Summary
Xenorhabdus bovienii (SS-2004) bacteria reside in the intestine of the infective-juvenile (IJ) stage of the entomopathogenic nematode, Steinernema jollieti. The recent sequencing of the X. bovienii genome facilitates its use as a model to understand host-symbiont interactions. To provide a biological foundation for such studies, we characterized X. bovienii in vitro and host-interaction phenotypes. Within the nematode host X. bovienii was contained within a membrane bound envelope that also enclosed the nematode-derived intravesicular structure. S. jollieti nematodes cultivated on mixed lawns of X. bovienii expressing green or DsRed fluorescent proteins were predominantly colonized by one or the other strain, suggesting the colonizing population is founded by a few cells. X. bovienii exhibits phenotypic variation between orange-pigmented primary form and cream-pigmented secondary form. Each form can colonize IJ nematodes when cultured in vitro on agar. However, IJs did not develop or emerge from Galleria mellonella insects infected with secondary form. Unlike primary-form infected insects that were soft and flexible, secondary-form infected insects retained a rigid exoskeleton structure. X. bovienii primary and secondary form isolates are virulent toward Manduca sexta and several other insects. However, primary form stocks present attenuated virulence, suggesting that X. bovienii, like X. nematophila may undergo virulence modulation.
Keywords: bacteria, microbe-higher organism interactions, symbionts, insect pathogen, nematode mutualist, virulence modulation, makes caterpillars floppy
Introduction
Entomopathogenic nematodes in the genus Steinernema are mutualistically associated with Gamma-proteobacteria in the genus Xenorhabdus. Together, Steinernema-Xenorhabdus complexes infect, kill, and reproduce in the larval stage of a wide range of insect hosts, including those in the orders Lepidoptera, Diptera, and Coleoptera (Ehlers, 2001). The Steinernema nematode harbors Xenorhabdus bacteria in a modified portion of the intestine, and the bacteria are released by defecation when the nematode infects an insect and reaches the insect’s body cavity (hemocoel) (Poinar, 1966; Martens et al., 2004; Snyder et al., 2007). After reproduction, nematode progeny become colonized by Xenorhabdus bacteria and emerge from the cadaver to seek a new host.
Since Xenorhabdus spp. are mutualists (of nematodes) and pathogens (of insects) they have become a model for studying both types of associations (Herbert and Goodrich-Blair, 2007; Richards and Goodrich-Blair, 2009) from cellular, molecular and evolutionary perspectives. Furthermore, unlike many current model systems to study host-microbe interactions, the mutualistic and pathogenic traits of Xenorhabdus bacteria are conserved among all members of the genus, facilitating comparative insights into the biology and evolution of these processes.
Twenty species of Xenorhabdus are currently recognized (Lee and Stock, 2010b; Tailliez et al., 2010) and the genomes of two, X. nematophila (ATCC 19061: genome accession #NC_014228, plasmid accession #NC_014170) and X. bovienii (SS-2004: accession #NC_013892), have been sequenced by our group. X. nematophila is known to colonize three nematode species from two clades, while X. bovienii strains appear to be more widely distributed, colonizing at least eight Steinernema spp. from three clades (Akhurst and Boemare, 1988; Fischer-Le Saux et al., 1998; Fischer-Le Saux et al., 1999; Spiridonov et al., 2004; Mrácek et al., 2006; Emelianoff et al., 2008; Chapuis et al., 2009; Lee and Stock, 2010a). However, X. bovienii comprises multiple phylotypes (Fischer-Le Saux et al., 1998; Lee and Stock, 2010b) each of which may have a distinct host range. Indeed, variations have been observed in the ability of X. bovienii strains to colonize the nematode S. feltiae (Chapuis et al., 2009). The Steinernema-Xenorhabdus mutualistic association that has been most extensively studied at the molecular and cellular level is that between X. nematophila and S. carpocapsae (Goodrich-Blair, 2007). This study is focused on the relationship between X. bovienii and the nematode Steinernema jollieti.
Generally, both Steinernema nematodes and Xenorhabdus bacteria contribute to pathogenesis. The nematode produces a factor that can degrade inducible immune proteins (Götz et al., 1981) but most Xenorhabdus bacteria are pathogenic if injected alone (without the nematode host) directly into the hemocoel of a susceptible host (Akhurst and Boemare, 1990). Xenorhabdus spp. are able to suppress immunity and produce virulence factors, including toxins that contribute to rapid killing of the insect host (reviewed in (Herbert and Goodrich-Blair, 2007)). Nematode reproduction is promoted in the insect by the presence of the bacterial symbiont (Poinar and Thomas, 1966; Sicard et al., 2003; Mitani et al., 2004; Richards and Goodrich-Blair, 2010), with X. nematophila producing lipase and hemolysin activities necessary for nematode reproduction (Richards and Goodrich-Blair, 2010) and virulence in the insect (Cowles and Goodrich-Blair, 2005; Vigneux et al., 2007), respectively. In addition, Xenorhabdus promotes nematode development by protecting the insect cadaver from scavengers and other microbes (Zhou et al., 2002; Morales-Soto and Forst, 2011) through the production of a wide array of antimicrobials (Akhurst, 1982; Park et al., 2009; Fang et al., 2011; Fuchs et al., 2011) including bacteriocins that inhibit invasion of the cadaver by non-specific symbionts (Hawlena et al., 2010; Morales-Soto and Forst, 2011).
Steinernema and Xenorhabdus reproduce within the insect cadaver until nutrient depletion and high nematode population density triggers development of the nematode into a non-feeding stage known as the Infective Juvenile (IJ) (Popiel et al., 1989). The IJs become colonized by the bacterial symbionts in the anterior intestinal lumen, a region known as the receptacle (Bird and Akhurst, 1983; Snyder et al., 2007). In S. carpocapsae, the receptacle comprises the lumen between the two most anterior intestinal cells, which are morphologically distinct from other intestinal cells (Martens and Goodrich-Blair, 2005; Flores-Lara et al., 2007; Snyder et al., 2007). The X. nematophila population in the S. carpocpasae receptacle is founded by 1–2 individual cells that grow to fill the space (Martens et al., 2003), where they adhere to a cluster of spheres termed the intravesicular structure (IVS) that is associated with a glycan-containing mucous material (Martens and Goodrich-Blair, 2005). Once formed, IJ progeny emerge from the spent insect cadaver to search for new hosts to infect (San-Blas et al., 2008).
All Xenorhabdus species reported to date undergo phenotypic variation characterized by the switching between two cell types known as primary and secondary forms. Although the phenotypic differences between primary and secondary forms can vary depending on strain and species (Akhurst and Boemare, 1990), typically the primary, but not secondary form cells are motile, pigmented, agglutinate red blood cells and produce fimbriae, hemolysins, proteases, antimicrobials, and crystalline inclusion bodies (Boemare and Akhurst, 1988; Volgyi et al., 1998; Forst and Clarke, 2002; Smits et al., 2006). Xenorhabdus bacteria isolated from nematode hosts typically are in the primary form, but in laboratory culture conditions some cells convert to the secondary form. Although again it varies depending on strain and species, some secondary form isolates can revert to the primary form, while others appear to be stable (Campos-Herrera et al., 2009; Goodrich-Blair, unpublished data). Studies to date indicate the mechanism underlying phenotypic variation does not involve DNA rearrangements (Akhurst et al., 1992) changes in plasmid content (Leclerc and Boemare, 1991), or recombination-dependent mechanisms (Pinyon et al., 2000). In X. nematophila the transcription factor Lrp is a positive regulator of primary form traits; lrp mutants are phenotypically secondary form (Cowles et al., 2007), suggesting phenotypic variation may be an epigenetic phenomenon.
The sequenced strain of X. bovienii (SS-2004) (Chaston, Suen et al., in press) was isolated from the nematode S. jollieti (Spiridonov et al., 1991), but to date there is no published literature describing this strain. A primary goal of our research is to identify molecular mechanisms underlying the X. bovienii-S. jollieti association using the genome sequence as a resource. We anticipate that comparing this system to the more extensively studied X. nematophila-S. carpocapsae association and to other X. bovienii host associations will provide insights into the common and diverse features of Steinernema-Xenorhabdus interactions with each other and with insect hosts. Toward these goals, in this study we investigated general characteristics of X. bovienii SS-2004 as well as its interactions with its S. jollieti nematode and insect hosts.
Results and Discussion
To characterize X. bovienii (SS-2004) host-interaction phenotypes, in 2007 we isolated individual bacterial colonies from the same S. jollieti nematode host lineage, and compared them to X. bovienii stocks isolated from S. jollieti in 2000, at the time of sequencing. Both stocks contained two colony morphologies, cream and orange, on LB agar (Fig. 1). 16S rRNA sequencing confirmed that all isolates were X. bovienii (data not shown). When isolated cream and orange colonies were re-streaked, each gave rise to a mixed population of orange and cream colonies. For both orange and cream colonies, the stability of the colony type increased with increasing number of re-streaks. From each X. bovienii SS-2004 stock individual stable orange and cream colonies were isolated and a variety of phenotypes were analyzed (Table 2). Small colony variants were observed in the 2007, but not the 2001 orange isolate after growth on LB medium (Fig. 1). Both cell types had similar growth rates in LB broth, defined medium, and hemolymph, and exhibited motility, antibiotic activity against Bacillus subtilis, and hemolysis toward rabbit and horse red blood cells. Orange but not cream colony isolates bound bromothymol blue dye, lysed sheep red blood cells, agglutinated horse red blood cells, and produced antibiotic activities against Escherichia coli. Conversely, cream colony isolates but not orange demonstrated lipase activity against Tween 20 substrate. Based on these tests we concluded that orange colony isolates are the primary form while cream color isolates are the secondary form (Akhurst, 1980; Boemare and Akhurst, 1988). Hereafter, isolates will be referred to by the stock from which they were isolated (2000 or 2007) and their phenotypic form (orange, primary [1°]; cream, secondary [2°]). The majority of stationary phase LB-grown primary and secondary cells were rod shaped, but 1° form cultures contained a higher frequency than secondary of long rods (Fig. 1).
Figure 1.
Colony and cell morphology of X. bovienii primary and secondary variants. When streaked on LB agar, X. bovienii primary form colonies produce an orange pigment, causing both colonies and the surrounding agar to appear orange, while secondary form colonies are cream colored (size bars: 2 mm). Cultures were grown overnight in LB broth, and DIC microscopy revealed rod shaped cells, with primary form variant cultures containing a higher frequency of elongated cells (insets) (size bars: 10 µM).
Table 2.
Phenotypes of X. bovienii SS-2004
| Strain | Colony Colora |
Dye binding (NBTA) |
Growth Rate (h/generation)b | Motilityc | Lipased | Lecithinasee | Proteasef | Hemolysinf | Hem- agglutinationg |
Antibiotic Productionh |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luria Broth | Minimal | Hemolymph | Sheep/Rabbit/ Horse |
Sheep/Rabbit/ Horse |
E. coli |
B. subtilis |
|||||||
| 2000-1° | Orange | + | 1.27 +/− 0.09 | 5.7 +/− 1.8 | 4.9 +/− 0.6 | + | − | − | + | +/+/+ | −/−/+ | + | + |
| 2000-2° | Cream | − | 1.27 +/− 0.07 | 5.2 +/− 1.1 | 4.4 +/− 1.2 | + | + | + | +/− | −/+/+ | −/−/− | − | + |
| 2007-1° | Orange | + | 1.16 +/−0.10 | 6.4 +/− 2.5 | 4.1 +/− 1.1 | + | − | − | + | NT | NT | + | + |
| 2007-2° | Cream | − | 1.24 +/−0.10 | 5.9 +/−0.3 | 4.2 +/− 1.1 | + | + | + | +/− | NT | NT | − | + |
Colony color was observed on LB plates after 24 h incubation at 30°C.
Growth rate in LB, minimal medium, and hemolymph presented as the slope of log10-transformed OD values from exponential part of growth curve.
Size of colony (mm), 24 h after inoculation on swim plates (0.25% agar).
Presence (+) or absence (−) of halo surrounding the bacterial colony, 3 days after inoculation on Tween 20-containing plates
Presence (+) or absence (−) of halo surrounding the bacterial colony, 4 days after inoculation on egg yolk-containing plates
Subjective evaluation of halo surrounding the bacterial colony, 3 days after inoculation on milk (protease) or blood (hemolysin) containing plates. NT; Not tested
Ability to keep red blood cells in suspension (+) or not (−). NT; Not tested
Presence (+) or absence (−) of halo surrounding the bacterial colony, 3 days after inoculation of the indicated tester bacterium.
X. bovienii (SS-2004) colonization of S. jollieti nematodes
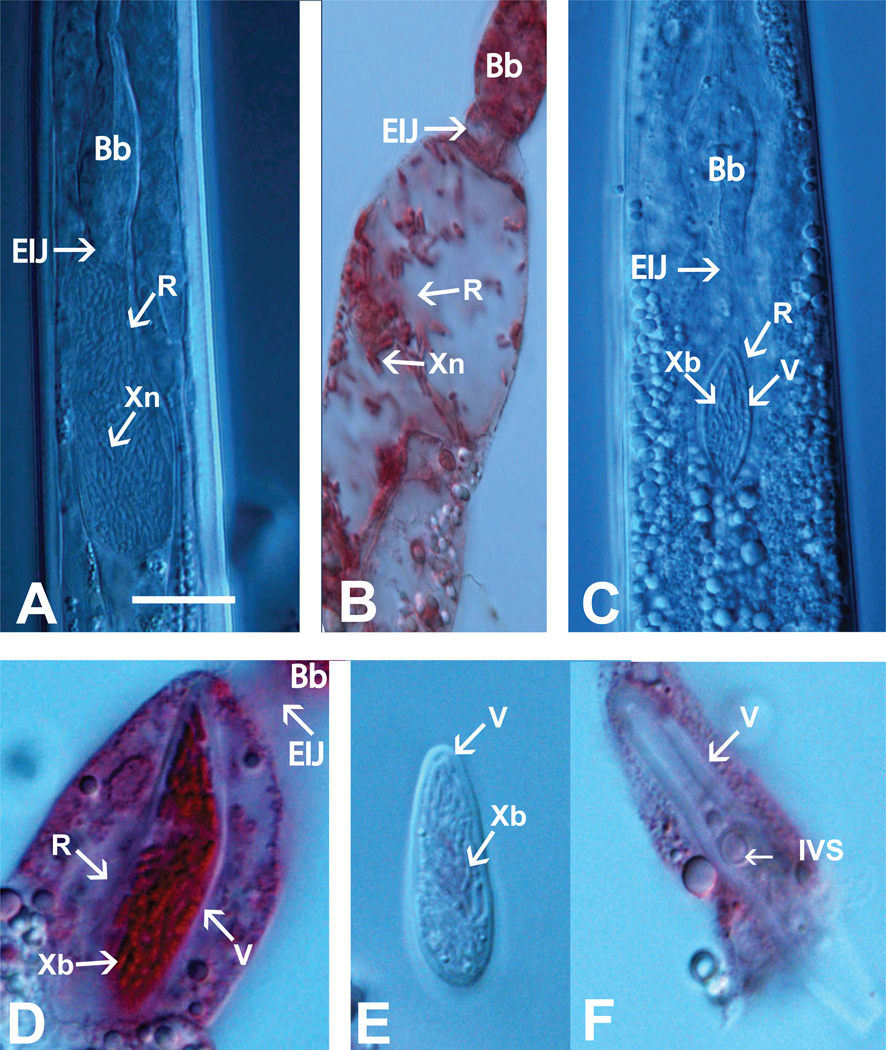
X. bovienii (2000-1°) was visualized within the S. jollieti colonization site in both in situ and extruded receptacles (Fig. 2). The S. jollieti colonization site shares several features in common with that of S. carpocapsae. In both nematodes, the bacteria colonize the nematode’s intestinal receptacle (Fig. 2A–D). The receptacle of these two nematode species also possesses a cluster of nematode-derived spheres, termed the intravesicular structure (IVS) (Fig 2F and data not shown). However, the S. jollieti receptacle has a non-cellular, cellophane-like envelope or membrane that is not apparent in S. carpocapsae. We refer to this novel structure as the ‘vesicle’, by definition “a small sac.” This vesicle is resistant to mechanical disruption and contains X. bovienii bacterial cells as well as the IVS (Fig. 2 D–F). Vesicle formation does not depend on the presence of bacteria, since it is present in axenically cultured S. jollieti nematodes (Fig. 2F) as well as in S. feltiae nematodes that are phylogenetically closely related to S. jollieti (Lee and Stock, 2010a). However, both the receptacle and the vesicle can stretch to accommodate the bacterial load (data not shown). At present there is no information about the chemical composition, physical nature, or function of this vesicle. We speculate it may serve to restrict bacterial growth, to facilitate timed release of bacteria into insect prey, or to protect symbiont bacteria from stress within the receptacle or insect hemolymph.
Figure 2.
A. S. carpocapsae (A–B) and S. jollieti (C–F) intestinal receptacle. A in situ colonized S. carpocapsae receptacle; B. Extruded colonized S. carpocapsae receptacle; C. in situ colonized S. jollieti receptacle; D. Extruded receptacle of S. jollieti showing colonized vesicle with thick membrane; E. Extruded colonized vesicle of S. jollieti; F. Uncolonized vesicle with thick membrane and IVS, extruded from an S. jollieti IJ cultured on liver-kidney agar in the absence of bacteria. .References: Bb; basal bulb, EIJ: esophago-intestinal junction, R: receptacle, V: vesicle, Xn: X. nematophila bacteria, Xb: X. bovienii bacteria, IVS: intra vesicular structure. Scale bar for all images as in A: A–C= 20 µm; D–E= 7 µm; F= 15 µm.
X. bovienii SS-2004 primary and secondary form colonization of S. jollieti nematodes was monitored in IJ progeny from nematodes cultivated on bacterial lawns (in vitro co-cultivation). To assess colonization levels, we initially used a method developed for X. nematophila, in which the average number of bacteria per IJ is calculated by sonicating and dilution plating approximately ~104 surface sterilized IJs. However, we found that this method yielded a very low (~4–7) average CFU/IJ of X. bovienii from S. jollieti nematodes. Since numerous bacteria within individual S. jollieti IJs were visible by microscopy (Fig. 2) we considered the possibility that the larger size of S. jollieti IJs relative to S. carpocapsae (Spiridonov et al., 1991; Flores-Lara et al., 2007), or the presence of the thick-walled vesicle, might reduce the efficiency of bacterial release during sonication. Efficiency was improved by limiting the number of sonicated nematodes to 100. Using this revised method, we determined that primary and secondary X. bovienii SS-2004 isolates each colonized S. jollieti nematodes at similar levels to each other (2000-1°, 59.7 ± 28.2; 2000-2°, 55.3 ± 22.4 average CFU/IJ±SEM, P>0.05, n=3), similar to the average X. nematophila CFU/ S. carpocapsae nematode IJ obtained using the sonication method (Martens et al., 2003). It has been reported that X. bovienii strains do not colonize its nematode hosts at as high a level as X. nematophila, as determined by crushing nematodes and plating the resulting suspension (Emelianoff et al., 2008; Chapuis et al., 2009). Our data suggest that X. bovienii loads may be higher than estimated by this method.
To calculate the distribution of X. bovienii SS-2004 within S. jollieti populations (colonization efficiency), X. bovienii SS-2004 form variants were engineered to express the green fluorescent protein (GFP) (see Materials and Methods) (Fig. 3). The average CFU/IJ (determined by sonication and plating) achieved by the fluorescent strains (Fig. 3) was not significantly different from their parental strains (P>0.05). For each variant, approximately 200 (2 sets of 100) nematodes per experiment were randomly selected and the frequency of colonization (indicated by fluorescence within the colonization site) was determined. Primary and secondary form cells were not significantly different (P=0.18, Student's t-test) in their frequency of occurrence within nematodes: X. bovienii 2000-1°/GFP colonized 97.3 ± 1.2%of nematodes and 2000-2°/GFP cells were visible in 71.5 ± 41.3% of nematodes (Fig. 3) and did not switch forms within the IJ (data not shown). Within the vesicle, the bacteria are tightly packed together longitudinally (Fig. 3). In contrast, in S. carpocapsae nematodes, X. nematophila cells are more loosely packed within the receptacle and are not bound by a vesicle (Snyder et al., 2007).
Figure 3.

X. bovienii colonization frequency. X. bovienii primary (1°) or secondary (2°) cells expressing green fluorescent protein (GFP) or DsRed protein (DsRed) were visualized after (A) growth in liquid culture or (B and C) within nematode intestines using an epifluorescence or confocal microscope respectively. In (B) confocal images overlay DIC images. Size bars for A, B, and C represent 5 microns. (D) Nematodes were cultivated on lawns of each bacterial strain, and the average CFU per infective juvenile (CFU/IJ) was calculated by sonication and plating and (E) the percentage of colonized nematodes (% colonized nematodes) were determined by microscopy. The experiment was conducted three independent times. Standard error of the mean and range (in parentheses) are provided. (F) Mixed lawns of X. bovienii expressing GFP or DsRed were cultivated with nematodes. Nematodes colonized by GFP-expressing cells, DsRed-expressing cells, or both were apparent within the population. Shown are confocal images of each type, with the size bar representing 5 microns.
Since the IJ is a non-feeding stage, the events necessary for intestinal colonization initiation must precede its development. Experimental evidence suggests that 1–2 X. nematophila bacterial cells initiate colonization in S. carpocapsae then grow within the receptacle after IJ formation (Martens et al., 2003; Martens et al., 2005). To begin to assess the colonization process in the S. jollieti-X. bovienii mutualism we determined the monoclonal and bi-clonal colonization frequency of IJs cultured on mixed lawns of 2000-1° X. bovienii expressing either GFP or DsRed fluorescent protein. As in X. nematophila (Martens et al., 2003), expression of DsRed significantly lowered the frequency of colonization (P<0.05, Student's t-test), with only 8 ± 2.6% and 1.5 ± 0.9% of nematodes being colonized by DsRed-expressing primary and secondary form cells (Fig. 3). Furthermore, DsRed expressing cells did not pack the receptacle as tightly as GFP-expressing cells, and appeared filamentous (Fig. 3). Despite the dramatically low frequency of colonization, the average DsRed expressing X. bovienii SS-2004 CFU/IJ was not significantly different from either X. bovienii SS-2004 without fluorescent protein, or expressing GFP. One possible explanation for this result is that in some nematodes the bacteria are no longer expressing DsRed and are not readily apparent by microscopy. In addition, the maximum CFU/IJ we obtain with our modified sonication method may be a gross under-estimate of the actual number of bacteria in nematodes colonized by wild type cells (see above), and is insufficient to detect differences in colonization level for X. bovienii SS-2004.
Although the DsRed-expressing strains are at a colonization disadvantage relative to GFP-expressing strains, we visually determined the frequency of nematodes colonized by GFP, DsRed, or both, after cultivation on a mixed (50:50) lawn of green and red primary form cells (Fig. 3F). Of the colonized nematodes derived from such lawns, 90.4 ± 8.2% fluoresced either all green (72.4%) or all red (18.0%), while nematodes visibly colonized by both types of bacteria represented only 9.6% of the population. Based on this frequency we calculated that, like X. nematophila (Martens et al., 2003), the final population of X. bovienii SS-2004 within an individual infective juvenile nematode is derived from 1–2 individual cells (see experimental procedures).
The colonization assays described above were conducted in vitro (i.e. on agar plates rather than within insects. Although colonization trends of S. carpocapsae IJs cultured in vitro on lawns of X. nematophila bacteria generally mirror those from in insecta cultivations (Cowles and Goodrich-Blair, 2008), S. carpocapsae IJs that develop within insects have higher bacterial loads than those that develop on bacterial agar lawns (Goetsch et al., 2006; Flores-Lara et al., 2007). To determine if in vivo cultivation within a host influences X. bovienii SS-2004 colonization of S. jollieti nematodes, we injected Galleria mellonella insects with X. bovienii SS-2004 bacteria and S. jollieti nematodes, and calculated average CFU/IJ for emerging progeny nematodes. X. bovienii 2000-primary colonized nematodes cultured in G. mellonella at 19.7 ± 10.9 CFU/IJ ± S.D. (n=2). Nematode IJs did not emerge from insects infected with secondary-phase cells, even after 4 weeks of incubation (data not shown). Dissection of insects at various time points after injection revealed that nematodes developed in both primary- and secondary-injected cadavers (Fig. 4A), but that IJ formation occurred only in the former (Fig. 4 C and E), not in the latter (Fig. 4 B and D) indicating that specifically in insects (not in vitro on lipid agar plates), secondary form cells lack an activity necessary for, or produce an activity inhibitory to development of infective juveniles.
Figure 4.
Nematode-infected G. mellonella with primary form, but not secondary form X. bovienii produce infective juveniles and are flaccid and liquefied.
At 7 d post-infection with nematodes and secondary form X. bovienii (HGB1054) G. mellonella insect cadavers (white arrow) are filled with non-emergent juvenile nematodes that can be seen in the water surrounding the cadaver upon dissection (an individual nematode among many is indicated by a white arrowhead) (A). However, phase contrast microscopy revealed that none of these is in the IJ stage (B and D) as indicated by body morphology and the presence of an open mouth (arrow). In contrast, juveniles from primary-form infected cadavers (C and E) are infective juveniles with closed mouths (arrow). Scale bars in B and C are 200 microns, and in D and E, 100 microns. G. mellonella insects 3 d post-infection with nematodes and bacteria (F) are flaccid if the injected bacteria are primary, but rigid if the injected bacteria are secondary. The flaccid insect cadaver phenotype associated with primary-form infection requires the presence of nematodes, since G. mellonella insects injected with either primary or secondary form bacteria, but without nematodes, are rigid (G). Both primary and secondary form bacteria are able to reproduce within the cadaver, as indicated by in vivo imaging system visualization of fluorescence in cadavers 7 days post-injection with secondary (HGB1245) or primary (HGB1263) X. bovienii strains expressing the green fluorescent protein (H). Images were taken using GFP-appropriate excitation and emission filters. Color corresponds to varying levels of emitted fluorescence, with a color spectrum of corresponding fluorescence levels shown on the right. Control insects without bacteria expressing green fluorescent protein showed no detectable fluorescence at these settings (not shown).
In four independent experiments, primary cell-infected insects reproducibly had a soft and spongy texture 3–5 days post-infection, while the secondary-form infected insects maintained a rigid exoskeleton (Fig. 4F), even at 14 d post-infection, relative to a time of emergence of 8–10 days from insects infected with primary form cells. One possible explanation for this finding is that secondary form X. bovienii SS-2004 may lack one or more activities (e.g. secreted enzymes) necessary for degradation of the insect cadaver exoskeleton. If so, this activity appears to be expressed specifically in the presence of the nematode and only by primary form cells, since insect cadavers injected with primary or secondary bacteria alone (in the absence of nematodes) were rigid (Fig. 4G), even at high bacterial loads (Fig. 4H).
Candidates for a bacterial factor contributing to the flaccid cadaver phenotype are the "Makes caterpillars floppy" (Mcf) toxins, Mcf1 (PLU4142, NP_931332) and Mcf2 (PLU3128, AAR21118), discovered in the entomopathogenic nematode symbiont Photorhabdus luminescens. E. coli expressing mcf1 is toxic when injected into M. sexta larvae, and the larval cadavers lose body turgor and become "floppy" (Daborn et al., 2002). Mcf2 has an N-terminal truncation relative to Mcf1, but is similar in its toxicity (Waterfield et al., 2003). Pseudomonas fluorescens encodes an mcf1 homolog (fitD), deletion of which decreases toxicity, melanization, and "floppiness" in Manduca sexta and G. mellonella insects (Pechy-Tarr et al., 2008). The X. bovienii SS-2004 genome encodes one mcf homolog (XBJ1_2410, YP_003468304, 2533 aa) in contrast to X. nematophila which encodes two: mcf1 (XNC1_2028, YP_003712268) and mcf2 (XNC1_2265, YP_003712501). XBJ1_2410 and P. luminescens Mcf2 share 76% and 31% identity in the N- and C-terminal thirds of the proteins, respectively. However, the middle third of XBJ1_2410 is distinct from P. luminescens Mcf2 and contains a cysteine peptidase domain (aa945–1100) (pfam11713, E-value: 8.5e-34) (Marchler-Bauer and Bryant, 2004; Marchler-Bauer et al., 2009; Marchler-Bauer et al., 2011). This domain is also present in X. nematophila mcf2. It will be of interest to determine if XBJ1_2410 expression phenotypically varies and if its expression or product activity is influenced by the presence of the nematode, as would be expected if Mcf2 is responsible for the flaccid phenotype observed in insects injected with primary-form X. bovienii and S. jollieti nematodes.
Our data are also consistent with the possibility that the nematode produces one or more activities necessary for insect bioconversion, but only in the presence of primary form bacteria. Indeed, S. carpocapsae expresses proteases specifically in the infective juvenile stage that have activity against insect tissues (Jing et al., 2010; Toubarro et al., 2010). Expression of these proteases may be triggered by primary form bacteria.
X. bovienii (SS-2004) virulence in M. sexta and other agricultural insect pests
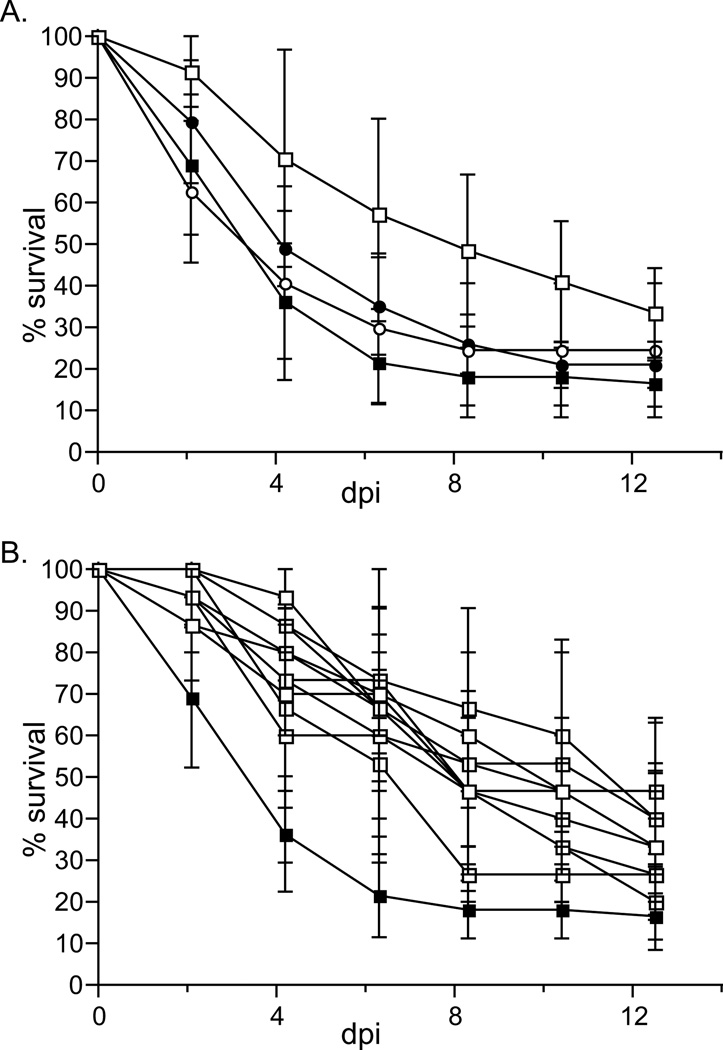
Virulence of X. bovienii (SS-2004) variants was measured as the percent survival of 4th instar M. sexta larvae after injection with approximately 100 cfu of bacteria (Fig. 5A). X. bovienii 2000-2°, 2007-1°, and 2007-2° each killed ~59% of insects by 96 h post-injection. However, 2000-1° cells killed only 20% of insects by that time, and both the percent mortality and time to death of insects injected with this strain were significantly different from that caused by the other three strains (p<0.0001). We considered the possibility that we had inadvertently chosen an attenuated variant from the 2000 stock and therefore examined the virulence of ten additional 2000-1° colonies. None were able to kill insects to the same levels as 2000-2°, 2007-1°, and 2007-2° (Fig. 5B). These data indicate that X. bovienii primary form cells can become attenuated for virulence, and that the majority of, if not all the primary form cells in the X. bovienii 2000 stock, but not the 2007 stock, have done so. Recently, it was reported that variants of X. nematophila arise within a population that are heritably and reversibly attenuated for virulence and immune suppression (Park et al., 2007). These virulence modulated (vmo) strains arise in laboratory stocks of X. nematophila but the mechanism underlying the modulation and the conditions that trigger modulation are unknown (Park et al., 2007). It is possible that the 2000 stock of X. bovienii was exposed to conditions (e.g. prolonged freezer storage) that promoted virulence modulation. Alternatively, some other phenomenon, such as a genetic mutation that altered virulence, may have occurred. The latter possibility is less likely since the genetic mutation would have had to selectively impact only the primary form cells in the population (secondary form colonies are virulent). We therefore favor the idea that laboratory-derived conditions promoted virulence modulation of the X. bovienii 2000 stock, and the intriguing corollary that primary form cells are more susceptible than secondary form cells to this modulation.
Figure 5.
X. bovienii virulence in M. sexta larvae.
Approximately 100 CFU of bacteria in late logarithmic phase growth (OD600=~0.8) were injected into each 4th instar Manduca sexta larvae (10 insects per treatment) and the percent of insects surviving each treatment (% survival) was monitored for approximately 13 days post-injection (dpi). A) Insects were injected with primary (squares) or secondary (circles) X. bovienii isolates from 2000 (open symbols) or 2007 (closed symbols). B) Individual primary form colonies were isolated from the parent stock and each was injected into insects as above (open squares). Closed squares show the survival curve of insects injected with X. bovienii 2007-1° (same as in A). Each experiment was conducted at least 3 independent times.
We also tested the oral toxicity of primary forms of both X. bovienii and X. nematophila against a number of known insect pathogens of plants (Table 3). X. bovienii exhibited a broader host range of secreted toxicity, with concentrated supernatants (>10 kDa) causing mortality in the Western Tarnished Plant Bug, Corn Ear Worm, and Western Corn Rootworm. In contrast, X. nematophila supernatants caused mortality only in Corn Ear Worm, and at lower levels (11% mortality) than that caused by X. bovienii (87%). In light of these results it is curious that the X. bovienii genome encodes a narrower repertoire of insecticidal Tc toxins than does the X. nematophila genome. Tc toxins are large molecular weight complexes of A, B, and C subunits (ffrench-Constant and Waterfield, 2006). X. bovienii encodes 3 A subunits, 2 B subunits, and 2 C subunits, while X. nematophila encodes 7, 3, and 3 of each type respectively (see supplemental Text S5 in (Chaston et al.). The apparently broader host range of X. bovienii may be due to broader target specificity of its TC toxins (Sergeant et al., 2003). Alternatively, X. bovienii may encode one or more additional toxins that expand its target host range.
Table 3.
Toxicity of X. bovienii concentrated protein samples against selected insect pests of plants.
| Percent Mortalitya | ||
|---|---|---|
| Insect | X. bovienii | X. nematophila |
| Black Cutworm | 0 | 0 |
| Corn Earworm | 87 ± 12 | 11 ± 9.6 |
| Southern Corn Rootworm | 0 | 0 |
| Western Corn Rootworm | 45.8 ± 12.3 | 0 |
| Western Tarnished Plant Bug | 100 | 0 |
Percent mortality ± standard deviation across three microtiter dish columns of each treatment
Conclusions
Although X. bovienii (SS-2004) and X. nematophila (ATCC 19061) colonize nematodes from different clades, we found many aspects of their physiology and host interactions to be similar. Both form mono- or bi-clonal populations within the nematode, undergo phenotypic variation of multiple activities, and exhibit heritable virulence variability. Also, the secondary forms of both strains express more lipase and lecithinase activities than their primary counterparts, are virulent in insects and colonize nematodes. Characteristics that distinguish X. bovienii from X. nematophila include that X. bovienii primary form colonies and cultures are pigmented orange, while those of X. nematophila are cream. Also, X. bovienii exhibits antimicrobial activities against E. coli and B. subtilis, and only the former is subject to phenotypic variation. In contrast only X. nematophila activity against B. subtilis is subject to phenotypic variation. The X. nematophila xcn locus is necessary for the production of the antibiotic xenocoumacin, which has activity against Micrococcus luteus (Park et al., 2009). The genome of X. bovienii lacks xcn gene homologs, indicating X. bovienii produces antibiotics that are distinct from those of X. nematophila. A further distinction between X. bovienii and X. nematophila is that the secondary form of the former, but not the latter, has a defect in supporting infective juvenile development within insect cadavers, and also fails to cause cadaver flaccidity. It remains to be determined whether or not these two defects are related, and what the molecular bases are for these phenotypes. Finally, a striking difference exists in the colonization site occupied by these two species of bacteria: when colonizing the nematode X. bovienii cells are bound within an envelope structure (vesicle) while X. nematophila are freely distributed in the receptacle. The fact that the host nematode tissues with which X. bovienii and X. nematophila cells interact suggests that the molecular requirements necessary to persist in this environment may differ between the two symbionts.
Experimental Procedures
Organisms and growth conditions
Strains used are listed in Table 1. The X. bovienii strain used in this study was deposited on Jun. 28, 2000 with the Agriculture Research Culture Collection (NRRL) International Depository Authority at 1815 North University Street, in Peoria, Ill. 61604 U.S.A., according to the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purpose of Patent Procedures and was designated as NRRL-30311. Permanent stocks of all cultures were stored in Luria-Bertani (LB) broth (Miller, 1972) supplemented with 20% glycerol at −80°C. Unless otherwise stated, cultures were grown at 30°C, in LB broth that had not been exposed to light or on LB agar that was supplemented with 0.1% pyruvate (Xu and Hurlbert, 1990). When appropriate, media were supplemented with antibiotics, including ampicillin (150 µg/ml), kanamycin (50 µg/ml), chloramphenicol (30 µg/ml), erythromycin (200 µg/ml), or tetracycline (15 µg/ml) were added as indicated. X. bovienii (SS-2004), like other Xenorhabdus spp. is catalase negative and is resistant to ampicillin, but is sensitive to the other antibiotics listed above. However, the genome of X. bovienii does encode a homolog of chloramphenicol transacetylase that confers resistance to 30 µg/ml chloramphenicol when present in high copy (data not shown). Lipid agar (LA), used to co-culture all strains of nematodes on respective bacterial lawns was prepared as previously described (Vivas and Goodrich-Blair, 2001). All nematode strains were propagated as previously described (Martens et al., 2003) through last instar Galleria mellonella larvae. In vitro, aposymbiotic cultivation of nematodes to produce uncolonized IJs of both S. jollieti and S. carpocapsae, was performed using liver-kidney agar as previously described (Sicard et al., 2003; Martens and Goodrich-Blair, 2005). X. bovienii growth rates in insect hemolymph were performed by harvesting Manduca sexta hemolymph as previously described (Orchard and Goodrich-Blair, 2004), performing a 1:100 subculture of rinsed X. bovienii cells into insect hemolymph and growing the cells at 30°C with shaking. The optical density (OD600) of the hemolymph culture was recorded every hour for 24 hours using a Beckman Coulter DTX880 Multimode Detector. Similarly, X. bovienii growth rates in defined medium broth were performed by subculturing 1:100 rinsed X. bovienii cells into defined medium broth made as previously described (Orchard and Goodrich-Blair, 2004) except that amino acids and agar were omitted and 0.68 g MgCl2(H2O)6 L-1 and 0.91% glucose were added after autoclaving and growing the cells at 30°C with shaking. The optical density (OD600) of the defined medium broth culture was recorded every hour for 24 hours.
Table 1.
Strains and plasmids used in this study
| Lab Strain Number | Description/Use | Reference/Source |
|---|---|---|
| Xenorhabdus strains | ||
| HGB800 | X. nematophila ATCC 19061, wild-type | American Type Culture Collection (ATCC) |
| HGB1053 | X. bovienii SS-2004 Jollieti 2000 stock | B. Goldman (Monsanto) |
| HGB1054 | X. bovienii SS-2004 Jollieti 2000-2° | This study |
| HGB1055 | X. bovienii SS-2004 Jollieti 2000-1° | This study |
| HGB1269 | X. bovienii Jollieti 2007 stock | This study |
| HGB1267 | X. bovienii Jollieti 2007-2° | This study |
| HGB1268 | X. bovienii Jollieti 2007-1° | This study |
| HGB1245 | HGB1054-GFP (2000-2°;GFP) | This study |
| HGB1263 | HGB1055-GFP (2000-1°;GFP) | This study |
| HGB1281 | HGB1054-DsRed (2000-2°;DsRed) | This study |
| HGB1285 | HGB1055-DsRed (2000-1°;DsRed) | This study |
| HGB1282 | HGB1267-GFP (2007-2°;GFP) | This study |
| HGB1283 | HGB1268-GFP (2007-1°;GFP) | This study |
| HGB1286 | HGB1267-DsRed (2007-2°;DsRed) | This study |
| HGB1287 | HGB1268-DsRed (2007-1°;DsRed | This study |
| Other bacterial strains | ||
| HGB1006 | E. coli K12 test strain for antibiotic | J. Imlay |
| HGB005 | E. coli DH5α test strain for antibiotic | Bethesda Research Laboratory |
| HGB006 | E. coli S17-1 λ pir test strain for antibiotic | (Simon et al., 1983) |
| BW29427 | E. coli (thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::[erm pir (wt)]); Donor for conjugations: StrepR | K.A. Datsenko and B.L. Wanner |
| HGB1262 | BW29427+pURR25; StrepR, KanR | D. Lies and D. Newman |
| HGB1266 | BW29427+pBK-mini-Tn7(Gm)PA1/04/03-DsRed; StrepR, GmR | This study |
| HGB1284 | BW29427+ pUX-BF13; StrepR, AmpR | D. Lies and D. Newman |
| HGB009 | Bacillus subtilis AD623 test strain for antibiotic | A. Driks |
| Plasmids | ||
| pURR25 | MTn7 PA1/03/04gfpmut3*Mini-Tn7-KSGFP | D. Lies and D. Newman |
| pUX-BF13 | Tn7 transposase, AmpR | (Bao et al., 1991) |
| pBK-mini-Tn7(Gm)PA1/04/03-DsRed | Mini-Tn7-DsRed | L. Lambertsen |
Phenotypic assays
X. bovienii colony color and morphology were observed after growth for 24–36 h at 30°C in or on LB medium. Haemolysin activity (Rowe and Welch, 1994) against mammalian erythrocytes (Colorado Serum Company, Denver, CO), Tween 20 lipolytic activity (Sierra, 1957), lecithinase activity (Boemare, 1997; Thaler et al., 1998), proteolytic activity (Boemare et al., 1997), haemagglutination (Moureaux et al., 1995), motility (Vivas and Goodrich-Blair, 2001), antibiotic activity (Akhurst, 1982; Herbert et al., 2007) and dye binding assays (Boemare and Akhurst, 1988) were conducted as described in the literature.
Determination of 16S rDNA sequences
Crude DNA extracts from fresh overnight bacterial cultures were used as templates in ExTaq (Takara, Otsu, Shiga, Japan) polymerase chain reactions (PCR) with the universal primers 27F: 5’-AGAGTTTGATCATGGCTCAG-3’, and 1492R: 5’-TACGGTTACCTTGTTACGACTT-3’ (Lane, 1991). Amplified products were then sequenced at UW-Madison Biotechnology Center using Big Dye version 3.1 (Applied Biosystems, Foster City, CA) with the primers above in addition to Xn850R: 5’-CATTTGAGTTTTAACCTTGCG-3’. Sequence similarity to 16S rRNA sequences in the national database were determined using BLAST (Altschul et al., 1997).
Construction and visualization of fluorescent-protein expressing X. bovienii strains
To generate X. bovienii strains expressing GFP or DsRed, pURR25 (mini-Tn7-KSGFP) or pBK-mini-Tn7(Gm)PA1/04/03-DsRed respectively were conjugated from BW29427, with the helper strain BW29427 (pUX-BF13) (Bao et al., 1991) according to previously described methods (Vivas and Goodrich-Blair, 2001), except the donor and helper strains were eliminated by selection of colonies on media without diaminopimelic acid (80 µg/ml) and X. bovienii exconjugants were selected on pyruvate (0.1%), ampicillin (150 µg/ml), and kanamycin (50 µg/ml). The resulting colonies were confirmed to be X. bovienii based on 16S rRNA sequencing.
Microscopy
Phase-contrast, differential interference contrast, and fluorescence microscopy were performed using a Nikon Eclipse TE300 inverted microscope. Fluorescence microscopy was performed using fluorescein isothiocyanate, tetramethyl rhodamine isocyanate (FITC), tetramethyl rhodamine isothiocyanate (TRITC) and triple-band DAPI (4,6-diamidino-2- phenylindole)-FITC-TRITC filter sets (Chroma, Brattleboro, Vt.; items 31001, 31002, and 82000, respectively). Images were recorded using an ORCA digital camera (model C4742-95-10R; Hamamatsu, Hamamatsu City, Japan) and Metamorph version 4.5r6 software (Universal Imaging Corporation, West Chester, PA). Additional images were obtained using a Zeiss LSM 510 Axioplan II confocal microscope (Zeiss, New York, NY). For cell morphology analysis, bacterial cells were immobilized on 3% agarose pads and viewed using DIC microscopy at 100× magnification. When visualizing X. bovienii (SS-2004) within whole nematodes, nematodes were paralyzed and immobilized with 1% levamisole in agarose as previously described (Martens et al., 2003) using confocal microscopy between 400× and 1000×. For microscopy of progeny nematodes, nematodes were immobilized as mentioned above and viewed using phase contrast microscopy under 40× and 400× magnification. To visualize the receptacle and vesicle (Fig. 2), nematode intestines were extruded by cutting the nematode head with a razor blade, then were maintained in M9 buffer and stained with 0.001% Neutral Red in M9 buffer. The photograph shown in Fig. 2 was taken with Olympus BX51 equipped with digital camera. Extended focal length images were taken and stacked by using Microsuite image analyzing software.
Photography and in vivo imaging
Additional photography for colony morphology and insect cadavers was done using a Canon Powershot camera. Scale bars were inserted using Metamorph version 4.5r6 software (Universal Imaging Corporation, West Chester, PA). Seven days after injection, 1 to 10 insects per experiment were visualized by using an IVIS Imaging System 200 (Xenogen Corp., Alameda, CA). Fluorescence was quantified by using Living Image software v2.6 (Xenogen).
Host interaction assays
Axenic S. jollieti eggs were isolated from adult female nematodes as previously described for S. carpocapsae (Cowles and Goodrich-Blair, 2004) and were stored in 5 ml LB broth supplemented with ampicillin, kanamycin, and chloramphenicol. Axenic eggs were collected and rinsed with sterile LB broth prior to inoculation onto LA plates (in vitro cultivation) or injection into G. mellonella (in vivo cultivation). X. bovienii co-cultivation with S. jollieti nematodes on lipid agar plates was as described for X. nematophila and S. carpocapsae co-cultivations (Martens et al., 2005). For in vivo colonization assays ~700 µl of fresh overnight bacterial cultures was mixed with ~500–1500 axenic nematode eggs and/or J1 juveniles and 12.5 µl of the mixture was injected into the haemocoel of Galleria mellonella larvae (Vanderhorst Wholesale, NJ) with a 30-gauge syringe (Hamilton, Reno, NV). Approximately 15 G. mellonella larvae were injected per bacterial treatment and once injected, the insects were placed in modified White traps as previously described (Kaya and Stock, 1997).
Collected nematodes were assessed by sonication for X. bovienii colonization as previously described for X. nematophila (Heungens et al., 2002) except that S. jollieti were surface sterilized for 3 min. with 0.5% NaOCl. Approximately 102 surface sterilized nematodes were sonicated (Branson Ultrasonics, Danbury, CT) for 60–90 seconds each and the sonicate solution was diluted and plated to enumerate colonies. The number of individual cells that are the founders of the final population of X. bovienii bacteria within a single S. jollieti IJ was calculated from the percentage of nematodes carrying either green or red fluorescence (90.4%) using the mathematical model described by Wollenberg and Ruby (Wollenberg and Ruby, 2009): p(red)x + p(green)x = p(single). In this formula, x is the initiating cell number, p(single) is the probability of observing a nematode with only red or green fluorescence, and p(red) and p(green) are the initial proportions of each bacterium in the input lawn (0.5 each). Based on our observations that 90.4% of S. jollieti nematodes were colonized by either red or green fluorescent bacteria (but not both): 0.5x + 0.5x = 0.94x and x = 1.1.
X. bovienii injection virulence assays in Manduca sexta insect larvae were performed as previously described (Herbert et al., 2007). For feeding toxicity screens against multiple insects, X. bovienii and X. nematophila (ATCC19061) were streaked onto LB agar plates from frozen glycerol stocks and incubated for 16 h at 25°C. Individual colonies were used to inoculate 4 × 2 ml TB media each and were grown on a roller drum incubator for 45 h at 25°C. After pelleting the bacteria, supernatants from each strain were combined (8 ml total) and concentrated in an Amicon 10 MWCO to 1.2 ml (6×, protein fraction). The flow-through was also collected. Both fractions were tested in a feeding assay for toxicity against 20–24 insects. The flow-through did not cause mortality in any insect. Artificial diet feeding assays were conducted as described by (Baum et al., 2007) except that assays were run for 5 days. Feeding assays were conducted with neonate larvae (<24 hr post hatch) of the coleopteran species Diabrotica virgifera virgifera (Western corn rootworm, WCR) and Diabrotica undecimpunctata howardii (Southern corn rootworm, SCR) (both obtained from Crop Characteristics, Inc.; Farmington, MN) using corn rootworm artificial diet and the lepidopteran species: Agrotis ipsilon [Hufnagel] (black cutworm, BCW) and Helicoverpa zea [Boddie] (corn earworm, CEW) (both obtained from Benzon, Inc.; Carlisle, PA) using Multiple Species diet (Southland, Lake Village, AK). Feeding assays with the hemipteran species Lygus hesperus were based on a 96 well micro-titer plate format using a sachet system as previously described (Habibi et al., 2002). The Lygus hesperus (Western tarnished plant bug, WT) artificial diet was obtained from Bio-Serv® (Bio-Serv® Diet F9644B, Frenchtown, NJ) and prepared as previously described (Habibi et al., 2001). Samples were prepared by mixing one hundred microliters of the test sample with one hundred microliters of blended diet (1:1). A sheet of Parafilm® (Pechiney Plastic Packing, Chicago, IL) was placed over a vacuum manifold designed for 96-well format (Analytical Research Systems, Gainesville, FL) and a vacuum of approximately −20 millimeters mercury was applied, sufficient to cause extrusion of the Parafilm® into the wells. Twenty or forty microliters of test sample were added to the Parafilm® wells. A sheet of Mylar film (Clear Lam Packaging, Inc., Elk Grove Village, IL) was placed over the Parafilm® and sealed gently with a tacking iron (Bienfang Sealector II, Hunt Corporation, Philadelphia, PA). The Parafilm® sachets were placed over a flat-bottom 96-well plate containing Lygus eggs suspended in a 0.19% agar solution. Upon hatching, Lygus nymphs were allowed to feed on the sachet diet for 5 days. Stunting and mortality scores were determined on day 5 and compared to the untreated control. Data were analyzed using JMP4 statistical software.
Statistical analysis
Colonization assays were analyzed using a paired, two-tailed Student’s t-test assuming unequal variance. Survival curves of M. sexta injected with different treatments were analyzed using a proportional hazards model and the final survival values were analyzed with a logistic regression model.
Acknowledgements
We wish to thank Dr. Sergei Spiridonov, Dr. Maurice Moens, and Dr. Kurt Heungens for helping us obtain Steinernema jollieti nematodes and Ting-Li Lin from the UW-Madison CALS Statistics Consulting Center for analyzing the virulence data. We would also like to thank Dr. Elizabeth A. Hussa for her assistance in using the in vivo imaging system, and Sam-Kyu Kim for technical help. This work was supported by collaborative grants from the National Science Foundation awarded to H.G.-B. (NSF IBN-0416783; IOS-0920631), S.F. (NSF IOB-0416747; IOS-0919912), and S.P.S. (NSF IOB-0146644; IOS- 0919565), an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund (H.G.B.), and the National Institutes of Health Grant GM59776 (H.G.B.). J.M.C. and K.E.M. were supported by National Institutes of Health (NIH) National Research Service Award T32 AI55397 and J.M.C was also supported by a National Science Foundation (NSF) Graduate Research Fellowship. G.R.R. was supported by a National Institutes of Health National Research Service Award T32 G07215. L.dL. was funded by the NSF Research Experience for Microbiology Project 0552809.
References
- Akhurst RJ. Morphological and functional dimorphism in Xenorhabdus spp. bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–310. [Google Scholar]
- Akhurst RJ. Antibiotic activity of Xenorhabdus spp. bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982;128:3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Boemare NE. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of Xenorhabdus nematophilus to species. J Gen Microbiol. 1988;134:1835–1846. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Boemare N. Biology and Taxonomy of Xenorhabdus. In: Gaugler R, Kaya HK, editors. Entomopathogenic Nematodes in Biological Control. Boca Raton: CRC Press, Inc; 1990. pp. 75–87. [Google Scholar]
- Akhurst RJ, Smigielski AJ, Mari J, Boemare N, Mourant RG. Restriction analysis of phase variation in Xenorhabdus spp. (Enterobacteriaceae), entomopathogenic bacteria associated with nematodes. Syst Appl Microbiol. 1992;15:469–473. [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Lies DP, Fu H, Roberts GP. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of Gram-negative bacteria. Gene. 1991;109:167–168. doi: 10.1016/0378-1119(91)90604-a. [DOI] [PubMed] [Google Scholar]
- Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, et al. Control of coleopteran insect pests through RNA interference. Nat Biotechnol. 2007;25:1322–1326. doi: 10.1038/nbt1359. [DOI] [PubMed] [Google Scholar]
- Bird AF, Akhurst RJ. The nature of the intestinal vesicle in nematodes of the family Steinernematidae. Int J Parasitol. 1983;13:599–606. [Google Scholar]
- Boemare N, Thaler JO, Lanois A. Simple bacteriological tests for phenotypic characterization of Xenorhabdus and Photorhabdus phase variants. Symbiosis. 1997;22:167–175. [Google Scholar]
- Boemare N, Thaler JO, Lanois A. Simple bacteriological tests for phenotypic characterization of Xenorhabdus and Photorhabdus phase variants. Symbiosis. 1997;22:167–175. [Google Scholar]
- Boemare NE, Akhurst RJ. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae) J Gen Microbiol. 1988;134:751–761. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- Campos-Herrera R, Tailliez P, Pages S, Ginibre N, Gutierrez C, Boemare NE. Characterization of Xenorhabdus isolates from La Rioja (Northern Spain) and virulence with and without their symbiotic entomopathogenic nematodes (Nematoda: Steinernematidae) J Invertebr Pathol. 2009;102:173–181. doi: 10.1016/j.jip.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Chapuis E, Emelianoff V, Paulmier V, Le Brun N, Pages S, Sicard M, Ferdy JB. Manifold aspects of specificity in a nematode-bacterium mutualism. J Evol Biol. 2009;22:2104–2117. doi: 10.1111/j.1420-9101.2009.01829.x. [DOI] [PubMed] [Google Scholar]
- Chaston JM, Suen G, Tucker SL, Andersen AW, Bhasin A, Bode E, et al. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: Convergent lifestyles from divergent genomes. PLoS One. doi: 10.1371/journal.pone.0027909. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CE, Goodrich-Blair H. Characterization of a lipoprotein, NilC, required by Xenorhabdus nematophila for mutualism with its nematode host. Mol Microbiol. 2004;54:464–477. doi: 10.1111/j.1365-2958.2004.04271.x. [DOI] [PubMed] [Google Scholar]
- Cowles CE, Goodrich-Blair H. The Xenorhabdus nematophila nilABC genes confer the ability of Xenorhabdus spp. to colonize Steinernema carpocapsae nematodes. J Bacteriol. 2008;190:4121–4128. doi: 10.1128/JB.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles KN, Goodrich-Blair H. Expression and activity of a Xenorhabdus nematophila haemolysin required for full virulence towards Manduca sexta insects. Cell Microbiol. 2005;2:209–219. doi: 10.1111/j.1462-5822.2004.00448.x. [DOI] [PubMed] [Google Scholar]
- Cowles KN, Cowles CE, Richards GR, Martens EC, Goodrich-Blair H. The global regulator Lrp contributes to mutualism, pathogenesis and phenotypic variation in the bacterium Xenorhabdus nematophila. Cell Microbiol. 2007;9:1311–1323. doi: 10.1111/j.1462-5822.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- Daborn PJ, Waterfield N, Silva CP, Au CP, Sharma S, ffrench-Constant RH. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc Natl Acad Sci USA. 2002;99:10742–10747. doi: 10.1073/pnas.102068099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers R-U. Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol. 2001;56:623–633. doi: 10.1007/s002530100711. [DOI] [PubMed] [Google Scholar]
- Emelianoff V, Le Brun N, Pagés S, Stock SP, Tailliez P, Moulia C, Sicard M. Isolation and identification of entomopathogenic nematodes and their symbiotic bacteria from Hérault and Gard (Southern France) J Invertebr Pathol. 2008;99:20–27. doi: 10.1016/j.jip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Fang XL, Li ZZ, Wang YH, Zhang X. In vitro and in vivo antimicrobial activity of Xenorhabdus bovienii YL002 against Phytophthora capsici and Botrytis cinerea. J Appl Microbiol. 2011;111:145–154. doi: 10.1111/j.1365-2672.2011.05033.x. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant R, Waterfield N. An ABC guide to the bacterial toxin complexes. Adv Appl Microbiol. 2006;58:169–183. [PubMed] [Google Scholar]
- Fischer-Le Saux M, Arteaga-Hernández E, Mrácek Z, Boemare N. The bacterial symbiont Xenorhabdus poinarii (Enterbacteriaceae) is harbored by two phylogenetic related host nematodes: the entomopathogenic species Steinernema cubanum and Steinernema glaseri (Nematoda: Steinernematidae) FEMS Microbiol Ecol. 1999;29:149–157. [Google Scholar]
- Fischer-Le Saux M, Mauleon H, Constant P, Brunel B, Boemare N. PCR-ribotyping of Xenorhabdus and Photorhabdus isolates from the Caribbean region in relation to the taxonomy and geographic distribution of their nematode hosts. Appl Environ Microbiol. 1998;64:4246–4254. doi: 10.1128/aem.64.11.4246-4254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Lara Y, Renneckar D, Forst S, Goodrich-Blair H, Stock P. Influence of nematode age and culture conditions on morphological and physiological parameters in the bacterial vesicle of Steinernema carpocapsae (Nematoda: Steinernematidae) J Invertebr Pathol. 2007;95:110–118. doi: 10.1016/j.jip.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Forst S, Clarke D. Bacteria-nematode symbioses. In: Gaugler R, editor. Entomopathogenic Nematology. Wallingford, UK: CABI Publishing; 2002. pp. 57–77. [Google Scholar]
- Fuchs SW, Proschak A, Jaskolla TW, Karas M, Bode HB. Structure elucidation and biosynthesis of lysine-rich cyclic peptides in Xenorhabdus nematophila. Organic & biomolecular chemistry. 2011;9:3130–3132. doi: 10.1039/c1ob05097d. [DOI] [PubMed] [Google Scholar]
- Goetsch M, Owen H, Goldman B, Forst S. Analysis of the PixA inclusion body protein of Xenorhabdus nematophila. J Bacteriol. 2006;188:2706–2710. doi: 10.1128/JB.188.7.2706-2710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Blair H. They've got a ticket to ride: Xenorhabdus nematophila-Steinernema carpocapsae symbiosis. Curr Op Microbiol. 2007;10:225–230. doi: 10.1016/j.mib.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Götz P, Bowman A, Bowman HG. Interactions between insect immunity and an insect-pathogenic nematode with symbiotic bacteria. Proc Royal Soc London. 1981;B 212:333–350. [Google Scholar]
- Habibi J, Backus EA, Coudron TA, Brandt SL. Effect of different host substrates on hemipteran salivary protein profiles. Entomol Exp Appl. 2001;98:369–375. [Google Scholar]
- Habibi J, Brandt SL, Coudron TA, Wagner RM, Wright MK, Backus EA, Huesing JE. Uptake, flow, and digestion of casein and green fluorescent protein in the digestive system of Lygus hesperus Knight. Arch Insect Biochem Physiol. 2002;50:62–74. doi: 10.1002/arch.10029. [DOI] [PubMed] [Google Scholar]
- Hawlena H, Bashey F, Lively CM. The evolution of spite: population structure and bacteriocin-mediated antagonism in two natural populations of xenorhabdus bacteria. Evolution; international journal of organic evolution. 2010;64:3198–3204. doi: 10.1111/j.1558-5646.2010.01070.x. [DOI] [PubMed] [Google Scholar]
- Herbert EE, Goodrich-Blair H. Friend and foe: the two faces of Xenorhabdus nematophila. Nat Rev Microbiol. 2007;5:634–646. doi: 10.1038/nrmicro1706. [DOI] [PubMed] [Google Scholar]
- Herbert EE, Cowles KN, Goodrich-Blair H. CpxRA regulates mutualism and pathogenesis in Xenorhabdus nematophila. Appl Environ Microbiol. 2007;73:7826–7836. doi: 10.1128/AEM.01586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heungens K, Cowles CE, Goodrich-Blair H. Identification of Xenorhabdus nematophila genes required for mutualistic colonization of Steinernema carpocapsae nematodes. Mol Microbiol. 2002;45:1337–1353. doi: 10.1046/j.1365-2958.2002.03100.x. [DOI] [PubMed] [Google Scholar]
- Jing Y, Toubarro D, Hao Y, Simoes N. Cloning, characterisation and heterologous expression of an astacin metalloprotease, Sc-AST, from the entomoparasitic nematode Steinernema carpocapsae. Mol Biochem Parasitol. 2010;174:101–108. doi: 10.1016/j.molbiopara.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. London: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. Oxford: Blackwell; 1991. pp. 115–175. [Google Scholar]
- Leclerc MC, Boemare NE. Plasmids and phase variation in Xenorhabdus spp. Appl Environ Microbiol. 1991;57:2597–2601. doi: 10.1128/aem.57.9.2597-2601.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Stock SP. A multilocus approach to assessing co-evolutionary relationships between Steinernema spp. (Nematoda: Steinernematidae) and their bacterial symbionts Xenorhabdus spp. (gamma-Proteobacteria: Enterobacteriaceae) Syst Parasitol. 2010a;77:1–12. doi: 10.1007/s11230-010-9256-9. [DOI] [PubMed] [Google Scholar]
- Lee MM, Stock SP. A multigene approach for assessing evolutionary relationships of Xenorhabdus spp. (gamma-Proteobacteria), the bacterial symbionts of entomopathogenic Steinernema nematodes. J Invertebr Pathol. 2010b;104:67–74. doi: 10.1016/j.jip.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:W327–W331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, et al. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009;37:D205–D210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Goodrich-Blair H. The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell Microbiol. 2005;7:1723–1735. doi: 10.1111/j.1462-5822.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Martens EC, Heungens K, Goodrich-Blair H. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J Bacteriol. 2003;185:3147–3154. doi: 10.1128/JB.185.10.3147-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Russell FM, Goodrich-Blair H. Analysis of Xenorhabdus nematophila metabolic mutants yields insight into stages of Steinernema carpocapsae nematode intestinal colonization. Mol Microbiol. 2005;51:28–45. doi: 10.1111/j.1365-2958.2005.04742.x. [DOI] [PubMed] [Google Scholar]
- Martens EC, Vivas EI, Heungens K, Cowles CE, Goodrich-Blair H. Investigating mutualism between entomopathogenic bacteria and nematodes. Nematol Monographs Persp. 2004;2:447–462. [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, N. Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- Mitani DK, Kaya HK, Goodrich-Blair H. Comparative study of the entomopathogenic nematode, Steinernema carpocapsae, reared on mutant and wild-type Xenorhabdus nematophila. Biol Control. 2004;29:382–391. [Google Scholar]
- Morales-Soto N, Forst SA. The xnp1 P2-like tail synthesis gene cluster encodes xenorhabdicin and is required for interspecies competition. J Bacteriol. 2011;193:3624–3632. doi: 10.1128/JB.00092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moureaux N, Karjalainen T, Givaudan A, Bourlioux P, Boemare N. Biochemical characterization and agglutinating properties of Xenorhabdus nematophilus F1 fimbriae. Appl Environ Microbiol. 1995;61:2707–2712. doi: 10.1128/aem.61.7.2707-2712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrácek Z, Nguyen KB, Tailliez P, Boemare N, Chen S. Steinernema sichuanense n. sp. (Rhabditida, Steinernematidae), a new species of entomopathogenic nematode from the province of Sichuan, east Tibetan Mts., China. J Invertebr Pathol. 2006;93:157–169. doi: 10.1016/j.jip.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Orchard SS, Goodrich-Blair H. Identification and functional characterization of a Xenorhabdus nematophila oligopeptide permease. Appl Environ Microbiol. 2004;70:5621–5627. doi: 10.1128/AEM.70.9.5621-5627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Ciezki K, van der Hoeven R, Singh S, Reimer D, Bode HB, Forst S. Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol Microbiol. 2009;73:938–949. doi: 10.1111/j.1365-2958.2009.06817.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Herbert EE, Cowles CE, Cowles KN, Menard ML, Orchard SS, Goodrich-Blair H. Clonal variation in Xenorhabdus nematophila virulence and suppression of Manduca sexta immunity. Cell Microbiol. 2007;9:645–656. doi: 10.1111/j.1462-5822.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- Pechy-Tarr M, Bruck DJ, Maurhofer M, Fischer E, Vogne C, Henkels MD, et al. Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environ Microbiol. 2008;10:2368–2386. doi: 10.1111/j.1462-2920.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Pinyon RA, Hew FH, Thomas CJ. Xenorhabdus bovienii T228 phase variation and virulence are independent of RecA function. Microbiol. 2000;146(Pt 11):2815–2824. doi: 10.1099/00221287-146-11-2815. [DOI] [PubMed] [Google Scholar]
- Poinar GO. The presence of Achromobacter nematophilus in the infective stage of a Neoaplectana sp. (Steinernematidae: Nematoda) Nematologica. 1966;12:105–108. [Google Scholar]
- Poinar GOJ, Thomas GM. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae) Parasitol. 1966;56:385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- Popiel I, Grove DL, Friedman MJ. Infective juvenile formation in the insect parasitic nematode Steinernema feltiae. Parasitol. 1989;99:77–81. [Google Scholar]
- Richards GR, Goodrich-Blair H. Masters of conquest and pillage: Xenorhabdus nematophila global regulators control transitions from virulence to nutrient acquisition. Cell Microbiol. 2009;11:1025–1033. doi: 10.1111/j.1462-5822.2009.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards GR, Goodrich-Blair H. Examination of Xenorhabdus nematophila lipases in pathogenic and mutualistic host interactions reveals a role for xlpA in nematode progeny production. Appl Environ Microbiol. 2010;76:221–229. doi: 10.1128/AEM.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe GE, Welch RA. Assays of hemolytic toxins. Methods Enzymol. 1994;235:657–667. doi: 10.1016/0076-6879(94)35179-1. [DOI] [PubMed] [Google Scholar]
- San-Blas E, Gowen SR, Pembroke B. Steinernema feltiae: ammonia triggers the emergence of their infective juveniles. Exp Parasitol. 2008;119:180–185. doi: 10.1016/j.exppara.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Sergeant M, Jarrett P, Ousley M, Morgan JA. Interactions of insecticidal toxin gene products from Xenorhabdus nematophilus PMFI296. Appl Environ Microbiol. 2003;69:3344–3349. doi: 10.1128/AEM.69.6.3344-3349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard M, Le Brun N, Pages S, Godelle B, Boemare N, Moulia C. Effect of native Xenorhabdus on the fitness of their Steinernema hosts: contrasting types of interactions. Parasitol Res. 2003;91:520–524. doi: 10.1007/s00436-003-0998-z. [DOI] [PubMed] [Google Scholar]
- Sierra G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie van Leeuwenhoek. 1957;23:15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnol. 1983;1:784–791. [Google Scholar]
- Smits WK, Kuipers OP, Veening JW. Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol. 2006;4:259–271. doi: 10.1038/nrmicro1381. [DOI] [PubMed] [Google Scholar]
- Snyder HA, Stock SP, Kim SK, Flores-Lara Y, Forst S. New insights into the colonization and release process of Xenorhabdus nematophila and the morphology and ultrastructure of the bacterial receptacle of its nematode host, Steinernema carpocapsae. Appl Environ Microbiol. 2007;73:5338–5346. doi: 10.1128/AEM.02947-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiridonov SE, Akhmedov EN, Belostotskaya FN. Proliferation of symbiotic bacteria in the intestinal vesicles of invasive larvae of Neoaplectana spp. (Nematoda, Steinernematidae) Helminthologia. 1991;28:141–142. [Google Scholar]
- Spiridonov SE, Krasomil-Osterfeld K, Moens M. Steinernema jollieti sp. n. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from the American Midwest. Russ J Nematol. 2004;12:85–95. [Google Scholar]
- Tailliez P, Laroui C, Ginibre N, Paule A, Pages S, Boemare N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int J Syst Evol Microbiol. 2010;60:1921–1937. doi: 10.1099/ijs.0.014308-0. [DOI] [PubMed] [Google Scholar]
- Thaler JO, Duvic B, Givaudan A, Boemare N. Isolation and entomotoxic properties of the Xenorhabdus namatophilus F1 Lecithinase. Appl Environ Microbiol. 1998;64:2367–2373. doi: 10.1128/aem.64.7.2367-2373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubarro D, Lucena-Robles M, Nascimento G, Santos R, Montiel R, Verissimo P, et al. Serine protease-mediated host invasion by the parasitic nematode Steinernema carpocapsae. J Biol Chem. 2010;285:30666–30675. doi: 10.1074/jbc.M110.129346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneux F, Zumbihl R, Jubelin G, Ribeiro C, Poncet J, Baghdiguian S, et al. The xaxAB genes encoding a new apoptotic toxin from the insect pathogen Xenorhabdus nematophila are present in plant and human pathogens. J Biol Chem. 2007;282:9571–9580. doi: 10.1074/jbc.M604301200. [DOI] [PubMed] [Google Scholar]
- Vivas EI, Goodrich-Blair H. Xenorhabdus nematophilus as a model for host-bacterium interactions: rpoS is necessary for mutualism with nematodes. J Bacteriol. 2001;183:4687–4693. doi: 10.1128/JB.183.16.4687-4693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi A, Fodor A, Szentirmai A, Forst S. Phase variation in Xenorhabdus nematophilus. Appl Environ Microbiol. 1998;64:1188–1193. doi: 10.1128/aem.64.4.1188-1193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield N, Dabord PJ, Dowling AJ, Yang G, Hares M, ffrench-Constant RH. The insecticidal toxin makes caterpillars floppy 2 (Mcf2) shows similarity to HrmA, an avirulence protein from a plant pathogen. FEMS Microbiol Lett. 2003;229:265–270. doi: 10.1016/S0378-1097(03)00846-2. [DOI] [PubMed] [Google Scholar]
- Wollenberg MS, Ruby EG. Population structure of Vibrio fischeri within the light organs of Euprymna scolopes squid from two Oahu (Hawaii) populations. Appl Environ Microbiol. 2009;75:193–202. doi: 10.1128/AEM.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hurlbert RE. Toxicity of irradiated media for Xenorhabdus spp. Appl Environ Microbiol. 1990;56:815–818. doi: 10.1128/aem.56.3.815-818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Kaya HK, Heungens K, Goodrich-Blair H. Response of ants to a deterrent factor(s) produced by the symbiotic bacteria of entomopathogenic nematodes. Appl Environ Microbiol. 2002;68:6202–6209. doi: 10.1128/AEM.68.12.6202-6209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]