Abstract
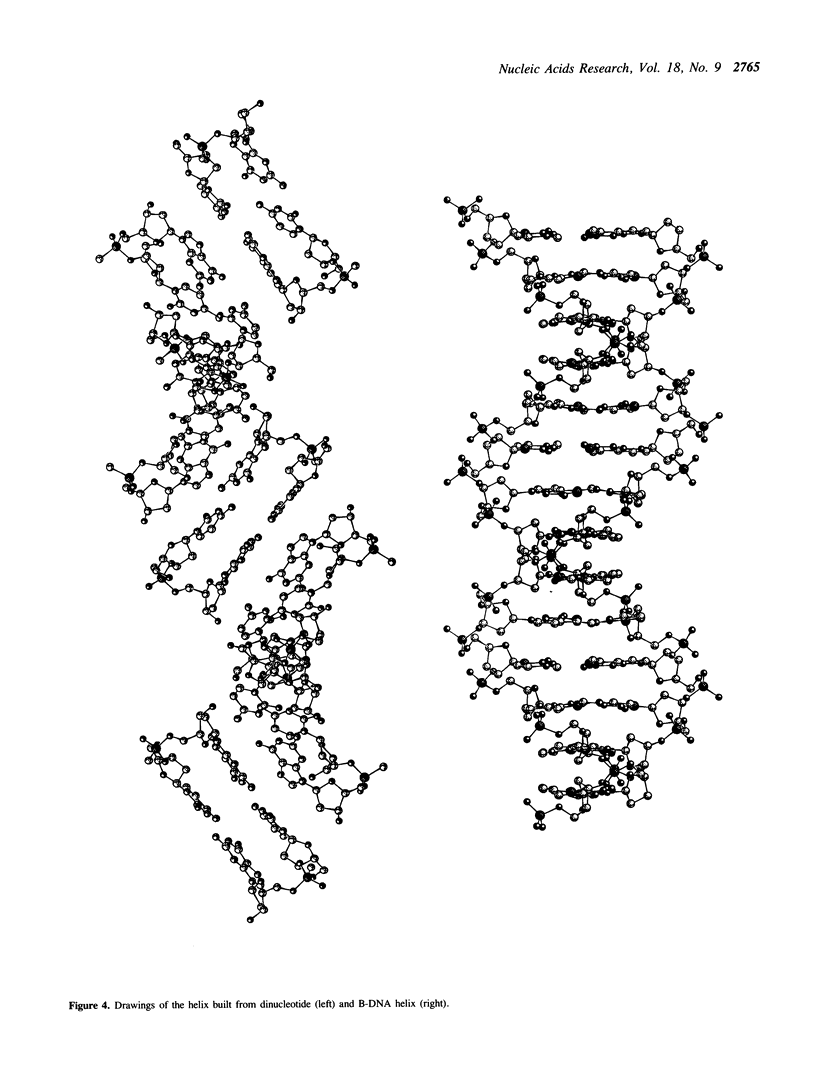
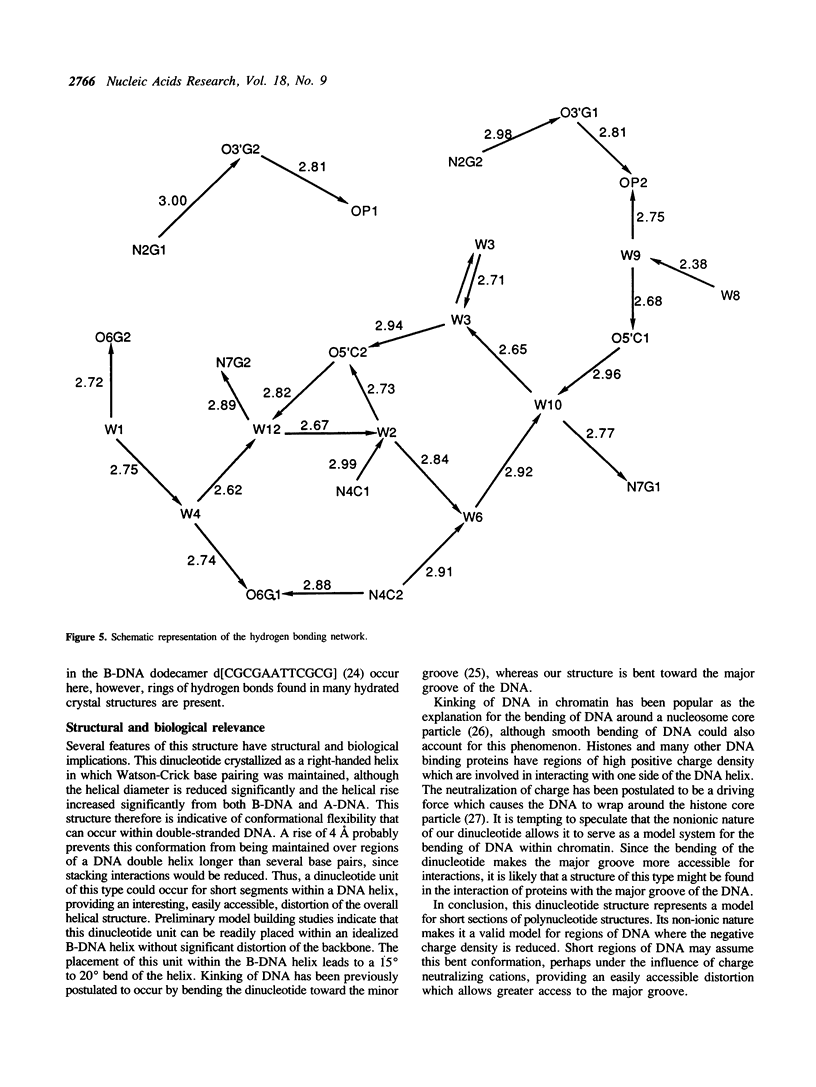
The crystal structure of d[Cp(CH3)G] has been determined as part of a project to study the mechanism of the B----Z transition in DNA. The asymmetric unit contains two dinucleotides and the equivalent of 7.5 water molecules, partially disordered over 12 definable positions. The two symmetry-independent dinucleotides form a duplex with Watson-Crick base-pairing and a right-handed helical sense. Comparison with previously determined structures of the B and A conformation showed that this duplex is closer to B than to A but significantly different from B. It corresponds to a stretched out helix with a 4 A rise per base pair and a helical twist of 32 degrees. This structure may serve as a model for the bending of DNA in certain situations. The configuration at the methyl phosphonate is RP, and a mechanism, based on this assignment, is presented for the B----Z transition in DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Callahan L., Han F. S., Watt W., Duchamp D., Kézdy F. J., Agarwal K. B- to Z-DNA transition probed by oligonucleotides containing methylphosphonates. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1617–1621. doi: 10.1073/pnas.83.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko K. K., Lindner K., Saenger W., Miller P. S. Molecular structure of deoxyadenylyl-3'-methylphosphonate-5'-thymidine dihydrate, (d-ApT x 2H2O), a dinucleoside monophosphate with neutral phosphodiester backbone. An X-ray crystal study. Nucleic Acids Res. 1983 May 11;11(9):2801–2814. doi: 10.1093/nar/11.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Crick F. H., Klug A. Kinky helix. Nature. 1975 Jun 12;255(5509):530–533. doi: 10.1038/255530a0. [DOI] [PubMed] [Google Scholar]
- Cruse W. B., Egert E., Kennard O., Sala G. B., Salisbury S. A., Viswamitra M. A. Self base pairing in a complementary deoxydinucleoside monophosphate duplex: crystal and molecular structure of deoxycytidylyl-(3'-5')-deoxyguanosine. Biochemistry. 1983 Apr 12;22(8):1833–1839. doi: 10.1021/bi00277a014. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. The influence of the purine 2-amino group on DNA conformation and stability. Synthesis and conformational analysis of d[T(2-aminoA)]3. Nucleic Acids Res. 1982 Jul 24;10(14):4351–4361. doi: 10.1093/nar/10.14.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Shick V. V., Belyavsky A. V., Bavykin S. G. Primary organization of nucleosome core particle of chromatin: sequence of histone arrangement along DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4184–4188. doi: 10.1073/pnas.75.9.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. M., Seeman N. C., Day R. O., Rich A. RNA double-helical fragments at atomic resolution. II. The crystal structure of sodium guanylyl-3',5'-cytidine nonahydrate. J Mol Biol. 1976 Jun 14;104(1):145–167. doi: 10.1016/0022-2836(76)90006-1. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Suddath F. L., Kim J. J., Rich A. RNA double-helical fragments at atomic resolution. I. The crystal and molecular structure of sodium adenylyl-3',5'-uridine hexahydrate. J Mol Biol. 1976 Jun 14;104(1):109–144. doi: 10.1016/0022-2836(76)90005-x. [DOI] [PubMed] [Google Scholar]
- Shakked Z., Rabinovich D., Kennard O., Cruse W. B., Salisbury S. A., Viswamitra M. A. Sequence-dependent conformation of an A-DNA double helix. The crystal structure of the octamer d(G-G-T-A-T-A-C-C). J Mol Biol. 1983 May 15;166(2):183–201. doi: 10.1016/s0022-2836(83)80005-9. [DOI] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]


