Background: Mice lacking integrins α2β1 and α11β1 are dwarfs.
Results: Bones are shorter and less mineralized in absence of osteoblast-specific defects. Severely reduced IGF-1, GH, and GHRH levels result in proportional dwarfism.
Conclusion: Integrins α2β1 and α11β1 crucially regulate IGF-1 levels.
Significance: We present a novel concept for the role of integrins in growth control, thereby coupling ECM signaling to endocrine homeostasis.
Keywords: Bone, Collagen, Extracellular Matrix, Growth Hormone, Integrin, Dwarfism, IGF-1
Abstract
Mice with a combined deficiency in the α2β1 and α11β1 integrins lack the major receptors for collagen I. These mutants are born with inconspicuous differences in size but develop dwarfism within the first 4 weeks of life. Dwarfism correlates with shorter, less mineralized and functionally weaker bones that do not result from growth plate abnormalities or osteoblast dysfunction. Besides skeletal dwarfism, internal organs are correspondingly smaller, indicating proportional dwarfism and suggesting a systemic cause for the overall size reduction. In accordance with a critical role of insulin-like growth factor (IGF)-1 in growth control and bone mineralization, circulating IGF-1 levels in the sera of mice lacking either α2β1 or α11β1 or both integrins were sharply reduced by 39%, 64%, or 81% of normal levels, respectively. Low hepatic IGF-1 production resulted from diminished growth hormone-releasing hormone expression in the hypothalamus and, subsequently, reduced growth hormone expression in the pituitary glands of these mice. These findings point out a novel role of collagen-binding integrin receptors in the control of growth hormone/IGF-1-dependent biological activities. Thus, coupling hormone secretion to extracellular matrix signaling via integrins represents a novel concept in the control of endocrine homeostasis.
Introduction
Nearly all cells in multicellular organisms are surrounded by an extracellular matrix (ECM)4 that provides to tissues structural support, organization, and orientation, which are indispensable for tissue morphogenesis, maintenance, and homeostasis (1–4). Extensive communication between cells and the surrounding ECM is mediated mainly by receptors of the integrin family (5–8). Integrins are heterodimeric transmembrane receptors of one α and one β subunit in non-covalent association that bind ligands with their extracellular domains, whereas their intracellular tails associate via linker proteins with the actin cytoskeleton. Signaling via integrins can proceed bidirectionally, from the outside in and the inside out, thus regulating virtually all cellular functions, including adhesion, polarity, differentiation, and survival.
Collagens represent the most abundant fibrous component of interstitial ECM, accounting for up to 30% of total protein mass in multicellular animals (2, 9). The 28 different collagen types can be grouped according to the supramolecular arrangement occurring in tissues (10). The fibril-forming collagen I is the most abundant type in interstitial ECM, supporting cell adhesion and migration and providing tensile strength. Collagen I fibrils are recognized most efficiently by two of four collagen-binding integrins, α2β1 (11) and α11β1 (12, 13), whereas network-forming collagens are preferred ligands for integrins α1β1 and α10β1 (14).
We aimed to delineate the functions of integrins α2β1 and α11β1 in tissues rich in fibrillar collagen I by analyzing mice deficient for either α2β1 (15–19) or α11β1 (20–23). These studies yielded distinct functions in spatial collagen reorganization in vitro and in vivo. Both integrin receptors confer high avidity cell binding to the sequence GFOGER (O = hydroxyproline) in triple helical collagen I (13, 24), and both receptors have been implicated in the organization of interstitial collagen fibrils (20, 25, 26). Integrin α2β1 is widely distributed, including expression by epithelial cells, mesenchymal cells such as fibroblasts, endothelial cells, chondrocytes, inflammatory and immune cells, as well as platelets (18, 27, 28). Mice ablated for the receptor are viable and fertile because of extensive functional compensation by the other collagen-binding integrins but display defects in homeostasis (15, 29), enhanced wound and tumor angiogenesis (17, 19), and diminished mammary gland branching morphogenesis (30). By contrast, α11β1 is restricted to subsets of ectomesenchymal and mesenchymal cells and detected abundantly during development in tissues adjacent to forming cartilage and bone (21, 31). In mice deficient for α11β1 the incisors fail to erupt normally because of malfunction of fibroblasts in the periodontal ligament that express α11β1 as the sole collagen receptor (21).
Here we concentrated on the role of integrins α2β1 and α11β1 in bone development, function, and homeostasis in mice deficient for either one or both integrins. Bone is highly enriched in collagen I that is continuously remodeled to maintain mineral homeostasis and structural integrity (32). This is accomplished by osteoclasts resorbing the mineralized matrix and osteoblasts forming new bone matrix in a tightly coordinated process (33). Several integrins have been suggested to play a role in bone development. Aszodi et al. (34) reported the essential role of the integrin β1 subunit for endochondral bone formation. Apparently two β1 receptors recognizing collagen are involved. Ekholm et al. (35) demonstrated the involvement of integrin α1β1 in proper callus formation in the healing process following bone fracture, and Bengtsson et al. (36) highlighted the importance of integrin α10β1, which is a collagen receptor with cartilage-restricted expression, for growth plate morphogenesis. Further functions for αvβ3 and β1 integrins were implicated in bone modeling and remodeling (37, 38) and in transducing mechanical signals (39–41).
Mice constitutively ablated for integrin α11β1 are significantly smaller in size than littermate controls, implicating this collagen receptor in bone development (21), whereas no such difference was noted in α2β1 deficient mice (15, 30). Bone growth and metabolism, and hence body size, are regulated by growth factors, including insulin-like growth factor (IGF)-1 (42). Its crucial role is illustrated by various IGF-1 knockout mouse models with phenotypes ranging from embryonic lethality to mild defects in skeletal development (43–46). Serum IGF-1 is predominantly produced by liver cells upon stimulation of the growth hormone receptor by growth hormone (GH). GH is synthesized by the pituitary gland, and its release is controlled by growth hormone-releasing hormone (GHRH), which is secreted by the hypothalamus (47). In serum, IGF-1 is detected in a complex with IGF-binding protein 3 (IGFBP-3) and acid-labile subunit (ALS), which prolongs the half-life of IGF-1 (48).
Integrins can engage in reciprocal cross-talk with growth factor receptors and are able to regulate their phosphorylation (49). Interestingly, signaling via the IGF-1 receptor can be modulated by β1 integrins in response to IGF-1 stimulation (50). Disruption of the interaction between integrins and the IGF-1 receptor blocks IGF-1-induced signaling, thereby influencing bone remodeling (51).
The results presented here demonstrate that the combined loss of integrins α2β1 and α11β1 severely compromises growth as well as structural and mechanical bone properties. In all aspects analyzed, loss of α11β1 seems to elicit more severe effects than that of α2β1. In vitro functional tests show that these alterations are not osteoblast autonomous but result from a systemic defect characterized by sharply reduced serum IGF-1 levels linked to dysregulated hypothalamic GHRH and pituitary gland-derived GH expression.
EXPERIMENTAL PROCEDURES
Mice
Mice deficient for integrin α2β1 (15) and integrin α11β1 (15, 21) were backcrossed into the C57Bl6 background for six generations. Integrin α2β1-deficient and α11β1-deficient mice were bred to generate double-deficient animals. Animals were housed in specific pathogen-free facilities. All animal protocols were approved by the local veterinary authorities. Genotyping was performed by PCR of tail genomic DNA as described (15, 21).
X-ray Analysis
Anesthetized mice were examined using a bench x-ray unit (HP cabinet x-ray system, Faxitron series, model 43855A, Hewlett-Packard), with single-side emulsion film (Agfa-Ts Structurix D4DW, Non-Destructive Testing (NDT) system) at 50 kV with an exposure time of 48 s.
Peripheral Quantitative Computed Tomography (pQCT) Measurements
Right femora of 1- and 3-month-old mice were scanned by pQCT using an XCT Research M scanner and software 5.50 (Stratec Medizintechnik GmbH) as described previously (52). Bones were scanned at the distal femoral metaphysis (at 15%, 17.5%, and 20% of total bone length measured from the distal joint line) and at the midshaft (at 50% of total bone length). Trabecular parameters (area, bone mineral density, and bone mineral content) were determined as the mean of the three slices at the distal femoral metaphysis. Cortical parameters (area, bone mineral density, and bone mineral content) as well as cortical thickness were evaluated at the midshaft.
Mechanical Testing
Femora were loaded until failure by a three-point bending test using a materials testing machine (Z2.5/TN1S, Zwick GmbH & Co.) (53). Ultimate load (Fmax, N), deformation (d, mm), energy (U, mJ) and stiffness (S, N/mm) were determined from the load-deformation curve. Ultimate stress (σ, MPa), strain (ϵ, %) and elastic modulus (E, MPa) were calculated as described (54). The cross-sectional moment of inertia was obtained from the midshaft pQCT scan.
Determination of Circulating IGF-1 and IGFBP3 Levels
Blood collected by cardiac puncture was centrifuged (1000 × g, 30 min at 4 °C). Plasma was collected and stored at −80 °C, and IGF-1 and IGFBP3 levels were analyzed by Radio-I Immuno-assay kit and ELISA, respectively (Mediagnost).
Histology
Tibiae of newborn mice were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4) and embedded in paraffin. Sections of 5 μm were deparaffinized, rehydrated, and stained with Alcian blue/hematoxylin-eosin according to standard protocols.
Cell Culture
Primary osteoblasts were isolated from calvariae of P2-P5 mice by two 30-min digestion steps in 0.1% collagenase IV (Sigma), seeded at 104/cm2, and cultured in DMEM supplemented with 10% fetal bovine serum (PAA Laboratories). Primary fibroblasts were isolated from trunk skin of newborn animals and cultured as described (18).
Determination of Mineralization
Osteoblasts were seeded in microwell plates at 104/cm2 and cultured in presence of 10 mm phosphoglycerate and 5 mm phosphoascorbate to induce osteogenic differentiation and mineralization (day 0) for up to 18 days. At days 4, 7, 11, 14, and 18 triplicate wells of each culture were fixed in methanol and washed in water, and mineralization was quantified in a microplate reader after Alizarin red S staining as described (56). In three individual experiments, cells from at least five animals per genotype were analyzed in triplicates.
Quantitative RT-PCR
cDNA was generated using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and amplified using TaqMan Universal PCR Master Mix, No AmpErase Uracil N-glycosylase (UNG) with TaqMan assay-on-demand kits for IGF-1, GHRH, glucoronidase β, and HPRT (Applied Biosystems). Further primer/probe sequences used were integrin α2 (α2 probe, GCT-GCT-AAT-GCT-AGT-TCA-AG; α2 sense primer, AGA-GAA-CTC-CTC-CGT-ACA-GT; α2 antisense primer, CTG-GGA-GGC-CAA-CAT-TAT-AC), integrin α11 (α11 probe, GCA-ACT-GCA-CCA-AGC-TCA-ACC-TGG; α11 sense primer, GCT-GCC-TTC-TTT-GGC-TAC-ACA-GTA-C; α11 antisense primer, TTG-GGG-TTG-GTG-GCA-AGG-CT) and GH (GH probe, GTT-CGA-GCG-TGC-CTA-CAT-TC; GH sense primer GCC-CTT-GTC-CAG-TCT-GTT-TTC; GH antisense primer GAT-GGT-CTC-TGA-GAA-GCA-GAA-AG). Total RNA input for generation of cDNA was adjusted using a Nanodrop (ThermoScientific) for each sample. Relative expression in samples was adjusted for total RNA content by glucoronidase β or hypoxanthine guanine phosphoribosyl transferase expression. Calculations were performed by a comparative method 2−ΔΔCT (69). Quantitative RT-PCR was performed on an ABI Prism 7700 sequence detector (Applied Biosystems).
Statistical Analysis
Results are presented as mean ± S.D. Statistical differences between wild-type and knockout mice were assessed by unpaired Student́s t test. Differences were considered to be significant at p < 0.05. Statistical analysis of mineralization assays was conducted using the Wilcoxon rank-sum test. One-way analysis of variance was used to test for significant differences between wild-type and integrin-deficient mice in pQCT measurements and a three-point bending test. To detect significant differences between groups, significant analysis of variance were followed by a post hoc Tukey test for multiple comparisons. Differences were considered significant at α < 0.05. Statistical calculations were performed using SPSS for Windows (SPSS 18.0, SPSS Inc., Chicago, IL).
RESULTS
Decreased Bone Mineral Density and Bone Strength in Mice Deficient for Integrin α11β1 or α2β1 and α11β1
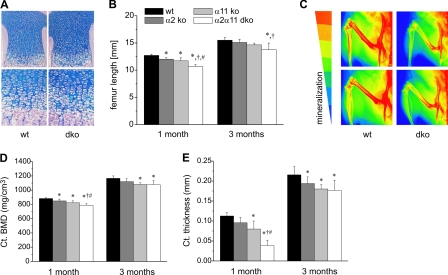
Mice deficient in α2β1, α11β1, or both integrin receptors were born without any conspicuous difference in size or bone phenotype. This observation was confirmed by histological analysis of newborn tibiae showing unaltered columnar arrangement of chondrocytes in epiphyseal cartilage of double-deficient mice compared with wild-type controls (Fig. 1A). However, during the first 4 weeks of postnatal life, growth of long bones, as exemplified for femurs, was significantly attenuated especially in mutants lacking integrin α11β1 and in double-deficient mice (Fig. 1B). Significant femoral growth retardation in double-deficient mice persisted during the first 3 months of age (Fig. 1B), whereas animals lacking either integrin α2β1 or α11β1 showed a tendency toward reduced femur length (Fig. 1B and supplemental Fig. S1A). In addition to length, bone density of femurs and tibiae in double-deficient animals was severely reduced, as shown by x-ray analysis confecting two-dimensional false color images of the hind limbs (Fig. 1C).
FIGURE 1.
Cartilage morphology and structural/geometrical bone parameters. A, hematoxylin/eosin staining of tibiae of newborn wild-type and double-deficient (dko) mice reveal no obvious histological defects in cartilage morphology. B, femur length of male wild-type and integrin mutants was measured at the age of 1 and 3 months, revealing reduced femur length in double-deficient animals. C, x-ray analysis confecting two-dimensional false color images of the hind limbs illustrates the degree of mineralization from red, indicating high, to blue, indicating low mineralization. Particularly femurs of mice lacking α2β1 and α11β1 integrins are mineralized to a lesser extent than wild-type controls. D and E, pQCT was carried out using male wild-type and integrin-deficient mice at the age of 1 and 3 months. D, mean ± S.D. cortical bone mineral density (ct. BMD). E, mean cortical (ct.) thickness of male mice is most severely reduced in α2β1 and α11β1-deficient animals. *, significant difference to WT, p < 0.05; †, significant difference to α2 KO, p < 0.05; #, significant difference to α11 KO, p < 0.05.
To confirm this bone defect in double-deficient mice and to dissect the contribution of each integrin, wild-type, α2β1, α11β1, and double-deficient mice (aged 1 or 3 months) were subjected to detailed bone geometrical and structural analysis using pQCT. Scans through the femoral midshaft allowed evaluation of cortical bone parameters, whereas scans through the distal epiphyseal bone were performed to quantify trabecular determinants. Cortical bone mineral density (Ct. BMD) was significantly reduced in 1- and 3-month-old animals lacking α11β1 or both α2β1 + α11β1 integrins, corroborating the false color images (Fig. 1D and supplemental Fig. S1B). Cortical thickness was also diminished, with the most significant changes seen in double-deficient mice (Fig. 1E and supplemental Fig. S1C). α2β1 deficiency resulted in mild reduction of cortical bone mineral density and cortical thickness. In contrast to cortical parameters, trabecular bone mineral density and content were only affected in double-deficient mice at 4 weeks but did not differ significantly from controls at 3 months (supplemental Table S1).
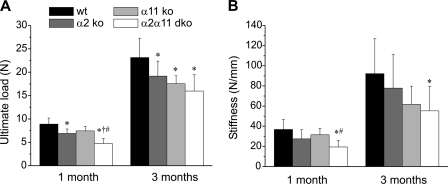
To test whether these altered bone geometrical and structural parameters translate into functional impairment, we subjected femurs of control and mutant mice of 1 and 3 months to three-point bending tests used to measure the maximal load taken by a bone before it breaks (defined as ultimate load). Femurs of integrin-deficient mice broke at significantly lower load than that applied to wild-type femurs (Fig. 2A and supplemental Fig. S1D). Moreover, bone stiffness was particularly reduced in mice lacking α2β1 and α11β1 (Fig. 2B and supplemental Fig. S1E). Energy and bending moment were decreased, whereas the elastic modulus was unaffected in mutant mice (supplemental Fig. S2).
FIGURE 2.
Functional bone parameters. A, mean ± S.D. ultimate load. B, mean stiffness of femora of male wild-type and integrin-deficient mice were measured using a three-point bending test. Integrin-deficient femora show a reduction in ultimate load and bone stiffness with a gradual decrease from α2β1 < α11β1 < double-deficient (dko) animals, which show the most severe phenotype. *, significant difference to WT, p < 0.05; †, significant difference to α2 KO, p < 0.05; #, significant difference to α11 KO, p < 0.05.
Taken together, these results showed most severe alterations in femur properties in mice deficient for integrins α2β1 + α11β1, followed by severe alterations in α11β1-deficient and mildest defects in α2β1-deficient mice.
Normal Osteoblast Function in Integrin-deficient Mice
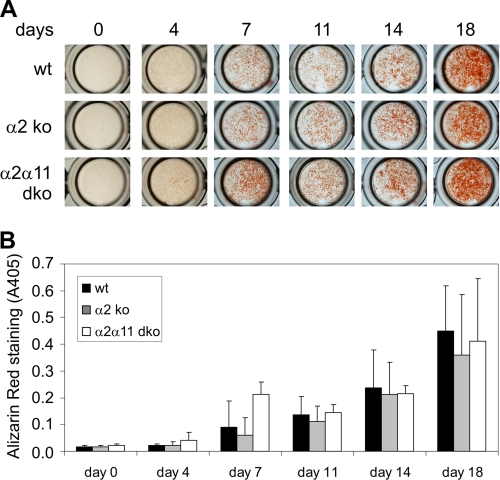
As mineralization of bone tissue is accomplished by osteoblasts, we analyzed whether the loss of integrins α2β1 and/or α11β1 has a direct influence on osteoblast function. Therefore, differentiation of primary osteoblasts, isolated from calvaria of newborn mice deficient in α2β1 or α2β1 and α11β1 as well as from wild-type control animals, was induced by adding differentiation-stimulating media containing 2-phosphoascorbic acid and β-phospho-glycerate. Expression of both collagen receptors was detected in wild-type osteoblasts in culture (supplemental Fig. S2A). Integrin α2 transcript levels increased with extent of differentiation, whereas α11 expression was not modulated by differentiation status.
Mineralization of osteoblast cultures was assessed after 0, 4, 7, 11, 14, and 18 days using Alizarin red solution, which stains calcium-containing hydroxyapatite deposits. After 7 days, all cultures displayed clearly visible Alizarin red-stained mineral deposits, and mineralization progressed during the time course of the experiment (Fig. 3A, one representative culture per genotype shown). Staining intensities were comparable between the different genotypes at all time points analyzed and quantification of the incorporated dye after acidic extraction did not reveal statistically significant alterations in mineralization (Fig. 3B). No significant alterations were detected in osteoblast differentiation as visualized by determining alkaline phosphatase levels nor in osteoblast proliferation (supplemental Fig. S2, B and C).
FIGURE 3.
Functional analysis of primary osteoblasts. Osteoblasts were isolated from calvaria of newborn wild-type (wt) mice and those deficient in α2β1 (α2 ko) or double-deficient (α2α11 dko) and cultured for up to 14 days with and without differentiation medium containing 2-phosphoascorbic acid and β-phosphoglycerate. A, osteoblast cultures were stained with Alizarin red at various time points. B, amounts of incorporated dye (mean ± S.D.) reflecting the degree of mineralization, quantified by assessing absorption at 405 nm, revealed no obvious differences between genotypes. n ≥ 5 animals (= cultures) per genotype. Each culture was analyzed in triplicate wells per assay.
This result clearly indicated that α2β1 osteoblasts and α2β1 and α11β1-deficient osteoblasts are fully competent in depositing a calcified bone matrix. Taken together, our results did not reveal an osteoblast-specific phenotype caused by altered osteoblast function.
Mice Lacking Integrins α2β1 and α11β1 Are Dwarfs
Mice with combined ablation of integrins α2β1 and α11β1 were easily detectable among their littermates by reduced body size (Fig. 4A). To assess the contribution to this effect by the individual integrins, detailed weight and size recordings were taken for animals deficient in integrin α2β1 or α11β1 and for double-deficient mice at 1 and 3 months. At the age of 1 month, mutants were smaller (Fig. 4C and supplemental S1, F and G) and weighed less (Fig. 4D and supplemental Fig. S1, H and I) when compared with wild-type controls, with the mildest phenotype observed in α2-null and the most severe one in double-deficient animals. These growth defects persisted until adulthood, as illustrated by size and weight recordings of animals at 3 months, pointing to growth impairment in integrin mutants rather than to growth delay. The difference accounted for 20–25% in double deficient animals versus wild-type controls and was similar to the reduction in size reported for α11β1 single mutants (21). Mass and size of inner organs were also reduced in α2β1 and α11β1 double mutants and in α11β1-null animals versus controls (Fig. 4B, upper and center panel). However, correlating organ weight with overall body weight abrogated these differences (Fig. 4B, lower panel). Hence, these mice developed severe proportional dwarfism after birth.
FIGURE 4.
Integrin-deficient mice are dwarfs. A, mice with combined ablation of integrins α2β1 and α11β1 were easily detectable among their littermates by reduced body size. B, reduced size of inner organs (heart, spleen, kidney) of wild-type and integrin-deficient mice. Bar charts display wet weight of organs (center panel) as well as wet weight of organs normalized to body weight (lower panel). In α11β1 and double-deficient (dko) mice, wet weight of heart, spleen, and liver is reduced, whereas in relation to body weight, the difference is abrogated, illustrating a proportional dwarfism in these animals. Body size from nose to anus (C) and weight (D) of male wild-type and integrin-deficient mice at the age of one and three months. Integrin α11β1 and double-deficient mice are smaller and weigh less when compared with wild-type controls with the most prominent differences observed in animals lacking both integrin subunits. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
Severely Decreased IGF-1 Levels in Sera of Integrin-deficient Mice
The dwarfism as well as structural and geometrical bone properties observed, especially in α2β1 and α11β1-deficient mice, could obviously not be attributed to growth plate alterations nor to osteoblast dysfunction. To identify the underlying mechanisms, systemic alterations, e.g. in IGF-1 levels and IGF-1 signaling, were explored.
First, we tested whether integrin-deficient cells were capable of responding to IGF-1 stimulation in vitro by assessing phosphorylation of the serine/threonine protein kinase AKT, a well established downstream signaling target of IGF-1. Cell lysates harvested from cultured primary skin fibroblasts and osteoblasts after stimulation with recombinant IGF-1 were subjected to Western blot analysis using antibodies directed against phosphorylated AKT. Stimulation of phosphorylated AKT by insulin was included as positive control. Similar phosphorylated AKT signals were detected upon stimulation with IGF-1 or insulin regardless of cell type and genotype (supplemental Fig. S3, A and B), clearly demonstrating that cells lacking α2β1 and/or α11β1 integrins were fully competent to activate the endogenous PI3K-AKT signaling cascade in response to IGF-1 in vitro.
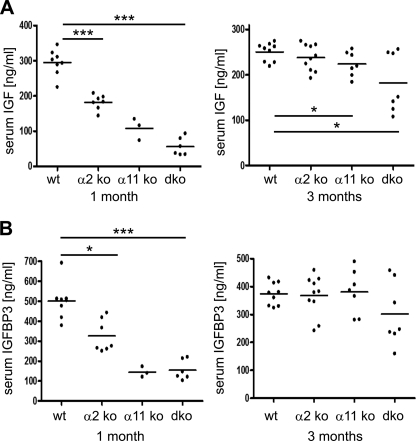
We then assessed circulating levels of IGF-1 and IGFBP3 in the sera of 1- and 3-month old mice. Dramatic alterations were observed at the age of 1 month, with significantly reduced serum levels in integrin-deficient mice (Fig. 5, A and B, and supplemental Fig. S1, J–M). The most prominent reduction of both IGF-1 and IGFBP-3 was seen in α11β1 and α2β1 and α11β1 double-deficient animals at the age of 1 month, with up to 85% reduction of IGF-1 and up to 70% reduction of IGFBP-3. By 3 months, IGF-1 levels ameliorated but remained markedly lower in double-deficient compared with wild-type animals.
FIGURE 5.
IGF-1 and IGFBP-3 protein levels. Levels of IGF-1 (A) and IGFBP-3 (B) were assessed in sera of male mice at the age of one and three months. Mutant mice show decreased IGF-1 and IGFBP-3 levels, with the most severe reduction observed in 1-month-old double-deficient (dko) mice.
Reduced Hepatic IGF-1 Expression in Integrin-deficient Mice
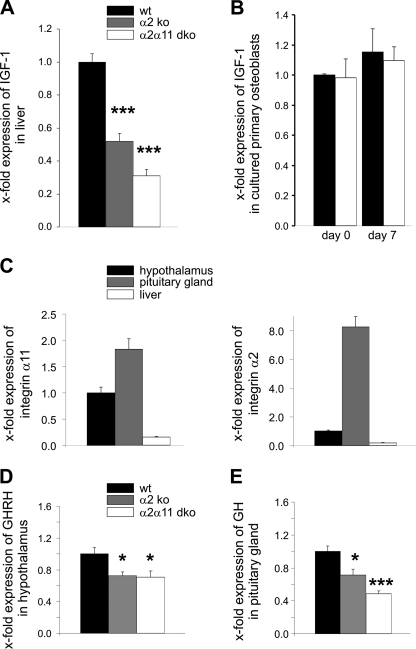
Reduced serum IGF-1 levels indicated that hepatic IGF-1 production was affected, particularly in mice lacking integrins α2β1 and α11β1. Impaired hepatic IGF-1 expression was confirmed by real-time analysis of liver extracts from wild-type, α2β1, and α2β1 and α11β1 double-deficient animals (Fig. 6A). In contrast to low systemic IGF-1 produced in the liver, locally produced IGF-1 mRNA by primary cultured osteoblasts derived from double-deficient integrin mutants was not altered (Fig. 6B). These results demonstrated that the phenotype of integrin-deficient mice is caused by insufficient hepatic IGF-1 production.
FIGURE 6.
Analysis of the GH/IGF-1 axis. A, IGF-1 mRNA expression in liver extracts of α2β1 and double-deficient female mice was significantly decreased in comparison to the wild type. B, similar amounts of locally produced IGF-1 mRNA levels were assessed in cultured primary wild-type and double-deficient osteoblasts at the beginning (day 0) and after 7 days in culture with osteogenic media. C, qRT-PCR analysis of integrin α11 and α2 transcripts demonstrates expression in hypothalamus and pituitary glands of wild-type mice. D, real-time analysis of hypothalamic GHRH mRNA expression revealed significantly reduced levels in α2β1 as well as double-deficient (dko) male mice. E, expression of GH mRNA in the pituitary gland was significantly lower in male integrin-deficient mice.
Reduced GHRH and GH Expression in Integrin-deficient Mice
The next experiments were aimed at elucidating whether impaired hepatic IGF-1 production might be caused by alterations in GHRH or GH production. Circulating IGF-1 levels produced by the liver are indirectly controlled by GHRH, which is secreted by specialized hypothalamic neurons and acts on so-called somatotrophs in the pituitary gland to produce and release GH. GH in turn acts on GH receptors in the liver, inducing IGF-1 transcription and release.
To identify at which step of the GH/IGF-1 axis the regulation may be impaired, we micro-dissected hypothalami and pituitary glands of wild-type as well as α2β1 and α2β1 and α11β1 double-deficient mutants at the age of 6 weeks. Expression of both integrins was verified in microdissected wild-type tissues (Fig. 6C). Hypothalami of mice lacking α2β1 and of double mutants were characterized by significantly reduced GHRH expression (Fig. 6D). In addition, GH expression in pituitary glands was significantly reduced in these mice (Fig. 6E). Correlating with decreased GH mRNA transcripts, GH serum levels were clearly diminished in 6-week-old double-deficient mice (supplemental Fig. S3C). To rule out a generalized loss of hypothalamic functions by integrin deficiency, we determined the expression of several other hypothalamic neuropeptides, including corticotrophin-releasing hormone, thyrotropin-releasing hormone, gonadotropin-releasing hormone, and somatostatin, which are involved in regulating glucocorticoid synthesis, energy expenditure, sex hormone synthesis, as well as antagonism of GHRH signaling. Unchanged expression of these peptides in comparison to controls demonstrated that overall hypothalamic function was not affected by integrin deficiency (supplemental Fig. S4). This result clearly implicates reduced GHRH production regulating systemic IGF-1 levels as a specific defect resulting from loss of α2β1 and α11β1.
DISCUSSION
Mice with constitutive ablation of integrins α2β1 + α11β1, the two major integrins binding to collagen I, were born without any conspicuous abnormalities but failed to grow to normal size. This defect developed within the first 4 weeks of life and persisted but was not amplified with age (e.g. at 3 months). To assess the contribution of each individual integrin, we also characterized mice lacking either integrin α2β1 or α11β1. All results obtained suggested that lack of α2β1 created the mildest defects, whereas lack of α11β1 was more severe, and double-deficiency resulted in most severe defects. Small size in double-deficient mice was illustrated by significantly shortened and functionally weakened femurs but was not limited to skeletal dwarfism. Rather, their internal organs are also smaller, indicating that these animals display proportional dwarfism. This conclusion is supported by normal growth plate architecture and unimpaired osteoblast proliferation, differentiation, and ability to deposit a mineralized bone matrix. Osteoclast activity has not been assessed and can therefore not be excluded.
Proportional dwarfism could result from malnutrition caused by tooth defects that were reported earlier (21). However, several findings speak against this mechanism. First, size differences were striking before weaning (data not shown), and the most severe defects were observed in mice at 4 weeks of age. Thus, defects developed already during the time when pups were fed milk by their mothers. Therefore, malnutrition because of impaired tooth development appears unlikely. Second, overall metabolism in integrin mutants was normal as reflected by respiratory exchange rates, which were comparable with the wild type (supplemental Table S3). Third, the dramatic size reduction of mice lacking integrins α2β1 and α11β1 persisted even when fed soft food (21) and was not amplified with age (between 1 and 3 months), arguing against generalized malnutrition.
As osteoblast-specific defects and malnutrition appear unlikely as mechanisms causing dwarfism in integrin-deficient mice, global or systemic metabolic modifications were explored. Here we demonstrate severely reduced levels of systemic IGF-1 in mice lacking integrins α2β1 and α11β1. Consistent with the well established role of IGF-1 in controlling bone mass and strength and body size, reduced IGF-1 explains the bone alterations as well as the dwarfism seen in these mice.
Low IGF-1 levels resulted from impaired hypothalamic GHRH expression in the absence of integrin α2β1 and α11β1. Decreased GHRH expression is a specific defect in integrin-mutant hypothalamus, as expression of other hormones was unaffected. Low GHRH levels, in turn, correlated with correspondingly reduced GH expression in integrin-mutant pituitary glands. In line with the finding that integrin double mutants are born with normal size, GH was reported as not being essential for intra-uterine growth and development, as illustrated by normal-sized infants with deletions of the genes encoding GH or GH receptor (57), and GH deficiency is a well characterized condition that results in postnatal growth retardation (58).
GH released by the pituitary gland in response to hypothalamic GHRH and then acts on the liver to produce the bulk of systemic IGF-1. The crucial importance of the GH/IGF-1 axis for bone mineralization has been demonstrated in several rodent models and was implicated in the pathogenesis of osteopenia and osteoporosis (59). Mice carrying a mutation of the GH receptor show very low levels of systemic IGF-1 and display osteopenia with reduced cortical but normal trabecular bone (60). In contrast, increased bone formation and increased trabecular bone formation was detected in mice overexpressing IGF-1 in osteoblasts (61). The role of locally produced IGF-1 for trabecular bone integrity was further strengthened using mice with an osteoblast-restricted ablation of the IGF-1 receptor that showed reduced trabecular bone volume (45). By contrast, a global knockout of IGF-1 as well as IGF-1 haploinsufficiency lead to low cortical bone mineral density (43, 62), which was also observed in liver-specific IGF-1-deficient mice that are characterized by reduced serum IGF-1 levels (44). Collectively, these reports show that local skeletal IGF-1 regulates trabecular bone formation and integrity, whereas systemic IGF-1 controls cortical bone structure (63). Thus, our results on impaired cortical bone properties and mineralization, but less or unaffected trabecular bone in integrin double mutants, agree with the observed low levels of systemic IGF-1 and normal osteoblast-derived IGF-1 production.
The crucial role of IGF-1 as key growth regulator has been documented by various mouse models that indicated that a threshold level of circulating IGF-1 is required for normal body size (46): Absence of serum IGF-1 in global IGF-1 knockout mice results in severe growth retardation (43), as does residual IGF-1, amounting to about 10% of normal levels, observed in mice with simultaneous gene disruption of liver-specific IGF-1 and ALS (48). By contrast, IGF-1 amounting to 25% of normal levels is sufficient to permit normal body size, as was demonstrated by liver IGF-1-deficient (LID) mice (44, 64). Absence of integrins α2β1 and α11β1 reduces serum IGF-1 to 15% of normal levels, clearly showing that the threshold level required for normal growth is above this value.
As suggested by the phenotype of LID mice, a lacking negative feedback loop of liver-derived IGF-1 on GH secretion leads to a compensatory increase in serum GH levels in mice (64, 65). In addition, LID mice show increased expression of pituitary GHRH and ghrelin receptors indicating at least some feedback to the pituitary (66). However, the target sites of IGF-1 for regulating GH release remain unclear and might either be located in the hypothalamus and/or the pituitary. Recently, a cell-specific knockout mouse in which the IGF-1 receptor was ablated from the somatotroph, the so-called somatotroph IGF-1 receptor knockout mouse, has been generated (67). The phenotype of the somatotroph IGF-1 receptor knockout mouse includes increased GH expression and secretion as well as increased serum IGF-1 levels (67). The feedback mechanisms to the hypothalamus resulted in decreased GHRH and increased GHIH mRNA levels. Furthermore, decreased growth hormone-releasing hormone receptor expression was observed in the anterior pituitary of these mice (67). However, these changes were not able to reduce GH secretion in the somatotroph IGF-1 receptor knockout mouse, indicating a role of IGF-1R signaling in the pituitary in addition to the hypothalamus in regulating GH secretion (67). Thus, IGF-1 acts in the hypothalamus and the pituitary to regulate GH secretion.
Collectively, our study implicates decreased GHRH and GH expression as the crucial defect in α2β1 + α11β1 integrin-deficient mice, leading to dramatically reduced GH and IGF-1 serum levels.
Having unraveled this mechanism, further work will now be aimed at understanding how integrins α2β1 + α11β1 regulate hypothalamic GHRH and pituitary gland-derived GH. This will require detailed analysis of the precise integrin repertoire on the cell types involved in hormone production and release. Two models appear feasible. First, the integrins are directly involved in modulating production of GHRH or GH by being expressed on the respective cell types. Alternatively, integrin-dependent signals from the environment of hormone-producing cells are required to induce proper hormone release. Similar examples have been demonstrated for different cell types that release defined mediators only if placed in a collagen environment that activates integrin-dependent signaling (for review, see Ref. 68).
In conclusion, the results presented here suggest a novel function for integrins in the GH/IGF-1 axis and in growth control, thus, coupling of hormone secretion to ECM signaling via integrins may represent a novel concept in control of endocrine homeostasis.
Supplementary Material
Acknowledgments
We thank Gabriele Scherr, Ute Hillebrand, Susanne Neumann, and Angelika Arora (Dermatology, Cologne) for excellent technical assistance; Prof. Jürgen Koebke and Jutta Knifka (Anatomy II, Cologne) for helpful assistance with the x-ray analysis; and Prof. Wilhelm Stoffel (Molecular Neurosciences, Cologne) for providing us with metabolic cages.
This project was supported in part by Deutsche Forschungsgemeinschaft Grant SFB 829 (to B. E. and T. K.) and BR1492/7-1 (to J. C. B.), by Research Council of Norway Grants 1823258/S10 and 197066/V40 (to D. G.), by Norwegian Cancer Association Grant 536711-2010 (to D. G.), and by Else Kröner-Fresenius-Stiftung Grant 2010_A93 (to M. S.).

This article contains supplemental Figs. S1–S4 and Tables S1–S3.
- ECM
- extracellular matrix
- IGF
- insulin-like growth factor
- GH
- growth hormone
- GHRH
- growth hormone-releasing hormone
- IGFBP
- insulin-like growth factor binding protein
- ALS
- acid-labile subunit
- pQCT
- peripheral quantitative computed tomography.
REFERENCES
- 1. Eckes B., Nischt R., Krieg T. (2010) Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frantz C., Stewart K. M., Weaver V. M. (2010) The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hynes R. O. (2009) The extracellular matrix. Not just pretty fibrils. Science 326, 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barczyk M., Carracedo S., Gullberg D. (2010) Integrins. Cell Tissue Res. 339, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harburger D. S., Calderwood D. A. (2009) Integrin signalling at a glance. J. Cell Sci. 122, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humphries J. D., Byron A., Humphries M. J. (2006) Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 8. Wickström S., Radovanac K., Fässler R. (2011) in Extracellular Matrix Biology (Hynes R. O., Yamada K. M., eds) pp. 223–244, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 9. Rozario T., DeSimone D. W. (2010) The extracellular matrix in development and morphogenesis. A dynamic view. Dev. Biol. 341, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadler K. E., Baldock C., Bella J., Boot-Handford R. P. (2007) Collagens at a glance. J. Cell Sci. 120, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 11. Emsley J., Knight C. G., Farndale R. W., Barnes M. J., Liddington R. C. (2000) Structural basis of collagen recognition by integrin α2β1. Cell 101, 47–56 [DOI] [PubMed] [Google Scholar]
- 12. Velling T., Kusche-Gullberg M., Sejersen T., Gullberg D. (1999) cDNA cloning and chromosomal localization of human α(11) integrin. A collagen-binding, I domain-containing, β(1)-associated integrin α-chain present in muscle tissues. J. Biol. Chem. 274, 25735–25742 [DOI] [PubMed] [Google Scholar]
- 13. Zhang W. M., Kapyla J., Puranen J. S., Knight C. G., Tiger C. F., Pentikainen O. T., Johnson M. S., Farndale R. W., Heino J., Gullberg D. (2003) α11β 1 integrin recognizes the GFOGER sequence in interstitial collagens. J. Biol. Chem. 278, 7270–7277 [DOI] [PubMed] [Google Scholar]
- 14. Tulla M., Pentikäinen O. T., Viitasalo T., Käpylä J., Impola U., Nykvist P., Nissinen L., Johnson M. S., Heino J. (2001) Selective binding of collagen subtypes by integrin α 1I, α 2I, and α 10I domains. J. Biol. Chem. 276, 48206–48212 [DOI] [PubMed] [Google Scholar]
- 15. Holtkötter O., Nieswandt B., Smyth N., Müller W., Hafner M., Schulte V., Krieg T., Eckes B. (2002) Integrin α 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem. 277, 10789–10794 [DOI] [PubMed] [Google Scholar]
- 16. Nyström A., Shaik Z. P., Gullberg D., Krieg T., Eckes B., Zent R., Pozzi A., Iozzo R. V. (2009) Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity. Blood 114, 4897–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woodall B. P., Nyström A., Iozzo R. A., Eble J. A., Niland S., Krieg T., Eckes B., Pozzi A., Iozzo R. V. (2008) Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 283, 2335–2343 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z. G., Bothe I., Hirche F., Zweers M., Gullberg D., Pfitzer G., Krieg T., Eckes B., Aumailley M. (2006) Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins. Contribution of α2β1 integrin. J. Cell Sci. 119, 1886–1895 [DOI] [PubMed] [Google Scholar]
- 19. Zweers M. C., Davidson J. M., Pozzi A., Hallinger R., Janz K., Quondamatteo F., Leutgeb B., Krieg T., Eckes B. (2007) Integrin α2β1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J. Invest. Dermatol 127, 467–478 [DOI] [PubMed] [Google Scholar]
- 20. Carracedo S., Lu N., Popova S. N., Jonsson R., Eckes B., Gullberg D. (2010) The fibroblast integrin α11β1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J. Biol. Chem. 285, 10434–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Popova S. N., Barczyk M., Tiger C. F., Beertsen W., Zigrino P., Aszodi A., Miosge N., Forsberg E., Gullberg D. (2007) α11 β1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol. Cell Biol. 27, 4306–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Popova S. N., Rodriguez-Sánchez B., Lidén A., Betsholtz C., Van Den Bos T., Gullberg D. (2004) The mesenchymal α11β1 integrin attenuates PDGF-BB-stimulated chemotaxis of embryonic fibroblasts on collagens. Dev. Biol. 270, 427–442 [DOI] [PubMed] [Google Scholar]
- 23. Zhu C. Q., Popova S. N., Brown E. R., Barsyte-Lovejoy D., Navab R., Shih W., Li M., Lu M., Jurisica I., Penn L. Z., Gullberg D., Tsao M. S. (2007) Integrin α 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc. Natl. Acad. Sci. U.S.A. 104, 11754–11759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knight C. G., Morton L. F., Onley D. J., Peachey A. R., Messent A. J., Smethurst P. A., Tuckwell D. S., Farndale R. W., Barnes M. J. (1998) Identification in collagen type I of an integrin α2 β1-binding site containing an essential GER sequence. J. Biol. Chem. 273, 33287–33294 [DOI] [PubMed] [Google Scholar]
- 25. Langholz O., Röckel D., Mauch C., Kozlowska E., Bank I., Krieg T., Eckes B. (1995) Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α 1 β 1 and α 2 β 1 integrins. J. Cell Biol. 131, 1903–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Velling T., Risteli J., Wennerberg K., Mosher D. F., Johansson S. (2002) Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins α 11β 1 and α 2β 1. J. Biol. Chem. 277, 37377–37381 [DOI] [PubMed] [Google Scholar]
- 27. Klein C. E., Dressel D., Steinmayer T., Mauch C., Eckes B., Krieg T., Bankert R. B., Weber L. (1991) Integrin α 2 β 1 is up-regulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J. Cell Biol. 115, 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J. E., Santoro S. A. (1994) Complex patterns of expression suggest extensive roles for the α 2 β 1 integrin in murine development. Dev. Dyn. 199, 292–314 [DOI] [PubMed] [Google Scholar]
- 29. Grüner S., Prostredna M., Aktas B., Moers A., Schulte V., Krieg T., Offermanns S., Eckes B., Nieswandt B. (2004) Anti-glycoprotein VI treatment severely compromises hemostasis in mice with reduced α2β1 levels or concomitant aspirin therapy. Circulation 110, 2946–2951 [DOI] [PubMed] [Google Scholar]
- 30. Chen J., Diacovo T. G., Grenache D. G., Santoro S. A., Zutter M. M. (2002) The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am. J. Pathol. 161, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tiger C. F., Fougerousse F., Grundström G., Velling T., Gullberg D. (2001) α11β1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 237, 116–129 [DOI] [PubMed] [Google Scholar]
- 32. Seeman E., Delmas P. D. (2006) Bone quality. The material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261 [DOI] [PubMed] [Google Scholar]
- 33. Karsdal M. A., Martin T. J., Bollerslev J., Christiansen C., Henriksen K. (2007) Are nonresorbing osteoclasts sources of bone anabolic activity? J. Bone Miner. Res. 22, 487–494 [DOI] [PubMed] [Google Scholar]
- 34. Aszodi A., Hunziker E. B., Brakebusch C., Fässler R. (2003) β1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ekholm E., Hankenson K. D., Uusitalo H., Hiltunen A., Gardner H., Heino J., Penttinen R. (2002) Diminished callus size and cartilage synthesis in α 1 β 1 integrin-deficient mice during bone fracture healing. Am. J. Pathol. 160, 1779–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bengtsson T., Aszodi A., Nicolae C., Hunziker E. B., Lundgren-Akerlund E., Fässler R. (2005) Loss of α10β1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J. Cell Sci. 118, 929–936 [DOI] [PubMed] [Google Scholar]
- 37. Duong L. T., Lakkakorpi P., Nakamura I., Rodan G. A. (2000) Integrins and signaling in osteoclast function. Matrix Biol. 19, 97–105 [DOI] [PubMed] [Google Scholar]
- 38. Nakayamada S., Okada Y., Saito K., Tamura M., Tanaka Y. (2003) β1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor κB ligand on osteoblasts and osteoclast maturation. J. Biol. Chem. 278, 45368–45374 [DOI] [PubMed] [Google Scholar]
- 39. Bikle D. D. (2008) Integrins, insulin like growth factors, and the skeletal response to load. Osteoporos. Int. 19, 1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katsumi A., Orr A. W., Tzima E., Schwartz M. A. (2004) Integrins in mechanotransduction. J. Biol. Chem. 279, 12001–12004 [DOI] [PubMed] [Google Scholar]
- 41. Phillips J. A., Almeida E. A., Hill E. L., Aguirre J. I., Rivera M. F., Nachbandi I., Wronski T. J., van der Meulen M. C., Globus R. K. (2008) Role for β1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol 27, 609–618 [DOI] [PubMed] [Google Scholar]
- 42. Canalis E. (2009) Growth factor control of bone mass. J. Cell Biochem. 108, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 [PubMed] [Google Scholar]
- 44. Yakar S., Canalis E., Sun H., Mejia W., Kawashima Y., Nasser P., Courtland H. W., Williams V., Bouxsein M., Rosen C., Jepsen K. J. (2009) Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J. Bone Miner. Res. 24, 1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang M., Xuan S., Bouxsein M. L., von Stechow D., Akeno N., Faugere M. C., Malluche H., Zhao G., Rosen C. J., Efstratiadis A., Clemens T. L. (2002) Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 277, 44005–44012 [DOI] [PubMed] [Google Scholar]
- 46. Shane E., Burr D., Ebeling P. R., Abrahamsen B., Adler R. A., Brown T. D., Cheung A. M., Cosman F., Curtis J. R., Dell R., Dempster D., Einhorn T. A., Genant H. K., Geusens P., Klaushofer K., Koval K., Lane J. M., McKiernan F., McKinney R., Ng A., Nieves J., O'Keefe R., Papapoulos S., Sen H. T., van der Meulen M. C., Weinstein R. S., Whyte M. (2010) Atypical subtrochanteric and diaphyseal femoral fractures. Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 25, 2267–2294 [DOI] [PubMed] [Google Scholar]
- 47. Le Roith D., Bondy C., Yakar S., Liu J. L., Butler A. (2001) The somatomedin hypothesis. 2001. Endocr. Rev. 22, 53–74 [DOI] [PubMed] [Google Scholar]
- 48. Yakar S., Rosen C. J., Beamer W. G., Ackert-Bicknell C. L., Wu Y., Liu J. L., Ooi G. T., Setser J., Frystyk J., Boisclair Y. R., LeRoith D. (2002) Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 110, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamada K. M., Even-Ram S. (2002) Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75–76 [DOI] [PubMed] [Google Scholar]
- 50. Goel H. L., Fornaro M., Moro L., Teider N., Rhim J. S., King M., Languino L. R. (2004) Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J. Cell Biol. 166, 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Long R. K., Halloran B. P., Bikle D. D. (2008) Integrin regulation of the IGF-I receptor in bone, and the response to load. Clin. Rev. Bone Miner. Metab. 5, 222–233 [Google Scholar]
- 52. Schmitz M., Niehoff A., Miosge N., Smyth N., Paulsson M., Zaucke F. (2008) Transgenic mice expressing D469Δ mutated cartilage oligomeric matrix protein (COMP) show growth plate abnormalities and sternal malformations. Matrix Biol. 27, 67–85 [DOI] [PubMed] [Google Scholar]
- 53. Baur A., Henkel J., Bloch W., Treiber N., Scharffetter-Kochanek K., Brüggemann G. P., Niehoff A. (2011) Effect of exercise on bone and articular cartilage in heterozygous manganese superoxide dismutase (SOD2)-deficient mice. Free Radic. Res. 45, 550–558 [DOI] [PubMed] [Google Scholar]
- 54. Turner C. H., Burr D. B. (1993) Basic biomechanical measurements of bone. A tutorial. Bone 14, 595–608 [DOI] [PubMed] [Google Scholar]
- 55. Deleted in proof.
- 56. Gregory C. A., Gunn W. G., Peister A., Prockop D. J. (2004) An Alizarin red-based assay of mineralization by adherent cells in culture. Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 329, 77–84 [DOI] [PubMed] [Google Scholar]
- 57. Takahashi Y., Kaji H., Okimura Y., Goji K., Abe H., Chihara K. (1996) Brief report. Short stature caused by a mutant growth hormone. N. Engl. J. Med. 334, 432–436 [DOI] [PubMed] [Google Scholar]
- 58. Gluckman P. D., Grumbach M. M., Kaplan S. L. (1981) The neuroendocrine regulation and function of growth hormone and prolactin in the mammalian fetus. Endocr. Rev. 2, 363–395 [DOI] [PubMed] [Google Scholar]
- 59. Giustina A., Mazziotti G., Canalis E. (2008) Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 29, 535–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sims N. A., Clément-Lacroix P., Da Ponte F., Bouali Y., Binart N., Moriggl R., Goffin V., Coschigano K., Gaillard-Kelly M., Kopchick J., Baron R., Kelly P. A. (2000) Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J. Clin. Invest. 106, 1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao G., Monier-Faugere M. C., Langub M. C., Geng Z., Nakayama T., Pike J. W., Chernausek S. D., Rosen C. J., Donahue L. R., Malluche H. H., Fagin J. A., Clemens T. L. (2000) Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice. Increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141, 2674–2682 [DOI] [PubMed] [Google Scholar]
- 62. He J., Rosen C. J., Adams D. J., Kream B. E. (2006) Postnatal growth and bone mass in mice with IGF-I haploinsufficiency. Bone 38, 826–835 [DOI] [PubMed] [Google Scholar]
- 63. Ahmed S. F., Farquharson C. (2010) The effect of GH and IGF1 on linear growth and skeletal development and their modulation by SOCS proteins. J. Endocrinol. 206, 249–259 [DOI] [PubMed] [Google Scholar]
- 64. Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. U.S.A. 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sjögren K., Liu J. L., Blad K., Skrtic S., Vidal O., Wallenius V., LeRoith D., Törnell J., Isaksson O. G., Jansson J. O., Ohlsson C. (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. U.S.A. 96, 7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ohlsson C., Mohan S., Sjögren K., Tivesten A., Isgaard J., Isaksson O., Jansson J. O., Svensson J. (2009) The role of liver-derived insulin-like growth factor I. Endocr. Rev. 30, 494–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Romero C. J., Ng Y., Luque R. M., Kineman R. D., Koch L., Bruning J. C., Radovick S. (2010) Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol. Endocrinol. 24, 1077–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Larsen M., Artym V. V., Green J. A., Yamada K. M. (2006) The matrix reorganized. Extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463–471 [DOI] [PubMed] [Google Scholar]
- 69. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.