), which transports imatinib into cells [187]. Of note, SALL4, discussed above, has also been implicated in CML cell proliferation and survival [188].
Imatinib and newer BCR-ABL inhibitors were found to be both substrates and inhibitors of both BCRP and Pgp. A number of studies demonstrated that imatinib is an inhibitor and/or a substrate of BCRP [30, 32, 189–192]. With regard to newer-generation BCR-ABL inhibitors, nilotinib was found to be both a substrate [192] and an inhibitor [112] of BCRP, and dasatinib was found to be a substrate of BCRP, as well as Pgp [193]. Dohse et al. [194] compared imatinib, nilotinib and dasatinib interactions with BCRP, and found that all three were both substrates and inhibitors, and that nilotinib was the most potent inhibitor, followed by imatinib, then dasatinib. All three BCR-ABL inhibitors were also shown to inhibit both BCRP and Pgp in murine and human hematopoietic stem cells, also with the potency order of nilotinib, then imatinib, then dasatinib. Thus BCR-ABL inhibitors may lessen the potential of BCRP and Pgp both to limit their oral absorption and confer resistance. The magnitude of these effects is difficult to quantify [46]. BCR-ABL inhibitors may have a more important effect in increasing absorption of co-administered oral medications, and possibly sensitivity of malignant cells to co-administered chemotherapeutic agents, whether parenteral or oral.
Two additional BCR-ABL inhibitors in current development were also studied. Bosutinib was found not to be a substrate of BCRP, nor of Pgp [195]. However, BCRP was reported to cause resistance to danusertib, which is a recently characterized inhibitor active against BCR-ABL with many active-site mutations including T315I, which confers resistance to imatinib, nilotinib, dasatinib and bosutinib. Danusertib is also a pan-aurora kinase inhibitor [25].
BCRP SNPs have also been studied in relation to imatinib dosing and response in CML. It was recently reported that to attain the plasma threshold of approximately 1,000 ng/ml, the daily dose for patients with the ABCG2 421C/C genotype should be 400 mg, and for patients with the 421C/A or 421A/A genotype, it should be 300 mg, an effect attributed to variability in BCRP excretion into bile [196]. In another study, the GG genotype of BCRP rs2231137, in relation to the heterozygous (AG) (G34A, encoding V12M) or homozygous (AA) variant genotypes, was significantly associated with a lower rate of complete cytogenetic response to imatinib [197]. As noted above, the GG genotype has been associated with higher BCRP levels in tissues [175].
Multiple myeloma
BCRP does not appear to play an important role in multiple myeloma drug resistance at presentation [198], but is regulated by promoter methylation and is up-regulated in response to chemotherapy [86]. Recently a myeloma side population with BCRP expression and functional activity was found to be a target of the immunomodulatory agents lenalidomide and thalidomide [199].
Lymphoma
The importance of BCRP in lymphoma subtypes is not well defined. In one recent study, BCRP was detected by immunohistochemistry in 78% of mature T/NK cell lymphoma cases [200]. BCRP has also recently been found to transport chemotherapy drugs used to treat lymphomas, including fludarabine and cladribine [162].
With regard to BCRP SNPs, among 145 Korean patients with DLBCL treated with the R-CHOP regimen, there was no influence of BCRP SNPs on clinical characteristics or treatment outcomes, but patients with the Q141K polymorphism (QK or KK), but not the V12M polymorphism discussed above for AML and CML, had more chemotherapy-related diarrhea [201].
Section 5. Recent findings in solid tumors
Summary of findings through 2008 [1]
As with the hematologic malignancies, our last review found data emerging in solid tumors associating BCRP expression with adverse outcomes. At that time, it was not known whether the adverse outcome caused by BCRP expression was directly related to resistance mediated by the efflux function of BCRP or whether BCRP expression served as a marker for the presence of other mediators of poor-risk cancers such as activity of signaling pathways controlling cellular proliferation, self-renewal, metastasis, genomic instability, and down-regulation of programmed cell death.
Update of literature since 2008
The past three years have seen a rise in the number of papers concerning BCRP expression in solid tumors in subpopulations of cells with “stem-like” properties: quiescence, drug resistance, enhanced self-renewal capacity and tumorigenicity, and expression of other markers characteristic of stem cells. The extent to which BCRP contributes to drug resistance in these subpopulations is currently under active investigation.
Cancer stem cells
Frequently, normal and/or cancer stem cells can be identified as side population (SP) cells based on their low accumulation of Hoechst 33342 dye. SP cells can be identified in many primary tumors obtained from patients, and in certain cancer cell lines grown in vitro. BCRP/ABCG2 is a major component of the ABC transporters responsible for the SP phenotype in a variety of cancers and cancer cell lines. Not all cancer stem cells manifest a SP, however [202]. Interestingly, human embryonic stem cells do not display a SP, nor do they exhibit BCRP/ABCG2 protein expression or function [203].
Dofequidar, an oral quinolone ABC-transporter inhibitor, inhibits ABCB1 (Pgp), ABCC1 (MRP1), and ABCG2 (BCRP). In a variety of cultured cancer cell lines, including cervical, breast, pancreatic, colon, and gastric carcinoma, dofequidar was observed to reduce the SP and to diminish in vivo SP-derived tumor growth following treatment with irinotecan [204]. These investigators found that amongst the three transporters ABCB1, ABCC1 and ABCG2, BCRP/ABCG2 had the highest expression in SP cells.
Many of the recent reports of BCRP expression in solid tumors found BCRP expression in SP cells and/or in cells with increased self-renewal capacity and tumorigenicity that also co-expressed other stem cell markers such as CD133, Nrf2, Notch1, and Oct-4. Subpopulations of stem-like cells expressing BCRP were found in cell lines or primary tumor samples from a wide assortment of solid tumors, including head and neck cancer [205–207], breast carcinoma [202], small cell and non-small cell lung cancer [208–212], gastrointestinal cancers including pancreatic [213, 214], colon [215, 216] and hepatocellular [217–219], ovarian cancer [220], gliomas [221, 222], malignant peripheral nerve sheath tumors [223], osteosarcoma [224, 225], prostate cancer [226], Ewing’s sarcoma [227], odontogenic tumors [228], transitional cell carcinoma of the bladder [229], and neuroblastoma [230]. Details of these findings will be discussed within individual tumor types in the ensuing paragraphs.
Head and neck carcinomas
The human oral squamous cell carcinoma cell line Ho-1-N-1 was found to have a SP that comprised approximately 3% of the total cells. The SP cells had self-renewal properties, displayed upregulation of BCRP as well as the anti-apoptotic proteins CFLAR, Bcl2, and Bcl2A1, and were resistant to chemotherapeutic agents such as 5-fluorouracil and carboplatin [206].
CD133 is a membrane-bound glycoprotein of unknown function that is expressed in a variety of stem cells and in tumors. There is some evidence that CD133 may be a marker for cancer stem cells in laryngeal carcinoma, based on its expression in a small subpopulation of cells with enhanced tumorigenicity in the human laryngeal epidermoid carcinoma cell line Hep-2 [231]. Analysis of the CD133+ subset of Hep-2 cells revealed that this subset has enhanced chemoresistance, tumorigenicity, invasiveness, and higher expression of BCRP compared to CD133-negative cells [205].
Akt kinase activity is often high in head and neck cancers [232]. Imatinib was found to diminish phospho-Akt levels and Akt kinase activity in the head and neck squamous cell carcinoma cell lines UMSCC10B and HN30 [207]. Imatinib treatment also diminished the SP in these cells, and reduced efflux and enhanced cytotoxicity of doxorubicin. This effect was attributed to reduction in BCRP function caused by loss of Akt pathway activity; however, a contributing factor to the effect on the SP and doxorubicin cytotoxicity could be that imatinib is also known to be potent inhibitor of BCRP-mediated transport [30]. BCRP protein levels were not affected by imatinib treatment; however, the treated cells showed localization of BCRP in an inactive (cytoplasmic) location [207]. Akt pathway involvement in the cellular localization of BCRP was first observed in Akt knockout mice, which despite having normal protein levels of BCRP, do not display a SP and express BCRP in a cytoplasmic distribution [233]. An exception to this phenomenon was seen in our own studies In K562 cells, where imatinib or LY294002 treatment diminished phospho-Akt, which in turn decreased BCRP protein expression by a post-transcriptional mechanism [190].
Breast cancer
Typically, BCRP must have plasma membrane residence to be an effective efflux pump; there is also evidence that its expression in cytoplasmic vesicles can result in drug sequestration and cancer drug resistance [234]. Recent work finds that multidrug resistant MCF-7 breast cancer cells generate extracellular vesicles containing ABCG2, ABCB1 and ABCC2 which sequester anticancer drugs, preventing them from reaching their intracellular targets. This represents a new modality of anticancer drug compartmentalization [235–237]. The extracellular vesicles were found mostly in cell-to-cell attachment zones between adjacent cells. These vesicles accumulate a green fluorescent substance [236] which was subsequently found to be riboflavin [237]. Whether this phenomenon occurs in primary breast cancer patient tumor samples awaits further study.
A number of studies examined BCRP effects on drugs used to treat breast cancer. The EGFR/HER2 blocker lapatinib (Tykerb®) used for treatment of advanced breast cancer was found to inhibit BCRP/ABCG2-mediated efflux of doxorubicin, another drug used for breast cancer chemotherapy [238]; lapatinib also inhibits Pgp and MRP7/ABCC10 [239, 240]. BCRP was implicated in resistance to 5-fluorouracil (5-FU) treatment in a Chinese breast cancer patient population (140 tissue specimens) [241]. Forty seven percent of the sampled breast cancers had detectable BCRP mRNA or protein expression as measured by RT-PCR or immunohistochemistry, respectively. Low intracellular retention of 5-FU measured by high-performance liquid chromatography (HPLC) correlated with expression of BCRP. Other investigators examined 5-FU intracellular uptake and retention by HPLC in BCRP-expressing cell lines [242] and found that 5-FU is indeed a substrate for BCRP. BCRP was also implicated in causing resistance to 5-FU in colon cancer cells, as will be discussed [215].
BCRP polymorphisms were not found to alter breast cancer treatment in recent literature; however, polymorphisms of other genes were found to affect BCRP expression. For example, polymorphism of codon 72 of the p53 gene affects breast cancer treatment outcomes. Patients with metastatic breast cancer homozygous for the arginine allele (72AA) had significantly lower progression-free survival and overall survival than did patients homozygous for the proline allele (72PP)[243]. In vitro transfection of p53-null breast cancer cells with the arginine allele resulted in better survival under hypoxic conditions than cells transfected with the proline allele. A factor contributing to enhanced survival of the 72AA-transfected cells under hypoxia was at least partially ascribed to upregulation of BCRP [243]. In other investigations, allelic polymorphisms of CYP2D6 and MRP2 (ABCC2), but not of MDR1 (ABCB1) or BCRP, were found to affect clinical outcomes of adjuvant tamoxifen treatment for breast cancer [244].
A variety of biologic factors have been found to alter BCRP function or expression in breast cancer. Mosaffa et al. studied the effects of pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 on BCRP expression in MCF-7 cells. IL-1β and TNF-α increased BCRP mRNA and protein expression and function. IL-6 increased BCRP protein but not mRNA expression in these cells. In mitoxantrone-selected MCF-7/MX cells which have overexpression of BCRP, IL-1β and TNF-α increased BCRP protein expression and activity without a further increase in mRNA expression [137]. These findings contrast with a study by Poller et al. cited earlier (Section 3) which presented evidence that inflammation in the brain decreases ABCG2 expression in the BBB [136]. Pradhan and coworkers in Chicago studied the ability of E2 to increase BCRP mRNA and protein levels in MCF-7 cells [74]. They found that proinflammatory cytokines (TNF-α, IL-1β) could markedly potentiate the E2-stimulated increase in BCRP expression, but did not affect BCRP expression in the absence of E2. This effect was found to be mediated by cytokine activation of NF-κB. The presence of ER at the ERE in the BCRP promoter allows recruitment of the NF-κB family member p65 to a latent NF-κB response element (NFκBRE) adjacent to and upstream of the ERE. The combined binding of ER and p65 to the ERE and NFκBRE respectively resulted in markedly enhanced transcription of BCRP [74]. Interestingly, at approximately the same time Wang and colleagues in China [76] identified an NFκBRE in the BCRP promoter at essentially the same location as was reported by the Chicago group. The Chinese investigators found that occupancy of this NFκBRE by NFκB-p50 (activated by transfection of wild-type p53) suppressed BCRP expression in MCF-7 cells [76]. E2 treatment does not always increase BCRP mRNA or protein levels, however. For example, recent work described in Section 3 by Mahringer et al [141], found that E2 decreased Bcrp1 expression and increased Bcrp1 protein degradation in rat BBB.
Liu and associates found that human MCF-7/MX cells (BCRP overexpressing cells selected with mitoxantrone) hyperexpress cytokeratin 8 (CK8). Enforced expression of CK8 or BCRP in NIH3T3 fibroblasts conferred resistance to mitoxantrone, but expression of both caused even greater resistance. Hence, CK8 may cooperate with BCRP in causing resistance [245].
Nakanishi and colleagues in our laboratories observed that HER2 signaling upregulates BCRP expression and expands the side population in luminal type breast cancer cells [202]. In contrast to luminal type cancers in which stem cells predominantly displayed the SP phenotype, basal type breast cancers had generally lower levels of SP cell subpopulations; stem cells in basal type cancers were more often SP-negative, CD44-, CD24-, and Aldefluor®-positive [202]. Murine breast tumors with a basal molecular signature can, however, upregulate Bcrp1/Abcg2 when challenged with topotecan, a BCRP substrate [246].
BCRP expression has been studied in mammary adenocarcinomas of dogs. The canine homologue of BCRP (cBCRP) was detected immunohistochemically in over 85% (36 cases) of canine mammary adenocarcinomas; a moderate correlation (r=0.35, p<0.05) was found between BCRP expression and grade of adenocarcinoma [247]. Another study of 103 canine mammary tumors using RT-PCR found expression of canine MRP1, MRP3, MRP6, MRP7 and cBCRP mRNA in most tumor samples; cBCRP function was demonstrated by resistance to doxorubicin in cells transfected to express cBCRP [248].
Lung Cancer
Small cell lung cancer - SCLC
In our previous review, there were reports cited that found an association of high BCRP expression prior to treatment with platinum-containing regimens with poor response to therapy and lower progression-free survival in 130 patients with SCLC [249], and with lower overall survival in 156 non-small cell lung cancer (NSCLC) patients with stage IV disease [250]. It is of interest that of the platinum agents currently approved for clinical use, only oxaliplatin may have an interaction with BCRP [242, 251].
Polymorphisms of the ABCG2 (G34A and C421A), ABCC3 and CNT1 genes were studied in 349 Caucasian patients with primary lung cancer (161 with SCLC, 187 with NSCLC, and 1 mixed) and were correlated with treatment outcome. Drug regimens varied, but primarily included platinum-based drugs (for both SCLC and NSCLC), gemcitabine (for NSCLC patients) and etoposide (for SCLC patients). None of the polymorphisms altered treatment response after 2 courses of treatment. However, worse overall survival was observed for patients with the BCRP C421A allele in a subset of 256 patients treated with platinum-containing regimens (hazard ratio: 1.6) [252].
In a variety of SCLC cell lines in vitro, SP stem cells were identified by Salcido and associates that have increased self-renewal capacity and overexpress genes typically found in primitive or stem cell populations, including BCRP [208]. Investigators in China found SP cells in H446 SCLC cells, which expressed CD133 and BCRP, showed enhanced tumorigenic and stem cell properties. It was suggested that CD133 and BCRP may serve as markers for stem cells in SCLC [209].
Non-small cell lung cancer - NSCLC
Mutations of the epithelial growth factor receptor (EGFR) in NSCLC have been associated with resistance to gefitinib. However, BCRP upregulation was reported to be associated with acquired resistance to gefitinib in a case of NSCLC in a non-smoking woman without evidence of a mutation in EGFR [253]. The mechanism of acquired resistance to gefitinib in cells with wild-type EGFR was examined recently in human epidermoid carcinoma A431 cells [254]. In these studies, Akt-mediated translocation of EGFR to the nucleus was observed in the resistant cells, resulting in enhanced transcription of BCRP [254]. In contrast to these studies, the EGFR tyrosine kinase inhibitor AG1478 caused downregulation of BCRP expression in AG1478-resistant cells, along with collateral sensitivity to a topoisomerase I inhibiting agent, Hoechst 33342 [255].
The abstracts of two papers from a group in China (we were unable to obtain the full articles at this writing) did not report an association of BCRP mRNA expression (using semiquantitative RT-PCR) with response to chemotherapy (platinum-based) or survival in patients with advanced or locally advanced NSCLC [256, 257]. These reports contrast with two earlier studies cited in our previous review [1]. The first, by Yoh et al. published in 2004, found that BCRP protein expression (measured by immunohistochemistry) correlated with lower response rate, shorter overall survival and progression-free survival following treatment with platinum-based regimens [258]. The second work, published in 2009 by Ota et al. and mentioned above in the section for SCLC, found that BCRP protein expression measured by immunohistochemistry had adverse impact on survival in 156 Japanese NSCLC patients treated with platinum-based therapy [250].
BCRP affected the outcome of photodynamic therapy with Photofrin-PDT but not the secondgeneration photosensitizer NPe6-PDT in tumor samples from 81 patients with early centrally located lung cancers [259].
In a study of xenografts from 26 well characterized patient-derived NSCLCs, the known chemosensitivity of these tumors to etoposide, carboplatin, gemcitabine, paclitaxel and erlotinib was compared with mRNA expression of BCRP, LRP, MDR1 and MRP1. Only a borderline correlation of chemosensitivity to etoposide with BCRP mRNA expression was found. The conclusion was that the “MDR proteins” do not play an important role in chemoresistance of NSCLC in vivo [260].
As found for specimens of SCLC, subpopulations of cells with a stem cell gene expression signature, including expression of BCRP, have been found in NCLC cell lines and patient samples. Primary NCLC cell lines treated in vitro with cisplatin showed enriched populations of highly tumorigenic CD133+/BCRP+/CXCR4+ cells [210]. A trend to lower progression-free survival was seen in patients with CD133+ tumors (N=32 CD133-, N=10 CD133+ patients) [210]. A study of CD133 expression in tumor samples from 10 NSCLC patients and in 5 NSCLC cell lines found high expression of Oct-4 and BCRP to be associated with chemotherapeutic drug resistance, and self-renewal capacity. Knockdown of Oct-4 caused the lung cancer cells to lose tumorigenicity and drug resistance [211]. SP cells with a stem cell-like phenotype including BCRP/ABCG2 expression were found in human NSCLC-derived A549 cells [212].
GI cancers
Pancreatic cancer
The association of SNPs in multidrug resistance transporter genes with clinical outcome in patients with potentially resectable pancreatic cancers was evaluated by a group at the MD Anderson Cancer Center. The study included SNPs of 7 ABC transporters: MDR1/ABCB1, MRP1–5, and the BCRP exon 5 (C421A/Q141K) SNP. Only the MRP5 A-2G AA genotype had a significant association with overall survival; the MRP2 G40A AA genotype was weakly associated with lower overall survival. The BCRP exon 5 SNP did not alter clinical outcomes in this study [261].
Many of the investigations probing the influence of BCRP expression on pancreatic cancer have centered on its role in cancer stem cells. The expression of the homeobox gene MSX2 is known to induce epithelial-mesenchymal transition in human pancreatic cells. MSX2 was found to correlate with resistance to chemotherapeutic agents and presence of a SP in a variety of human pancreatic cell lines. As mentioned in Section 2, MSX2 expression led to enhanced transcription of BCRP in these lines via recruitment of SP1 to its response element in the BCRP promoter [262]. Investigators in China found that nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and BCRP expression correlated with drug resistance in a number of pancreatic cancer cell lines [213]. Another study from China characterized the SP cells in the PANC-1 cell line. The SP cells displayed stem-like characteristics including upregulation of BCRP, CD133, Notch1, increased tumorigenicity, and drug resistance in vitro [214].
Colon carcinoma
As with pancreatic cancers, a number of studies investigated BCRP in terms of its role in the cancer stem cell phenotype. For example, Wnt signaling was found to expand the SP in SW480 colon cancer cells in vitro. These SP cells expressed Pgp and BCRP and displayed resistance to 5-FU and irinotecan, two BCRP substrates [215]. Inhibition of Wnt signaling diminished SP cells. The authors concluded that targeting the Wnt pathway may reduce chemotherapy resistance in colon cancer [215]. Colon cancer cells derived from 13 patient biopsy or resection samples were propagated as solid tumor spheroids in vitro. These spheroids display cancer stem cell properties, including self-renewal, resistance to irinotecan, and expression of BCRP/ABCG2, CD133 and CD44 [216].
A study of the effects of FOLFOX regimen components (folinic acid, 5-FU, and oxaliplatin) on expression of multidrug resistance transporters in LS180 colorectal cells grown in vitro revealed that 5-FU suppressed expression of ATP7B and human organic cation transporter 2, but increased expression of MRP2. This alteration sensitized the cells to oxaliplatin. Furthermore, upregulation of ABCG2 (and MRP2, MRP3) was found to enhance the cytotoxic efficacy of oxaliplatin [263]. In this study, an 8–10 fold increase in resistance to 5-FU was seen in MDCKII cells transfected to overexpress BCRP or MRP1 or 2. In contrast, the enforced expression of BCRP, MRP2 or MRP3 in MDCKII cells sensitized these cells to the cytotoxic effects of oxaliplatin [264]. These investigators did not do studies with the combination of folinic acid + 5-FU, which has previously been reported to be synergistic because folinic acid stabilizes the 5-FdUMP-thymidylate synthase enzyme-inhibitor complex [265].
Hepatocellular carcinoma (HCC)
Recent reports of BCRP expression in HCC have primarily concerned cancer stem cells. SP stem cells expressing BCRP were reported in a variety of HCC cell lines [219]. Oct-4, a transcription factor associated with stem cells and self-renewal, was found to be expressed in tumor samples from 60 HCC patients; Oct-4 expression correlated strongly with BCRP and phospho-Akt protein expression. It was proposed that Oct-4 increases BCRP expression by activation of the Akt pathway [217]. Other investigators found a SP in the HCC cell line MHCC-97L; this SP and ABCG2 expression were regulated by Akt signaling [218].
In our previous review [1], we cited a report that in hepatoblastoma, 7/7 biopsy specimens had increased BCRP expression following chemotherapy treatment [266].
Gastric cancer
In the gastric cancer cell line SGC7901, siRNAs were used to identify genes whose suppression led to resistance to epirubicin. This approach identified the GAS1 gene (growth arrest-specific 1) as playing a key role in epirubicin resistance. Enforced expression of GAS1 resulted in upregulation of Bcl-2, Pgp, and BCRP, but not MRP1 [267].
Esophageal cancer
Cancer stem-like cells were isolated and characterized in the esophageal carcinoma cell lines EC9706 and ED109. The stem-like cells displayed a SP phenotype, including expression of BCRP and ABCA5, expression of Oct-4, SOX2, BMI-1, ZFX, notch pathway genes, and increased tumorigenicity in NOD/SCID mice [268].
Brain Tumors
Much of the recent work in brain tumors concerns studies designed to understand how BCRP expressed in brain capillary endothelial cells plays a role in the BBB, preventing penetration of chemotherapeutic drugs into brain tumor tissue. The bulk of this work was discussed in Section 3. In addition to studies of the BBB, there is ongoing research focusing on how BCRP expression in brain tumors themselves, and particularly in cancer stem cell populations, results in drug resistance. The discussion here will center on this latter aspect.
Evidence is accumulating that murine and human gliomas are propagated by a stem cell population characterized as a highly tumorigenic, BCRP-expressing SP. Chua and colleagues identified SP cells in two glioma cell lines (U87MG AND T98G); the SP cells were tumorigenic and displayed stem cell markers such as nestin and BCRP, and were resistant to temozolomide. Furthermore, temozolomide treatment caused an increase in the SP. Knockdown of ABCG2 did not alter the SP response to temozolomide, suggesting coexistence of other forms of chemoresistance [221]. In human and mouse gliomas, Bleau et al. found that BCRP function was diminished in glioma capillary endothelial cells, consistent with disruption of the BBB within the tumors; however, SP cells isolated from the gliomas formed highly tumorigenic neurospheres, and expressed stem cell markers. BCRP activity in the neurospheres was driven by the PI3K/Akt pathway, independent of mTOR. Treatment with temozolomide and/or loss of the tumor suppressor gene PTEN resulted in augmentation of the SP phenotype [269]. Using immunostaining, Jin and coworkers found BCRP expression to increase with increasing grade of glioma (68 cases studied, 31 grade I-II, 37 high grade III-IV). ABCG2 expression also correlated with expression of the stem cell marker CD133, and with resistance to mitoxantrone [270]. The role of BCRP in resistance in glioblastoma is summarized in a review paper entitled “The ABCG2 resistance network of glioblastoma” [271].
Brain tumors with high expression of the angiopoietin receptor tyrosine kinase Tie2 display a high malignant potential [272]. Angiopoietin 1 (Ang1) is highly expressed in malignant gliomas. Glioma cell lines with high Tie2 expression become chemoresistant when treated with Ang 1, and upregulate ABCG2 and ABCC2 [273].
In diffuse pontine gliomas and spinal cord astrocytomas, hyaluronan, an extracellular glycosaminoglycan, was found to potentiate drug resistance and invasiveness. Hyaluronan interacts with BCRP, extracellular matrix metalloproteinase inducer, and CD44 (the hyaluronan receptor, a stem cell marker often associated with breast cancer). It was suggested that hyaluronan may be a useful target to attenuate brain tumor resistance/aggressiveness [222]. Similar findings were observed in a malignant peripheral nerve sheath tumor cell line, which is discussed below.
A study demonstrating that dexamethasone induces functional upregulation of Bcrp1 and Abcb1 in primary rat brain microvascular endothelial cells via pathways involving the glucocorticoid receptor and PXR raises concern that dexamethasone, commonly used to control cerebral edema in brain tumor patients, may increase drug resistance and prevent BBB penetration by chemotherapeutics in such patients [274]. In some brain tumors, BCRP expression appears confined to microvasculature. For example, in ependymoma, Pgp and BCRP appear to localize in microvessels [275].
Other cancers
Malignant peripheral nerve sheath tumors (MPNSTs) are a major cause of mortality and morbidity in patients with neurofibromatosis type-1 (von Recklinghausen’s disease). MPNSTs exhibit multidrug resistance, with a poor response to chemotherapy. In human MPNST cell lines, CD44 (the hyaluronan receptor) forms complexes with ABCG2 and ABCB1. Treatment with hyaluronan oligomers causes disruption of these complexes, with inactivation (internalization) of the ABC transporters, and sensitization to doxorubicin. In vivo, the hyaluronan oligomers inhibited growth of MPNST xenografts. Since CD44 is frequently expressed in cancer stem cells, hyaluronan oligomers may be useful in cancer therapy [223].
In osteosarcomas, tumor-initiating cells with high tumor forming and metastatic potential were identified in both human and murine osteosarcoma cells which express the mesenchymal stem cell markers CD117 and Stro-1; these tumor-initiating cells also upregulate ABCG2/Abcg2 and were doxorubicin-resistant [224]. A cancer stem-like cell line was produced by culturing human osteosarcoma MG-63 cells with 3-aminobenzamide. These cells showed high expression of stem cell markers (Oct-3/4, hTERT, Nanog), CD133, and ABCG2 [225].
Highly tumorigenic stem-like cells were identified in a subpopulation of the prostate cancer cell line 22RV1 that have high surface expression of CD117 and ABCG2. This subpopulation expresses other stem cell markers, and is resistant to taxol, platinum, doxorubicin, and methotrexate [226]. BCRP and Pgp can transport biclutamide and cause resistance to this drug in prostate cancer cell lines [276].
SP cells were identified in the Ewing’s sarcoma cell line SK-ES-1 [227]. In malignant amelioblastic (odontogenic) tumors, CD133, BMI-1 and ABCG2 were found to be expressed; BCRP expression was higher in the neoplastic tissue than in tooth germ tissues [228]. Evidence for SP cells that overexpress BCRP/ABCG2 was found in the bladder transitional cell carcinoma cell line T24 [229]. Evidence for a stem cell population was found in 4 of 8 neuroblastoma cell lines which expressed the stem cell markers CD133, ABCG2 and nestin [230]. Self-renewing, tumorigenic, drug-resistant SP cells with increased BCRP expression were observed in ascites from patients and in ovarian cancer cell lines [220].
Section 6. Final discussion
The past three years have seen significant strides in elucidating the role of BCRP in cancer drug resistance. It is clear that BCRP exerts a negative effect on cancer treatment outcomes both at the level of the neoplastic cell itself and by virtue of its effects on anticancer drug ADME in the host.
Data are starting to accumulate defining the role that common polymorphisms of BCRP play in the toxicity of antineoplastic drugs and treatment outcome; although some studies found enhanced drug toxicity or more favorable tumor response in patients with certain BCRP alleles [174, 175], other studies did not substantiate these findings [176]. Further work in this area is clearly needed. Given this, in the foreseeable future, drug treatment may be guided by individualized genotype databases – which include BCRP polymorphisms – that can enable customized drug dosing to minimize toxicity and to enhance therapeutic effect.
One area that has undergone intense scrutiny in the past three years is detailed evaluation of the role that BCRP and other ABC transporters play in maintenance of the BBB. Indeed, BCRP is evolving as a major contributor to this barrier. Hence, future use of BCRP as a therapeutic target may involve BCRP inhibitors to allow/enhance chemotherapeutic drug penetration into brain tumors.
With regard to the influence of BCRP expression within tumor cells themselves, the last three years have produced a marked increase in reports of the expression of BCRP as a manifestation of the presence of self-renewing, highly drug-resistant cell populations – putative cancer stem cells – amongst cells derived from a wide variety of malignancies. Targeted inhibition of BCRP as a means of sensitizing cancer stem cells to cancer therapeutics is currently an experimental approach under investigation; such studies must consider the effects of BCRP reversal on toxicity to normal tissue stem cells.
The past three years have provided mounting evidence for expression of BCRP in hematologic malignancies and a wide variety of solid tumors; in these studies, BCRP expression frequently correlates with chemotherapy-resistant disease, or poor outcomes in terms of shortened survival. In some cases, BCRP expression predicted poor outcome for chemotherapeutics that were not known substrates for the transporter, leading to the notion that BCRP expression may indicate the presence of a host of other cellular xenobiotic defenses that result in failure to cure. Such cells with redundant xenobiotic defense mechanisms could be cancer stem cells.
Acknowledgement
Dr. Ross supported in part and Dr. Xie is supported by a Merit Review grant from the Department of Veterans Affairs to Dr. Ross.
List of Abbreviations
- ABC
ATP binding cassette family of transporter proteins. This superfamily consists of seven subgroups, designated A–G
- ABCB1
The first member of the B subgroup of human ABC transporter proteins, also known as P-glycoprotein
- Abcb1a/1b
Murine equivalent of P glycoprotein, encoded by the Mdr1a and Mdr1b genes, also known as Abcb1/b2
- ABCB6
The 6th member of the C subgroup of human ABC transporter proteins
- ABCC1
The first member of the C subgroup of human ABC transport proteins, also known as MRP1
- ABCC10
The 10th member of the C subgroup of ABC transporters; also known as MRP7
- Abcc2
Murine Abcc2; also known as Mrp2
- Abcc3
Murine Abcc3; also known as Mrp3
- ABCC3
The 3rd member of the C subgroup of ABC transporters, also known as MRP 3
- Abcg2
The murine orthologue of ABCG2 (BCRP), also known as Bcrp1
- ABCG2
The second member of the G subgroup of human ABC transporter proteins, also known as BCRP
- ADME
Absorption, Distribution, Metabolism and Excretion
- AhR
Aryl hydrocarbon receptor
- AhRE
Aryl hydrocarbon response element
- Akt
A family of serine-threonine protein kinases that regulate downstream effectors controlling cell survival, protein and glucose metabolism. Akts can be activated by phosphorylation by phosphatidylinositol-3-kinases
- Aldefluor®
A fluorometric method for assessing for stem cells based on their content of aldehyde dehydrogenase
- ALL
Acute lymphoblastic leukemia
- AML
Acute myelogenous leukemia
- Ang1
Angiopoietin 1
- ARE
Anti-oxidant response element
- AUC
Area under the concentration-time curve
- BBB
Blood brain barrier
- Bcl-2
B-cell lymphoma 2 protein. An apoptosis regulating protein that opposes caspase activation
- Bcl-2A1
Bcl-2-related protein A1: A Bcl-2 family member that can block caspase activation
- BCR-ABL
A chimeric tyrosine kinase oncogene commonly found in chronic myelogenous leukemias and sometimes in acute lymphocytic and acute myelogenous leukemias
- BCRP
Breast Cancer Resistance Protein, ABCG2
- Bcrp1
The murine orthologue of BCRP
- BMI-1 gene
B lymphoma Mo-MLV insertion region 1 homolog gene. The gene product is an oncogene that plays a crucial role in stem cell self-renewal
- BTB
Blood testis barrier
- cAMP
Cyclic AMP
- CD
Clusters of differentiation
- CFLAR
CASP8 and FADD-like apoptosis regulator. It is also known as FLICE-like inhibitory protein (FLIP). A gene product involved in the regulation of apoptosis
- CK8
Cytokeratin 8
- CML
Chronic myelogenous leukemia
- c-Myc
A transcription factor that regulates many genes by altering chromatin structure by recruiting histone acetyltransferases to DNA
- CNS
Central nervous system
- CNT1 gene
Concentrative nucleoside transporter 1
- CpG islands
Regions of DNA rich in cytosine and guanine often located in the promoter of a gene; Methylation of the cytosine residues in the CpG island results in gene repression
- CR
Complete remission
- CRE
Cyclic-AMP response element
- CREB
Cyclic-AMP response element binding (protein)
- CSF
Cerebrospinal fluid
- DFS
Disease-free survival
- DLBCL
Diffuse large B cell lymphoma
- E2
Estradiol
- EGFR
Epithelial growth factor receptor
- ERE
Estrogen response element
- ERα
Estrogen receptor α, a transcription factor
- ES cells
Embryonic stem cells
- 5-FU
5-fluorouracil, a chemotherapeutic drug
- FLT3-ITD
Fms-like tyrosine kinase 3 internal tandem duplication
- FTC
Fumitremorgin C
- GAS1 gene
A gene that encodes the growth arrest-specific protein 1
- Gli
Zinc finger transcription factors that are effectors of Hedgehog signaling
- GR
Glucocorticoid receptor, a transcription factor activated by cortisol
- HDAC
Histone deacetylase
- HER2
Human epidermal growth factor receptor 2; its expression is associated with aggressiveness in breast cancers
- Hh
Hedgehog
- Hif-1α
Hypoxia-inducible factor 1 transcription factor
- HIF2α
Hypoxia inducible factor 2 alpha, a transcription factor
- HPLC
High-performance liquid chromatography
- HRE
Hypoxia response element
- HSP90
Heat shock protein 90
- hTERT
Human telomerase reverse transcriptase
- HUGO
Human Genome Organization
- IL-1, IL-1β, IL-6
Interleukins 1, 1β and 6, respectively
- LRP
Lung resistance protein, also known as the major vault protein. It is not an ABC transporter
- MBD2, MECP2
Methyl [CpG] binding domain protein 2: Members of a family of proteins that bind specifically to methylated DNA; can repress transcription in methylated gene promoters
- MDR1
The gene which encodes human P-glycoprotein (ABCB1)
- Mdr1a/1b
Murine equivalent of P glycoprotein, encoded by the Mdr1a and Mdr1b genes, also known as Abcb1/b2
- miR
Micro RNA: RNAs that bind to sequences in the 3' untranslated region of mRNA and affect mRNA stability or interfere with RNA translation
- MPNST
Malignant peripheral nerve sheath tumor
- MRE
Micro RNA response element
- MRP1
Human Multidrug Resistance-associated Protein 1, also known as ABCC1
- Mrp2
Murine multidrug resistance protein 2, also known as Abcc2
- Mrp3
Murine multidrug resistance protein 3, also known as Abcc3
- MRP7
Multidrug resistance-associated protein 7; also known as ABCC10
- mSin3A
A co-repressor protein that interacts with methyl binding domain proteins, histone deacetylase I and other proteins to repress transcription of genes with methylated promoters
- MSX2
A homeobox protein that serves as a transcriptional repressor by regulating the binding of other transcription factors
- mTOR
Mammalian target of rapamycin, a serine-threonine kinase that is involved with regulation of cell nutrition, proliferation and survival
- MTX
Methotrexate
- Nanog
A transcription factor that is involved with self-renewal of stem cells
- NBD
Nucleotide binding domain
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells: a heterodimers transcription factor that often is involved in the cellular response to stress
- NF-κBRE
NF-κB response element
- NOD/SCID mice
Non-obese diabetic severe combined immunodeficient mice
- Notch1
A cell membrane localized receptor involved with developmental processes; may be expressed in stem cells
- Nrf2
Also known as nuclear factor (erythroid-derived 2)-like 2: a transcription factor that may play a role in the regulation of oxidative stress
- NSCLC
Non-small cell lung cancer
- OCT1
Human organic cation transporter 1
- Oct-4
Octamer-binding transcription factor 4. This protein is involved with control of self-renewal; often expressed in embryonic stem cells; can be a marker for primitive undifferentiated cells
- OS
Overall survival
- p50
A 50 kilodalton protein that sometimes is a component of the NF-κB heterodimer
- p53
A53 kilodalton tumor suppressor protein that plays a central role in regulation of the cell cycle
- p65
A 65 kilodalton protein that sometimes is a component of the NF-κB heterodimer
- p-CREB
Phospho-cAMP response element binding protein
- PFS
Progression-free survival
- Pgp
P-glycoprotein
- Ph
Philadelphia chromosome
- PhIP
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen commonly found in cooked meat
- PI3K
Phosphatidyl inositol-3-kinase. A family of proteins involved in regulation of cell growth, survival, differentiation and motility
- Pim-1
A serine-threonine protein kinase protooncogene encoded by the PIM1 gene
- PPARγ
Peroxisome proliferator-activated receptor gamma, a transcription factor that forms a heterodimer with the retinoid X receptor (RXR)
- PPIs
Proton pump inhibitors
- PRA
Progesterone receptor isoform A
- PRB
Progesterone receptor isoform B
- PRE
Progesterone response element
- PTEN
Phosphatase and tensin homolog protein. A phosphatase that acts as a tumor suppressor by inactivating pro-proliferative signaling effectors by dephosphorylation, such as dephosphorylating phosphatidylinositol (3,4,5)-trisphosphate
- PXR
Pregnane X receptor, a nuclear transcription factor activated by steroids or xenobiotics; Forms a heterodimer with the retinoid X receptor
- R-CHOP
A chemotherapy regimen commonly used for B-cell lymphomas comprised of rituxan, cyclophosphamide, doxorubicin, vincristine, and prednisone
- SALL4
Sal-like protein 4, a putative zinc-finger transcription factor
- SCLC
Small cell lung cancer
- smad2/3
Intracellular proteins involved in transduction of signals from TGF-β ligands to the nucleus
- SNP
Single nucleotide polymorphism
- SOX2 gene
Also known as the SRY (sex determining region Y)-box 2) gene, SOX2 is a transcription factor essential for maintaining self-renewal in stem cells
- SP
Side population
- SP1
Specificity protein 1, a transcription factor; it interacts with MSX2
- TGF-β
Transforming growth factor beta
- Tie2
Angiopoietin receptor tyrosine kinase 2
- TKI
Tyrosine kinase inhibitor
- TMD
Transmembrane binding domain
- TNF-α
Tumor necrosis factor alpha
- UTR
Untranslated region
- VEGF
Vascular endothelial growth factor
- ZFX gene
Encodes the zinc finger X-chromosomal protein, which regulates transcription in embryonic and hematopoietic stem cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ross DD, Nakanishi T. Impact of breast cancer resistance protein on cancer treatment outcomes. In: Zhou J, editor. Methods in Molecular Biology. New York: Humana Press, Springer; 2010. pp. 251–290. [DOI] [PubMed] [Google Scholar]
- 2.Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44:152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi YA, Morris ME. Structure-activity relationships and quantitative structure-activity relationships for breast cancer resistance protein (ABCG2) AAPS J. 2009;11:541–552. doi: 10.1208/s12248-009-9132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99:598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 5.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, et al. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robey RW, Ierano C, Zhan Z, Bates SE. The Challenge of Exploiting ABCG2 in the Clinic. Curr Pharm Biotechnol. 2011;12:595–608. doi: 10.2174/138920111795163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwabedissen HE, Kroemer HK. In vitro and in vivo evidence for the importance of breast cancer resistance protein transporters (BCRP/MXR/ABCP/ABCG2) Handb Exp Pharmacol. 2011;201:325–371. doi: 10.1007/978-3-642-14541-4_9. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer. 2011 doi: 10.5732/cjc.011.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross DD, Doyle LA, Schiffer CA, Lee EJ, Grant CE, Cole SP, et al. Expression of multidrug resistance-associated protein (MRP) mRNA in blast cells from acute myeloid leukemia (AML) patients. Leukemia. 1996;10:48–55. [PubMed] [Google Scholar]
- 11.Chen YN, Mickley LA, Schwartz AM, Acton EM, Hwang JL, Fojo AT. Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J Biol Chem. 1990;265:10073–10080. [PubMed] [Google Scholar]
- 12.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 14.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 15.Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97:626–630. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi T, Doyle LA, Hassel B, Wei Y, Bauer KS, Wu S, et al. Functional characterization of human breast cancer resistance protein (BCRP, ABCG2) expressed in the oocytes of Xenopus laevis. Mol Pharmacol. 2003;64:1452–1462. doi: 10.1124/mol.64.6.1452. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Liu Y, Yang Y, Bates S, Zhang J. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem. 2005;280:36926–36934. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi K, Nakagawa H, Tamura A, Koshiba S, Hoshijima K, Komada M, et al. Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein. J Biol Chem. 2007;282:27841–27846. doi: 10.1074/jbc.C700133200. [DOI] [PubMed] [Google Scholar]
- 20.McDevitt C, Collins R, Conway M, Modok S, Storm J, Kerr I, et al. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Diop N, Hrycyna C. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry. 2005;44:5420–5429. doi: 10.1021/bi0479858. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa H, Wakabayashi-Nakao K, Tamura A, Toyoda Y, Koshiba S, Ishikawa T. Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J. 2009;276:7237–7252. doi: 10.1111/j.1742-4658.2009.07423.x. [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi-Nakao K, Tamura A, Furukawa T, Nakagawa H, Ishikawa T. Quality control of human ABCG2 protein in the endoplasmic reticulum: ubiquitination and proteasomal degradation. Adv Drug Deliv Rev. 2009;61:66–72. doi: 10.1016/j.addr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Sanz G, Barrera B, Guijarro A, d'Elia I, Otero JA, Alvarez AI, et al. The ABC membrane transporter ABCG2 prevents access of FAAH inhibitor URB937 to the central nervous system. Pharmacol Res. 2011;64:359–363. doi: 10.1016/j.phrs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balabanov S, Gontarewicz A, Keller G, Raddrizzani L, Braig M, Bosotti R, et al. Abcg2 Overexpression Represents a Novel Mechanism for Acquired Resistance to the Multi-Kinase Inhibitor Danusertib in BCR-ABL-Positive Cells In Vitro. PLoS One. 2011;6:e19164. doi: 10.1371/journal.pone.0019164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 27.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 28.Cuestas ML, Sosnik A, Mathet V. Poloxamines display a multiple inhibitory activity of ATP-Binding Cassette (ABC) transporters in cancer cell lines. Mol Pharm. 2011;8:1152–1164. doi: 10.1021/mp2000132. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki R, Nishiyama Y, Furuta T, Hatano H, Igarashi Y, Asakawa N, et al. Novel acrylonitrile derivatives, YHO-13177 and YHO-13351, reverse BCRP/ABCG2-mediated drug resistance in vitro and in vivo. Mol Cancer Ther. 2011;10:1252–1263. doi: 10.1158/1535-7163.MCT-10-0874. [DOI] [PubMed] [Google Scholar]
- 30.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, et al. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64:2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 31.Ding PR, Tiwari AK, Ohnuma S, Lee JW, An X, Dai CL, et al. The Phosphodiesterase-5 Inhibitor Vardenafil Is a Potent Inhibitor of ABCB1/P-Glycoprotein Transporter. PLoS One. 2011;6:e19329. doi: 10.1371/journal.pone.0019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Laterra J, Pomper M. Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp. Neoplasia. 2009;11:96–101. doi: 10.1593/neo.81264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 35.Wulf GG, Luo KL, Jackson KA, Brenner MK, Goodell MA. Cells of the hepatic side population contribute to liver regeneration and can be replenished with bone marrow stem cells. Haematologica. 2003;88:368–378. [PubMed] [Google Scholar]
- 36.Lin KK, Goodell MA. Detection of hematopoietic stem cells by flow cytometry. Methods Cell Biol. 2011;103:21–30. doi: 10.1016/B978-0-12-385493-3.00002-4. [DOI] [PubMed] [Google Scholar]
- 37.Fatima S, Zhou S, Sorrentino BP. Abcg2 Expression Marks Tissue-Specific Stem Cells in Multiple Organs in a Mouse Progeny Tracking Model. Stem Cells. 2011 Dec 1; doi: 10.1002/stem.1002. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 39.An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert Opin Drug Metab Toxicol. 2009;5:1529–1542. doi: 10.1517/17425250903228834. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S, et al. Suppression of ABCG2 inhibits cancer cell proliferation. Int J Cancer. 2010;126:841–851. doi: 10.1002/ijc.24796. [DOI] [PubMed] [Google Scholar]
- 41.Maliepaard M, Scheffer G, Faneyte I, van Gastelen M, Pijnenborg A, Schinkel A, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 42.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 43.Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, et al. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Kitano T, Iizasa H, Hwang IW, Hirose Y, Morita T, Maeda T, et al. Conditionally immortalized syncytiotrophoblast cell lines as new tools for study of the blood-placenta barrier. Biol Pharm Bull. 2004;27:753–759. doi: 10.1248/bpb.27.753. [DOI] [PubMed] [Google Scholar]
- 45.Asashima T, Hori S, Ohtsuki S, Tachikawa M, Watanabe M, Mukai C, et al. ATP-binding cassette transporter G2 mediates the efflux of phototoxins on the luminal membrane of retinal capillary endothelial cells. Pharm Res. 2006;23:1235–1242. doi: 10.1007/s11095-006-0067-2. [DOI] [PubMed] [Google Scholar]
- 46.Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs. 2009;27:31–40. doi: 10.1007/s10637-008-9138-z. [DOI] [PubMed] [Google Scholar]
- 47.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnamurthy P, Ross D, Nakanishi T, Bailey-Dell K, Zhou S, Mercer K, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 49.Susanto J, Lin Y, Chen Y, Shen C, Yan Y, Tsai S, et al. Porphyrin homeostasis maintained by ABCG2 regulates self-renewal of embryonic stem cells. PLoS One. 2008;3:e4023. doi: 10.1371/journal.pone.0004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desuzinges-Mandon E, Arnaud O, Martinez L, Huche F, Di Pietro A, Falson P. ABCG2 transports and transfers heme to albumin through its large extracellular loop. J Biol Chem. 285:33123–33133. doi: 10.1074/jbc.M110.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnamurthy P, Xie T, Schuetz J. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol Ther. 2007;114:345–358. doi: 10.1016/j.pharmthera.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Solazzo M, Fantappie O, D'Amico M, Sassoli C, Tani A, Cipriani G, et al. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009;69:7235–7242. doi: 10.1158/0008-5472.CAN-08-4315. [DOI] [PubMed] [Google Scholar]
- 53.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–4054. [PubMed] [Google Scholar]
- 54.Lemos C, Kathmann I, Giovannetti E, Beliën J, Scheffer G, Calhau C, et al. Cellular folate status modulates the expression of BCRP and MRP multidrug transporters in cancer cell lines from different origins. Mol Cancer Ther. 2009;8:655–664. doi: 10.1158/1535-7163.MCT-08-0768. [DOI] [PubMed] [Google Scholar]
- 55.Lemos C, Kathmann I, Giovannetti E, Dekker H, Scheffer GL, Calhau C, et al. Folate deprivation induces BCRP (ABCG2) expression and mitoxantrone resistance in Caco-2 cells. Int J Cancer. 2008;123:1712–1720. doi: 10.1002/ijc.23677. [DOI] [PubMed] [Google Scholar]
- 56.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 57.Woodward O, Köttgen A, Coresh J, Boerwinkle E, Guggino W, Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dehghan A, Köttgen A, Yang Q, Hwang S, Kao W, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakanishi T, Bailey-Dell KJ, Hassel BA, Shiozawa K, Sullivan DM, Turner J, et al. Novel 5' untranslated region variants of BCRP mRNA are differentially expressed in drug-selected cancer cells and in normal human tissues: implications for drug resistance, tissue-specific expression, and alternative promoter usage. Cancer Res. 2006;66:5007–5011. doi: 10.1158/0008-5472.CAN-05-4572. [DOI] [PubMed] [Google Scholar]
- 60.Zong Y, Zhou S, Fatima S, Sorrentino BP. Expression of mouse Abcg2 mRNA during hematopoiesis is regulated by alternative use of multiple leader exons and promoters. J Biol Chem. 2006;281:29625–29632. doi: 10.1074/jbc.M606314200. [DOI] [PubMed] [Google Scholar]
- 61.Natarajan K, Xie Y, Nakanishi T, Beck WT, Bauer KS, Ross DD. Identification and characterization of the major alternative promoter regulating Bcrp1/Abcg2 expression in the mouse intestine. Biochim Biophys Acta. 2011;1809:295–305. doi: 10.1016/j.bbagrm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell PK, Zong Y, Yang S, Zhou S, Rubnitz JE, Sorrentino BP. Identification of a novel, tissuespecific ABCG2 promoter expressed in pediatric acute megakaryoblastic leukemia. Leuk Res. 2011;35:1321–1329. doi: 10.1016/j.leukres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta. 2001;1520:234–241. doi: 10.1016/s0167-4781(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 64.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 65.Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64:1247–1251. doi: 10.1158/0008-5472.can-03-3583. [DOI] [PubMed] [Google Scholar]
- 66.Nakamichi N, Morii E, Ikeda J, Qiu Y, Mamato S, Tian T, et al. Synergistic effect of interleukin-6 and endoplasmic reticulum stress inducers on the high level of ABCG2 expression in plasma cells. Lab Invest. 2009;89:327–336. doi: 10.1038/labinvest.2008.157. [DOI] [PubMed] [Google Scholar]
- 67.Vore M, Leggas M. Progesterone acts via progesterone receptors A and B to regulate breast cancer resistance protein expression. Mol Pharmacol. 2008;73:613–615. doi: 10.1124/mol.107.044289. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Lee EW, Zhou L, Leung PC, Ross DD, Unadkat JD, et al. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol Pharmacol. 2008;73:845–854. doi: 10.1124/mol.107.041087. [DOI] [PubMed] [Google Scholar]
- 69.Singh RR, Kunkalla K, Qu C, Schlette E, Neelapu SS, Samaniego F, et al. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene. 2011 May 30; doi: 10.1038/onc.2011.195. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh A, Wu H, Zhang P, Happel C, Ma J, Biswal S. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol Cancer Ther. 2010;9:2365–2376. doi: 10.1158/1535-7163.MCT-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.To KK, Robey R, Zhan Z, Bangiolo L, Bates SE. Upregulation of ABCG2 by romidepsin via the aryl hydrocarbon receptor pathway. Mol Cancer Res. 2011;9:516–527. doi: 10.1158/1541-7786.MCR-10-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tompkins LM, Li H, Li L, Lynch C, Xie Y, Nakanishi T, et al. A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem Pharmacol. 2010;80:1754–1761. doi: 10.1016/j.bcp.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, Hoshino Y, et al. Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells. Oncogene. 2011;30:1693–1705. doi: 10.1038/onc.2010.546. [DOI] [PubMed] [Google Scholar]
- 74.Pradhan M, Bembinster LA, Baumgarten SC, Frasor J. Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NF{kappa}B cooperativity at adjacent response elements. J Biol Chem. 2010;285:31100–31106. doi: 10.1074/jbc.M110.155309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamada S, Satoh K, Hirota M, Kanno A, Umino J, Ito H, et al. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2012;227:729–738. doi: 10.1002/jcp.22781. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Wu X, Wang C, Zhang W, Ouyang Y, Yu Y, et al. Transcriptional suppression of breast cancer resistance protein (BCRP) by wild-type p53 through the NF-kappaB pathway in MCF-7 cells. FEBS Lett. 2010;584:3392–3397. doi: 10.1016/j.febslet.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2011;334:147–155. doi: 10.1124/jpet.110.167601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, et al. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 79.Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, et al. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281:23812–23823. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- 80.Honorat M, Mesnier A, Di Pietro A, Lin V, Cohen P, Dumontet C, et al. Dexamethasone down-regulates ABCG2 expression levels in breast cancer cells. Biochem Biophys Res Commun. 2008;375:308–314. doi: 10.1016/j.bbrc.2008.07.149. [DOI] [PubMed] [Google Scholar]
- 81.Kang KW, Im YB, Go WJ, Han HK. C-myc amplification altered the gene expression of ABC- and SLC-transporters in human breast epithelial cells. Mol Pharm. 2009;6:627–633. doi: 10.1021/mp800116f. [DOI] [PubMed] [Google Scholar]
- 82.Naspinski C, Gu X, Zhou GD, Mertens-Talcott SU, Donnelly KC, Tian Y. Pregnane X receptor protects HepG2 cells from BaP-induced DNA damage. Toxicol Sci. 2008;104:67–73. doi: 10.1093/toxsci/kfn058. [DOI] [PubMed] [Google Scholar]
- 83.Jeong HW, Cui W, Yang Y, Lu J, He J, Li A, et al. SALL4, a Stem Cell Factor, Affects the Side Population by Regulation of the ATP-Binding Cassette Drug Transport Genes. PLoS One. 2011;6:e18372. doi: 10.1371/journal.pone.0018372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porro A, Iraci N, Soverini S, Diolaiti D, Gherardi S, Terragna C, et al. c-MYC oncoprotein dictates transcriptional profiles of ATP-binding cassette transporter genes in chronic myelogenous leukemia CD34+ hematopoietic progenitor cells. Mol Cancer Res. 2011;9:1054–1066. doi: 10.1158/1541-7786.MCR-10-0510. [DOI] [PubMed] [Google Scholar]
- 85.Ding S, Gong BD, Yu J, Gu J, Zhang HY, Shang ZB, et al. Methylation profile of the promoter CpG islands of 14 "drug-resistance" genes in hepatocellular carcinoma. World J Gastroenterol. 2004;10:3433–3440. doi: 10.3748/wjg.v10.i23.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, et al. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood. 2006;108:3881–3889. doi: 10.1182/blood-2005-10-009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.To K, Zhan Z, Bates S. Aberrant promoter methylation of the ABCG2 gene in renal carcinoma. Mol Cell Biol. 2006;26:8572–8585. doi: 10.1128/MCB.00650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.To KK, Polgar O, Huff LM, Morisaki K, Bates SE. Histone modifications at the ABCG2 promoter following treatment with histone deacetylase inhibitor mirror those in multidrug-resistant cells. Mol Cancer Res. 2008;6:151–164. doi: 10.1158/1541-7786.MCR-07-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calcagno AM, Fostel JM, To KK, Salcido CD, Martin SE, Chewning KJ, et al. Single-step doxorubicin-selected cancer cells overexpress the ABCG2 drug transporter through epigenetic changes. Br J Cancer. 2008;98:1515–1524. doi: 10.1038/sj.bjc.6604334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3' untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28:5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.To K, Robey R, Knutsen T, Zhan Z, Ried T, Bates S. Escape from hsa-miR-519c enables drugresistant cells to maintain high expression of ABCG2. Mol Cancer Ther. 2009;8:2959–2968. doi: 10.1158/1535-7163.MCT-09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang F, Xue X, Wei J, An Y, Yao J, Cai H, et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, -519c and-520h) and their differential expression in stemlike ABCG2+ cancer cells. Biochem Pharmacol. 2011;81:783–792. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie Y, Xu K, Linn DE, Yang X, Guo Z, Shimelis H, et al. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem. 2008;283:3349–3356. doi: 10.1074/jbc.M707773200. [DOI] [PubMed] [Google Scholar]
- 96.Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 97.Takada T, Suzuki H, Gotoh Y, Sugiyama Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos. 2005;33:905–909. doi: 10.1124/dmd.104.003228. [DOI] [PubMed] [Google Scholar]
- 98.Zhang W, Ding W, Chen Y, Feng M, Ouyang Y, Yu Y, et al. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim Biophys Sin (Shanghai) 2011;43:647–653. doi: 10.1093/abbs/gmr050. [DOI] [PubMed] [Google Scholar]
- 99.Cascorbi I, Haenisch S. Pharmacogenetics of ATP-binding cassette transporters and clinical implications. Methods Mol Biol. 2010;596:95–121. doi: 10.1007/978-1-60761-416-6_6. [DOI] [PubMed] [Google Scholar]
- 100.Nakagawa H, Tamura A, Wakabayashi K, Hoshijima K, Komada M, Yoshida T, et al. Ubiquitinmediated proteasomal degradation of non-synonymous SNP variants of human ABC transporter ABCG2. Biochem J. 2008;411:623–631. doi: 10.1042/BJ20071229. [DOI] [PubMed] [Google Scholar]
- 101.Cusatis G, Sparreboom A. Pharmacogenomic importance of ABCG2. Pharmacogenomics. 2008;9:1005–1009. doi: 10.2217/14622416.9.8.1005. [DOI] [PubMed] [Google Scholar]
- 102.Murakami T, Takano M. Intestinal efflux transporters and drug absorption. Expert Opin Drug Metab Toxicol. 2008;4:923–939. doi: 10.1517/17425255.4.7.923. [DOI] [PubMed] [Google Scholar]
- 103.Vlaming ML, Pala Z, van Esch A, Wagenaar E, de Waart DR, van de Wetering K, et al. Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin Cancer Res. 2009;15:3084–3093. doi: 10.1158/1078-0432.CCR-08-2940. [DOI] [PubMed] [Google Scholar]
- 104.Vlaming ML, van Esch A, Pala Z, Wagenaar E, van de Wetering K, van Tellingen O, et al. Abcc2 (Mrp2), Abcc3 (Mrp3), and Abcg2 (Bcrp1) are the main determinants for rapid elimination of methotrexate and its toxic metabolite 7-hydroxymethotrexate in vivo. Mol Cancer Ther. 2009;8:3350–3359. doi: 10.1158/1535-7163.MCT-09-0668. [DOI] [PubMed] [Google Scholar]
- 105.Yang JJ, Milton MN, Yu S, Liao M, Liu N, Wu JT, et al. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab Lett. 2010;4:201–212. doi: 10.2174/187231210792928279. [DOI] [PubMed] [Google Scholar]
- 106.Li H, Jin HE, Kim W, Han YH, Kim DD, Chung SJ, et al. Involvement of P-glycoprotein, multidrug resistance protein 2 and breast cancer resistance protein in the transport of belotecan and topotecan in Caco-2 and MDCKII cells. Pharm Res. 2008;25:2601–2612. doi: 10.1007/s11095-008-9678-0. [DOI] [PubMed] [Google Scholar]
- 107.Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/− /Mdr1a/1b−/− (triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 108.Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64:5804–5811. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]
- 109.Whelan J, Hoare D, Leonard P. Omeprazole does not alter plasma methotrexate clearance. Cancer Chemother Pharmacol. 1999;44:88–89. doi: 10.1007/s002800050949. [DOI] [PubMed] [Google Scholar]
- 110.Joerger M, Huitema AD, van den Bongard HJ, Baas P, Schornagel JH, Schellens JH, et al. Determinants of the elimination of methotrexate and 7-hydroxy-methotrexate following high-dose infusional therapy to cancer patients. Br J Clin Pharmacol. 2006;62:71–80. doi: 10.1111/j.1365-2125.2005.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67:44–49. doi: 10.1111/j.1365-2125.2008.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR, Jr, Chen X, et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009;78:153–161. doi: 10.1016/j.bcp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Wang JS, Zhu HJ, Markowitz JS, Donovan JL, Yuan HJ, Devane CL. Antipsychotic drugs inhibit the function of breast cancer resistance protein. Basic Clin Pharmacol Toxicol. 2008;103:336–341. doi: 10.1111/j.1742-7843.2008.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, et al. Sildenafil Reverses ABCB1- and ABCG2-Mediated Chemotherapeutic Drug Resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, et al. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66:4802–4807. doi: 10.1158/0008-5472.CAN-05-2915. [DOI] [PubMed] [Google Scholar]
- 116.Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–7499. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 117.Azzariti A, Porcelli L, Simone GM, Quatrale AE, Colabufo NA, Berardi F, et al. Tyrosine kinase inhibitors and multidrug resistance proteins: interactions and biological consequences. Cancer Chemother Pharmacol. 2010;65:335–346. doi: 10.1007/s00280-009-1039-0. [DOI] [PubMed] [Google Scholar]
- 118.Noguchi K, Kawahara H, Kaji A, Katayama K, Mitsuhashi J, Sugimoto Y. Substrate-dependent bidirectional modulation of P-glycoprotein-mediated drug resistance by erlotinib. Cancer Sci. 2009;100:1701–1707. doi: 10.1111/j.1349-7006.2009.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–7991. doi: 10.1158/0008-5472.CAN-10-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37:359–365. doi: 10.1124/dmd.108.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nicolazzo JA, Katneni K. Drug transport across the blood-brain barrier and the impact of breast cancer resistance protein (ABCG2) Curr Top Med Chem. 2009;9:130–147. doi: 10.2174/156802609787521580. [DOI] [PubMed] [Google Scholar]
- 122.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 123.Poller B, Wagenaar E, Tang SC, Schinkel AH. Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm. 2011;8:571–582. doi: 10.1021/mp1003898. [DOI] [PubMed] [Google Scholar]
- 124.Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010;9:319–326. doi: 10.1158/1535-7163.MCT-09-0663. [DOI] [PubMed] [Google Scholar]
- 125.Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336:223–233. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res. 2011;17:89–99. doi: 10.1158/1078-0432.CCR-10-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs. 2010 Oct 21; doi: 10.1007/s10637-010-9569-1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 128.Shen J, Carcaboso AM, Hubbard KE, Tagen M, Wynn HG, Panetta JC, et al. Compartment-specific roles of ATP-binding cassette transporters define differential topotecan distribution in brain parenchyma and cerebrospinal fluid. Cancer Res. 2009;69:5885–5892. doi: 10.1158/0008-5472.CAN-09-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethy l]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016) Drug Metab Dispos. 2009;37:439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 130.Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab Dispos. 2009;37:946–955. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]
- 131.Salphati L, Lee LB, Pang J, Plise EG, Zhang X. Role of P-glycoprotein and breast cancer resistance protein-1 in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos. 2010;38:1422–1426. doi: 10.1124/dmd.110.034256. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956–963. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- 133.Poller B, Iusuf D, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Differential Impact of P-Glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) on Axitinib Brain Accumulation and Oral Plasma Pharmacokinetics. Drug Metab Dispos. 2011;39:729–735. doi: 10.1124/dmd.110.037317. [DOI] [PubMed] [Google Scholar]
- 134.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333:788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 135.Zhao R, Raub TJ, Sawada GA, Kasper SC, Bacon JA, Bridges AS, et al. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier. Drug Metab Dispos. 2009;37:1251–1258. doi: 10.1124/dmd.108.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol. 2010;30:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mosaffa F, Lage H, Afshari JT, Behravan J. Interleukin-1 beta and tumor necrosis factor-alpha increase ABCG2 expression in MCF-7 breast carcinoma cell line and its mitoxantrone-resistant derivative, MCF-7/MX. Inflamm Res. 2009;58:669–676. doi: 10.1007/s00011-009-0034-6. [DOI] [PubMed] [Google Scholar]
- 138.Kuo MT. Redox regulation of multidrug resistance in cancer chemotherapy: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2009;11:99–133. doi: 10.1089/ars.2008.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sakata S, Fujiwara M, Ohtsuka K, Kamma H, Nagane M, Sakamoto A, et al. ATP-binding cassette transporters in primary central nervous system lymphoma: decreased expression of MDR1 P-glycoprotein and breast cancer resistance protein in tumor capillary endothelial cells. Oncol Rep. 2011;25:333–339. doi: 10.3892/or.2010.1102. [DOI] [PubMed] [Google Scholar]
- 140.Tang SC, Lagas JS, Lankheet NA, Poller B, Hillebrand MJ, Rosing H, et al. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer. 2012;130:223–333. doi: 10.1002/ijc.26000. [DOI] [PubMed] [Google Scholar]
- 141.Mahringer A, Fricker G. BCRP at the Blood-Brain Barrier: Genomic Regulation by 17beta-Estradiol. Mol Pharm. 2010;7:1835–1847. doi: 10.1021/mp1001729. [DOI] [PubMed] [Google Scholar]
- 142.Suvannasankha A, Minderman H, O'Loughlin KL, Nakanishi T, Greco WR, Ross DD, et al. Breast cancer resistance protein (BCRP/MXR/ABCG2) in acute myeloid leukemia: discordance between expression and function. Leukemia. 2004;18:1252–1257. doi: 10.1038/sj.leu.2403395. [DOI] [PubMed] [Google Scholar]
- 143.Nakanishi T, Karp JE, Tan M, Doyle LA, Peters T, Yang W, et al. Quantitative analysis of breast cancer resistance protein and cellular resistance to flavopiridol in acute leukemia patients. Clin Cancer Res. 2003;9:3320–3328. [PubMed] [Google Scholar]
- 144.Plasschaert SL, van der Kolk DM, de Bont ES, Kamps WA, Morisaki K, Bates SE, et al. The role of breast cancer resistance protein in acute lymphoblastic leukemia. Clin Cancer Res. 2003;9:5171–5177. [PubMed] [Google Scholar]
- 145.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- 146.Stam RW, van den Heuvel-Eibrink MM, den Boer ML, Ebus ME, Janka-Schaub GE, Allen JD, et al. Multidrug resistance genes in infant acute lymphoblastic leukemia: Ara-C is not a substrate for the breast cancer resistance protein. Leukemia. 2004;18:78–83. doi: 10.1038/sj.leu.2403168. [DOI] [PubMed] [Google Scholar]
- 147.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16:1443–1447. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- 148.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, et al. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 149.Benderra Z, Faussat AM, Sayada L, Perrot JY, Tang R, Chaoui D, et al. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–7772. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 150.Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A, et al. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res. 2005;29:141–146. doi: 10.1016/j.leukres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 151.Abbott BL, Colapietro AM, Barnes Y, Marini F, Andreeff M, Sorrentino BP. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100:4594–4601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- 152.van der Kolk DM, Vellenga E, Scheffer GL, Müller M, Bates SE, Scheper RJ, et al. Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood. 2002;99:3763–3770. doi: 10.1182/blood.v99.10.3763. [DOI] [PubMed] [Google Scholar]
- 153.Raaijmakers MH, de Grouw EP, Heuver LH, van der Reijden BA, Jansen JH, Scheper RJ, et al. Breast cancer resistance protein in drug resistance of primitive CD34+38− cells in acute myeloid leukemia. Clin Cancer Res. 2005;11:2436–2444. doi: 10.1158/1078-0432.CCR-04-0212. [DOI] [PubMed] [Google Scholar]
- 154.de Figueiredo-Pontes LL, Pintao MC, Oliveira LC, Dalmazzo LF, Jacomo RH, Garcia AB, et al. Determination of P-glycoprotein, MDR-related protein 1, breast cancer resistance protein, and lung-resistance protein expression in leukemic stem cells of acute myeloid leukemia. Cytometry B Clin Cytom. 2008;74:163–168. doi: 10.1002/cyto.b.20403. [DOI] [PubMed] [Google Scholar]
- 155.Ma Y, Cui W, Yang J, Qu J, Di C, Amin HM, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108:2726–2735. doi: 10.1182/blood-2006-02-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aguila JR, Liao W, Yang J, Avila C, Hagag N, Senzel L, et al. SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood. 2011;118:576–585. doi: 10.1182/blood-2011-01-333641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Damiani D, Tiribelli M, Calistri E, Geromin A, Chiarvesio A, Michelutti A, et al. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica. 2006;91:825–828. [PubMed] [Google Scholar]
- 158.Galimberti S, Guerrini F, Palumbo GA, Consoli U, Fazzi R, Morabito F, et al. Evaluation of BCRP and MDR-1 co-expression by quantitative molecular assessment in AML patients. Leuk Res. 2004;28:367–372. doi: 10.1016/j.leukres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 159.Ho MM, Hogge DE, Ling V. MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp Hematol. 2008;36:433–442. doi: 10.1016/j.exphem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 160.van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, Knauf WU, Fey MF, Verhoef GE, et al. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol. 2007;86:329–337. doi: 10.1007/s00277-007-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tiribelli M, Geromin A, Michelutti A, Cavallin M, Pianta A, Fabbro D, et al. Concomitant ABCG2 overexpression and FLT3-ITD mutation identify a subset of acute myeloid leukemia patients at high risk of relapse. Cancer. 2011;117:2156–2162. doi: 10.1002/cncr.25753. [DOI] [PubMed] [Google Scholar]
- 162.de Wolf C, Jansen R, Yamaguchi H, de Haas M, van de Wetering K, Wijnholds J, et al. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7:3092–3102. doi: 10.1158/1535-7163.MCT-08-0427. [DOI] [PubMed] [Google Scholar]
- 163.Damiani D, Tiribelli M, Michelutti A, Geromin A, Cavallin M, Fabbro D, et al. Fludarabine-based induction therapy does not overcome the negative effect of ABCG2 (BCRP) over-expression in adult acute myeloid leukemia patients. Leuk Res. 2010;34:942–945. doi: 10.1016/j.leukres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 164.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–152. [PubMed] [Google Scholar]
- 165.Burcu M, O'Loughlin KL, Ford LA, Baer MR. Amonafide L-malate is not a substrate for multidrug resistance proteins in secondary acute myeloid leukemia. Leukemia. 2008;22:2110–2115. doi: 10.1038/leu.2008.87. [DOI] [PubMed] [Google Scholar]
- 166.Stone RM, Allen SL, Pigneux A, Stuart RK, Wetzler M, Rizzieri D, Erba HP, Damon LEJ, Jang H, Tallman MS, Warzocha K, Masszi T, Sekeres MA, Miklos E, Horst H, Selleslag DLD, Solomon SR, Venugopal P, Lundberg AS, Powell BL. A phase III, open label, randomized comparison of AS1413 (amonafide L-malate) plus cytarabine with daunorubicin plus cytarabine in secondary acute myeloid leukemia (ACCEDE) J Clin Oncol. 2011;29 suppl doi: 10.1200/JCO.2014.57.0952. abstr 6520. [DOI] [PubMed] [Google Scholar]
- 167.Qadir M, O'Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 168.List AF, Kopecky KJ, Willman CL, Head DR, Persons DL, Slovak ML, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98:3212–3220. doi: 10.1182/blood.v98.12.3212. [DOI] [PubMed] [Google Scholar]
- 169.Ahmed-Belkacem A, Pozza A, Macalou S, Perez-Victoria JM, Boumendjel A, Di Pietro A. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2) Anticancer Drugs. 2006;17:239–243. doi: 10.1097/00001813-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 170.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 171.Allen JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2) Mol Cancer Ther. 2002;1:427–434. [PubMed] [Google Scholar]
- 172.Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, et al. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7:935–941. [PubMed] [Google Scholar]
- 173.Hauswald S, Duque-Afonso J, Wagner MM, Schertl FM, Lubbert M, Peschel C, et al. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin Cancer Res. 2009;15:3705–3715. doi: 10.1158/1078-0432.CCR-08-2048. [DOI] [PubMed] [Google Scholar]
- 174.Hampras SS, Sucheston L, Weiss J, Baer MR, Zirpoli G, Singh PK, et al. Genetic polymorphisms of ATP-binding cassette (ABC) proteins, overall survival and drug toxicity in patients with Acute Myeloid Leukemia. Int J Mol Epidemiol Genet. 2010;1:201–207. [PMC free article] [PubMed] [Google Scholar]
- 175.Poonkuzhali B, Lamba J, Strom S, Sparreboom A, Thummel K, Watkins P, et al. Association of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphisms. Drug Metab Dispos. 2008;36:780–795. doi: 10.1124/dmd.107.018366. [DOI] [PubMed] [Google Scholar]
- 176.Wang F, Liang YJ, Wu XP, Chen LM, To KK, Dai CL, et al. Prognostic value of the multidrug resistance transporter ABCG2 gene polymorphisms in Chinese patients with de novo acute leukaemia. Eur J Cancer. 2011;47:1990–1999. doi: 10.1016/j.ejca.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 177.Sauerbrey A, Sell W, Steinbach D, Voigt A, Zintl F. Expression of the BCRP gene (ABCG2/MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br J Haematol. 2002;118:147–150. doi: 10.1046/j.1365-2141.2002.03550.x. [DOI] [PubMed] [Google Scholar]
- 178.Suvannasankha A, Minderman H, O'Loughlin KL, Nakanishi T, Ford LA, Greco WR, et al. Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br J Haematol. 2004;127:392–398. doi: 10.1111/j.1365-2141.2004.05211.x. [DOI] [PubMed] [Google Scholar]
- 179.Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–5040. [PubMed] [Google Scholar]
- 180.Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63:5538–5543. [PubMed] [Google Scholar]
- 181.Kourti M, Vavatsi N, Gombakis N, Sidi V, Tzimagiorgis G, Papageorgiou T, et al. Expression of multidrug resistance 1 (MDR1), multidrug resistance-related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. Int J Hematol. 2007;86:166–173. doi: 10.1532/IJH97.E0624. [DOI] [PubMed] [Google Scholar]
- 182.Cortez MA, Scrideli CA, Yunes JA, Valera ET, Toledo SR, Pavoni-Ferreira PC, et al. mRNA expression profile of multidrug resistance genes in childhood acute lymphoblastic leukemia. Low expression levels associated with a higher risk of toxic death. Pediatr Blood Cancer. 2009;53:996–1004. doi: 10.1002/pbc.22220. [DOI] [PubMed] [Google Scholar]
- 183.Fedasenka UU, Shman TV, Savitski VP, Belevcev MV. Expression of MDR1, LRP, BCRP and Bcl-2 genes at diagnosis of childhood all: comparison with MRD status after induction therapy. Exp Oncol. 2008;30:248–252. [PubMed] [Google Scholar]
- 184.Semsei AF, Erdélyi DJ, Ungvári I, Kámory E, Csókay B, Andrikovics H, et al. Association of some rare haplotypes and genotype combinations in the MDR1 gene with childhood acute lymphoblastic leukaemia. Leuk Res. 2008;32:1214–1220. doi: 10.1016/j.leukres.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 185.Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 186.Jordanides NE, Jorgensen HG, Holyoake TL, Mountford JC. Functional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood. 2006;108:1370–1373. doi: 10.1182/blood-2006-02-003145. [DOI] [PubMed] [Google Scholar]
- 187.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21:926–935. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- 188.Lu J, Ma Y, Kong N, Alipio Z, Gao C, Krause DS, et al. Dissecting the role of SALL4, a newly identified stem cell factor, in chronic myelogenous leukemia. Leukemia. 2011;25:1211–1213. doi: 10.1038/leu.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485–1495. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 190.Nakanishi T, Shiozawa K, Hassel BA, Ross DD. Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL-expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinib-induced reduction of BCRP expression. Blood. 2006;108:678–684. doi: 10.1182/blood-2005-10-4020. [DOI] [PubMed] [Google Scholar]
- 191.Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 192.Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, et al. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–1275. doi: 10.1038/sj.leu.2404638. [DOI] [PubMed] [Google Scholar]
- 193.Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 194.Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010;38:1371–1380. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hegedus C, Ozvegy-Laczka C, Apati A, Magocsi M, Nemet K, Orfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Takahashi N, Miura M. Therapeutic Drug Monitoring of Imatinib for Chronic Myeloid Leukemia Patients in the Chronic Phase. Pharmacology. 2011;87:241–248. doi: 10.1159/000324900. [DOI] [PubMed] [Google Scholar]
- 197.Kim DH, Sriharsha L, Xu W, Kamel-Reid S, Liu X, Siminovitch K, et al. Clinical relevance of a pharmacogenetic approach using multiple candidate genes to predict response and resistance to imatinib therapy in chronic myeloid leukemia. Clin Cancer Res. 2009;15:4750–4758. doi: 10.1158/1078-0432.CCR-09-0145. [DOI] [PubMed] [Google Scholar]
- 198.Raaijmakers MH, de Grouw EP, Heuver LH, van der Reijden BA, Jansen JH, Scheffer G, et al. Impaired breast cancer resistance protein mediated drug transport in plasma cells in multiple myeloma. Leuk Res. 2005;29:1455–1458. doi: 10.1016/j.leukres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 199.Jakubikova J, Adamia S, Kost-Alimova M, Klippel S, Cervi D, Daley JF, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117:4409–4419. doi: 10.1182/blood-2010-02-267344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Saglam A, Hayran M, Uner AH. Immunohistochemical expression of multidrug resistance proteins in mature T/NK-cell lymphomas. Acta Pathol Microbiol Immunol Scand. 2008;116:791–800. doi: 10.1111/j.1600-0463.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 201.Kim IS, Kim HG, Kim DC, Eom HS, Kong SY, Shin HJ, et al. ABCG2 Q141K polymorphism is associated with chemotherapy-induced diarrhea in patients with diffuse large B-cell lymphoma who received frontline rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone chemotherapy. Cancer Sci. 2008;99:2496–2501. doi: 10.1111/j.1349-7006.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakanishi T, Chumsri S, Khakpour N, Brodie AH, Leyland-Jones B, Hamburger AW, et al. Sidepopulation cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. Br J Cancer. 2010;102:815–826. doi: 10.1038/sj.bjc.6605553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zeng H, Park JW, Guo M, Lin G, Crandall L, Compton T, et al. Lack of ABCG2 expression and side population properties in human pluripotent stem cells. Stem Cells. 2009;27:2435–2445. doi: 10.1002/stem.192. [DOI] [PubMed] [Google Scholar]
- 204.Katayama R, Koike S, Sato S, Sugimoto Y, Tsuruo T, Fujita N. Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export. Cancer Sci. 2009;100:2060–2068. doi: 10.1111/j.1349-7006.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang JP, Liu Y, Zhong W, Yu D, Wen LJ, Jin CS. Chemoresistance of CD133+ cancer stem cells in laryngeal carcinoma. Chin Med J (Engl) 2011;124:1055–1060. [PubMed] [Google Scholar]
- 206.Yajima T, Ochiai H, Uchiyama T, Takano N, Shibahara T, Azuma T. Resistance to cytotoxic chemotherapy-induced apoptosis in side population cells of human oral squamous cell carcinoma cell line Ho-1-N-1. Int J Oncol. 2009;35:273–280. [PubMed] [Google Scholar]
- 207.Chu TS, Chen JS, Lopez JP, Pardo FS, Aguilera J, Ongkeko WM. Imatinib-mediated inactivation of Akt regulates ABCG2 function in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134:979–984. doi: 10.1001/archotol.134.9.979. [DOI] [PubMed] [Google Scholar]
- 208.Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102:1636–1644. doi: 10.1038/sj.bjc.6605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang B, Yang H, Huang YZ, Yan RH, Liu FJ, Zhang JN. Biologic characteristics of the side population of human small cell lung cancer cell line H446. Chin J Cancer. 2010;29:254–260. doi: 10.5732/cjc.009.10330. [DOI] [PubMed] [Google Scholar]
- 210.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sung JM, Cho HJ, Yi H, Lee CH, Kim HS, Kim DK, et al. Characterization of a stem cell population in lung cancer A549 cells. Biochem Biophys Res Commun. 2008;371:163–167. doi: 10.1016/j.bbrc.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 213.Hong YB, Kang HJ, Kwon SY, Kim HJ, Kwon KY, Cho CH, et al. Nuclear factor (erythroid-derived 2)- like 2 regulates drug resistance in pancreatic cancer cells. Pancreas. 2010;39:463–472. doi: 10.1097/MPA.0b013e3181c31314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang YH, Li F, Luo B, Wang XH, Sun HC, Liu S, et al. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma. 2009;56:371–378. doi: 10.4149/neo_2009_05_371. [DOI] [PubMed] [Google Scholar]
- 215.Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, et al. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30:2041–2048. [PubMed] [Google Scholar]
- 216.Fang DD, Kim YJ, Lee CN, Aggarwal S, McKinnon K, Mesmer D, et al. Expansion of CD133(+) colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br J Cancer. 2010;102:1265–1275. doi: 10.1038/sj.bjc.6605610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, et al. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology. 2010;52:528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- 218.Hu C, Li H, Li J, Zhu Z, Yin S, Hao X, et al. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29:2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 219.Shi GM, Xu Y, Fan J, Zhou J, Yang XR, Qiu SJ, et al. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J Cancer Res Clin Oncol. 2008;134:1155–1163. doi: 10.1007/s00432-008-0407-1. [DOI] [PubMed] [Google Scholar]
- 220.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276–1283. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chua C, Zaiden N, Chong KH, See SJ, Wong MC, Ang BT, et al. Characterization of a side population of astrocytoma cells in response to temozolomide. J Neurosurg. 2008;109:856–866. doi: 10.3171/JNS/2008/109/11/0856. [DOI] [PubMed] [Google Scholar]
- 222.Maria BL, Gupta N, Gilg AG, Abdel-Wahab M, Leonard AP, Slomiany M, et al. Targeting hyaluronan interactions in spinal cord astrocytomas and diffuse pontine gliomas. J Child Neurol. 2008;23:1214–1220. doi: 10.1177/0883073808321771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Slomiany MG, Dai L, Bomar PA, Knackstedt TJ, Kranc DA, Tolliver L, et al. Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. 2009;69:4992–4998. doi: 10.1158/0008-5472.CAN-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, et al. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Di Fiore R, Santulli A, Ferrante RD, Giuliano M, De Blasio A, Messina C, et al. Identification and expansion of human osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide treatment. J Cell Physiol. 2009;219:301–313. doi: 10.1002/jcp.21667. [DOI] [PubMed] [Google Scholar]
- 226.Liu T, Xu F, Du X, Lai D, Zhao Y, Huang Q, et al. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340:265–273. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- 227.Yang M, Zhang R, Yan M, Ye Z, Liang W, Luo Z. Detection and characterization of side population in Ewing's sarcoma SK-ES-1 cells in vitro. Biochem Biophys Res Commun. 2010;391:1062–1066. doi: 10.1016/j.bbrc.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 228.Kumamoto H, Ohki K. Detection of CD133, Bmi-1, and ABCG2 in ameloblastic tumors. J Oral Pathol Med. 2010;39:87–93. doi: 10.1111/j.1600-0714.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 229.Ning ZF, Huang YJ, Lin TX, Zhou YX, Jiang C, Xu KW, et al. Subpopulations of stem-like cells in side population cells from the human bladder transitional cell cancer cell line T24. J Int Med Res. 2009;37:621–630. doi: 10.1177/147323000903700304. [DOI] [PubMed] [Google Scholar]
- 230.Mahller YY, Williams JP, Baird WH, Mitton B, Grossheim J, Saeki Y, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS One. 2009;4:e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, Maccallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck. 2009;31:94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 232.Ongkeko WM, Altuna X, Weisman RA, Wang-Rodriguez J. Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am J Clin Pathol. 2005;124:71–76. doi: 10.1309/BTLN5WTMJ3PCNRRC. [DOI] [PubMed] [Google Scholar]
- 233.Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 234.Rajagopal A, Simon SM. Subcellular localization and activity of multidrug resistance proteins. Mol Biol Cell. 2003;14:3389–3399. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Goler-Baron V, Assaraf YG. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS One. 2011;6:e16007. doi: 10.1371/journal.pone.0016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ifergan I, Scheffer GL, Assaraf YG. Novel extracellular vesicles mediate an ABCG2-dependent anticancer drug sequestration and resistance. Cancer Res. 2005;65:10952–10958. doi: 10.1158/0008-5472.CAN-05-2021. [DOI] [PubMed] [Google Scholar]
- 237.Ifergan I, Goler-Baron V, Assaraf YG. Riboflavin concentration within ABCG2-rich extracellular vesicles is a novel marker for multidrug resistance in malignant cells. Biochem Biophys Res Commun. 2009;380:5–10. doi: 10.1016/j.bbrc.2008.12.168. [DOI] [PubMed] [Google Scholar]
- 238.Vannini I, Zoli W, Fabbri F, Ulivi P, Tesei A, Carloni S, et al. Role of efflux pump activity in lapatinib/caelyx combination in breast cancer cell lines. Anticancer Drugs. 2009;20:918–925. doi: 10.1097/CAD.0b013e32833179bf. [DOI] [PubMed] [Google Scholar]
- 239.Kuang YH, Shen T, Chen X, Sodani K, Hopper-Borge E, Tiwari AK, et al. Lapatinib and erlotinib are potent reversal agents for MRP7 (ABCC10)-mediated multidrug resistance. Biochem Pharmacol. 2010;79:154–161. doi: 10.1016/j.bcp.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yuan JH, Cheng JQ, Jiang LY, Ji WD, Guo LF, Liu JJ, et al. Breast cancer resistance protein expression and 5-fluorouracil resistance. Biomed Environ Sci. 2008;21:290–295. doi: 10.1016/S0895-3988(08)60044-6. [DOI] [PubMed] [Google Scholar]
- 242.Yuan J, Lv H, Peng B, Wang C, Yu Y, He Z. Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009;63:1103–1110. doi: 10.1007/s00280-008-0838-z. [DOI] [PubMed] [Google Scholar]
- 243.Vannini I, Zoli W, Tesei A, Rosetti M, Sansone P, Storci G, et al. Role of p53 codon 72 arginine allele in cell survival in vitro and in the clinical outcome of patients with advanced breast cancer. Tumour Biol. 2008;29:145–151. doi: 10.1159/000143400. [DOI] [PubMed] [Google Scholar]
- 244.Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M, et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28:1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liu F, Fan D, Qi J, Zhu H, Zhou Y, Yang C, et al. Co-expression of cytokeratin 8 and breast cancer resistant protein indicates a multifactorial drug-resistant phenotype in human breast cancer cell line. Life Sci. 2008;83:496–501. doi: 10.1016/j.lfs.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 246.Zander SA, Kersbergen A, van der Burg E, de Water N, van Tellingen O, Gunnarsdottir S, et al. Sensitivity and acquired resistance of BRCA1; p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res. 2010;70:1700–1710. doi: 10.1158/0008-5472.CAN-09-3367. [DOI] [PubMed] [Google Scholar]
- 247.Nowak M, Madej JA, Dziegiel P. Expression of Breast Cancer Resistance Protein (BCRP-1) in canine mammary adenocarcinomas and adenomas. In Vivo. 2009;23:705–709. [PubMed] [Google Scholar]
- 248.Honscha KU, Schirmer A, Reischauer A, Schoon HA, Einspanier A, Gabel G. Expression of ABC-transport proteins in canine mammary cancer: consequences for chemotherapy. Reprod Domest Anim. 2009;44 Suppl 2:218–223. doi: 10.1111/j.1439-0531.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 249.Kim YH, Ishii G, Goto K, Ota S, Kubota K, Murata Y, et al. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer. 2009;65:105–111. doi: 10.1016/j.lungcan.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 250.Ota S, Ishii G, Goto K, Kubota K, Kim YH, Kojika M, et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 251.Ceckova M, Vackova Z, Radilova H, Libra A, Buncek M, Staud F. Effect of ABCG2 on cytotoxicity of platinum drugs: interference of EGFP. Toxicol In Vitro. 2008;22:1846–1852. doi: 10.1016/j.tiv.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 252.Muller PJ, Dally H, Klappenecker CN, Edler L, Jager B, Gerst M, et al. Polymorphisms in ABCG2, ABCC3 and CNT1 genes and their possible impact on chemotherapy outcome of lung cancer patients. Int J Cancer. 2009;124:1669–1674. doi: 10.1002/ijc.23956. [DOI] [PubMed] [Google Scholar]
- 253.Usuda J, Ohira T, Suga Y, Oikawa T, Ichinose S, Inoue T, et al. Breast cancer resistance protein (BCRP) affected acquired resistance to gefitinib in a "never-smoked" female patient with advanced non-small cell lung cancer. Lung Cancer. 2007;58:296–299. doi: 10.1016/j.lungcan.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 254.Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, et al. Nuclear Translocation of Epidermal Growth Factor Receptor by Akt-dependent Phosphorylation Enhances Breast Cancer-resistant Protein Expression in Gefitinib-resistant Cells. J Biol Chem. 2011;286:20558–20568. doi: 10.1074/jbc.M111.240796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ohtsuka K, Ohnishi H, Morii T, Fujiwara M, Kishino T, Ogura W, et al. Downregulated ABCG2 enhances sensitivity to topoisomerase I inhibitor in epidermal growth factor receptor tyrosine kinase inhibitor-resistant non-small cell lung cancer. J Thorac Oncol. 2010;5:1726–1733. doi: 10.1097/JTO.0b013e3181f0b6af. [DOI] [PubMed] [Google Scholar]
- 256.Li J, Li ZN, Du YJ, Li XQ, Bao QL, Chen P. Expression of MRP1, BCRP, LRP, ERCC1 in advanced non-small-cell lung cancer: correlation with response to chemotherapy and survival. Clin Lung Cancer. 2009;10:414–421. doi: 10.3816/CLC.2009.n.078. [DOI] [PubMed] [Google Scholar]
- 257.Li J, Li ZN, Yu LC, Bao QL, Wu JR, Shi SB, et al. Association of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Cancer. 2010;69:116–122. doi: 10.1016/j.lungcan.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 258.Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 259.Usuda J, Tsunoda Y, Ichinose S, Ishizumi T, Ohtani K, Maehara S, et al. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer. 2010;67:198–204. doi: 10.1016/j.lungcan.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 260.Rolff J, Dorn C, Merk J, Fichtner I. Response of Patient-Derived Non-Small Cell Lung Cancer Xenografts to Classical and Targeted Therapies Is Not Related to Multidrug Resistance Markers. J Oncol. 2009;2009:814140. doi: 10.1155/2009/814140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tanaka M, Okazaki T, Suzuki H, Abbruzzese JL, Li D. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 2011;117:744–751. doi: 10.1002/cncr.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hamada S, Satoh K, Hirota M, Kanno A, Umino J, Ito H, et al. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2011 doi: 10.1002/jcp.22781. [DOI] [PubMed] [Google Scholar]
- 263.Theile D, Grebhardt S, Haefeli WE, Weiss J. Involvement of drug transporters in the synergistic action of FOLFOX combination chemotherapy. Biochem Pharmacol. 2009;78:1366–1373. doi: 10.1016/j.bcp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 264.Konig SK, Herzog M, Theile D, Zembruski N, Haefeli WE, Weiss J. Impact of drug transporters on cellular resistance towards saquinavir and darunavir. J Antimicrob Chemother. 65:2319–2328. doi: 10.1093/jac/dkq324. [DOI] [PubMed] [Google Scholar]
- 265.Moran RG, Keyomarsi K. Biochemical rationale for the synergism of 5-fluorouracil and folinic acid. NCI Monogr. 1987:159–163. [PubMed] [Google Scholar]
- 266.Vander Borght S, van Pelt J, van Malenstein H, Cassiman D, Renard M, Verslype C, et al. Up-regulation of breast cancer resistance protein expression in hepatoblastoma following chemotherapy: A study in patients and in vitro. Hepatol Res. 2008;38:1112–1121. doi: 10.1111/j.1872-034X.2008.00381.x. [DOI] [PubMed] [Google Scholar]
- 267.Zhao L, Pan Y, Gang Y, Wang H, Jin H, Tie J, et al. Identification of GAS1 as an epirubicin resistance-related gene in human gastric cancer cells with a partially randomized small interfering RNA library. J Biol Chem. 2009;284:26273–26285. doi: 10.1074/jbc.M109.028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Huang D, Gao Q, Guo L, Zhang C, Jiang W, Li H, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev. 2009;18:465–473. doi: 10.1089/scd.2008.0033. [DOI] [PubMed] [Google Scholar]
- 269.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jin Y, Bin ZQ, Qiang H, Liang C, Hua C, Jun D, et al. ABCG2 is related with the grade of glioma and resistance to mitoxantone, a chemotherapeutic drug for glioma. J Cancer Res Clin Oncol. 2009;135:1369–1376. doi: 10.1007/s00432-009-0578-4. [DOI] [PubMed] [Google Scholar]
- 271.Bleau AM, Huse JT, Holland EC. The ABCG2 resistance network of glioblastoma. Cell Cycle. 2009;8:2936–2944. [PubMed] [Google Scholar]
- 272.Lee OH, Xu J, Fueyo J, Fuller GN, Aldape KD, Alonso MM, et al. Expression of the receptor tyrosine kinase Tie2 in neoplastic glial cells is associated with integrin beta1-dependent adhesion to the extracellular matrix. Mol Cancer Res. 2006;4:915–926. doi: 10.1158/1541-7786.MCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 273.Martin V, Xu J, Pabbisetty SK, Alonso MM, Liu D, Lee OH, et al. Tie2-mediated multidrug resistance in malignant gliomas is associated with upregulation of ABC transporters. Oncogene. 2009;28:2358–2363. doi: 10.1038/onc.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Narang VS, Fraga C, Kumar N, Shen J, Throm S, Stewart CF, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–C450. doi: 10.1152/ajpcell.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ginguene C, Champier J, Maallem S, Strazielle N, Jouvet A, Fevre-Montange M, et al. P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) localize in the microvessels forming the blood-tumor barrier in ependymomas. Brain Pathol. 2010;20:926–935. doi: 10.1111/j.1750-3639.2010.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Colabufo NA, Pagliarulo V, Berardi F, Contino M, Inglese C, Niso M, et al. Bicalutamide failure in prostate cancer treatment: involvement of Multi Drug Resistance proteins. Eur J Pharmacol. 2008;601:38–42. doi: 10.1016/j.ejphar.2008.10.038. [DOI] [PubMed] [Google Scholar]