Summary
In both vertebrate and invertebrate visual systems, neurons form multiple-contact synapses at which a single presynaptic site releases neurotransmitter upon a discrete combination of different postsynaptic cells. Recognition mechanisms underlying the assembly of such synapses are not known. In Drosophila, photoreceptor terminals form tetrad synapses that incorporate an invariable pair of postsynaptic elements, one each from lamina interneuron L1 and L2, and two elements from other cells. Here, we demonstrate that Drosophila Dscam1 and Dscam2, genes encoding homophilic repulsive proteins, act redundantly to ensure the invariable combination of L1 and L2 postsynaptic elements at all tetrads. We demonstrate that this strict pairing is lost in Dscam1;Dscam2 double mutants. Thus, removing these two repulsive proteins allows elements from the same cell to incorporate into the same postsynaptic tetrad, altering the specificity of photoreceptor transmission. We propose that Dscams regulate synaptic specificity by excluding inappropriate partners at multiple-contact synapses.
Introduction
How neurites select synaptic partners during development remains a central issue in neurobiology. While axon guidance cues may place neurites close to their synaptic partners, axon guidance alone is insufficient for target-specific synapse formation (Vogt et al., 2005), because neurites must choose between many potential targets. Even in the relatively simple brain of Drosophila melanogaster, discrete synapses between specific cells form within a sea of processes from many different cell types (Fröhlich and Meinertzhagen, 1983; Gao et al., 2008). It is generally believed that specific cell recognition molecules allow neurites to discriminate each another, allowing pairing of appropriate synaptic partners or alternatively preventing inappropriate pairings. Understanding how this occurs at a molecular level is, at best, poorly understood. Here we describe one mechanism regulating the assembly of precise synaptic connections in the fly's visual system and the cellular recognition proteins that underlie this process.
The structural composition of synapses varies greatly between the brains of different species and between different regions of the same species (Peters et al., 1991; Prokop and Meinertzhagen, 2006). Variation is greater for the postsynaptic side, and although a typical synapse is often presented as a presynaptic terminal releasing neurotransmitter upon a single postsynaptic element, alternative arrangements are common. For example, in Drosophila (Prokop and Meinertzhagen, 2006; Takemura et al., 2008) and C. elegans (Hall and Russell, 1991; White J G et al., 1986), CNS synapses comprise a single presynaptic site abutting multiple postsynaptic elements, an arrangement also found within both the outer and inner plexiform layers of the vertebrate retina (Dowling and Boycott, 1966).
Photoreceptor synapses in the first optic neuropil, the lamina, are the best-characterized multiple-contact synapses of the fly's brain (Meinertzhagen, 1993). The lamina is organized into synaptic modules called cartridges, each comprising six photoreceptor, or R-cell input terminals (R1–R6), five individual lamina neurons (L1–L5), and several other cell types (Clandinin and Zipursky, 2002) (Figure 1A,B). A cartridge typically contains a single representative of each cell type, and is enwrapped by a glial sheath separating it from neighboring cartridges (Meinertzhagen and O'Neil, 1991). Each R1–R6 terminal forms about 50 output synapses (Meinertzhagen and Sorra, 2001) each comprising a single presynaptic site that releases a neurotransmitter, histamine (Hardie, 1987), upon four postsynaptic elements. These are: an invariant pair of contacts from L1 and L2 (Figure 1C, D) and a pair of variable contributions from lamina amacrine, L3, or glial cells (Meinertzhagen and O'Neil, 1991). Thus, each presynaptic site relays visual information to a tetrad of postynaptic elements that invariably incorporates paired L1 and L2 contributions. The invariable pairing of L1 and L2 at all tetrads ensures that these cells receive matched inputs from R1–R6 (Meinertzhagen and Sorra, 2001). L1 and L2, in turn, provide the inputs to two motion-sensing channels, which can respond differentially to motion in opposite directions (Rister et al., 2007). The obligate pairing of L1 and L2 has been highly conserved through insect evolution (Shaw and Meinertzhagen, 1986), so this feature of synaptic organization probably plays an essential computational function in fly vision. Here, we demonstrate using serial-section electronmicroscopy (EM) that Dscam proteins, members of the immunoglobulin (Ig) superfamily, ensure the postsynaptic pairing of L1 and L2 at all tetrad synapses.
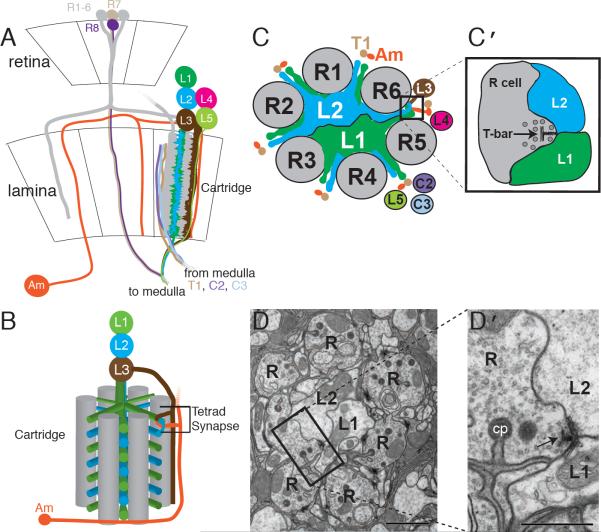
Figure 1. Lamina neurons L1 and L2 pair at tetrad synapses.
(A) Retina and lamina of the fly's visual system. R1–6 photoreceptor axons (gray) innervate the lamina where they form synapses with a subset of lamina cells. For clarity, only two R-cell axon terminals that project to the same cartridge are shown encircling lamina neuron L1 and L2 dendrites. Axons of R7, R8, and lamina neurons (L1–L5) innervate the medulla neuropil (to medulla). Axons from medulla neurons (T1, C2 and C3) also make connections in the lamina (from medulla). Am=amacrine cell. (B) Single cartridge showing a single tetrad synapse. Six R-cell terminals (gray) encircle L1 and L2, which extend dendrites radially from their axons to receive input at tetrad synapses from R cells. A tetrad comprised of L1/L2 and L3/Am pairs is shown. The L1/L2 pair is invariant (see methods); the other pair comprises combinations of elements from other cell types (L3, Am, and glia). (C,D) Cross-section of a cartridge showing the stereotypic positions of axons (schematic, C; EM, D; tetrad synapses are boxed). Scale bar (D) = 1μm. (C′ , D′) enlargements of the boxed regions. Presynaptic T-bar ribbons (arrows) lie opposite paired L1 and L2 elements; the other two elements of the tetrad oriented orthogonally to these lie above and below the plane illustrated. cp = capitate projection, glial invagination into the R cell terminal. Scale bar = 0.5μm.
Dscam proteins regulate brain wiring in both vertebrates and invertebrates. Alternative splicing of Drosophila Dscam1 generates more than 18,000 ectodomains tethered to the membrane by one of two alternative transmembrane domains. Biochemical studies support the argument that most of these exhibit exquisite isoform-specific homophilic binding (Wojtowicz et al., 2004; Wojtowicz et al., 2007). Every neuron is proposed to express a stochastic array of many Dscam1 isoforms, endowing each with a unique Dscam1 signature (Neves et al., 2004). Binding between Dscam1 proteins on axon branches from the same cell in the CNS and from dendritic branches from the same sensory cell in the periphery elicit neurite repulsion (Hughes et al., 2007; Matthews et al., 2007; Neves et al., 2004; Soba et al., 2007; Wang et al., 2002; Zhan et al., 2004), a process called self-avoidance. Dscam1 is unlikely to mediate binding between neurites of different cells because these express largely different isoforms. Self-avoidance promotes the segregation of axon branches to different pathways, and the repulsion of self-neurites ensures uniform coverage of receptive fields. Dscam2, like Dscam1, mediates homophilic repulsion (Millard et al., 2007). However, in contrast to Dscam1, it encodes only two alternative isoforms and therefore lacks the same molecular diversity. Dscam2 can presumably mediate repulsion both between neurites from the same cell (self-avoidance) and neurites from different cells (cell-type-specific avoidance), depending on which isoform or ratio of isoforms each neuron expresses. Indeed, we previously demonstrated that Dscam2 mediates cell-type-specific avoidance to restrict the terminals of L1 cells to single columns in the second neuropil, or medulla (Millard et al., 2007). While vertebrate Dscam proteins are not alternatively spliced, they exhibit functional similarities to fly Dscams. Retinal cells from knockout mice for the DSCAM and DSCAML1 genes exhibit self-avoidance and cell-type-specific avoidance phenotypes (Fuerst et al., 2009; Fuerst et al., 2008). Thus, Dscam's function in promoting homophilic repulsive interactions is highly conserved.
Dscam proteins, like many neuronal receptors, are multifunctional, with different ligands and context-dependent attraction or repulsion to the same ligand. In both vertebrates and flies, Dscam proteins act as attractive netrin receptors (Andrews et al., 2008; Liu et al., 2009; Ly et al., 2008). In addition, gain- and loss-of-function studies in the chick retina support the view that Dscam proteins promote the targeting of retinal ganglion cell dendrites and bipolar cell axons to the same layer, and may promote synaptogenesis between them (Yamagata and Sanes, 2008). Similarly, cultured Aplysia neurons require Dscam trans-synaptically to cluster AMPA-like receptors (Li et al., 2009). Thus the simplest interpretation of these data in both the chick and Aplysia is that Dscam proteins act adhesively at synapses.
In this report, we explore the role of Drosophila Dscam proteins in regulating synaptogenesis. We show that while photoreceptor tetrads appear structurally normal in flies that lack both Dscam1 and Dscam2, the composition of their postsynaptic elements revealed through serial-EM reconstruction is altered. These findings and previous developmental studies of tetrad assembly in normal animals support a model in which Dscam1 and Dscam2 act redundantly to promote the invariable postsynaptic partnership between L1 and L2 by preventing L1/L1 or L2/L2 pairings at presynaptic release sites through homophilic repulsion.
Results and Discussion
Tetrad synapses form normally in Dscam1 mutants
To investigate whether fly Dscam proteins play a role in photoreceptor synapse formation we analyzed synapses in mutant flies using serial EM. Antibodies to Dscam1 co-localized to markers for L1 and L2 during mid-pupal stages, demonstrating that this protein is expressed at the correct developmental time to regulate synapse formation (Figure 2A–C). Although Dscam1 mutant animals typically die as early larvae, they survive until late pupal stages if isolated from their heterozygous siblings and grown on rich media (Hattori et al., 2007). We were consequently able to analyze tetrad synapses in Dscam1 homozygous mutant flies.
Figure 2. Tetrads contain paired L2 elements in fused Dscam2 mutant cartridges.
(A–C) Co-localization of an L1/L2-specific marker (Rister et al., 2007) and Dscam1 protein. L1/L2-Gal4::UAS CD8GFP (A) and Dscam1 protein localization (B) in pupal brains at ~70% APF. C, merge. (D–F) Co-localization of an L1/L2 marker and Dscam2 protein at ~70% APF. There is clear overlap of Dscam2 immunoreactivity (E,F) with L1 and L2 (D,F), but compared with Dscam1 the distribution of Dscam2 is punctate. (G) Confocal section of a wild-type adult lamina array visualized with an R cell antibody (mAb24B10). Arrowheads indicate single cartridges. (H) Confocal section of a Dscam2 mutant array reveals single (arrowheads) and fused (encircled by dashed line) cartridges (see J and K for EM). Scale bars (A–H) = 5 μm. (I) Percentage of total synapses containing L2/L2 tetrads. Dscam2 mutant single cartridges (left, Single) and three other genotypes with fused cartridges (Fused). Asterisks: statistical significance calculated by Fisher's exact test, **=<10−6 , ***=<10−12. (J) EM of two single cartridges from a wild-type fly. Inset: schematic; g = epithelial glia that surround each cartridge. Scale bar = 2 μm. (K) EM of a fused Dscam2 mutant cartridge. Note that glial boundaries between the two cartridges are missing and that the fused cartridge contains two L1 and two L2 cells. Scale bar = 2 μm. (L) Tetrad composition of mutant fused cartridges determined from serial-EM reconstructions. Schematic (top) shows each synapse type and the origin of its L1 and L2 elements. Elements that originate from the same constituent cartridge: left three columns. Elements that originate from different cartridges within the fused cartridge: right columns. See inset in (K) for an illustration of a fused cartridge. EM data from three cartridges per genotype except flamingo (n = 1 cartridge).
Synapses in wild-type and mutant laminas were reliably identified from their characteristic presynaptic T-bar ribbons (Meinertzhagen and O'Neil, 1991). At the postsynaptic sites opposite these, L1 and L2 occupied the median positions of the postsynaptic tetrad, with the remaining elements paired at the polar positions. The median elements of each tetrad were followed through EM sections until they could be assigned to an L1 or L2 axon (see Figure 1B, C; note that in arthropod brains dendrites extend from specific domains of the axon, rather than the soma). L2 was discriminated from L1 based on its larger axon caliber, its position within each cartridge cross section with respect to the lamina's external coordinates, and its occasional feedback synapses (Meinertzhagen and O'Neil, 1991). Not all elements could be followed to their cell of origin. Here we include only tetrads in which both equatorial elements were successfully traced.
Lamina cartridges in Dscam1 homozygous mutants looked normal except for occasional fusions between neighboring cartridges (about 2% fusions, Table 1). Cartridge fusion occurred when the border between two cartridges was interrupted, and was rare in wild-type laminas (<1%) (Table 1). Synaptic defects were not observed in either single (data not shown; and see below) or fused (Figure 2L) mutant cartridges. Indeed, virtually all tetrads contained L1/L2 pairs, indistinguishable from wild type.
Table 1.
Fused cartridges in wild type, Dscam1, and Dscam2 mutant flies.
| Single | Fused | Total | % Fused | |
|---|---|---|---|---|
| wild-type | 592 | 3 | 595 | 1 ± 1 |
| Dscam1 | 529 | 12 | 541 | 2 ± 2 |
| Dscam2 | 150 | 60 | 210 | 29 ± 21 |
Percent fused was calculated by dividing the fused cartridges by the total ± SD. Data are derived from confocal sections from 7–9 flies of each genotype. p values calculated with Fisher's exact test: wild-type vs Dscam1 = 0.02; wild type vs Dscam2 = <<10−4.
Abnormal composition of postsynaptic elements in fused cartridges of Dscam2 mutants
Like Dscam1, Dscam2 is expressed in lamina neurons during synapse formation (Figure 2D–F) and Dscam2 homozygous mutants are viable (Millard et al., 2007). In contrast to Dscam1 mutants, however, the cartridge array in Dscam2 mutants was markedly disrupted by frequent (29%) cartridge fusions (Figure 2G, H, J, K, Table 1). These fused cartridges comprised double the number of constituents (i.e. 12 R cell terminals, 2 L1 neurons, 2 L2 neurons, etc.) and like single cartridges each fused cartridge was ensheathed by glia.
We analyzed synapses in three single and three fused Dscam2 mutant cartridges using serial EM. Out of 106 synapses from single cartridges, no synaptic defects were observed (Figure 2I). In contrast, fused cartridges exhibited a striking change in tetrad specificity. Instead of the invariant L1/L2 partnerships observed in wild-type flies, 40% (68/169) of Dscam2 mutant tetrads incorporated two L2 elements (Figure 2I, L). Interestingly, most (68 out of 70) L2/L2 tetrads contained one postsynaptic element from each of the two L2 cells within the fused cartridge, rather than two L2 elements from the same cell. The change in tetrad specificity was not a consequence of cartridge fusion itself, because tetrad composition was unchanged in fused cartridges from Dscam1 and flamingo (encoding a cadherin-like protein) mutants (Figure 2I, L). Thus, inappropriate pairing of L2 processes in fused cartridges was specific to Dscam2.
Why are synaptic phenotypes observed only in fused Dscam2 mutant cartridges?
Several observations led us to hypothesize that tetrad defects in fused cartridges had uncovered a redundant role for Dscam1 and Dscam2 in ensuring that only a single L2 element was incorporated into each wild-type tetrad. In fused Dscam2 mutant cartridges, abnormal tetrads almost always involved L2 elements from different cells. This phenotype reflects a deficiency in cell-type-specific repulsion (avoidance), a Dscam2-specific function. Since Dscam1 can only mediate repulsion between neurites from the same cell (because neurites from different cells express different Dscam1 isoforms), Dscam1 would not compensate for loss of Dscam2 between postsynaptic elements from different L2 neurons in a fused cartridge. However, Dscam1 would compensate for the loss of Dscam2 self-avoidance within these fused cartridges, leading to the paucity of L2/L2 tetrads from the same cell that we observed. In normal cartridges, then, containing one L2 cell, Dscam1 and Dscam2 would mediate self-avoidance between postsynaptic elements of L2 cells in a redundant fashion. In Dscam1 mutant single cartridges, Dscam2 could mediate self-avoidance between L2 postsynaptic elements from the same cell, thereby compensating for the loss of Dscam1. A similar rationale can explain why tetrads in Dscam2 mutant single cartridges were normal. Thus, if Dscam1 and Dscam2 act redundantly in L2 neurons, then in animals lacking both Dscam1 and Dscam2 many tetrads between cells in a single cartridge would contain two postsynaptic elements from the same L2 cell.
Dscam1 and Dscam2 act redundantly to control obligate pairing of L1 and L2 postsynaptic elements at tetrad synapses
To test whether Dscam1 and Dscam2 act redundantly to regulate the composition of tetrad synapses, we analyzed single cartridges lacking both Dscam1 and Dscam2. Because homozygous double-mutant flies did not survive we used MARCM (Lee and Luo, 1999) to generate clones of Dscam1 mutant tissue in the lamina of flies homozygous mutant for Dscam2. Dscam1 mutant L2 cells were generated by targeting expression of FLP recombinase to lamina precursor cells and were marked with HRP-CD2, a membrane-localized label compatible with EM analysis (Gao et al., 2008). Thus, every labeled L2 cell was mutant for both Dscam1 and Dscam2 in this experiment. The manner in which clones were generated and because HRP-CD2 was only expressed in L2 cells meant that other cells including L1, however, also lacked Dscam1 and Dscam2 but were unlabeled. In each genotype described below, at least three cartridges and over 100 synapses per genotype were analyzed by serial-EM reconstruction.
As a control, we first analyzed Dscam2 heterozygous animals with labeled L2 cells to assess whether the HRP-CD2 marker might affect tetrad composition. The marker clearly labeled the L2 axon in each cartridge and this label could be followed out to the distal tips of dendrites allowing us to identify L2 postsynaptic elements at tetrads unambiguously. The tetrad composition of these control flies was normal, with a single labeled L2 element paired with an unlabeled L1 (Figure 3A–C). As additional controls, we analyzed single cartridges with labeled L2 cells in flies homozygous mutant for either Dscam1 or Dscam2. We did not observe any phenotypes in Dscam1 mutant cartridges. However, we observed a few (8%) L2/L2 tetrads in single Dscam2 mutant cartridges (Figure 3J, K). The discrepancy between these results and those from unlabeled single mutant cartridges (see Figure 2I) likely reflected our increased ability to identify L2 processes when these were labeled. These data argue that Dscam2 is necessary to exclude multiple L2 elements from the same tetrad, but that other factors must also contribute to this exclusion.
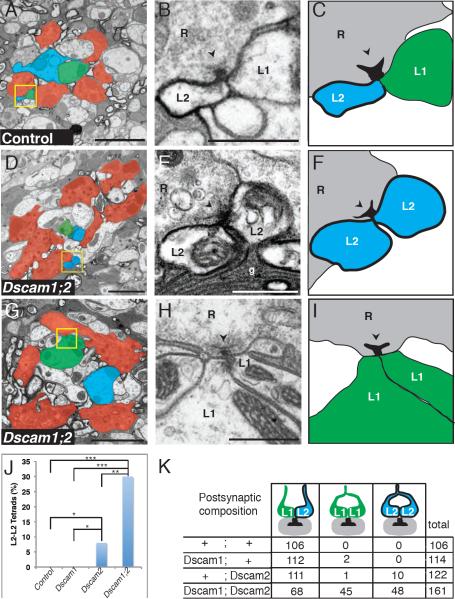
Figure 3. Dscam1 and Dscam2 are both required for synaptic exclusion.
(A) EM of a single cartridge from a control (Dscam2 heterozygous) fly with labeled L2 cells. Yellow box: a synapse containing an L1/L2 pair. R cells (red), L1 (green), L2 (blue). (B–C) Higher magnification of the boxed area in A shows a T-bar ribbon (arrowhead) in the presynaptic terminal. The L2 membrane is labeled with HRP, while L1 is unlabeled. (D) Single Dscam2 mutant cartridge containing a Dscam1 mutant L2 cell. Yellow box: a synapse with an L2/L2 pair. (E–F) Paired L2 elements opposite a presynaptic T-bar ribbon (arrowhead). Color scheme and arrowhead as in A–C. g = glial cell processes. (G) Single Dscam2 mutant cartridge containing a Dscam1 mutant L2 cell. Only four of six R cell profiles are visible in this section. Yellow box: unlabeled tetrad. (H–I) Higher magnification of the boxed region in (G) showing paired L1 elements opposite a T-bar ribbon (arrowhead). Scale bars = 2μm (A,D, G), 0.5 μm (B,E,H). (J) L2/L2 tetrads observed in each genotype with labeled L2 cells. P values calculated with Fisher's exact test. *=<10−3, **=<10−6, ***=<10−12 (see k for numbers). (K) Tetrad composition in single cartridges of different genotypes with labeled L2 cells (as in Figure 2L). Three cartridges were analyzed per genotype except for Dscam1 mutant (n = 2 cartridges). See methods for genotypes of flies.
We next analyzed three single cartridges containing L2 cells double-mutant for both Dscam1 and Dscam2. About 30% of the tetrads lacked an L1 profile and instead incorporated two labeled L2 elements from the same cell (Figure 3D–F, J, K). Thus, Dscam1 and Dscam2 are redundantly required to prevent two L2 elements from incorporating into the same tetrad.
Surprisingly, we also observed a second tetrad phenotype. About 28% of the tetrads in cartridges with double-mutant L2 cells lacked a labeled L2 process (Figure 3G–I, K). When these unlabeled elements could be traced back to the axon from which they originated, they were assigned to L1. In total, 22% of the paired, unlabeled elements traced back to the L1 axon. In the remaining unlabeled tetrads, the two processes could not both be followed through a sufficient number of sections to unambiguously assign them to L1 or any other neuron. As indicated above, the genetic mosaic scheme in this experiment did produce Dscam1 mutant L1 cells (which were also Dscam2 mutant), but these cells were not labeled because our marker is L2-specific. Indeed, because the mitotic recombination scheme used to generate double-mutant cells typically generates clones in which both L1 and L2 neurons are mutant, we expected most cartridges containing a double-mutant L2 neuron also to include a double-mutant L1 neuron. Thus, the simplest interpretation of these data is that Dscam1 and Dscam2 act in a redundant fashion in L1 and L2 to prevent inappropriate pairing of postsynaptic elements from the same cell. If self-recognition between prospective postsynaptic elements were abrogated completely in L1 and L2, tetrad composition would become randomized and the chance of a tetrad containing L1/L2, L1/L1, or L2/L2 pairs would be 50%, 25%, and 25%, respectively. Our results fit this prediction well with 42% L1/L2 (one HRP-labeled element), 28% L1/L1 (no HRP-labeled elements), and 30% L2/L2 (two HRP-labeled elements) in double-mutant cartridges.
Given that L1 and L2 have a similar phenotype in single cartridges mutant for both Dscam1 and Dscam2, why does L2 but not L1 have a phenotype in fused Dscam2 mutant cartridges? We speculate that this reflects differences in exploratory behavior between L1 and L2 dendrites. Indeed, in both EM and confocal micrographs of Dscam2 mutant cartridges, L2 dendrites spread to fill much of the fused cartridge whereas L1 dendrites tend to remain restricted to the half containing the L1 axon (Figure S1). Thus, L2 processes from the two different cells in the fused cartridge likely encountered each other more frequently than did processes of L1 neurons.
Concluding Remarks
Does the invariable pairing of L1 and L2 arise as a consequence of interactions between self-dendrites prior to tetrad assembly or is it achieved through interactions between prospective postsynaptic elements at developing synapses? Previous EM studies favor the latter view (Fröhlich and Meinertzhagen, 1983). About halfway through metamorphosis (50% after puparium formation, APF), filopodial dendrites from L1 and L2 cells begin extending towards the photoreceptor terminal. These processes intermingle extensively over the surface of the axon terminal with many L1/L1 and L2/L2 neighboring pairs. These dendrites later assume a strict alternating sequence (i.e. L1, L2, L1 and so on) along the length of the axon terminal. Their alternation coincides with a reduction in the number of dendrites at 70% APF and the maturation of tetrads. That is, as the total number of dendrites decreases, the L1/L2 alternating pattern is established and the number of mature tetrads increases. The selective regression of dendrites lacking the correct neighbor coincides with synapse formation (Figure 4A). Thus, these data favor the view that Dscam1 and Dscam2 mediate repulsive interactions between postsynaptic elements of the same cell thereby ensuring the correct composition of tetrad synapses. A phenomenon of this type has previously been proposed and coined synaptic exclusion (Fröhlich and Meinertzhagen, 1983); here we present its mechanism.
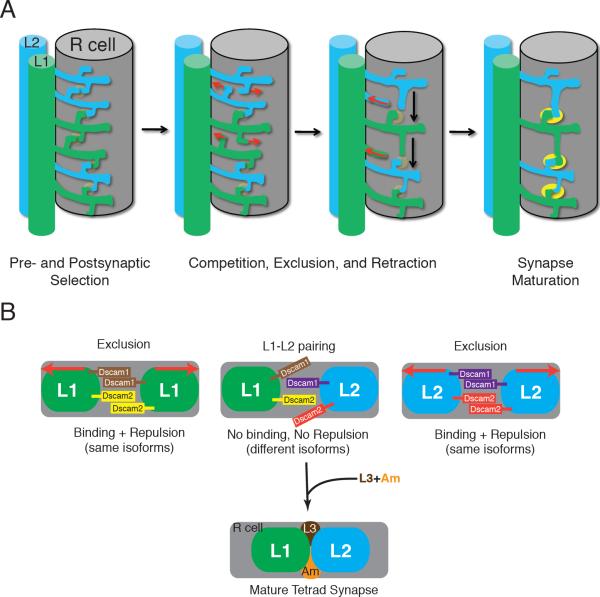
Figure 4. Dscam-mediated repulsion promotes obligate pairing of L1 and L2 postsynaptic elements at tetrad synapses.
(A) Model for tetrad assembly: Tetrad synapses form when neurites from L1 and L2 randomly explore the surface of an R-cell terminal, where they contact and recognize nascent presynaptic sites (pale yellow) and postsynaptic partners (left panel). L1/L1 or L2/L2 partnerships are excluded from the same site. We propose that competition between L1 and L2 postsynaptic elements selects those neurites with stable (heterotypic) partners on either side. Neurites denied postsynaptic access retract leading to the progressive strictness of alternation between L1 and L2 neurites down the length of the terminal and a reduction in their overall number (middle two panels). Gradually, and in parallel, each synaptic contact accumulates two other postsynaptic elements, and matures functionally as its organelles progressively differentiate (solid yellow, right panel). This schematic is based on the electronmicrographic studies of Meinertzhagen et al. (2000) on developing cartridges in Musca domestica and Drosophila melanogaster.
(B) Model for synaptic exclusion: We propose that L1 and L2 cells express different sets of Dscam1 proteins and also different Dscam2 proteins or distinct ratios of its two isoforms. When two postsynaptic elements from the same cell encounter each other, Dscam1 and Dscam2 promote self-avoidance (exclusion), preventing L1/L1 or L2/L2 pairs from incorporating into the same tetrad. Pairing of L1 and L2 may also require other, adhesive cell recognition molecules that mediate interactions between L1 and L2, between these elements and the presynaptic R cell terminal, or between the postsynaptic elements from other cells at the tetrad (e.g. amacrine (Am) or L3 neurons). Note that for clarity, a single bar in this schematic represents multiple Dscam1 isoforms. While there are undoubtedly other models for how Dscam1 and Dscam2 control synaptic exclusion, the model proposed here is the most parsimonious. Red arrows indicate Dscam-mediated homophilic repulsion.
In this paper we demonstrate that Dscam1 and Dscam2 play a key role in regulating the composition of tetrad synapses. We propose that each L1 and L2 cell in a wild-type cartridge expresses a unique combination or a different ratio of Dscam1 and Dscam2 isoforms (Figure 4B). Selective repulsive interactions between neurites from the same cell would then prevent inappropriate combinations of elements (i.e. L1/L1 or L2/L2) from assembling at tetrad synapses.
In conclusion, Dscam1 and Dscam2 proteins are widely expressed throughout the developing fly CNS, mediate homophilic repulsion, and function at multiple steps in circuit assembly. Dscam1 promotes repulsion between neurite branches promoting uniform coverage of receptive fields and segregation of axon branches during guidance. In the medulla of the fly's visual system Dscam2 promotes tiling of axon terminals, thereby restricting visual processing to non-overlapping regions of the visual field. And finally, as we report here, at the final step of circuit assembly, Dscam1 and Dscam2 utilize homophilic repulsive interactions between prospective postsynaptic dendrites of the same cell to prevent assembly of inappropriately configured multiple-contact synapses in the lamina. We speculate that L1 and L2's invariable coupling is required to ensure reliable processing of motion stimuli. Given that multiple-contact synapses are a common feature of the fly's brain, we anticipate that Dscam proteins play a widespread role in recruiting the appropriate combination of cellular contributors to postsynaptic elements at multiple-contact synapses. We further speculate that Dscam proteins may also play a role, perhaps in a redundant fashion as in flies, in controlling the composition of multiple-contact synapses in the vertebrate retina (Fuerst et al., 2008, 2009).
EXPERIMENTAL PROCEDURES
Fly Stocks
Dscam2 homologous recombination mutants have been described (Millard et al., 2007). The Dscam1 alleles we used were extensively backcrossed versions of those previously reported (Dscam21 and Dscam23) (Schmucker et al., 2000). For serial EM on flies with labeled L2s, the following lines were generated: For Dscam2 heterozygous mutants (w; FRT42, UAS HRP-CD2/CyO; Dscam2null-3, 21D-Gal4/21D-Gal4); for Dscam1 homozygous mutants (w; FRT42, Dscam121C3/FRT42, Dscam123C2, UAS HRP-CD2; +/21D-Gal4); for Dscam2 homozygous mutants (eyFLP/w; FRT42, UAS HRP-CD2/FRT42, Gal80; 21D-Gal4, Dscam2null-3/21D-Gal4, Dscam2null-2); for Dscam1 homozygous clones in a Dscam2 homozygous mutant background (eyFLP/w; FRT42, Dscam123C2, UAS HRP-CD2/FRT42, Gal80; 21D-Gal4, Dscam2null-3/21D-Gal4, Dscam2null-2). Gal4 lines used in this study were: L1/L2-Gal4 (labels both L1 and L2 at late pupal and adult stages), 21D-Gal4 (labels L2 in adults), both generous gifts from the Heisenberg lab, Würzburg (Rister et al., 2007).
Generating Dscam1 clones in a Dscam2 mutant background
Two crosses were performed to generate these flies. First, males from the stock (w; FRT42, Gal80/CyO; 21D-Gal4, Dscam2null-2) were crossed to females (eyFLP; +; TM2/TM6b) to generate males of the genotype (eyFLP/Y; FRT42, Gal80/+; 21D-Gal4, Dscam2null-2/TM6b). These flies were then crossed to females with the genotype (w; FRT42, Dscam123C2, UAS HRP-CD2/CyO; 21D-Gal4, Dscam2null-2/TM6b). In the resulting progeny, non-CyO, non-TM6b, females were selected (eyFLP/w; FRT42, Dscam123C2, UAS HRP-CD2/FRT42, Gal80 [or +]; 21D-Gal4, Dscam2null-3/21D-Gal4, Dscam2null-2).). The heads were dissected, fixed, and cut into 120μm Vibratome slices before revealing HRP activity in the presence of H2O2, diaminobenzidine and NiCl2 (Gao et al., 2008); DNA was prepared from the bodies. The bodies were genotyped for Gal80 using PCR and Gal80 positive heads were sectioned further for EM.
Homozygous Dscam1 mutant clones (in a Dscam2 mutant background) were identified using an L2 marker (as these cells, but not L1 cells, showed phenotypes in fused cartridges homozygous for Dscam2). The eyFLP driver used in these MARCM experiments is expressed in precursor cells giving rise to photoreceptors and lamina neurons. It is therefore very likely that other cells within cartridges analyzed, including L1, were also homozygous mutant. Neither Dscam1 (unpublished observations) nor Dscam2 (Millard et al., 2007) plays an autonomous role in photoreceptors. A detailed analysis of mosaic laminas using specific Gal4 markers and confocal microscopy to determine the likely genotype of clones analyzed by EM (i.e. the genotype of different lamina and photoreceptor neurons) was not undertaken. These caveats notwithstanding it seems most likely that the synaptic defects reported here are due to a cell-autonomous requirement for Dscam1 and Dscam2, acting in a redundant fashion, in both L1 and L2. Cartridges containing double mutant L2 neurons contained the normal composition of photoreceptor axons. As homozygous double mutant animals do not survive past early larval stages, it was not possible to compare the number of fused cartridges in these animals with those in single mutant animals.
Confocal microscopy
Confocal microscopy was performed using a Zeiss LSM 510 Meta.
Immunohistochemistry
Immunohistochemistry of Dscam1 and Dscam2 proteins was performed essentially as described (Lee et al., 2001). The Dscam1 antibody was mouse monoclonal 11D4 used at a 1:2 dilution. The Dscam2 antibody was rabbit polyclonal 565 used at a 1:1000 dilution. The rabbit polyclonal GFP antibody from Molecular Probes was used at a 1:1000 dilution.
Electron microscopy of unlabeled sections
Series of 50–60-nm sections were cut from the laminas beneath control and mutant eyes of homozygous flies prepared for EM as previously reported (Meinertzhagen, 1996). Digital montages collected from images obtained with a Philips Tecnai 12 using a Kodak Megaview II camera with software (AnalySIS: SIS GmbH, Münster, Germany), were used to trace profiles of lamina cells to presynaptic sites at tetrads. Our reanalysis of data from previous studies (Meinertzhagen and O'Neil, 1991; Meinertzhagen and Sorra, 2001) demonstrated that L1 and L2 constitute the median elements at essentially all photoreceptor synapses. Out of 283 synapses from a wild-type EM series, 210 had postsynaptic elements in which two L-cell components could be unambiguously identified by tracing them back to the axon of origin. Of these, 206 had L1 and L2 contacts, while only three had two L1s and one had two L2s (Meinertzhagen, I. A. and Sorra, K. E., unpublished observations). Several investigators traced postsynaptic elements either by labeling prints of consecutive profiles of lamina cell dendrites, as in a previous study of the wild-type (Meinertzhagen and O'Neil, 1991), or by reconstructing these in 3-D by means of software (Reconstruct(Fiala, 2005); downloaded from <http://synapses.bu.edu/tools/history.htm)>http://synapses.bu.edu/tools/history.htm). Departures from the normal wild-type pattern of partnerships at tetrads were confirmed by a second investigator.
The complete list of ~25 EM series used is given in Figures 2l and 3K. Each contained at least 60 consecutive micrographs and sampled at least 40 tetrads, in either fused or single cartridges from each genotype. We examined multiple series of cartridges with abnormal L2/L2 pairings at tetrads. Not all postsynaptic elements could be traced to each presynaptic site, because some dendrites spread from outside into the reconstructed depth of lamina neuropil, or because the dendrites were too fine. With the exception of fused cartridges in Dscam2, the postsynaptic participants at the tetrads had a uniform L1/L2 combination. Even at tetrads at which for technical reasons not all postsynaptic elements could be traced, those that were traced nevertheless conformed to the combinations of elements seen at tetrads with a full tetrad complement of identified elements.
Analysis of labeled sections
Labeled sections were aligned using Reconstruct software. L2 profiles were identified from their labeled membranes visible both on the axon at the cartridge axis and on its distal dendrites. Such label extended several sections from a postsynaptic site, providing evidence of its continuity and reliability. We assumed that all L2 dendrites carried visible label. L1s were identified from their proximity to the L2 axon and by their lack of label. All abnormal tetrads (containing a pair of labeled L2 or unlabeled L1 elements) were also traced back to their axon. Of the 10 L2/L2 tetrads in normal cartridges from Dscam2 mutants (i.e. those that were not fused) L2 postsynaptic elements were identified initially by HRP labeling, and 6 were traced to the L2 axon. Similarly, 26/48 of L2/L2 tetrads from Dscam1;Dscam2 double-mutants were traced back to the L2 axon and 10/45 of the unlabeled tetrads were traced back to L1. The inability to trace some postsynaptic elements was for technical reasons, chiefly the tortuousness of profiles or distorted sections, not because they originated from cells other than L1 and L2.
Supplementary Material
Acknowledgements
We thank Jim Clemens, Kelsey Martin, Carlos Portera-Cailliau, Alvaro Sagasti, and members of the Zipursky lab for critical comments on the manuscript and Shin-ya Takemura at Janelia Farm for technical help with serial EM. We thank Dorian Gunning for generating the Dscam1 monoclonal antibody and Susan Yee for technical help with figures and text. We acknowledge the technical help of Mss. Rita Kostyleva, Jane Anne Horne and Dorota Tarnogorska (Dalhousie), and the support of NIH (EY03592, to I.A.M.). S.L.Z. is an investigator of the Howard Hughes Medical Institute, and I.A.M. was a Killam Fellow of the Canada Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, Price H, Fernandez GC, Mastick GS, Charron F, Kidd T. Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development. 2008;135:3839–3848. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Fröhlich A, Meinertzhagen IA. Quantitative features of synapse formation in the fly's visual system. I. The presynaptic photoreceptor terminal. J Neurosci. 1983;3:2336–2349. doi: 10.1523/JNEUROSCI.03-11-02336.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Takemura SY, Ting CY, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong ST, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol A. 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, Hawkins RD, Bailey CH, Kandel ER. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci U S A. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA. Ultrastructure and quantification of synapses in the insect nervous system. J Neurosci Methods. 1996;69:59–73. doi: 10.1016/S0165-0270(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. The Development of Drosophila melanogaster. Vol 2. Cold Spring Harbor Laboratory Press; New York: 1993. [Google Scholar]
- Meinertzhagen IA, O'Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Sorra KE. Synaptic organization in the fly's optic lamina: few cells, many synapses and divergent microcircuits. Prog Brain Res. 2001;131:53–69. doi: 10.1016/s0079-6123(01)31007-5. [DOI] [PubMed] [Google Scholar]
- Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Zucker J, Daly M, Chess A. Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nat Genet. 2004;36:240–246. doi: 10.1038/ng1299. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H.d. The fine structure of the nervous system : neurons and their supporting cells. 3rd edn Oxford University Press; New York: 1991. [Google Scholar]
- Prokop A, Meinertzhagen IA. Development and structure of synaptic contacts in Drosophila. Semin Cell Dev Biol. 2006;17:20–30. doi: 10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Shaw SR, Meinertzhagen IA. Evolutionary progression at synaptic connections made by identified homologous neurones. Proc Natl Acad Sci U S A. 1986;83:7961–7965. doi: 10.1073/pnas.83.20.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura SY, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt AK, Brewer GJ, Decker T, Bocker-Meffert S, Jacobsen V, Kreiter M, Knoll W, Offenhausser A. Independence of synaptic specificity from neuritic guidance. Neuroscience. 2005;134:783–790. doi: 10.1016/j.neuroscience.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Wang J, Zugates CT, Liang IH, Lee CH, Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002;33:559–571. doi: 10.1016/s0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode C. elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.