Background: Integrin-β3 is important for the cell migration and proliferation linked to muscle regeneration.
Results: In mice with global integrin-β3 KO, an initial macrophage polarization impairs muscle regeneration and stimulates fibrosis via TGF-β1 production.
Conclusion: In bone marrow cells, integrin-β3 expression is necessary for macrophage-dependent processes of muscle repair.
Significance: Stimulating integrin-β3 could improve muscle regeneration.
Keywords: Fibrosis, Integrins, Macrophages, Muscle Regeneration, Myogenesis
Abstract
Following injury, skeletal muscle achieves repair by a highly coordinated, dynamic process resulting from interplay among numerous inflammatory, growth factors and myogenic regulators. To identify genes involved in muscle regeneration, we used a microarray analysis; there was a significant increase in the expression of a group of integrin genes. To verify these results, we used RT-PCR and Western blotting and found that 12 integrins were up-regulated from 3 h to 15 days following injury. Following muscle injury, integrin-β3 was initially expressed, mainly in macrophages. In integrin-β3 global KO mice, the expression of myogenic genes was decreased and muscle regeneration was impaired, whereas fibrosis was enhanced versus events in wild type (WT) mice. The mechanism for these responses in integrin-β3 KO mice included an infiltration of macrophages that were polarized into the M2 phenotype. These macrophages produced more TGF-β1 and increased TGF-β1/Smad signaling. In vitro, we confirmed that M2 macrophages lacking integrin-β3 produced more TGF-β1. Furthermore, transplantation of bone marrow cells from integrin-β3 KO mice into WT mice led to suppression of the infiltration and accumulation of macrophages into injured muscles. There was also impaired muscle regeneration with an increase in muscle fibrosis. Our results demonstrate that integrin-β3 plays a fundamental role in muscle regeneration through a regulation of macrophage infiltration and polarization leading to suppressed TGF-β1 production. This promotes efficient muscle regeneration. Thus, an improvement in integrin-β3 function could stimulate muscle regeneration.
Introduction
Postnatally, muscle growth and regeneration arise from satellite cells (muscle stem cells). They are in intimate contact with the extracellular matrix and basement membrane. Interactions between cells and extracellular matrix proteins are mediated primarily by integrin genes. Integrins are transmembrane receptors that bind the extracellular matrix and the intracellular cytoskeleton. Consequently, integrins, by transducing signals from outside of cells into cells and vice versa, could play important roles in regulating cell adhesion, spreading, migration, proliferation, and differentiation as well as tissue remodeling. The importance of integrin signaling in influencing the repair of injured muscle is emphasized by reports of muscular degenerative disorders in mice with specific integrin deficiencies (1, 2). Integrins can also affect muscle growth indirectly. In response to muscle overloading, integrin-β2 knock-out (KO) mice (integrin-β2 is exclusively expressed by hematopoietic cells) exhibited decreased myofiber size, a lower muscle mass, and decreased satellite cell activation and proliferation (3). The pathophysiological changes stimulated by muscle injury also involve integrins (4).
Muscle injury is followed by infiltration of inflammatory cells and removal of necrotic tissue plus initiation of satellite cell proliferation and muscle regeneration (5). Cells infiltrating to damaged muscles include neutrophils and macrophages, which may release cytokines and chemokines (6). Macrophages are a heterogeneous population of cells expressing different functions. Activated, proinflammatory M1 macrophages appear early following muscle injury and produce proinflammatory cytokines including TNFα and IL-1β; they also remove necrotic tissues. Anti-inflammatory macrophages (M2c) appear later to stimulate tissue repair (5). The alternative M2a macrophages can be identified by their expression of CD206 (a mannose receptor), and they are associated with the development of fibrosis (7, 8). Patients with Duchenne muscular dystrophy are often identified by the presence of increased numbers of M2a macrophages and a high TGF-β1 level in a muscle biopsy (9).
TGF-β1 is a potent stimulus for fibrosis and an inhibitor of muscle regeneration (10). It is highly expressed in regenerating muscles and in dystrophic muscles of Duchenne muscular dystrophy patients or mdx mice (10). TGF-β1 signaling involves transmembrane serine/threonine kinase receptors that phosphorylate Smad proteins, leading to translocation of the Smad complex into the nucleus. This stimulates the transcription of target genes, including those influencing fibrosis and muscle regeneration (11, 12). For example, treatment of C2C12 cells with TGF-β1 down-regulates the expression of myogenic genes and initiates the production of fibrosis-related proteins. Consistent with these findings, direct injection of recombinant TGF-β1 into skeletal muscles of mice stimulates scar tissue formation. When myoblasts transfected with the TGF-β1 gene were injected into mice, they differentiated into myofibroblasts (13). Thus, TGF-β1 could play an important role in the initiation of fibrotic cascades and the induction of myogenic cells to differentiate into myofibroblasts.
We found that increased expression of integrins in injured muscles was an early and persistent response to muscle injury. In integrin-β3 KO mice, we found fewer infiltrating macrophages plus impaired muscle regeneration and increased fibrosis. Our evaluation of macrophages revealed that integrin-β3 regulates macrophage polarization, which in turn affects muscle regeneration.
EXPERIMENTAL PROCEDURES
Muscle Regeneration Model
All animal experiments and procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. C57/BL6 and global integrin-β3 KO mice (C57/BL6 background) were purchased from The Jackson Laboratory (Bar Harbor, ME) and studied after 6–10 weeks of age. We used a standard model of muscle injury from cardiotoxin (Sigma-Aldrich) injection (6). Briefly, 80 μl of 10 μm cardiotoxin in saline was injected into one tibialis anterior (TA)2 mixed fiber muscle using a 27-gauge needle. The uninjured, contralateral muscle was injected with the same volume of PBS. For the gene microarray analyses, we injured another mixed fiber muscle, the gastrocnemius. Mice were anesthetized and perfused with PBS, and muscles were collected at 0–28 days after injury. Muscles were either mounted in tissue freezing medium and frozen in isopentane chilled with dry ice for histological analysis or stored in liquid nitrogen for protein or RNA isolation.
Gene Expression Analysis
Gene microarrays were performed in the Baylor Microarray Core facility. Total RNA was converted into sense-strand cDNA with the Ambion WT expression kit (Austin, TX). cDNAs were fragmented and labeled using the Affymetrix GeneChip® WT terminal labeling kit (Santa Clara, CA). A 200-μl hybridization mixture was loaded onto the GeneChip® mouse exon 1.0 ST array. The arrays were hybridized for 17 h at 45 °C with a 60-rpm rotation in the GeneChip® hybridization oven 640. After washing, arrays were stained with a streptavidin, R-phycoerythrin, via the GeneChip® fluidics station 450. The signal was assessed using biotinylated anti-streptavidin and scanned using the Affymetrix GeneChip® scanner 3000. The raw signal intensity data were preprocessed using the ITERPLIER and sketch quantile normalization algorithm (Expression Console Software, Affymetrix). The differential expression analysis was performed using the program for a paired, significant analysis of a microarray (14).
RT-PCR
RT-PCR was performed as described (15, 16); the relative gene expression was calculated from cycle threshold values using 18 S RNA or GAPDH as an internal control (Ct; relative expression = 2(sample Ct − 18 S Ct)). Primers and their sequences are listed in supplemental Table 1.
Immunohistochemical Analyses
Serial, transverse cryo-sections (8 μm thick) of the frozen midbelly region of TA muscles were stained for fibrosis in the muscle interstitium using Sirius Red (Sigma-Aldrich) (16). Antibodies used for immunohistochemistry staining, Western blotting, and FACS are listed in supplemental Table 2. To calculate the cross-sectional areas of individual myofibers that had been initially immunostained for laminin, we used the Nikon NIS-Elements BR 3.0 software (Melville, NY). The distribution of fiber sizes was expressed as a percentage of myofiber examined.
Bone Marrow Transplantation
Bone marrow (BM) cells were harvested by flushing femurs and tibias from anesthetized, integrin-β3 KO or WT mice (donors). Recipient WT mice (5–7 weeks old) were irradiated with 1100 radiation-absorbed doses, and 1 day later, 5 × 106 BM cells were injected into recipients by tail vein injection. After 30 days, TA muscles were injured by the injection of cardiotoxin, and the muscle samples were collected 0–14 days after injury and prepared for histological analysis or for protein or RNA isolation.
Macrophage Isolation and Polarization
Bone marrow-derived macrophages (BMDMs) were generated from BM of adult C57/BL6 mice (17). Briefly, the cells were washed and then cultured in RPMI 1640 media supplemented with 1% penicillin/streptomycin, 1% HEPES, 10% FBS, and 100 ng/ml macrophage colony-stimulating factor (M-CSF). After 7 days, BMDMs were differentiated into an M1 phenotype by incubating them with activated LPS (100 ng/ml) plus interferon-γ (IFNγ) (20 ng/ml) for 16 h. Other groups of BM cells were converted into the M2a phenotype by incubating with M-CSF (10 ng/ml) plus IL-4 (20 ng/ml) for 96 h. To convert M0 to M2c macrophages, cells were cultured with M-CSF (10 ng/ml) plus IL-10 (20 ng/ml) for 96 h. Cell characteristics were documented by flow cytometry and RT-PCR analysis. TGF-β1 in the media was measured using an ELISA kit from Promega (Madison, WI).
Flow Cytometry
Injured and uninjured muscles were minced before incubating with 0.2% Type II collagenase for 45 min at 37 °C. The mixture was passed through 100-μm and then 40-μm filters. Mononuclear cells (106 in 100 μl of PBS) were incubated with an Fc blocker (BD Biosciences) for 10 min on ice and then incubated with antibodies (APC-F4/80 or Alexa Fluor 488-CD206 or both) for 15 min on ice. Stained cells were washed with PBS, 0.5% BSA, resuspended in 0.5 ml of ice cold PBS, and analyzed with a BD Biosciences LSRII flow cytometer system.
Hydroxyproline Assay
The collagen content of injured or control muscles was assessed using a hydroxyproline assay kit (BioVision, Mountain View, CA) according to the accompanying instructions.
Statistical Analysis
Values are presented as means ± S.E. Data were compared across different mouse strains and time points using a two-way analysis of variance. Student's t test was performed with statistical significance set at the 0.05 confidence level.
RESULTS
Integrin Expression Is Up-regulated in Injured Muscles
Previously, we found that mononucleated cells infiltrate injured areas of muscles induced by cardiotoxin injection (6). This response continues for 3 days before the expression of myogenic genes (e.g. MyoD and myogenin) increases sharply and newly regenerated myofibers begin to appear (recognized by central nuclei) (6). To examine the pattern of gene expression occurring in regenerating muscle, we used a microarray analysis. Approximately 493 genes were differentially regulated (supplemental Table 3). The up-regulated mRNAs included those known to be involved in inflammation, immune response, cell cycles, growth factor, and some myogenic markers of satellite cells.
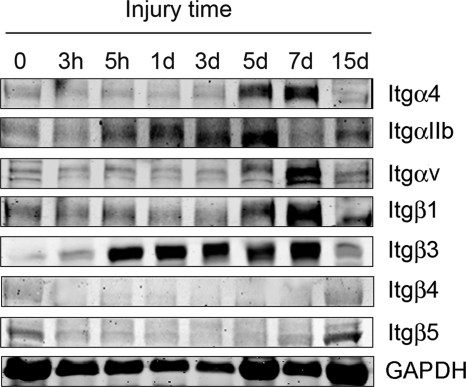
Several integrins were also up-regulated, prompting us to evaluate the temporal expression of integrin mRNAs and proteins in injured muscles. As early as 3 h after the injury, mRNAs of integrins αL, α5, αIIB, β2, and β3 were up-regulated, and at days 1, 3, and 6, the mRNA expression of nine of the α subunits and four of the β subunits of integrins was increased (Table 1). Integrin-β3 was one of the most prominently up-regulated subunits; it was increased at 5 h after injury, and this response persisted for up to 6 days after injury (Fig. 1). At 15 days after injury, the integrin-β3 had returned to control levels. These results suggest that integrins are stimulated in injured muscles during the early phases of recovery and repair. Thus, integrin-β3 exhibits an early and persistent response to muscle injury.
TABLE 1.
Up-regulated integrin subunits in regenerating skeletal muscle at different times after injury
| Integrins | Average -fold increase (injury times) |
Accession number | |||||
|---|---|---|---|---|---|---|---|
| 3 h | 5 h | 1 day | 3 day | 6 day | 15 day | ||
| Itgαl | 2.75 | 13.78 | 70.15 | 102.74 | 19.03 | 2.14 | NM_008400 |
| Itgαm | 0.37 | 0.81 | 6.91 | 238.30 | 12.56 | 0.55 | NM_001082960 |
| ItgαX | 0.42 | 1.21 | 20.23 | 78.09 | 109.54 | 4.61 | NM_021334 |
| Itgα4 | 0.92 | 2.10 | 5.16 | 19.78 | 7.33 | 2.63 | NM_0.0576 |
| Itgα5 | 1.46 | 2.81 | 34.48 | 56.00 | 26.39 | 1.79 | NM_010577 |
| Itgα6 | 0.80 | 1.48 | 4.10 | 8.10 | 1.84 | 0.88 | NM_008397 |
| Itgα7 | 0.59 | 0.66 | 1.92 | 13.26 | 4.97 | 1.26 | NM_008398 |
| ItgαV | 0.80 | 1.93 | 12.09 | 32.21 | 16.06 | 4.58 | NM_008402 |
| ItgαIIB | 2.06 | 8.75 | 11.79 | 22.16 | 18.73 | 2.48 | NM_010579 |
| Itgβ2 | 1.66 | 13.62 | 334.12 | 350.94 | 73.90 | 13.45 | NM_008404 |
| Itgβ3 | 3.00 | 3.35 | 24.73 | 36.93 | 6.03 | 1.61 | NM_008404 |
| Itgβ5 | 0.88 | 1.94 | 6.92 | 23.32 | 12.99 | 4.27 | NM_010580 |
| Itgβ7 | 0.36 | 1.50 | 9.59 | 22.15 | 4.00 | 1.62 | NM_013566 |
FIGURE 1.
Muscle injury increases expression of integrin subunits. TA muscles of three C57/BL6 mice were collected at different times after injury. Western blotting of integrins revealed the changes that are indicated on the right side of the blots (representative blotting from muscles of three mice at each time point after injury).
To determine which cells in the injured muscle express integrin-β3, we immunostained cryo-sections of TA muscles at 5 h and 3 days after injury with anti-integrin-β3 and anti-myogenin (a marker of satellite cells), anti-integrin-β3 and anti-F4/80 (or MAC2, macrophage markers), or anti-integrin-β3 and anti-CD41 (a platelet marker). At 5 h after injury, most integrin-β3-positive cells were positive for MAC2 (Fig. 2A, upper panel), and fewer integrin-β3-positive cells were positive for CD41 (Fig. 2A, lower panel); we found no integrin-β3 cells positive for myogenin (not shown). Thus, at early stages, macrophages and platelets infiltrate injured muscles and express integrin-β3. At 3 days after injury, most integrin-β3-positive cells were macrophages (F4/80-positive; Fig. 2B, upper panel). A few cells were myogenin-positive (Fig. 2B, middle panel), and very few integrin-β3-positive cells were CD41-positive (Fig. 2B, bottom panel). Thus, at 3 days after injury, macrophages were the most abundant cells expressing integrin-β3.
FIGURE 2.
Expression of integrin-β3 in injured muscles. A, at 5 h after injury, the cryo-sections of TA muscles were immunostained with integrin-β3 (red color) and MAC2 (green, upper panel) or CD41 (green, lower panel)). The yellow cells (right panel labeled Combined) demonstrate “double positive” cells (n = muscles of 3 mice). B, at 3 days after injury, the cryo-sections of TA muscles were immunostained with integrin-β3 (red color) and MAC2 or CD41 (green). The yellow cells (right panel labeled Combined) demonstrate double positive cells (n = muscles of 3 mice).
Muscle Regeneration Is Impaired in Mice Lacking Integrin-β3
To explore the role of the increase in integrin-β3 during muscle regeneration, we studied mice with a global KO of integrin-β3 and compared the results with those in WT mice treated similarly. As shown in Fig. 3A, muscle regeneration was significantly impaired in mice lacking integrin-β3. At 5 days after injury, muscle from integrin-β3 KO mice had few newly generated myofibers (identified by their central nuclei), and by days 8–14, there were still empty spaces between the regenerating myofibers versus findings in WT mice (Fig. 3A). At 28 days, regenerating myofibers in injured muscles of integrin-β3 KO mice were smaller, and the distribution of myofiber sizes was shifted to the left when compared with the distribution of myofibers from injured muscles of WT mice (Fig. 3, A and B). These results demonstrate that the absence of integrin-β3 impairs the capacity for muscle regeneration.
FIGURE 3.
Muscle regeneration is impaired in integrin-β3 KO mice. A, H&E staining of cryo-cross-sections of TA muscles at 3, 5, 8, 14, and 28 days following injury revealed impairment of muscle regeneration in integrin-β3 KO mice (n = muscles of 3 mice). B, at 28 days after injury, cryo-cross-sections of TA muscles were immunostained with laminin, and the areas of newly formed myofibers (i.e. those with central nuclei) were measured to calculate the distribution of myofiber sizes (n = muscles of 5 mice; *, p ≤ 0.05, difference between values from WT and integrin-β3 KO mice).
Suppression of Myogenesis in Mice Lacking Integrin-β3
During muscle regeneration, MyoD and myogenin are highly expressed in proliferating and differentiating satellite cells, reflecting their role in repair of muscle injury. However, at 3 or 5 h after injury, we found that the products of both myogenic genes in muscles of WT and integrin-β3 KO mice were lower than in uninjured muscles of the same mice (data not shown). At 1 day after injury, however, the mRNA of MyoD was significantly increased, reaching its highest level at 3 days and returning to control levels by 14 days after injury. Notably, MyoD mRNA levels in muscle of integrin-β3 KO mice were significantly lower than those in WT mice (Fig. 4A). Western blots of myogenin were consistent with its mRNA changes, indicating that KO of integrin-β3 decreases myogenin expression (Fig. 4, B and C). Consistent with these results, we found that KO integrin-β3 also exhibited reduced cell proliferation (BrdU incorporation) during muscle regeneration (supplemental Fig. 1).
FIGURE 4.
Myogenic gene expression is impaired in injured TA muscles of integrin-β3 KO mice. A and B, at different times after injury, TA muscles from WT (white columns) or integrin-β3 KO mice (black columns) were collected, and the mRNAs of MyoD (A) or myogenin (B) were evaluated by RT-PCR. Data are means ± S.E. (n = muscles of 4 mice, three repetitions by RT-PCR). Two-way analysis of variance revealed a significant difference in values between treatment groups (*, p ≤ 0.05, difference between WT and integrin-β3 KO mice). C, Western blots of myogenin from 5 h to 15 days were measured using GAPDH as the control. (Representative blots were from muscles of three mice at each time point after injury.)
Absence of Integrin-β3 Enhances Fibrosis in Regenerating Muscles
To determine whether the unstained areas among regenerating myofibers of integrin-β3 KO mice were related to the development of fibrosis, we performed Sirius Red staining of the muscle sections. As a control, we examined the uninjured, contralateral muscles of both strains of mice; no fibrosis was found (data not shown). In contrast, injured muscles of integrin-β3 KO mice exhibited increased Sirius Red staining, indicating that there was an increase in fibrosis at 28 days after injury versus results in WT mice (Fig. 5A). To evaluate this finding further, we immunostained specimens for α-SMA and found increased α-SMA in muscles of integrin-β KO mice at 6 days after injury (Fig. 5B). At other times after injury, muscles from integrin-β3 KO mice also exhibited higher levels of mRNAs of α-SMA (Fig. 5C) and collagen I (Fig. 5D). Finally, we measured hydroxyproline in muscles of integrin-β3 KO and WT mice at 0, 7, and 14 days after injury. The basal level of hydroxyproline in uninjured muscles was not different between the two groups of mice, but at 7 or 14 days after injury, hydroxyproline was significantly higher in muscle of integrin-β3 KO mice versus results in WT mice (Fig. 5E). Thus, the absence of integrin-β3 promotes fibrosis in regenerating muscles.
FIGURE 5.
Fibrosis markers are up-regulated in injured TA muscles of integrin-β3 KO mice. A, cryo-cross-sections of TA muscles obtained 28 days after injury were stained with Sirius Red. The dark red staining indicates collagen I deposition (n = muscles of 3 mice). B, cryo-cross-section of TA muscles obtained at 6 days after injury were immunostained with α-SMA (red), laminin (green), and DAPI (blue) (n = muscles of 3 mice). C and D, injured TA muscles from WT (white columns) and integrin-β3 KO mice (black columns) at different times after injury were subjected to RT-PCR to evaluate the mRNAs of α-SMA (C) and collagen 1 (D) (n = muscles of 4 mice; three repeats on RT-PCR analysis, means ± S.E.; *, p ≤ 0.05, difference between values from WT and integrin-β3 KO mice). E, hydroxyproline in uninjured and injured muscle at 7 or 14 days after injury (n = muscles from 6 mice with three repeat measurements for each muscle. Means ± S.E., *, p ≤ 0.05).
Absence of Integrin-β3 Increases TGF-β1 Expression in Injured Muscles
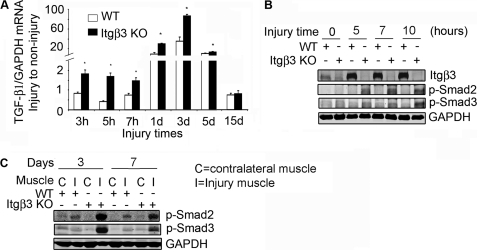
To elucidate the mechanism for enhanced fibrosis in regenerating muscles of integrin-β3 KO mice, we examined TGF-β1 signaling, a trigger for fibrosis. In injured muscles of integrin-β3 KO mice, there was a significant increase in TGF-β1 mRNA at 3 h, and the change persisted through day 5 when compared with results in WT mice (Fig. 6A). At 15 days after injury, TGF-β1 mRNA levels had returned to control levels in both integrin-β3 KO and WT mice. To examine the relevance of the increase in mRNA of TGF-β1, we measured phosphorylated Smad2 and Smad3 because they are downstream effectors of TGF-β1 signaling. At 5–10 h after injury, there were significant increases in phospho-Smad2 and phospho-Smad3 in integrin-β3 KO mice when compared with results in muscles of WT mice (Fig. 6B). The highest level was at day 3, and it gradually decreased to normal levels by day 15 after injury (Fig. 6C). These results were confirmed by immunofluorescent staining (data not shown).
FIGURE 6.
TGF-β1 and its signaling are increased in injured TA muscles of integrin-β3 KO mice. A, injured TA muscles from WT (white columns) and integrin-β3 KO mice (black columns) were collected at different times. TGF-β1 mRNA expression was evaluated by RT-PCR (n = muscles from 4 mice (means ± S.E., *, p ≤ 0.05, difference between values from WT and integrin-β3 KO mice). B and C, Western blots of phospho-Smad2 (p-Smad2) and phospho-Smad3 (p-Smad3) in injured (I) and uninjured (C) muscles from TAs of WT and integrin-β3 KO mice at hours (B) or days (C) following injury (n = muscles from 3 mice).
Macrophages from Integrin-β3 KO Mice Polarize into M2 Phenotype
To evaluate a mechanism whereby injured muscles of integrin-β3 mice produce more TGF-β1 (Fig. 6), we examined the types of cells infiltrating injured muscles. First, we studied macrophages because neutrophils do not express integrin-β3 (18). In uninjured muscles of WT and integrin-β3 KO mice, there were no differences in the number of macrophages. However, at different times after injury, the mRNA of CD68 (a marker of activated macrophages) was lower in muscles of integrin-β3 KO mice versus WT mice (Fig. 7A). Consistent with this analysis, at 5 h after injury, FACS analysis revealed that there were fewer F4/80-positive macrophages in injured muscles of integrin-β3 KO mice versus WT mice (16.6 ± 2.4% in integrin-β3 KO versus 25.9 ± 3.2% in WT) (Fig. 7B). In contrast, the ratio of M2a macrophages (CD206-positive cells) to F4/80-positive macrophages was significantly higher in injured muscles of integrin-β3 KO mice (6.5 ± 0.2% in integrin-β3 KO versus 2.9 ± 0.8% in WT) (Fig. 7B). Western blotting confirmed the increase in CD206 and CD163 in injured muscles of integrin-β3 KO mice at different times after injury (3 h to 5 days; Fig. 7C). Thus, the absence of integrin-β3 decreases the infiltration of activated macrophages into injured muscles; it also favors macrophage differentiation into the M2, and especially, the M2a phenotype.
FIGURE 7.
Macrophages lacking integrin-β3 develop characteristics of M2 phenotype. A, CD68 mRNA was evaluated by RT-PCR using injured TA muscles at different times (n = muscles from 4 mice; means ± S.E.; *, p ≤ 0.05, difference between values from WT and integrin-β3 KO mice). B, a representative FACS analysis of cells expressing CD206 and F4/80 is shown. The cells were obtained 5 h after gastrocnemius injury; the upper right area includes M2a macrophage cells. (Data are means ± S.E.; n = muscles of 5 mice.) C, Western blot analysis of the M2 macrophage markers, CD206 and CD163, in injured TA muscles of WT and integrin-β3 KO mice. The muscles were obtained at different times after injury. D, BMDMs from WT (white column) or integrin-β3 KO mice (black column) were used to assess the expression of mRNAs of cytokines or genes (means ± S.E.; *, p ≤ 0.05; n = 3 repeated experiments). E, media from a 24-h culture of BMDMs of the M0, M1, M2a, or M2c from WT or integrin-β KO mice were analyzed for total TGF-β1 using an ELISA kit from Promega (n = 3 repeat experiments). The percentage of increase of TGF-β1 in BMDMs of integrin-β3 KO over results of WT is indicated (*, p ≤ 0.05, difference between integrin-β3 KO and WT).
To support the importance of macrophages, and specifically, their characteristics, we isolated BM from WT and integrin-β3 KO mice and differentiated them into macrophages (BMDMs, see “Experimental Procedures”). Using these cells, we measured the mRNAs of IFNγ, IL-4, and IL-10 because IFNγ is associated with the M1 phenotype, whereas IL-4 and IL-10 are associated with the M2 phenotype. BMDMs lacking integrin-β3 produced significantly more of the mRNAs of IL-4, TGF-β, and IL-10 but lower values of IFNγ when compared with BMDMs isolated from WT mice (Fig. 7D). This in vitro result supports the conclusion that macrophages lacking integrin-β3 develop into an M2 phenotype consistent with their expression of a higher level of CD163 and CD206 (Fig. 7D).
Reportedly, the alternative activated M2a macrophages produce an excess of TGF-β1 (8). To test whether the absence of integrin-β3 in BMDMs is associated with TGF-β1 production, we polarized BMDMs derived from integrin-β3 KO and WT mice into different types of macrophages including M1, M2a, or M2c type, and their activation stages were verified by both flow cytometry and RT-PCR (supplemental Fig. 2, A and B). Cultured macrophages representing the different macrophage types were tested for TGF-β1 by measuring its release into the media. The M2a and M2c macrophages lacking integrin-β3 produced 30–50% more total TGF-β1 than the WT macrophages (Fig. 7E). Thus, BMDMs lacking integrin-β3 produce more TGF-β1. This response could enhance fibrosis in injured muscles of integrin-β3 KO mice.
Transplantation of BM from Integrin-β3 KO Mice to WT Mice Suppressed Injury-induced Muscle Regeneration and Increased Fibrosis
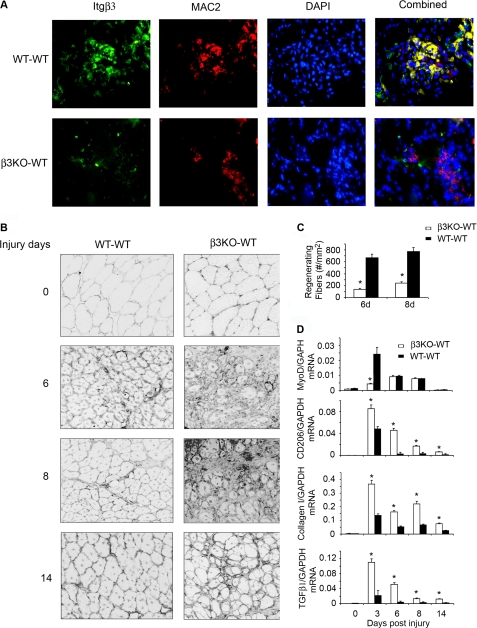
The hypothesis that integrin-β3 regulates macrophage migration, polarization, and TGF-β1 production was tested using a BM transplantation strategy. WT mice reconstituted with integrin-β3 KO BM (β3KO-WT) and WT mice with WT BM (WT-WT) were studied. As a control, we tested uninjured muscles in mice of the β3KO-WT transplant and found no morphologic differences from the WT-WT transplant (Fig. 8B, upper panel). However, infiltrating macrophages were decreased, as was the expression of integrin-β3 in injured muscles of β3KO-WT at 3 days after injury (Fig. 8A). Accompanying this change, MyoD expression in muscles of β3KO-WT mice was significantly lower (Fig. 8D), and there were fewer regenerating myofibers at 6–8 days after injury in muscles of β3KO-WT mice (Fig. 8, B and C). These findings were also present for up to 14 days after injury (Fig. 8B). Thus, the absence of integrin-β3 in BM cells suppressed macrophage infiltration as well as myogenic gene expression and muscle regeneration.
FIGURE 8.
BM transplant from integrin-β3 KO to WT mice decreases muscle regeneration following muscle injury. Muscles from WT mice bearing integrin-β3 KO BM were injured 30 days after the transplant. At 3, 6, 8, or 14 days after injury, the muscles from 4–6 mice were studied. A, at 3 days, the cryo-cross-section of muscles from WT transplanted with WT BM (WT-WT) or WT mice transplanted with integrin-β3 KO BM (β3KO-WT) were immunostained with integrin-β3 (green) or MAC2 (red; a macrophage marker). B, muscle morphology was visualized in laminin- and DAPI-stained cryo-sections. Over the entire period, there was decreased muscle regeneration in the β3KO-WT transplanted mice versus the WT-WT transplanted mice. C, the distribution of regenerating fibers also confirmed that there was a decrease in muscle regeneration in mice with the β3KO-WT transplant versus the WT-WT transplant. D, the mRNAs of MyoD, CD206, collagen-1, and TGF-β1 were examined by RT-PCR. Two-way analysis of variance showed a significant difference in the average values among treatment groups (p ≤ 0.05; *, β3KO-WT transplant versus the WT-WT transplant).
These results raise the question, why was satellite cell function decreased in the β3KO-WT transplant although the satellite cells in the WT mice that received the transplant were equivalent? One mechanism could involve increased TGF-β1 expression in injured muscles of β3KO-WT mice (Fig. 8D). This mechanism is suggested by the increase in CD206 macrophages present in the injured muscles (Fig. 8D). An increase in TGF-β1 could suppress satellite cell function and stimulate fibrosis in injured muscles of integrin-β3 KO mice. In fact, there was a significant increase in collagen 1 mRNA in injured muscles of the β3KO-WT transplanted mice (Fig. 8D). Taken together, BM transplantation from integrin-β3 KO to WT mice resulted in suppression of macrophage accumulation, proliferation of satellite cells, and regeneration of muscle plus an increase in fibrosis.
DISCUSSION
Our results indicate that following injury, critical responses of macrophages are regulated by integrin-β3, influencing the repair of injured muscle. We have concentrated on the role of integrin-β3 in muscle repair because our microarray analysis of mRNAs revealed increases in multiple integrin genes including integrin-β3 in response to muscle injury (Table 1, Fig. 1, supplemental Table 3). Specifically, integrin-β3 was undetectable in normal muscle, but from 5 h to 10 days after injury, its expression was dramatically increased. Using the integrin-β3 KO mice, we were able to identify a sequence of events in muscle that were stimulated by injury. In response to muscle injury, these mice exhibited incomplete regeneration (Fig. 3) plus increase in fibrosis. The latter was stimulated by increased production of TGF-β1 by macrophages. In exploring the mechanism for the increase in TGF-β1, we studied BMDMs from integrin-β3 KO mice and found that the cells polarized into the M2 macrophage phenotype and produced more TGF-β1. Because TGF-β1 will suppress the differentiation of satellite cells into myotubes, our finding that BMDMs from integrin-β3 KO mice produce more TGF-β1 explains why these mice have impaired muscle regeneration plus increased fibrosis.
There is evidence for similar events in other models of injury. For example, there is evidence that a crush injury stimulates the expression of integrin-α6 in muscle (19). In another study, integrin-β2 KO blocked overload-induced muscle hypertrophy while decreasing satellite cell proliferation (3). Finally, Liu et al. (4) reported that integrin-β3 is exclusively expressed in activated satellite cells, mediating their differentiation to contribute to muscle regeneration. Our results, however, indicate that the expression of integrin-β3 increased within 5–7 h after injury and not only preceded satellite cell activation but also occurred simultaneously with the infiltration of inflammatory cells into injured muscles. Consequently, our results suggest that the role of integrin-β3 in muscle regeneration is not limited to influencing satellite cell function but also stimulates changes in the types and responses of cells infiltrating the injured muscles. To prove this hypothesis, we performed BM transplants using BM from integrin-β3 KO mice into WT mice and compared the results with those of WT to WT BM transplants. Our results indicate that the absence of integrin-β3 in BM cells suppressed macrophage infiltration and increased the infiltration of macrophages of the M2 phenotype. These responses were accompanied by an expected increase in the expression of TGF-β1 and collagen 1 (Fig. 8) plus a decrease in MyoD expression and impaired regeneration of the injured muscles.
How does a decrease in macrophage infiltration impair muscle regeneration? The answer depends on the types of macrophages involved. For example, we found that KO of integrin-β3 impairs the accumulation of acute, proinflammatory M1 macrophages into injured muscles (Fig. 6). This is pertinent because others report that treating macrophages with anti-M-CSF to inhibit macrophages of the M1 type causes defective muscle regeneration (20). Likewise, depletion of circulating monocytes in transgenic, CD11b diphtheria toxin receptor mice prevents muscle regeneration, including a reduction in the diameter of regenerating myofibers (21). The depletion of intramuscular macrophages in this study was associated with reduction in the diameter of regenerating myofibers. Finally, depletion of monocytes or macrophages in mice was shown to delay the recovery of muscle force after injury (22). Taken together, the results suggest that there is a direct role of macrophages in promoting myofiber formation and growth.
To determine how macrophages lacking integrin-β3 change their phenotype in injured muscles, we studied BMDMs lacking integrin-β3. These cells initially produced an excess of IL-4 and IL-10 but lower amounts of IFNγ versus results from BMDMs of WT mice (Fig. 7D). This is relevant because the increase in IL-4 and IL-10 reflects the transition of M0 to the M2 macrophage phenotype and reflects the heterogeneity of macrophages. It is also consistent with reports that macrophages from interleukin-12-p40 KO mice exhibit a bias toward the M2 macrophage profile and secrete large amounts of TGF-β1 (23). Furthermore, proinflammatory M1 macrophages in injured muscles have been reported to be converted into an M2 phenotype (21). Conversely, activin A (a member of TGF-β superfamily) promotes an increase in the proinflammatory M1 phenotype but inhibits M2 macrophages (24).
In addition, an increase in TGF-β1 expression by macrophages can explain the fibrosis that occurs in injured muscles of integrin-β3 KO mice. Within 3–5 h after injury, the M2 macrophage markers CD206 and CD163 were expressed at high levels in muscles of integrin-β3 KO mice versus results in WT mice (Fig. 7C). Moreover, our in vivo and in vitro experimental results confirm that M2 macrophages produce more TGF-β1 (Fig. 7). The increase in TGF-β1 production is consistent with the report that mice lacking integrin-β3 produce excess TGF-β1 and will promote wound healing (25). In those experiments, the source of TGF-β1 was from platelets rather than macrophages. We tested for a similar result and did find accumulation of platelets in injured muscles of WT mice, but there were fewer platelets in injured muscles of integrin-β3 KO mice. This leads us to conclude that in our model of muscle injury, the increase in expression of TGF-β1 arises from macrophages rather than platelets.
In summary, our results provide new evidence that in response to muscle injury, the absence of integrin-β3 suppresses satellite cell activation and impairs muscle regeneration by stimulating the polarization of macrophages. The result is an increase in TGF-β1 production, providing a mechanism for reduced satellite cell function and increased fibrosis. Our results could aid the development of strategies to promote muscle growth in inflammatory diseases and conditions characterized by muscle wasting.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R37 DK37175 (to W. E. Mitch). This work was also supported by a Norman S. Coplon extramural research grant from the Satellite Health and American Diabetic Association (to L. Z.) and by the generous support of Dr. and Mrs. Harold Selzman.

This article contains supplemental Figs. 1 and 2 and Tables 1–3.
- TA
- tibialis anterior
- BMDM
- bone marrow-derived macrophage
- BM
- bone marrow
- α-SMA
- α-smooth muscle actin
- Itg
- integrin.
REFERENCES
- 1. Mayer U., Saher G., Fässler R., Bornemann A., Echtermeyer F., von der Mark H., Miosge N., Pöschl E., von der Mark K. (1997) Absence of integrin-α 7 causes a novel form of muscular dystrophy. Nat. Genet. 17, 318–323 [DOI] [PubMed] [Google Scholar]
- 2. Taverna D., Disatnik M. H., Rayburn H., Bronson R. T., Yang J., Rando T. A., Hynes R. O. (1998) Dystrophic muscle in mice chimeric for expression of α5-integrin. J. Cell Biol. 143, 849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marino J. S., Tausch B. J., Dearth C. L., Manacci M. V., McLoughlin T. J., Rakyta S. J., Linsenmayer M. P., Pizza F. X. (2008) β2-Integrins contribute to skeletal muscle hypertrophy in mice. Am. J. Physiol. Cell Physiol. 295, C1026–C1036 [DOI] [PubMed] [Google Scholar]
- 4. Liu H., Niu A., Chen S. E., Li Y. P. (2011) β3-Integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 25, 1914–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tidball J. G., Villalta S. A. (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang L., Ran L., Garcia G. E., Wang X. H., Han S., Du J., Mitch W. E. (2009) Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am. J. Pathol. 175, 2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vidal B., Serrano A. L., Tjwa M., Suelves M., Ardite E., De Mori R., Baeza-Raja B., Martínez de Lagrán M., Lafuste P., Ruiz-Bonilla V., Jardí M., Gherardi R., Christov C., Dierssen M., Carmeliet P., Degen J. L., Dewerchin M., Muñoz-Cánoves P. (2008) Fibrinogen drives dystrophic muscle fibrosis via a TGF-β/alternative macrophage activation pathway. Genes Dev. 22, 1747–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desguerre I., Mayer M., Leturcq F., Barbet J. P., Gherardi R. K., Christov C. (2009) Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J. Neuropathol. Exp. Neurol. 68, 762–773 [DOI] [PubMed] [Google Scholar]
- 10. Serrano A. L., Muñoz-Cánoves P. (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp. Cell Res. 316, 3050–3058 [DOI] [PubMed] [Google Scholar]
- 11. Shi Y., Massagué J. (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 12. Runyan C. E., Schnaper H. W., Poncelet A. C. (2003) Smad3 and PKCδ mediate TGF-β1-induced collagen I expression in human mesangial cells. Am. J. Physiol. Renal Physiol. 285, F413–FF422 [DOI] [PubMed] [Google Scholar]
- 13. Li Y., Foster W., Deasy B. M., Chan Y., Prisk V., Tang Y., Cummins J., Huard J. (2004) Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am. J. Pathol. 164, 1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tusher V. G., Tibshirani R., Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L., Du J., Hu Z., Han G., Delafontaine P., Garcia G., Mitch W. E. (2009) IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 20, 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L., Wang X. H., Wang H., Du J., Mitch W. E. (2010) Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J. Am. Soc. Nephrol. 21, 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longbrake E. E., Lai W., Ankeny D. P., Popovich P. G. (2007) Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J. Neurochem. 102, 1083–1094 [DOI] [PubMed] [Google Scholar]
- 18. Rieu P., Lesavre P., Halbwachs-Mecarelli L. (1993) Evidence for integrins other than β2 on polymorphonuclear neutrophils: expression of α6β1 heterodimer. J. Leukoc. Biol. 53, 576–582 [DOI] [PubMed] [Google Scholar]
- 19. Sorokin L. M., Maley M. A., Moch H., von der Mark H., von der Mark K., Cadalbert L., Karosi S., Davies M. J., McGeachie J. K., Grounds M. D. (2000) Laminin-α4 and integrin-α6 are up-regulated in regenerating dy/dy skeletal muscle: comparative expression of laminin and integrin isoforms in muscles regenerating after crush injury. Exp. Cell Res. 256, 500–514 [DOI] [PubMed] [Google Scholar]
- 20. Segawa M., Fukada S., Yamamoto Y., Yahagi H., Kanematsu M., Sato M., Ito T., Uezumi A., Hayashi S., Miyagoe-Suzuki Y., Takeda S., Tsujikawa K., Yamamoto H. (2008) Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp. Cell Res. 314, 3232–3244 [DOI] [PubMed] [Google Scholar]
- 21. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dumont N., Frenette J. (2010) Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am. J. Pathol. 176, 2228–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bastos K. R., Alvarez J. M., Marinho C. R., Rizzo L. V., Lima M. R. (2002) Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J. Leukoc. Biol. 71, 271–278 [PubMed] [Google Scholar]
- 24. Sierra-Filardi E., Puig-Kröger A., Blanco F. J., Nieto C., Bragado R., Palomero M. I., Bernabéu C., Vega M. A., Corbí A. L. (2011) Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 117, 5092–5101 [DOI] [PubMed] [Google Scholar]
- 25. Reynolds L. E., Conti F. J., Lucas M., Grose R., Robinson S., Stone M., Saunders G., Dickson C., Hynes R. O., Lacy-Hulbert A., Hodivala-Dilke K. (2005) Accelerated re-epithelialization in β3-integrin-deficient mice is associated with enhanced TGF-β1 signaling. Nat. Med. 11, 167–174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.